Abstract
Objective
The purpose of this study was to investigate if the levonorgestrel-impregnated intrauterine device (LNG-IUS, Mirena®) is safe and effective as therapy for low-risk and medium-risk endometrial hyperplasia compared with oral medroxyprogesterone (MPA).
Design
A multicentre randomised trial.
Setting
Norway.
Population
In all, 170 women aged 30–70 years with low- or medium-risk endometrial hyperplasia who met inclusion criteria.
Methods
Patients were randomly assigned to one of three treatment arms: LNG-IUS; oral MPA 10 mg administered for 10 days per cycle, or continuous oral MPA 10 mg daily, for 6 months.
Main outcome measures
The primary outcome measure was normalisation or persisting hyperplasia.
Results
After 6 months all three treatment regimens showed significant effect when the outcome was evaluated as therapy response or not (P < 0.001). Responses were obtained for all the women in the LNG-IUS group (53/53, 95% CI 0.93–1.0) and for 96% of the women in the continuous oral group (46/48, 95% CI 0.86–0.99). Only 69% of the women in the cyclic oral group were responders (36/52, 95% CI 0.55–0.81). Adverse effects were relatively common with minimal differences between therapy groups.
Conclusion
In the first trial of its kind, women treated with the LNG-IUS showed histologically normal endometrium after 6 months of therapy for endometrial hyperplasia. Cyclical progestogens are found to be less effective compared with continuous oral therapy and LNG-IUS and should not be used for this purpose.
Keywords: Endometrial hyperplasia, levonorgestrel-impregnated intrauterine device versus oral progestin, therapy
Introduction
Endometrial cancer is considered the most frequent gynaecological malignancy in the industrialised world and the incidence is still rising.1 In Norway a 50% increase in occurrence has been observed over the last 10 years.2 Because endometrial hyperplasia represents precursor lesions to endometrial cancer, it seems likely that adequate therapy of preliminary stages would contribute to reduce the rapid increase in endometrial cancer.3 While hysterectomy has been preferred as treatment for complex atypical hyperplasia, oral use of progestogens (norethisterone, megestrol and medroxyprogesterone [MPA]) has become routine therapy for endometrial hyperplasia not selected for surgery. However, former studies have shown too much variation in dose, treatment time, progestational agent and mode of distribution to be comparable and provide a basis for consensus for therapy.1,4–15. Hence, no professional therapy guidelines exist.
The levonorgestrel-impregnated intrauterine device (LNG-IUS) (Mirena®, Bayer HealthCare, Berlin, Germany) releasing 20 μg levonorgestrel per 24 hours was developed for the beneficial effects of intrauterine and hormonal contraception and for the treatment of menorrhagia.16,17 The endometrial progestin concentration observed in LNG-IUS-treated women has been found to be 100-fold higher compared with concentration in the endometrial mucosa after oral therapy.18 Over the past 30 years, although not included in the official list of indications, the LNG-IUS has been introduced as an alternative therapy for endometrial hyperplasia and cohort studies as well as case series have shown promising results.19–26 Unfortunately, these previous studies have been mostly observational studies that do not meet the necessary requirements to create novel treatment recommendations. Thus, no former studies have shown that the LNG-IUS is safe as therapy for endometrial hyperplasia. The main goal of our randomised controlled trial was to find out whether the LNG-IUS was more effective as therapy for endometrial hyperplasia compared with oral progestin treatment after 6 months of treatment. The lowest chosen cyclic oral regimen (10 mg MPA given for 10 days per cycle for 3–6 months) has been practiced in Norway as routine therapy for low- and medium-risk endometrial hyperplasia. In a former study we showed that only half of the women had a normal endometrium after 3 months on this regimen.23 In the present study our purpose was to investigate if a 6-month treatment period might improve the treatment result. As far as we are aware, this is the first randomised multicentre study comparing LNG-IUS and oral progestin as treatments for endometrial hyperplasia.
Methods
Trial design
This is a randomised, multicentre study with three parallel equally sized arms investigating the effect of progestogen on endometrial hyperplasia. We designed a study to include 200 women and enrolled 170 women who were randomly assigned to one of three treatment arms: LNG-IUS (20 μg levonorgestrel per 24 hours, Mirena®); oral MPA (10 mg administered for 10 days per cycle), or continuous oral MPA 10 mg daily. The treatment time was 6 months for each of the three regimens. The study was designed according to the CONSORT statement.27 No changes in design took place after trial commencement.
Participants
Women between 30 and 70 years of age, with histologically confirmed endometrial hyperplasia according to WHO94 classification and D-score (see Additional methods) were eligible; excluded were women with hypersensitivity to progestin, active genital infection, a history of genital or mammary cancer, undiagnosed vaginal bleeding, liver disease, serious thrombophlebitis, or pregnancy.
Study setting
The study inclusion period was from 1 January 2005–1 November 2011. During the study all participating women were investigated and treated by their own gynaecologist in 17 different gynaecological centres in Norway. Potential candidates for the study had been consulting their gynaecologist on their own initiative due to clinical symptoms (mostly irregular vaginal bleeding). Due to history and/or ultrasound-related signs of endometrial hyperplasia a biopsy (pipelle biopsy) was taken and routinely sent to the Department of Clinical Pathology, University Hospital of North Norway for histological investigation and D-score assessment. Only women with histologically confirmed endometrial hyperplasia according to a modified WHO94 classification1,3,28 and D-score >0 (low- and medium-risk hyperplasia) were potential candidates for the study. After the result of the index biopsy and D-score had been communicated back to the woman's gynaecologist, the woman met with a second consultation to discuss possible therapy and, if eligible, to be given a study invitation including detailed information regarding the study, as well as an informed consent form. If the woman decided to join the study, the form was signed. As long as the woman was enrolled in the study her gynaecologist performed each clinical consultation. All endometrial biopsies were sent for histological evaluation at the Department of Clinical Pathology, University Hospital of North Norway.
Enrolment and allocation
Only women with confirmed endometrial hyperplasia fulfilling the inclusion criteria were eligible, after having signed the informed consent during the second consultation. Randomisation was arranged by telephone contact between the woman's gynaecologist and the randomisation unit at the Clinical Research Centre, University Hospital of North Norway. A randomisation form was submitted along with a copy of the signed written informed consent to the Clinical Research Centre. For allocation of the participants, a computer-generated list of random numbers was used. The IT manager at the Clinical Research Centre, University Hospital of North Norway, made a computer random number generator with two strata and fixed block size. The people involved in the randomisation procedure were unaware of the block size used. To secure concealed allocation, central telephone randomisation at the Clinical Research Centre, University Hospital of North Norway was used.
Interventions
The women included were randomly assigned to one of three potential treatment arms: LNG-IUS, oral MPA 10 mg administered for 10 days per cycle, and continuous oral MPA 10 mg daily, treatment time being 6 months. Progestogen tablets and LNG-IUS were funded by the coordinator of the study (AØ) and were given free to the women for the entire treatment period. At inclusion, blood samples for investigation of estradiol, progesterone, follicle-stimulating hormone (FSH) and haemoglobin were routinely taken. In the present study we finally chose to define menopausal status according to serum levels of estradiol and FSH as follows: women with normal estradiol and normal FSH were defined as not menopausal, women with increased FSH but estradiol within normal limits were defined as perimenopausal, and women with low estradiol and high FSH level were defined as postmenopausal. Before the start of the study the women were also informed that all therapy was stopped after 6 months.
Additional interventions
A third and fourth clinical consultation including endometrial biopsy was undertaken by the gynaecologist after 3 and 6 months, respectively, the last at cessation of therapy. Occurrence and degree of adverse effects comprising irregular vaginal bleeding, nausea and headache were reported after 3 and 6 months. Ultrasound-estimated endometrial thickness was registered at each consultation. The gynaecologist at each consultation completed a separate form reporting this information during the study and copies were sent to the Clinical Research Centre, University Hospital of North Norway for electronic reading. The endometrial biopsies taken at each consultation (including index biopsy and biopsies taken after 3 and 6 months) were immediately soaked in a separate 10-ml glass with 10% formaldehyde (glasses labelled with woman's name and date of birth). All biopsies were sent for investigation by light microscopy based on modified WHO941,28 classification followed by the D-score assessment at the Department of Clinical Pathology, University Hospital of North Norway.
Outcome measures
The primary efficacy outcome after 6 months of therapy was endometrial hyperplasia or not, assessed by light microscopy. Regular proliferative endometrium or exaggerated progestogen effect with atrophic glands and pseudodecidualised stroma was considered as a therapy effect. Secondary outcomes included reported adverse effects experienced during therapy with focus on nausea (only sometimes and trivial versus often and annoying), pain (only sometimes and trivial versus often and annoying), vaginal bleeding (more or <10 days per month). No changes to outcomes were made after the trial commenced.
Blinding procedures
All clinical information (study form copies) from the study was sent by the gynaecologists to the Clinical Research Centre, University Hospital of North Norway to be stored there blinded to the main investigators (AØ, ABV, MA, IP and BS). This information was not given to the main investigator before the study was closed. Histological slides obtained during therapy were kept in the treatment database in the Department of Clinical Pathology, University Hospital North Norway. On investigation of the endometrial biopsies the pathologists and the engineers were always blinded to which treatment group the woman belonged. Treatment effect, i.e. presence of hyperplasia or not, was obtained after consensus between two different pathologists (AØ, who is a gynaecological pathologist, and one routine pathologist).
Statistical methods
The sample size estimation was based on a pilot study reporting that 50% of women given oral progestin were responders whereas all women treated by LNG-IUS were cured.23 The intended study population in the present study was estimated to 200 with an α-value of 0.05 and a β-value of 0.20, according to a difference in effect of 20% and a drop out frequency of 10%. For analyses of primary outcome of the present study the histological material of the endometrial specimens was analysed according the principle of intention to treat.
Main hypotheses were answered by comparing number of women with regressed hyperplasia in each of the three treatment groups at the end of therapy using simple univariate statistics.
Additional methods
Light microscopy
The histological material obtained from endometrial biopsies was sent to the Department of Pathology, University Hospital of North Norway for routine assessment by the modified WHO94 classification, which is still considered the reference standard for evaluation of endometrial hyperplasia.1,3,28 All endometrial biopsies showing hyperplasia were divided into one of three groups, simple hyperplasia, complex hyperplasia or atypical complex hyperplasia according to the modified WHO94 classification.1,3,28
Morphometric analysis D-score
As reproducibility of the WHO classification is considered rather poor we have introduced the morphometric image analyses algorithm D-score to improve the selection of risk groups. The D-score method represents an objective and highly reproducible procedure that has been implemented as routine analysis in our health region.29–32 Hence all women with D-score >0 are considered to have medium- and low-risk hyperplasia (recommendation is progestin therapy and follow up), and women with D-score <0 are considered at high risk of co-existent or future carcinoma (recommendation is hysterectomy).29–32 After investigation by light microscopy by pathologists, the D-score analyses were performed by trained engineers (IP, KH, KL). In the original computerised morphometric analysis study on endometrial hyperplasia, a total of ten nuclear features and 12 architectural features were analysed. Using a linear stepwise regression analysis and discriminant analysis, three of these quantitative features were selected as having significant independent prognostic value and were combined into the formula called D-score, as follows: D-score = 0.6229 + (0.0439 × [volume percentage stroma]) − (3.9934 ×Ln [standard deviation shortest nuclear axis]) − (0.1592 × [outer surface density glands]), Where Ln stands for natural logarithm. The measurements were performed with a Q-PRODIT image analysis system (version 6.1; Leica, Cambridge, UK). The method describing the performance of the D-score method is described in detail in previous studies.29,31,33 D-score measurements for hyperplasia have been implemented as routine investigations in our health region and include routines for follow up. The group with D-score <0 are recommended hysterectomy according to the established routine in our health region. Hence, only the women with D-score 0–1 or D-score >1 were eligible for the study.
Results
Between January 2005 and November 2011, 170 women with endometrial hyperplasia were enrolled and randomly assigned to one of the three treatment regimens; the last included woman completed therapy in May 2012. Due to the slow inclusion rate, the study was closed when the lowest desired number of included women was attained (November 2011). After randomisation, 17 withdrawals were reported from the total group, five in the cyclic oral group, nine in the continuous oral group, and three in the LNG-IUS group, respectively. The number of withdrawals was most frequent in the continuous oral group. The main reasons for withdrawal from the study were reported to be irregular bleeding, two in cyclic oral group, three in the continuous oral group, and one in the LNG-IUS group. One woman in the cyclic oral group dropped out due to depression. Withdrawal without reporting any specific reason occurred in all three groups, two in the cyclic oral, six in the continuous oral, and two in the LNG-IUS group (Figure 1). A simple sensitivity analysis was performed to ensure that withdrawals from the study were not influencing the main conclusions of the study (data not shown).
Figure 1.
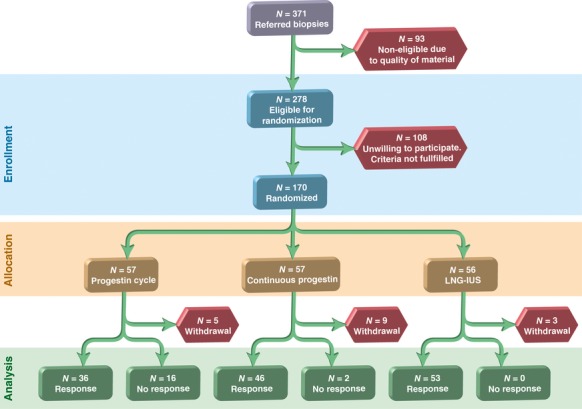
Flow diagram for the study.
Clinical and laboratory data
The three therapy groups seemed well balanced according to demographic data including age, parity, body mass index (BMI), menopausal status, hormones and haemoglobin (Table 1). The final results revealed that the majority of women with endometrial hyperplasia were between 45 and 51 years of age when included in the study (Table 1). Therapy response seemed independent of age group. More than 50% of the women included were overweight when BMI was considered with no difference between treatment groups (Table 1); however, BMI showed no influence on therapy response or not. The majority of women in all three therapy groups had two or more children (Table 1), but parity did not correlate with therapy response. Menopausal status was not correlated to therapy response but a majority of women belonged to the ‘not menopausal group’. Serum levels of estradiol, FSH (reported according to menopausal limits in our laboratory) and haemoglobin were evenly distributed among groups and without correlation to therapy response.
Table 1.
Baseline demographic data of the women in each treatment group at the time of inclusion and randomisation
| Therapy/demographic data | Cyclic oral MPA, n (%) | Cont. oral MPA, n (%) | LNG-IUS, n (%) |
|---|---|---|---|
| Age levels, years (n = 153) | |||
| <44 | 15 (37.5) | 11 (27.5) | 14 (35.0) |
| 45–48 | 12 (34.2) | 9 (25.7) | 14 (40.0) |
| 49–51 | 15 (37.5) | 15 (37.5) | 10 (37.5) |
| ≥52 | 10 (26.3) | 13 (34.2) | 15 (39.5) |
| BMI levels, kg/m2 (n = 151) | |||
| <20 | 2 (40.0) | 2 (40.0) | 1 (20.0) |
| 20–24 | 16 (26.3) | 22 (36.1) | 23 (37.7) |
| 25–29 | 12 (28.6) | 14 (33.3) | 16 (38.1) |
| ≥30 | 21 (48.8) | 10 (23.3) | 12 (27.9) |
| Parity* (n = 152) | |||
| 0 | 8 (36.4) | 6 (27.3) | 8 (36.4) |
| 1 | 8 (40.0) | 5 (25.0) | 7 (35.0) |
| 2 | 20 (35.1) | 21 (36.8) | 16 (28.1) |
| ≥3 | 16 (30.2) | 15 (28.3) | 22 (41.5) |
| Menopausal status (n = 146) | |||
| Not menopausal | 34 (35.4) | 28 (29.2) | 34 (35.4) |
| Perimenopausal | 11 (27.5) | 14 (35.0) | 15 (37.5) |
| Postmenopausal | 4 (40.0) | 2 (20.0) | 4 (40.0) |
| Estradiol, mIU/ml (n = 153) | |||
| <0.2 | 20 (32.8) | 19 (31.1) | 22 (36.1) |
| ≥0.2 | 32 (34.8) | 29 (31.5) | 31 (33.7) |
| (Mean) | (0.56) | (0.45) | (0.45) |
| FSH, mIU/ml (n = 153) | |||
| <26 | 36 (36.0) | 29 (29.0) | 35 (35.0) |
| ≥26 | 16 (30.2) | 19 (36.8) | 18 (33.9) |
| (Mean) | (21.3) | (21.5) | (12.3) |
| Haemoglobin, g/l (n = 153) | |||
| <11.5 | 4 (40.0) | 4 (40.0) | 2 (20.0) |
| ≥11.5 | 48 (33.6) | 44 (30.7) | 51 (36.7) |
| (Mean) | (13.1) | (13.3) | (13.4) |
Menopausal status is defined according to levels of estradiol and FSH explained in the text, see Methods.
Number of live children born.
Primary objective—effect of therapy
Each of the 153 women completed 6 months of therapy in one of the three different therapy groups. After 6 months the effect of all three treatment regimens was highly significant when the outcome was classified as therapy response or not (Figure 1). Response was obtained for all women in the LNG-IUS group (n = 53) and for 96% of women in the continuous oral group (n = 48). Only 69% of the women in the cyclic oral group were responders (n = 36). Hence, the LNG-IUS and continuous oral therapy were shown to be significantly superior to the cyclic oral regimen (P = 0.01). Therapy responses according to the modified WHO classification are shown in Table 2.1,28
Table 2.
Fraction of regression of hyperplasia and the confidence intervals (95% CI) in each category of endometrial hyperplasia in the three therapy groups
| Intervention | SH Fraction of regress. (95% CI) | CH Fraction of regress. (95% CI) | ACH Fraction of regress. (95% CI) |
|---|---|---|---|
| LNG-IUS | 6/6 = 1.0 (0.54–1.0) | 41/41 = 1.0 (0.91–1.0) | 6/6 = 1.0 (0.54–1.0) |
| Oral continuous | 6/6 = 1.0 (0.54–1.0) | 33/34 = 0.97 (0.84–1.0) | 7/8 = 0.88 (0.47–1.0) |
| Oral cyclic | 7/11 = 0.64 (0.31–0.89) | 26/36 = 0.72 (0.55–0.86) | 3/5 = 0.6 (0.14–0.95) |
| Total | 19/30 = 0.64 (0.44–0.80) | 100/111 = 0.90 (0.83–0.95) | 16/19 = 0.84 (0.60–0.97) |
Secondary objectives—adverse effects
The main registered adverse effects occurring during the 6 months of therapy, irregular vaginal bleeding, abdominal pain and nausea, were actively asked about by the gynaecologist and enrolled in the study at each consultation. Only 21 women reported no adverse effects at all, nine in the cyclic oral group, nine in the continuous oral group and three in the LNG-IUS group. Hence, the majority of the participating women reported some adverse effect during therapy. Irregular bleeding was significantly more frequent in the LNG-IUS group compared with the groups receiving oral therapy (Table 3). The quoted frequency of pain was evenly distributed among groups (Table 4). Although not significant, more women in the cyclic oral group reported nausea compared with the two other groups (Table 5). When adverse effects were weighted against therapy response or not, no difference was observed for haemorrhage (Table 3) and nausea (Table 5). As far as pain was concerned, Table 4 shows that pain was significantly more frequent in the response group compared with the group with persisting hyperplasia. A number of symptoms were not actively asked about by the gynaecologist but were occasionally reported by the woman such as weight gain, altered appetite, altered libido and sleep disturbances (data not shown).
Table 3.
The incidence and frequency of irregular vaginal bleedings were registered during the treatment period in all three therapy groups and related to therapy response
| No adverse effects, n (%) | Grade 1, n (%) | Grade 2, n (%) | Total, n (%) | |
|---|---|---|---|---|
| Therapy/irregular bleeding* | ||||
| Cyclic MPA | 16 (48.5) | 25 (37.3) | 11 (20.75) | 52 |
| Continuous MPA | 11 (33.3) | 19 (28.4) | 18 (33.9) | 48 |
| LNG-IUS | 6 (18.2) | 23 (34.3) | 24 (45.3) | 53 |
| Total | 33 (100.0) | 67 (100.0) | 53 (100.0) | 153 (100.0) |
| Therapy response/irregular bleeding** | ||||
| Normal | 27 (81.8) | 58 (86.6) | 50 (94.3) | 135 |
| Persisting hyperplasia | 6 (18.2) | 9 (13.4) | 3 (5.7) | 18 |
| Total | 33 (100.0) | 67 (100.0) | 53 (100.0) | 153 (100.0) |
Vaginal bleeding grade 1 and 2 correspond to observed bleedings lasting more or <10 days per month. Pearson chi-square = 9.65.
Vaginal irregular bleeding grade 1 and 2 correspond to observed bleedings lasting more or <10 days per month. Pearson chi-square = 3.39.
Table 4.
Incidence and grade of pain during therapy in the treatment groups and related to therapy response
| No adverse effects, n (%) | Grade 1, n (%) | Grade 2, n (%) | Total, n (%) | |
|---|---|---|---|---|
| Therapy/pain* | ||||
| Cyclic MPA | 24 (28.6) | 21 (36.2) | 7 (63.6) | 52 (100.0) |
| Continuous MPA | 28 (33.3) | 19 (32.8) | 1 (9.1) | 48 (100.0) |
| LNG-IUS | 32 (38.1) | 18 (31.0) | 3 (27.3) | 53 (100.0) |
| Total | 84 (100.0) | 58 (100.0) | 11 (100.0) | 153 (100.0) |
| Therapy response/pain** | ||||
| Normal | 75 (89.3) | 53 (91.4) | 7 (63.6) | 135 (88.2) |
| Persisting hyperplasia | 9 (10.7) | 5 (8.6) | 4 (36.4) | 18 (11.8) |
| Total | 126 (100.0) | 21 (100.0) | 11 (100.0) | 153 (100.0) |
Pain grade 1 corresponds to only sometimes and trivial. Pain grade 2 corresponds to frequent and/or annoying. Pearson chi-square = 6.61.
Pain grade 1 corresponds to only sometimes and trivial. Pain grade 2 corresponds to frequent and/or annoying. Pearson chi-square = 7.05.
Table 5.
Incidence and grade of nausea in the three treatment groups during therapy and related to therapy response
| No adverse effects, n (%) | Grade 1, n (%) | Grade 2, n (%) | Total, n (%) | |
|---|---|---|---|---|
| Therapy/sickness* | ||||
| Cyclic MPA | 26 (26.8) | 21 (43.7) | 5 (62.5) | 52 (33.9) |
| Continuous MPA | 33 (34.0) | 12 (25.0) | 3 (37.5) | 48 (31.4) |
| LNG-IUS | 38 (39.2) | 15 (31.3) | 0 (0.00) | 53 (34.6) |
| Total | 97 (100.0) | 48 (100.0) | 8 (100.0) | 153 (100.0) |
| Therapy response/sickness** | ||||
| Normal | 89 (91.8) | 40 (83.3) | 6 (75.0) | 135 (88.2) |
| Persisting hyperplasia | 8 (8.3) | 8 (16.7) | 2 (25.0) | 18 (11.8) |
| Total | 97 (100.0) | 48 (100.0) | 8 (100.0) | 153 (100.0) |
Grade 1 and 2 correspond to more or <10 days per month. Pearson chi-square 9.17.
Grade 1 and 2 correspond to more or <10 days per month. Pearson chi-square 3.62.
Therapy response related to the modified WHO classification and D-score
In Table 6 different classification systems for endometrial hyperplasia are compared for the three treatment groups before therapy. Most cases with simple and complex hyperplasia correspond to the D-score group >1.
Table 6.
The table shows modified WHO94 diagnoses and categories of D-score for the therapy groups before treatment
| D- score versus WHO | Cyclic MPA, n = 52 | Continuous MPA, n = 48 | LNG-IUS, n = 53 | Total | |||
|---|---|---|---|---|---|---|---|
| D-score 0–1 | D-score >1 | D-score 0–1 | D-score >1 | D-score 0–1 | D-score >1 | ||
| SH | 0 | 11 | 0 | 6 | 0 | 6 | 23 |
| CH | 6 | 30 | 3 | 31 | 1 | 40 | 111 |
| ACH | 5 | 0 | 7 | 1 | 6 | 0 | 19 |
| Total | 11 | 41 | 10 | 38 | 7 | 46 | 153 |
Of the 23 women with simple hyperplasia and D-score >1 before therapy, four of 11 in the cyclic group were nonresponders. All women in the continuous oral group and all in the LNG-IUS group responded. No women with simple hyperplasia had D-score 0–1. Among women with complex hyperplasia and D-score >1, all women responded to therapy in the continuous oral (n = 31) and in the LNG-IUS (n = 40) groups, respectively. In the cyclic oral group nine of 30 women with complex hyperplasia and D-score >1 were non-responders. For women with complex hyperplasia and D-score 0–1, one of six lacked a response in the cyclic oral group and one of three in the continuous oral group. Only one woman with atypical hyperplasia had D-score >1. This woman had continuous therapy and responded. For women with atypical hyperplasia and D-score 0–1, two of five in the oral group and one of seven in the continuous group were non-responders. All six women with atypical hyperplasia who had LNG-IUS responded.
Discussion
Main findings
Our study is the first randomised multicentre trial to show that the LNG-IUS is safe and efficient as therapy for simple, complex and atypical hyperplasia. Although continuous oral therapy was nearly as effective as intrauterine treatment, the cyclic oral dose was significantly less efficient compared with LNG-IUS and the continuous regimen. Adverse effects were common and reported in all three therapy groups independent of treatment regimen; however, vaginal bleeding was found to be more annoying for LNG-IUS users compared with those on oral therapy. Hence, adverse effects could hardly be decisive for choice of therapy in the treatment of endometrial hyperplasia.
Strengths and limitations
A main strength of the study is the design, which represents the first investigation to meet the mandatory criteria of a multicentre RCT comparing LNG-IUS with oral progestin regimens. The study is also performed according to the standards of the CONSORT criteria. The three treatment groups were equally sized and well balanced and the investigated variables were evenly distributed among the participants. Blinding of treatment to the investigators was performed during the study. It was also consistently accomplished during the randomisation procedure. When cases with endometrial hyperplasia were evaluated for eligibility for the study, the quality of endometrial biopsies was carefully considered. Therefore, specimens with fragmentation, those lacking preserved tissue architecture, and specimens with scant material were not included. High quality was also ensured when the two same trained pathologists, blinded to treatment group, independently investigated all endometrial samples in the study in the same laboratory.
The WHO classification system for endometrial hyperplasia, still regarded and used as a gold standard, was used as a primary criterion for tissue selection. As the WHO classification at present is heavily debated because of low reproducibility and lack of objectivity in diagnostics,34 the D-score was used as an additional procedure to compensate for this limitation. For all the women included, a D-score was performed after WHO classification to ensure objective stratification into low- and medium-risk categories. In previous studies the morphometric prognostic index for endometrial hyperplasia has proved capable of predicting more accurately the outcome of endometrial hyperplasia and has given reliable recommendations for treatment.30,31,33,35–40 Hence, the present results showed that women with simple, complex and atypical hyperplasia could safely choose the LNG-IUS but also continuous progestogen as therapy for this disease. Patients with D-score below zero were not included in the study.
One main weakness of the study was the long inclusion period of the women lasting for nearly 6 years, partly through the strict inclusion criteria. As shown in Figure 1, many women were not eligible for the study, most often because of the poor quality of endometrial biopsy material unsuitable for light microscopy or morphometry. The high number of participating centres recruiting women may have caused differences in questioning of the women and reporting routines of adverse effects, although, the study procedures were described in detail in the protocol. It may be open to discussion if such variations might have impaired the validity of the results. Age distribution is another limitation of the study. The data demonstrate that a proportionally low fraction of the women were older than 52 years or postmenopausal and differences in response due to hormonal status are not considered. No interim analyses were performed during the inclusion period to avoid bias, as the first included women had completed treatment before the last were included. Optimal procedures for the comparison between therapy regimens should have included placebo therapy but procedures including placebo medication were not performed in the present study. Participating gynaecologists as well as women in all three therapy groups might have been biased as blinding of the therapy was not performed. Although the possibility was evaluated before study start, it was concluded that the intrauterine and oral therapy were so principally different that placebo medication would have been difficult to implement. Construction of an intrauterine placebo device was considered a possible alternative; however, this was not within reach from an economical view. Of great importance is the fact that when dealing with premalignant diseases, treatment with placebo might be considered unethical.
Interpretations
In a previous study we showed that nearly 50% of the women had persisting hyperplasia after therapy with 10 mg MPA 10 days per cycle for 3 months.23 Comparably, the percentage of women with therapy failure in the present study had been reduced to 31% after 6 months of treatment. Hence, the low-dosed therapy seemed more effective when continued for 6 months compared with withdrawal after 3 months.23 A direct dose-dependent effect was also demonstrated for the continuous oral therapy, which was shown to be significantly superior to the cyclic oral treatment. Treatment time no <6 months to accurately assess response, was also recommended by Gunderson and colleagues 41 in a recent review of women receiving progestin therapy for atypical endometrial hyperplasia. However, when meta-analyses of studies are evaluated the results are less comparable because of variation in type, dose, regimen and duration of oral treatment.1,7–9,12–15,23,25,42
Only women with simple, complex and atypical hyperplasia have been included in the present study. However, successful treatment of women with complex atypical hyperplasia or highly differentiated endometrial carcinoma in stage I with LNG-IUS has been performed in a few studies.7,42,43 Permission to include cases with severe endometrial changes suspicious of endometrial carcinoma would be hard to obtain from the Committee of Ethics as these women are most often chosen for surgery. However, safe conservative therapy is important for women who want to preserve their fertility and for women who are inoperable due to serious illness. A study with sufficient power including only the mentioned categories would take too long as such women are few and difficult to recruit.
Conclusions
The current randomised multicentre study is the first ever to prove that the LNG-IUS can be used as therapy for endometrial hyperplasia and that the LNG-IUS is completely safe used in simple, complex and atypical hyperplasia. Continuous oral therapy proved to be nearly as effective as the LNG-IUS and can be used as an alternative for women who do not tolerate the LNG-IUS. Only 69% of the women obtained response after cyclic oral treatment, proving that this regimen cannot be recommended as treatment for endometrial hyperplasia. Adverse effects were relatively common with minimal differences between therapy groups and should be excluded as an argument for choice of therapy. Other characteristics such as BMI and menopausal status had no influence on response to therapy.
Acknowledgments
We are grateful to all the gynaecologists contributing to include women in this study: Senior resident Christine Hancke, MD, Department of Gynaecology and Obstetrics University Hospital North Norway, Tromsø, N-9038 Tromsø, Norway; Chief physician Hans Krogstad MD, Department of Gynaecology and Obstetrics, University Hospital North Norway, Harstad, N-9400 Norway; Chief Physician Kristen Olav Lind, MD, Department of Gynaecology and Obstetrics, Hospital of Nordland, Stokmarknes Hospital, Stokmarknes, N-8451, Norway; Chief Physician Kevin Sunde Oppegaard, MD, Department of Gynaecology and Obstetrics, Hospital of Finnmark, Hammerfest, N-9600 Hammerfest, Norway; Chief Physician Hemma Hegnauer, MD, Department of Gynaecology and Obstetrics, Hospital of Nordland, N- 8000 Bodø, Norway; Researcher Louise Reinertsen, Department of Gynaecology and Obstetrics, St. Olavs Hospital, Trondheim, N-7006 Norway; Chief Physician Gunn Fallås Dahl, MD, Department of Gynaecology and Obstetrics, Oslo, University Hospital-Ullevål and Faculty of Medicine, University of Oslo, Oslo, Norway; Chief Physician Hans Skjelle, MD, Department of Obstetrics and Gynaecology, Akershus University Hospital, Mailbox 24, N- 1478 Lørenskog, Norway; Chief Physician Jacob Nakling, Department of Obstetrics and Gynaecology, Innlandet Hospital HF, Lillehammer, Anders Sandvigs gt., N-172629 Lillehammer; Norway. Specialists in private practice: Gynaecologist Kari Anne Trosterud, Postboks 331, N-2403 Elverum, Norway; Gynaecologist Louise Silfverskiöld, Kirkeveien 64 B, N-0364 Oslo, Norway; Gynaecologist Fredrik Hancke, Spesialistsenteret Pilestredet Park, Pilestredet Park 70176 OSLO, Norway; Gynaecologist Randi Akerøy Lundgren, Postboks 300, N-2001 Lillestrøm, Norway; Gynaecologist Grete Riis-Johannessen, Kolbotnvn. 33, N-1410 Kolbotn, Norway; Gynaecologist Finn Forsdahl and Gynaecologist Nils Sørheim, Gyn Tromsø AS, Søndre Tollbugata 7B, N-9008 Tromsø, Norway; Gynaecologist Pia Christina Sillanpââ, Moelv Spesialistsenter, Storgt. 150—Miljøsenteret Sør H-bygget, N-2390 Moelv, Norway; Gynaecologist Berit Aune, Stortorget 2, N-9008, Tromsø, Norway; We are also grateful to the Engineers performing D-score analyses: Inger Pettersen, Karin Hanssen, Bjørn T.G. Moe, and Kurt Larsen in the Laboratory of Morphometry at the Department of Clinical Pathology, University Hospital of North Norway. We thank Advisor Dag Grønvoll, Clinical Research Centre, and University Hospital of North Norway, who has thoroughly monitored all data of this study.
Disclosure of interests
AØ has received fees from Bayer for invited lectures in scientific seminars for gynaecologists. For contributing authors ABV, MA, IP and BS no disclosure of interest exists.
Contributions to authorship
All the authors were involved in the planning and design of the study. All authors contributed to the writing of the report and can confirm the accuracy and completeness of the data reported. After the completion of the study all authors had full access to all of the results.
Details of ethics approval
The trial was conducted in accordance with national law and local regulations. Permission from The Regional Committee for Medical Research Ethics (P REK NORD 25/2004). Permission from the Medicines Agency (Statens Legemiddelverk, SLV) was granted on 13th of May 2005. Permission from the National Privacy Protection Committee was granted on 18th of March 2004. The Norwegian System of Compensation to Patients also insured each participating woman.
Funding
We are grateful to the institutions financing the present study. From 2005 to 2011 a research grant given by the Norwegian Cancer Association, the Regional Research Board of Northern Norway (Helse Nord), and the Bank of North Norway funded the study. Annual funding is also granted from the University of Tromsø.
References
- 1.Horn LC, Schnurrbusch U, Bilek K, Hentschel B, Einenkel J. Risk of progression in complex and atypical endometrial hyperplasia: clinicopathologic analysis in cases with and without progestogen treatment. Int J Gynecol Cancer. 2004;14:348–53. doi: 10.1111/j.1048-891x.2004.014220.x. [DOI] [PubMed] [Google Scholar]
- 2.Norwegian Cancer Registry. Annual report. Oslo: Norwegian Cancer Registry; 2009. [Google Scholar]
- 3.Kurman RJ, Kaminski PF, Norris HJ. The behavior of endometrial hyperplasia. A long-term study of “untreated” hyperplasia in 170 patients. Cancer. 1985;56:403–12. doi: 10.1002/1097-0142(19850715)56:2<403::aid-cncr2820560233>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 4.Ferenczy A, Gelfand M. The biologic significance of cytologic atypia in progestogen-treated endometrial hyperplasia. Am J Obstet Gynecol. 1989;160:126–31. doi: 10.1016/0002-9378(89)90103-8. [DOI] [PubMed] [Google Scholar]
- 5.Lindahl B, Alm P, Ferno M, Norgren A. Endometrial hyperplasia: a prospective randomized study of histopathology, tissue steroid receptors and plasma steroids after abrasio, with or without high dose gestagen treatment. Anticancer Res. 1990;10:725–30. [PubMed] [Google Scholar]
- 6.Lindahl B, Willen R. Steroid receptor concentrations as a prognostic factor in atypical endometrial hyperplasia. Anticancer Res. 1998;18:3793–5. [PubMed] [Google Scholar]
- 7.Randall TC, Kurman RJ. Progestin treatment of atypical hyperplasia and well-differentiated carcinoma of the endometrium in women under age 40. Obstet Gynecol. 1997;90:434–40. doi: 10.1016/s0029-7844(97)00297-4. [DOI] [PubMed] [Google Scholar]
- 8.Wheeler DT, Bristow RE, Kurman RJ. Histologic alterations in endometrial hyperplasia and well-differentiated carcinoma treated with progestins. Am J Surg Pathol. 2007;31:98898. doi: 10.1097/PAS.0b013e31802d68ce. [DOI] [PubMed] [Google Scholar]
- 9.Reed SD, Voigt LF, Newton KM, Garcia RH, Allison HK, Epplein M, et al. Progestin therapy of complex endometrial hyperplasia with and without atypia. Obstet Gynecol. 2009;113:655–62. doi: 10.1097/AOG.0b013e318198a10a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Minaguchi T, Waite KA, Eng C. Nuclear localization of PTEN is regulated by Ca2+ through a tyrosil phosphorylation-independent conformational modification in major vault protein. Cancer Res. 2006;66:11677–82. doi: 10.1158/0008-5472.CAN-06-2240. [DOI] [PubMed] [Google Scholar]
- 11.Kaku T, Kamura T, Hirakawa T, Sakai K, Amada S, Kobayashi H, et al. Endometrial carcinoma associated with hyperplasia–immunohistochemical study of angiogenesis and p53 expression. Gynecol Oncol. 1999;72:51–5. doi: 10.1006/gyno.1998.5230. [DOI] [PubMed] [Google Scholar]
- 12.Jobo T, Kawaguchi M, Imai M, Kuramoto H. Treatment for complex atypical hyperplasia of the endometrium. Eur J Gynaecol Oncol. 2001;22:365–8. [PubMed] [Google Scholar]
- 13.Jarvela IY, Santala M. Treatment of non-atypic endometrial hyperplasia using thermal balloon endometrial ablation therapy. Gynecol Obstet Invest. 2005;59:202–6. doi: 10.1159/000084142. [DOI] [PubMed] [Google Scholar]
- 14.Clark TJ, Neelakantan D, Gupta JK. The management of endometrial hyperplasia: an evaluation of current practice. Eur J Obstet Gynecol Reprod Biol. 2006;125:259–64. doi: 10.1016/j.ejogrb.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 15.Bese T, Vural A, Ozturk M, Dagistanli F, Demirkiran F, Tuncdemir M, et al. The effect of long-term use of progesterone therapy on proliferation and apoptosis in simple endometrial hyperplasia without atypia. Int J Gynecol Cancer. 2006;16:809–13. doi: 10.1111/j.1525-1438.2006.00533.x. [DOI] [PubMed] [Google Scholar]
- 16.Andersson JK, Rybo G. Levonorgestrel-releasing intrauterine device in the treatment of menorrhagia. Br J Obstet Gynaecol. 1990;97:690–4. doi: 10.1111/j.1471-0528.1990.tb16240.x. [DOI] [PubMed] [Google Scholar]
- 17.Luukkainen T, Allonen H, Haukkamaa M, Holma P, Pyorala T, Terho J, et al. Effective contraception with the levonorgestrel-releasing intrauterine device: 12-month report of a European multicenter study. Contraception. 1987;36:169–79. doi: 10.1016/0010-7824(87)90012-6. [DOI] [PubMed] [Google Scholar]
- 18.Nilsson CG, Haukkamaa M, Vierola H, Luukkainen T. Tissue concentrations of levonorgestrel in women using a levonorgestrel-releasing IUD. Clin Endocrinol (Oxf) 1982;17:529–36. doi: 10.1111/j.1365-2265.1982.tb01625.x. [DOI] [PubMed] [Google Scholar]
- 19.Perino A, Quartararo P, Catinella E, Genova G, Cittadini E. Treatment of endometrial hyperplasia with levonorgestrel releasing intrauterine devices. Acta Eur Fertil. 1987;18:137–40. [PubMed] [Google Scholar]
- 20.Rose G, Edmonds D. Levonorgestrel IUS-treatment for endometrial cystic hyperplasia. Gynecol Case reports. 1997;104:614–6. [Google Scholar]
- 21.Scarselli G, Tantini C, Colafranceschi M, Taddei GL, Bargelli G, Venturini N, et al. Levo-norgestrel-nova-T and precancerous lesions of the endometrium. Eur J Gynaecol Oncol. 1988;9:284–6. [PubMed] [Google Scholar]
- 22.Varma R, Soneja H, Bhatia K, Ganesan R, Rollason T, Clark TJ, et al. The effectiveness of a levonorgestrel-releasing intrauterine system (LNG-IUS) in the treatment of endometrial hyperplasia—a long-term follow-up study. Eur J Obstet Gynecol Reprod Biol. 2008;139:169–75. doi: 10.1016/j.ejogrb.2008.02.022. [DOI] [PubMed] [Google Scholar]
- 23.Vereide AB, Arnes M, Straume B, Maltau JM, Orbo A. Nuclear morphometric changes and therapy monitoring in patients with endometrial hyperplasia: a study comparing effects of intrauterine levonorgestrel and systemic medroxyprogesterone. Gynecol Oncol. 2003;91:526–33. doi: 10.1016/j.ygyno.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 24.Wildemeersch D, Janssens D, Pylyser K, De WN, Verbeeck G, Dhont M, et al. Management of patients with non-atypical and atypical endometrial hyperplasia with a levonorgestrel-releasing intrauterine system: long-term follow-up. Maturitas. 2007;57:210–3. doi: 10.1016/j.maturitas.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 25.Buttini MJ, Jordan SJ, Webb PM. The effect of the levonorgestrel releasing intrauterine system on endometrial hyperplasia: an Australian study and systematic review. Aust N Z J Obstet Gynaecol. 2009;49:316–22. doi: 10.1111/j.1479-828X.2009.00981.x. [DOI] [PubMed] [Google Scholar]
- 26.Gallos ID, Shehmar M, Thangaratinam S, Papapostolou TK, Coomarasamy A, Gupta JK. Oral progestogens v levonorges-trel-releasing intrauterine system for endometrial hyperplasia: a systematic review and metaanalysis. Am J Obstet Gynecol. 2010;203:e1–10. doi: 10.1016/j.ajog.2010.07.037. [DOI] [PubMed] [Google Scholar]
- 27.Schulz KF, Altman DG, Moher D. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMC Med. 2010;8:18. doi: 10.1186/1741-7015-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gallos ID, Krishan P, Shehmar M, Ganesan R, Gupta JK. Relapse of endometrial hyperplasia after conservative treatment: a cohort study with long-term follow-up. Hum Reprod. 2013;28:1231–6. doi: 10.1093/humrep/det049. [DOI] [PubMed] [Google Scholar]
- 29.Orbo A, Baak JP. Computer-based morphometric image analysis of endometrial hyperplasia. Tidsskr Nor Laegeforen. 2000;120:496–9. [PubMed] [Google Scholar]
- 30.Orbo A, Baak JP, Kleivan I, Lysne S, Prytz PS, Broeckaert MA, et al. Computerised morphometrical analysis in endometrial hyperplasia for the prediction of cancer development. A long-term retrospective study from northern Norway. J Clin Pathol. 2000;53:697–703. doi: 10.1136/jcp.53.9.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Orbo A, Arnes M, Hancke C, Vereide AB, Pettersen I, Larsen K. Treatment results of endometrial hyperplasia after prospective D-score classification A follow-up study comparing effect of LNG-IUD and oral progestins versus observation only. Gynecol Oncol. 2008;111:68–73. doi: 10.1016/j.ygyno.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 32.Orbo A, Kaino T, Arnes M, Larsen K, Pettersen I, Moe B. Prognostic markers for coexistent carcinoma in high-risk endometrial hyperplasia with negative D-score: significance of morphometry, hormone receptors and apoptosis for outcome prediction. Acta Obstet Gynecol Scand. 2009;88:1234–42. doi: 10.3109/00016340903281014. [DOI] [PubMed] [Google Scholar]
- 33.Baak JP, Nauta JJ, Wisse-Brekelmans EC, Bezemer PD. Architectural and nuclear morphometrical features together are more important prognosticators in endometrial hyperplasias than nuclear morphometrical features alone. J Pathol. 1988;154:335–41. doi: 10.1002/path.1711540409. [DOI] [PubMed] [Google Scholar]
- 34.Zaino RJ, Kauderer J, Trimble CL, Silverberg SG, Curtin JP, Lim PC, et al. Reproducibility of the diagnosis of atypical endometrial hyperplasia: a Gynecologic Oncology Group study. Cancer. 2006;106:804–11. doi: 10.1002/cncr.21649. [DOI] [PubMed] [Google Scholar]
- 35.Baak JP, Wisse-Brekelmans EC, Fleege JC, van der Putten HW, Bezemer PD. Assessment of the risk on endometrial cancer in hyperplasia, by means of morphological and morphometrical features. Pathol Res Pract. 1992;188:856–9. doi: 10.1016/S0344-0338(11)80244-X. [DOI] [PubMed] [Google Scholar]
- 36.Bergeron C, Nogales FF, Masseroli M, Abeler V, Duvillard P, Muller-Holzner E, et al. A multicentric European study testing the reproducibility of the WHO classification of endometrial hyperplasia with a proposal of a simplified working classification for biopsy and curettage specimens. Am J Surg Pathol. 1999;23:1102–8. doi: 10.1097/00000478-199909000-00014. [DOI] [PubMed] [Google Scholar]
- 37.Dunton CJ, Baak JP, Palazzo JP, van Diest PJ, McHugh M, Widra EA. Use of computerized morphometric analyses of endometrial hyperplasias in the prediction of coexistent cancer. Am J Obstet Gynecol. 1996;174:1518–21. doi: 10.1016/s0002-9378(96)70599-9. [DOI] [PubMed] [Google Scholar]
- 38.Baak JP, Orbo A, van Diest PJ, Jiwa M, de Bruin P, Broeckaert M, et al. Prospective multicenter evaluation of the morphometric D-score for prediction of the outcome of endometrial hyperplasias. Am J Surg Pathol. 2001;25:930–5. doi: 10.1097/00000478-200107000-00012. [DOI] [PubMed] [Google Scholar]
- 39.Baak JP, Mutter GL, Robboy S, van Diest PJ, Uyterlinde AM, Orbo A, et al. The molecular genetics and morphometry-based endometrial intraepithelial neoplasia classification system predicts disease progression in endometrial hyperplasia more accurately than the 1994 World Health Organization classification system. Cancer. 2005;103:2304–12. doi: 10.1002/cncr.21058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mutter GL, Baak JP, Crum CP, Richart RM, Ferenczy A, Faquin WC. Endometrial precancer diagnosis by histopathology, clonal analysis, and computerized morphometry. J Pathol. 2000;190:462–9. doi: 10.1002/(SICI)1096-9896(200003)190:4<462::AID-PATH590>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 41.Gunderson CC, Fader AN, Carson KA, Bristow RE. Oncologic and reproductive outcomes with progestin therapy in women with endometrial hyperplasia and grade 1 adenocarcinoma: a systematic review. Gynecol Oncol. 2012;125:477–82. doi: 10.1016/j.ygyno.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 42.Witkiewicz AK, McConnell T, Potoczek M, Emmons RV, Kurman RJ. Increased natural killer cells and decreased regulatory T cells are seen in complex atypical endometrial hyperplasia and well-differentiated carcinoma treated with progestins. Hum Pathol. 2010;41:26–32. doi: 10.1016/j.humpath.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 43.Tjalma W, Van ME, Weyler J, Dirix L, Van DA, Goovaerts G, et al. Quantification and prognostic relevance of angiogenic parameters in invasive cervical cancer. Br J Cancer. 1998;78:170–4. doi: 10.1038/bjc.1998.460. [DOI] [PMC free article] [PubMed] [Google Scholar]


