Abstract
Objectives
To determine the pharmacodynamic profile of serum total testosterone and luteinizing hormone (LH) levels in men with secondary hypogonadism after initial and chronic daily oral doses of enclomiphene citrate vs transdermal testosterone.
To determine the effects of daily oral doses of enclomiphene citrate in comparison with transdermal testosterone on other hormones and markers in men with secondary hypogonadism.
Patients and Methods
This was a randomized, single-blind, two-centre, phase II study to evaluate the effects of three different doses of enclomiphene citrate (6.25, 12.5 and 25 mg) vs transdermal testosterone on 24-h LH and total testosterone in otherwise normal healthy men with secondary hypogonadism.
Forty-eight men were enrolled in the trial (the intent-to-treat population), but four men had testosterone levels >350 ng/dL at baseline. Forty-four men completed the study per protocol. All subjects enrolled in this trial had serum total testosterone in the low range (<350 ng/dL) and had low to normal LH (<12 IU/L) on at least two occasions.
Total testosterone and LH levels were assessed each hour for 24 h to examine the effects at each of three treatment doses of enclomiphene citrate vs a standard dose (5 g) of transdermal testosterone. In the initial profile, total testosterone and LH were determined in a naïve population after a single initial oral or transdermal treatment (day 1). This was contrasted to that seen after 6 weeks of continuous daily oral or transdermal treatment (day 42).
The pharmacokinetics of enclomiphene citrate were assessed in a select subpopulation.
Serum samples were obtained over the course of the study to determine the levels of various hormones and lipids.
Results
After 6 weeks of continuous use, the mean (sd) concentration of total testosterone at day 42 was 604 (160) ng/dL for men taking the highest dose of enclomiphene citrate (enclomiphene citrate, 25 mg daily) and 500 (278) ng in those men treated with transdermal testosterone. These values were higher than day 1 values but not different from each other (P = 0.23, t-test).
All three doses of enclomiphene citrate increased the testosterone concentration at time 0 of each 24-h sampling period, and the mean, maximum, minimum and range of testosterone concentrations over the 24-h sampling period. Transdermal testosterone also raised total testosterone, albeit with more variability, and with suppressed LH levels.
The patterns of total testosterone over the 24-h period after 6 weeks of dosing could be fit to a nonlinear function with morning elevations, mid-day troughs, and rising night-time levels.
Enclomiphene citrate and transdermal testosterone increased levels of total testosterone within 2 weeks, but they had opposite effects on FSH and LH.
Treatment with enclomiphene citrate did not significantly affect levels of thyroid-stimulating hormone, adenocorticotropic hormone, cortisol, lipids or bone markers. Both transdermal testosterone and enclomiphene citrate decreased insulin-like growth factor-1 levels (P < 0.05) but suppression was greater in the enclomiphene citrate groups.
Conclusions
Enclomiphene citrate increased serum LH and total testosterone; however, there was not a temporal association between the peak drug levels and the maximum concentration levels of LH or total testosterone.
Enclomiphene citrate consistently increased serum total testosterone into the normal range and increased LH and FSH above the normal range. The effects on LH and total testosterone persisted for at least 1 week after stopping treatment.
Keywords: serum testosterone, LH, secondary hypogonadism, transdermal testosterone, testosterone restoration
Introduction
Clomiphene citrate is approved by the Federal Drug Administration (FDA) and is widely used in women for induction of ovulation. Clomiphene citrate has been used off-label to increase LH, FSH and testosterone levels in men with idiopathic infertility and/or secondary hypogonadism; however, it is not approved by the FDA for use in men. Clomiphene citrate is a mixture of two diastereoisomers, (cis)zuclomiphene citrate (38%) and (trans)enclomiphene citrate (62%). The commercial name for enclomiphene citrate is Androxal® [1]. Zuclomiphene is thought to cause some of the side effects that have been associated with clomiphene citrate. By contrast, enclomiphene citrate is primarily responsible for causing an increase in FSH and LH. One example is men treated with exogenous testosterone, as such treatment will relieve symptoms of secondary hypogonadism but will not maintain or restore sperm production in the testes [2,3].
Enclomiphene citrate is proposed for the treatment of some men who have secondary hypogonadism, especially that caused by dysfunctional, but reversible hypothalamus/pituitary activity. These men present with low total testosterone and low or inappropriately normal gonadotropin levels (LH and FSH). Treatment of these men with an anti-oestrogen or an aromatase inhibitor can increase gonadotropin and testosterone levels; however, enclomiphene citrate is not proposed for some forms of secondary hypogonadism such as pituitary tumours, craniopharingiomas, haemochromatsis or congenital GnRH deficiency.
The consequences of long-term secondary hypogonadism are recognized health problems typically associated with ageing. Indeed, secondary hypogonadism in men has been associated with increased mortality, obesity, metabolic syndrome and/or insulin resistance/type II diabetes [4–9]. Many studies have shown that serum total and free testosterone in levels in obese men are significantly lower than aged-matched healthy male controls [10]. The European Male Ageing Study showed that obesity and ageing are risk factors for secondary hypogonadism, while the prevalence of primary hypogonadism is also linked to age [11].
Biological rhythms dominate reproductive hormone release in both males and females. Classic experiments have shown that the long-term, pulsatile administration of GnRH can induce both puberty and fertility in GnRH-deficient males who do not initiate puberty spontaneously [12]. Patients with this hypothalamic defect have helped elucidate the feedback control of sex steroids at the level of the pituitary and the hypothalamus and to identify additional defects [13–15]. At the level of the hypothalamus, pituitary and testes there is a clear rhythm that results in the testicular production of testosterone in males, with healthy young men experiencing a morning peak, a trough in the early evening, followed by a gradual increase towards the morning peak [16]. In most ageing men there is an overall decrease in testosterone levels and a blunting in circadian androgen levels [17], perhaps caused by the disruption of feedback and feed-forward control mechanisms [18]. In addition, other factors may contribute, as shown by the suppressive effects of a standard glucose tolerance test on serum testosterone levels [19]. Oestrogens have also been shown to play a role in these feedback processes in men [20].
The present study was undertaken in men with secondary hypogonadism to better determine the profile of serum testosterone and LH after enclomiphene citrate administration for 6 weeks in comparison to that for transdermal testosterone treatment. In most studies with transdermal testosterone the determination of serum levels of total testosterone constitutes pharmacokinetics. In the present study, treatment with oral enclomiphene citrate constitutes pharmacodynamics, i.e. an effect of the drug on the body.
In an early phase II study we evaluated the potential of oral enclomiphene citrate to increase serum testosterone levels in 52 men with secondary hypogonadism [21]. Men who had low total testosterone values at baseline had significant increases in serum total testosterone levels after 2 weeks of treatment with enclomiphene citrate. The increase in total testosterone was accompanied by increases in LH and FSH. A more recent study provided evidence that enclomiphene citrate raises testosterone levels in men with secondary hypogonadism and also preserves or improves sperm counts [22,23]. This effect was associated with increased endogenous levels of LH and FSH. In the latter study, the randomized groups included treatment with Testim®, a transdermal testosterone gel. In contrast to the group receiving enclomiphene citrate, men receiving Testim had a reduction in LH and FSH levels and in sperm counts.
Patients and Methods
Patients
Forty-eight men were randomized to receive either: 6.25 mg enclomiphene citrate (Androxal®, Repros Therapeutics Inc., Houston, TX, USA) daily (n = 16), 12.5 mg enclomiphene citrate daily (n = 14), 25 mg enclomiphene citrate daily (n = 16) or transdermal testosterone daily (AndroGel®, AbbVie Inc., Chicago, IL, USA; n = 14). These men were defined as the intent-to treat (ITT) population. Four men (three in the enclomiphene citrate 12.5 mg group and one man in the enclomiphene citrate 25 mg group) were later found to have testosterone levels >350 ng/dL at baseline. Forty-four subjects completed the study per protocol (PP) and were defined as the PP population. The mean (sd) age and body mass index (BMI) for the transdermal testosterone group(n = 13) were 53.3 (10.2) years and 31.5 (5.9) kg/m2, respectively. The mean (sd) BMI for all men was 34.7 (7.2) kg/m2. There was no significant difference in age or BMI among the treatment groups.
Laboratories
All hormone assays and blood chemistries were performed by a central laboratory (Cetero Research, Houston, TX, USA). Total testosterone, estradiol, dihydrotestosterone, LH, FSH, IGF-1 and thyroid-stimulating hormone (TSH) were determined by immunoassay. Others variables were determined by standard laboratory methods. Analytical procedures to determine concentrations of enclomiphene citrate in human semen were performed at Cetero Research. Serum triglycerides, total and high and low density lipoprotein cholesterol levels were assayed using an ADVIA® 1650/2400 Chemistry system. Serum osteocalcin was assayed using an ELISA kit (ALPCO Diagnostics).
Protocol
The present study was a randomized, single-blind, phase II, four-arm study with a 6-week active dosing period. The objectives were to assess LH and testosterone levels in men with secondary hypogonadism. Two US sites participated in the study between July and October 2011. Institutional review board or independent ethics committee approvals were obtained at each centre, all patients provided written informed consent, and the study was conducted in accordance with the Declaration of Helsinki and principles of good clinical practice. Study participants were healthy males 18–65 years of age with morning testosterone levels ≤350 ng/dL and low or inappropriately normal LH levels and normal screening chemistries. Subjects agreed to use a condom or another form of contraception during the study. Subjects with diabetes, hyperprolactinaemia, HIV, cataracts, breast or prostate cancer, end-stage renal disease, cystic fibrosis, uncontrolled hypertension, abnormal haemoglobin or heamatocrit, or subjects using medications known to interfere with sex steroid synthesis or action were excluded. The exclusion criteria also included the use of testosterone <3 months before the study, the use of clomiphene citrate in the past year, the use of spironolactone, cimetidine, 5α-reductase inhibitors, hCG, oestrogen, glucocorticoids, narcotics, anabolic steroids, dehydroepiandrosterone or herbal hormone products, and a PSA level >3.6 ng/mL or BMI >42 kg/m2.
The men in each of the four study arms were randomized to one of three single-blind enclomiphene citrate doses or open label transdermal testosterone. The doses of enclomiphene citrate in the single-blind portion of the study were enclomiphene citrate 6.25, 12.5 and 25 mg (Rainbow Gold Products, Inc., Boaz, AL, USA). Capsules were supplied by Repros Therapeutics. Subjects receiving enclomiphene citrate were instructed to take one capsule of blinded study medication between 7:00 h and 10:00 h every day. Transdermal testosterone was applied as per the package insert. Subjects were instructed to apply the gel at the same time each day, and not to bathe or swim for 3–4 h after application. At visit 1 (or week 2) subjects underwent screening procedures, which included signing of the consent form, a complete medical history, medication history, physical examination, blood evaluation and a slit lamp eye examination and visual acuity test. Eligible subjects underwent a 24-h assessment of baseline total testosterone, LH and FSH (visit 2 or week 0). Blood samples were also collected to test for lipids and hormones and subjects were asked to follow a normal ad libitum diet. Subjects received a study medication kit, containing a 2-week supply of study medication. Men in the transdermal testosterone arm were instructed to apply the gel at the same time every day and to schedule their office visit ∼4 h after the time of application. Subjects in the enclomiphene citrate arms returned for visits at 2 and 4 weeks in the morning (before 09:00 h) for blood draws (before 10:00 h) to measure total testosterone, FSH, LH and trough levels of the study drug. At 6 weeks, subjects underwent a 24-h assessment of total testosterone and LH. During this time blood was also drawn for a pharmacokinetic assessment of serum enclomiphene citrate levels at 0, 1, 2, 3, 4, 6, 8, 12, 16 and 24 h after a single dose of enclomiphene citrate (6.25, 12.5, 25 mg enclomiphene citrate). Subjects returned at 7 weeks for a final blood draw after a 1-week washout (before 10:00 h).
Statistical Analysis
Statistical analysis of the primary efficacy variable was the change between day 1 and day 42 in the 24-h mean testosterone and LH levels. An overall comparison among the four treatment groups was performed using anova or the Kruskal–Wallis test. The secondary efficacy variables were the changes in total testosterone, LH and FSH over the course of the study. Changes in lipids and other hormones were assessed between the baseline and week 6. An analysis of pharmacokinetics for the enclomiphene citrate arms also was performed based on enclomiphene citrate concentrations in serum at 0, 1,2, 3, 4, 8, 12, 16 and 24 h drawn during week 6. Summaries for quantitative variables include the sample size and the mean, median, sd, minimum and maximum values. Summaries for categorical variables include the number and percent of patients for each outcome. Differences between groups were determined by t-test, paired t-test or Mann–Whitney–Wilcoxin (MWW) test. A regression analysis was conducted to determine whether morning testosterone was predictive of the mean, minimum and maximum concentrations of total testosterone (CTTmean, CTTmin and CTTmax, respectively) at various time points.
Results
The present study was intended to be a study in men with secondary hypogonadism. We screened men with two morning total testosterone measurements <350 ng/dL (<10.4 nmol/L) and an appropriate LH level (<12 IU/L). Forty-eight men qualified and were defined as the ITT population. Testosterone concentration at time 0 of each 24-h sampling period (CTT0h) values were determined before the drug was administered (i.e. the initial pharmacodynamics). Four subjects were removed from the analysis because their total testosterone levels were >350 ng/dL. These men were included for hormone and safety considerations. The remaining 44 men constituted the per protocol (PP) population.
Characteristics of the study arms are shown in Table 1. There were no differences between groups at baseline, and total testosterone levels increased in all treatment arms compared with pre-treatment levels. After daily treatment for 6 weeks, differences among treatment groups were significant according to anova. These observations were true whether the data were recorded as CTT0h, CTTmean, CTTmax, CTTmin values or as the range of total testosterone concentration (CTTrange). Increases from day 1 were significant for the 6.25 mg enclomiphene citrate group (P = 0.007, paired t-test), the 25 mg enclomiphene citrate group (P = 1 × 10−5, paired t-test) and for the transdermal testosterone group (P = 0.014 signed-rank test). The 6.25-mg and 25-mg enclomiphene citrate groups had an increase in serum LH concentration from pre-treatment levels, whereas transdermal testosterone decreased the mean serum LH level. These changes were significant for enclomiphene citrate 6.25 mg (P = 0.023, paired t-test), enclomiphene citrate 25 mg (P = 7 × 10−4, paired t-test) and for transdermal testosterone (P = 1 × 10−4, paired t-test). Changes in LH paralleled the increases in total testosterone in kind if not quantity for the enclomiphene citrate groups.
Table 1.
Effect of enclomiphene citrate on pharmacodynamic variables in the PP population
| Variable | Pharmacodynamic study part 1: after first dose | Pharmacodynamic study part 2: after last dose | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Enclomiphene citrate | Transdermal testosterone | Difference among groups*, P | Enclomiphene citrate | Transdermal testosterone | Difference among groups*, P | |||||
| 6.25 mg | 12.5 mg | 25 mg | 6.25 mg | 12.5 mg | 25 mg | |||||
| n = 12 | n = 7 | n = 12 | n = 13 | n = 12 | n = 7 | n = 12 | n = 13 | |||
| Total testosterone, ng/dL | ||||||||||
| CTT0h | 265 ± 93 | 295 ± 81 | 253 ± 80 | 268 ± 58 | 0.38 | 420 ± 212 | 404 ± 165 | 604 ± 160 | 500 ± 278 | 0.07 |
| CTTmean | 262 ± 80 | 280 ± 67 | 300 ± 88 | 325 ± 108 | 0.57 | 391 ± 222 | 426 ± 140 | 586 ± 145 | 546 ± 233 | 0.003 |
| CTTmax | 358 ± 120 | 391 ± 106 | 424 ± 108 | 581 ± 506 | 0.60 | 516 ± 340 | 554 ± 175 | 764 ± 164 | 908 ± 551 | 0.024 |
| CTTmin | 189 ± 56 | 194 ± 47 | 198 ± 72 | 228 ± 60 | 0.31 | 297 ± 148 | 308 ± 120 | 451 ± 128 | 318 ± 157 | 0.002 |
| CTTrange | 169 ± 72 | 197 ± 80 | 226 ± 81 | 334 ± 138 | 0.42 | 219 ± 204 | 246 ± 106 | 314 ± 65 | 590 ± 465 | <0.001 |
| LH, IU/L | ||||||||||
| CLH0h | 3.7 ± 1.8 | 3.9 ± 1.2 | 4.5 ± 3.9 | 3.6 ± 2.2 | 0.75 | 5.7 ± 2.8 | 6.7 ± 3.3 | 13.3 ± 10.4 | 1.3 ± 2.5 | 2 × 10−5 |
| CLHmean | 3.8 ± 1.5 | 3.6 ± 1.3 | 4.9 ± 2.3 | 3.7 ± 1.5 | 0.32 | 6.0 ± 2.7 | 6.9 ± 2.4 | 13.1 ± 7.0 | 1.5 ± 1.8 | 3 × 10−6 |
| CLHmax | 6.6 ± 2.2 | 5.6 ± 1.9 | 8.7 ± 4.1 | 6.3 ± 2.6 | 0.18 | 9.7 ± 3.8 | 10.2 ± 3.1 | 17.8 ± 8.9 | 3.3 ± 3.7 | 2 × 10−5 |
| CLHmin | 2.2 ± 1.0 | 2.1 ± 0.8 | 2.8 ± 1.7 | 2.2 ± 1.7 | 0.43 | 3.7 ± 1.8 | 4.4 ± 1.8 | 8.6 ± 6.1 | 0.7 ± 1.1 | 6 × 10−5 |
| CLHrange | 4.1 ± 1.8 | 2.5 ± 2.0 | 5.4 ± 3.1 | 4.1 ± 1.5 | 0.15 | 6.1 ± 2.8 | 5.9 ± 2.3 | 9.0 ± 2.3 | 2.6 ± 2.8 | 9 × 10−4 |
Values are mean ± sd.
anova or Kruskal–Wallis.
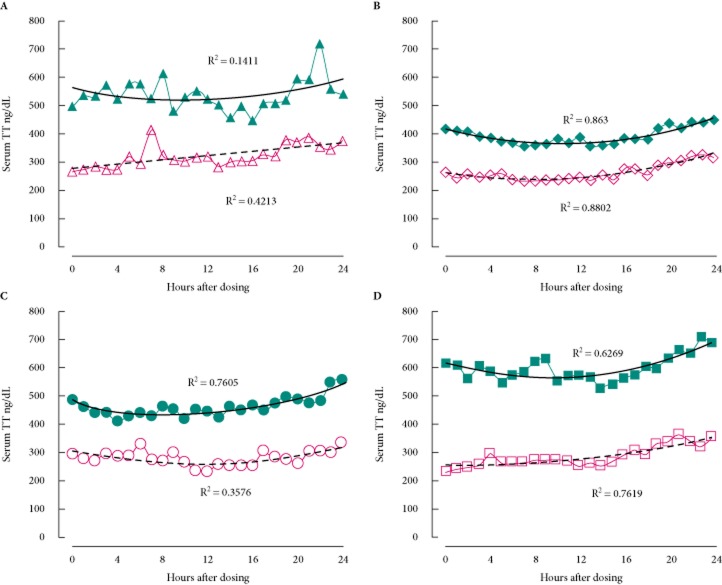
The results of the two 24-h pharmacodynamic studies provided useful comparisons between treatment with transdermal testosterone and enclomiphene citrate (Fig. 1). We observed an increase in concentration of testosterone in all treatment groups after 6 weeks of treatment in the PP population. On day 1 there were no differences between the groups in terms of mean CTT0h, or their average CTTmean, CmaxTT, CTTmin, or CTTrange values. On day 1 transdermal testosterone raised the CTTmean and CTTmax, although those increases were not significant among groups; probably because of the high variation in the mean values in the subjects who were treated with transdermal testosterone.
Fig. 1.
Effect of enclomiphene citrate on the pharmacodynamics of serum total testosterone compared with transdermal testosterone. The effects of various treatments are seen over 24-h period and compared between an initial dose and a dose given following 6 weeks of continuous treatment. Men with AIHH were treated with (A) transdermal testosterone according to label instructions initially (open triangles and dashed line) and after daily dosing (filled triangles and solid line); (B) enclomiphene citrate 6.25 mg initially (open diamonds and dashed line) and after daily oral dosing (filled diamond and solid line); (C) enclomiphene citrate 12.5 mg initially (open circles and dashed line) and after daily oral dosing (filled circles and solid line); and (D) enclomiphene citrate 25 mg initially (open squares and dashed line) and after daily oral dosing (filled squares and solid line). A second order polynomial is fitted to the data points and the R2 value is given next to the line. TT, total testosterone.
Mean testosterone levels increased in all treatment groups at 6 weeks. Transdermal testosterone was associated with a wider range of total testosterone values than we found for the enclomiphene citrate groups, as shown in Table 1. For example, after 6 weeks on treatment, the mean ± sd CTTrange was 590 ± 465 ng/dL (20.5 ± 16.1 nmol/L) for the subjects in the transdermal testosterone group and 314 ± 65 ng/dL (10.9 ± 2.2 nmol/L) for subjects in the enclomiphene citrate 25 mg group. The coefficients of variation of those two groups were 79 and 21%, respectively. We speculate that the wide variation of total testosterone in the topical treatment group may result from application issues (i.e. rushing application, not applying a full dose by leaving gel in vial, washing dose away from hands before fully absorbed), from skin thickness differences between patients, or from the timing of application.
Figure 2 shows the number of outlier values associated with each treatment during the 24-h sampling. Outlier values are those serum total testosterone values below the 300 ng/dL (10.4 nmol/L) or above the 1000 ng/dL (34.7 nmol/L) level found within each 24-h study sampling for the PP population. When we looked at the effects of each of the treatments before and after the 6-week treatment regimen, it was determined that there were many fewer values below 300 ng/dL before treatment, as expected, but after treatment there was a higher percentage of morning total testosterone values in the 300–1000 ng/dL range. There was a clear shift in the morning total testosterone values from <300 ng/dL to the 300–1000 ng/dL range for the enclomiphene citrate 25 mg group. This shift also appeared to be dose-dependent for enclomiphene citrate treatment. Transdermal testosterone was found to have more outlier values, i.e. not in the 300–1000 ng/dL range. To further assess the effects of each treatment we evaluated the data in relation to responders (Table 2).
Fig. 2.
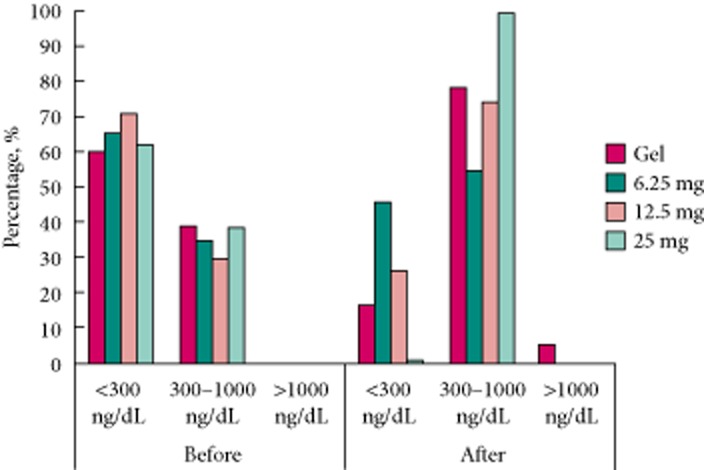
Outlier values of serum total testosterone found during the 24-h pharmacodynamic studies' PP population. The distribution of morning TT values determined during the two pharmacodynamic studies which were conducted before and after 6 weeks of continuous treatment. Subjects randomized to the transdermal testosterone (Gel) group are shown by solid bars; lightly stippled bars represent the enclomiphene citrate 6.25 mg group, darkly stippled bars represent the enclomiphene citrate 12.5 mg group and the vertical lined bars represent the enclomiphene citrate 25 mg group. TT, total testosterone.
Table 2.
Graded responder analysis: PP population
| Transdermal testosterone | Enclomiphene citrate 25 mg | Enclomiphene citrate 12.5 mg | Enclomiphene citrate 6.25 mg | |
|---|---|---|---|---|
| N = 13 | N = 12 | n = 7 | n = 12 | |
| Any increase in total testosterone, n (%) | 13 (100) | 12 (100) | 7 (100) | 9 (75) |
| CTTmean >300 ng/dL, n (%) | 11 (85) | 12 (100) | 5 (71) | 7 (58) |
| CTTmean >350 ng/dL, n (%) | 11 (85) | 12 (100) | 4 (57) | 6 (50) |
| CTTmean >400 ng/dL, n (%) | 10 (77) | 12 (100) | 3 (43) | 5 (42) |
| CTTmean >500 ng/dL, n (%) | 6 (46) | 8 (67) | 3 (43) | 3 (25) |
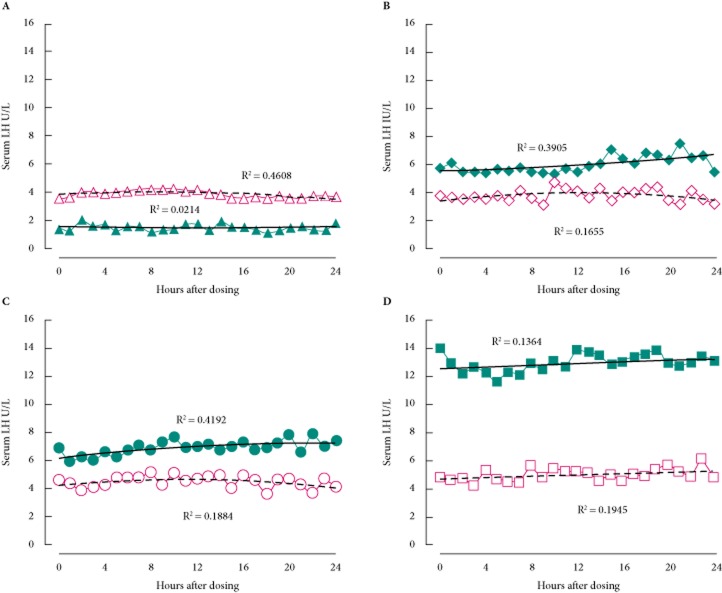
After 6 weeks of enclomiphene citrate and transdermal testosterone treatment, serum LH levels were altered in opposite ways. All enclomiphene citrate dose groups were found to have increased LH levels, whereas the transdermal testosterone treatment arm had suppressed LH levels (Fig. 3). There was considerable variability in LH values hour-to-hour, probably because of the pulsatile nature of its pituitary secretion. To discern a pulsatility pattern would require that samples be drawn every 10 min for at least 4–6 h.
Fig. 3.
Effect of enclomiphene citrate on the pharmacodynamics of serum LH compared with transdermal testosterone. The effects of various treatments are seen over 24 h and compared between an initial dose and a dose given after 6 weeks of continuous treatment. Men with AIHH were treated with (A) transdermal testosterone according to label instructions initially (open triangles and dashed line) and after daily dosing (filled triangles and solid line); (B) enclomiphene citrate 6.25 mg initially (open diamonds and dashed line) and after daily oral dosing (filled diamond and solid line); (C) enclomiphene citrate 12.5 mg initially (open circles and dashed line) and after daily oral dosing (filled circles and solid line); and (D) enclomiphene citrate 25 mg initially (open squares and dashed line) and after daily oral dosing (filled squares and solid line.) A second order polynomial is fitted to the data points and the R2 value is given next to the line.
The pharmacokinetics of enclomiphene citrate after a single enclomiphene citrate dose during week 6 are shown in Fig. 4 for a subset of the study population. The individual and overall pharmacokinetic profiles showed essentially a rapid rise of the drug in the serum with a CTTmax of 2–3 h, followed by a loss that was typical of an apparent first order process. Calculations of the mean area under the curve (AUC)0–24h show that the value for the 25 mg dose was ∼4.5 times and 7.5 times higher, respectively, than the values for the 12.5 and 6.25 mg doses. There was also evidence of an accumulation of drug in the serum because serum levels did not return to baseline 24 h after taking the drug. The pharmacokinetic profile of enclomiphene citrate did not match the serum LH patterns, which fluctuated considerably across the 24-h observation period but were uniformly elevated.
Fig. 4.
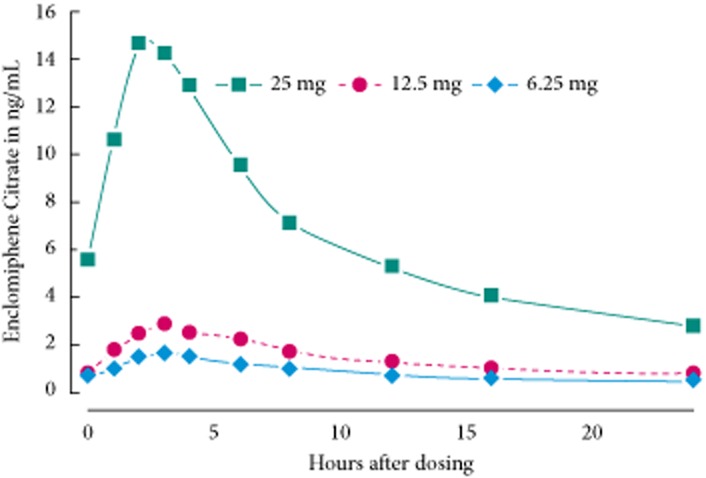
Pharmacokinetics of serum enclomiphene citrate. After 6 weeks of continuous oral dosing at various dosages of enclomiphene citrate, serum samples were obtained at various time points for the assessment of serum drug levels. The levels of serum enclomiphene citrate are given for subjects taking 6.25 mg (filled diamonds), 12.5 mg (filled circles), and 25 mg (filled squares) of study drug.
One of our objectives was to determine whether there were clear relationships between the various ways of measuring serum total testosterone when using enclomiphene citrate as a treatment. We compared the various values of serum total testosterone after both a single acute dose and a dose taken after 6 weeks of treatment, i.e. in a steady state. We were most interested in whether, at steady state, there was a clear relationship with serum total testosterone over time. We further explored the relationship between a morning serum total testosterone (i.e. CTT0h) value and the average serum total testosterone value returned over 24 h. This relationship was shown for the enclomiphene citrate 25 mg group. When we compared CTT0h with CTTmean values for each subject, we determined that there was a clear linear relationship between the values (Fig. 5). For the men treated for 6 weeks, the linear relationship was strong (R = 0.93), had a slope near unity (1.025) and an intercept that was low (4.96 ng/dL or 0.17 nmol/L) compared with the physiological range interrogated (100–900 ng/dL or 3.5–31.2 nmol/L). When we compared the other serum total testosterone measures with CTT0h,we determined strong correlations for CTTmax, CTTmin and CTTrange as well (Table 3). The relationships were not as strong after a single dose of enclomiphene citrate (data not shown) and transdermal testosterone showed a poorer relationship.
Fig. 5.
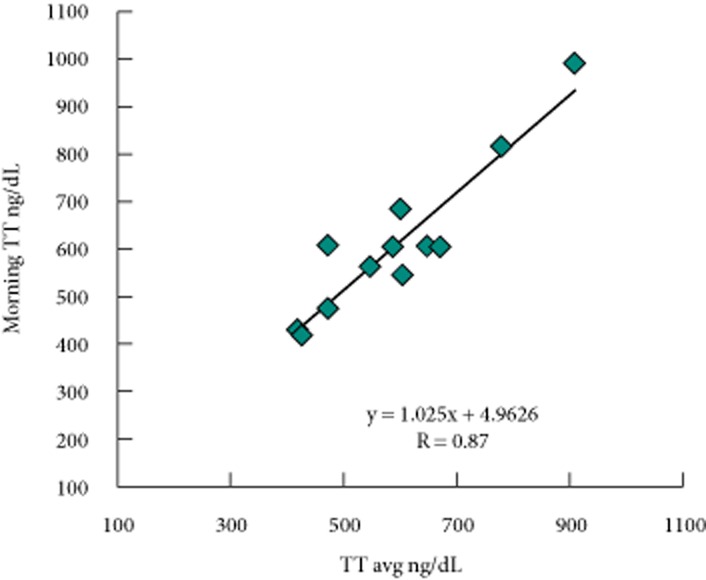
Relationship in morning total testosterone concentration to average total testosterone concentration for the enclomiphene citrate 25 mg group. An example of the relationship between a morning total testosterone value and the average total testosterone value over a 24-h period is given. Subjects were all men with AIHH who had been treated for 6 weeks with enclomiphene citrate and the pharmacodynamics of their total testosterone was determined (pharmacodynamic study part 2); thus, they were assessed for total testosterone every hour for 24 h and the mean hourly total testosterone was determined. TT, total testosterone.
Table 3.
Correlation coefficients comparing various assessments of serum total testosterone during the final 24-h pharmacodynamic study: PP population
| Treatment | n | Correlation coefficient | ||
|---|---|---|---|---|
| CTT0h vs CTTmean | CTT0h vs CTTmax | CTT0h vs CTTmin | ||
| Transdermal testosterone | 13 | 0.56 | 0.58 | 0.62 |
| Enclomiphene citrate 25 mg | 12 | 0.93 | 0.86 | 0.87 |
| Enclomiphene citrate 12.5 mg | 7 | 0.82 | 0.76 | 0.90 |
| Enclomiphene citrate 6.25 mg | 12 | 0.87 | 0.82 | 0.82 |
The time course of the serum total testosterone over the entire study is shown in Fig. 6 for the ITT population. There was no difference among the groups at the first or second qualifying visits (week 2, P = 0.74, anova; week 0, P = 0.23, Kruskal–Wallis test, respectively). Total testosterone levels after 2 weeks of treatment for the two highest doses of enclomiphene citrate and transdermal testosterone were similar. Total testosterone levels increased significantly in all groups at 2 weeks when compared with baseline, but the serum total testosterone for the transdermal testosterone group and the enclomiphene citrate 25 mg group were significantly higher than for the enclomiphene citrate 6.25 mg group (P = 0.025 and P = 0.016, t-test). A steady state, defined here by serum total testosterone values >400 ng/dL, appeared after 4 weeks of treatment with both agents. There were no significant changes from week 2 to 4 or from week 4 to 6 for any group.
Fig. 6.
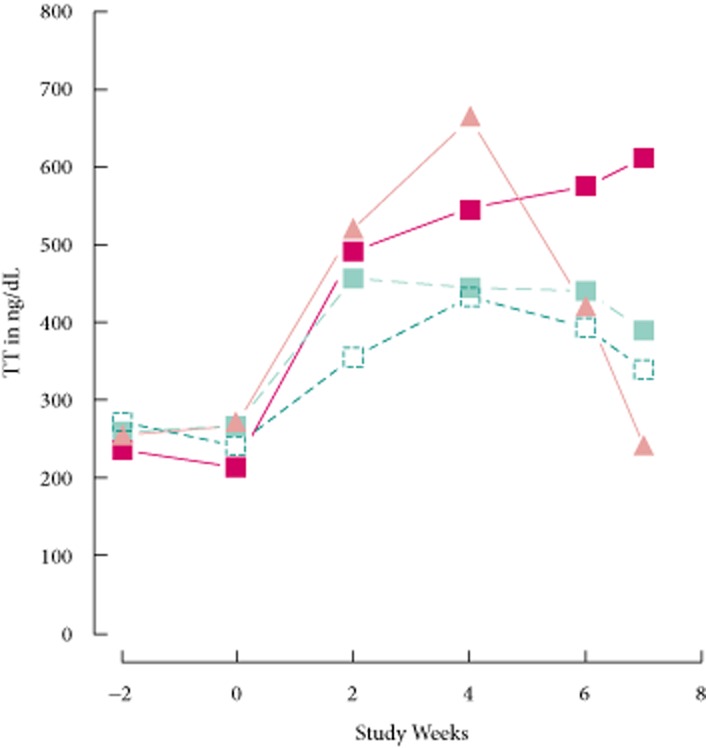
Time course of effects on serum total testosterone. Figure shows the levels of serum total testosterone found before and after treatment with daily doses of enclomiphene citrate 6.25 mg (open squares), enclomiphene citrate 12.5 mg (green squares), enclomiphene citrate 25 mg (red squares) or transdermal testosterone (orange triangles). TT, total testosterone.
Seven days after cessation of treatment, total testosterone levels in men receiving enclomiphene citrate 25 mg were higher than those in men receiving enclomiphene citrate 12.5 mg (P = 0.012, t-test), and total testosterone levels in the enclomiphene citrate 12.5 mg group were higher than those in the men on transdermal testosterone (P = 0.003, t-test). There was no significant difference between the serum total testosterone for the groups on any of the enclomiphene citrate doses over the last 7 days (at least P > 0.4 for all groups, t-test). The greatest preservation of serum total testosterone was found in the enclomiphene citrate 25 mg group, in which it was found to be slightly increased.
The time course of LH over the study is shown in Fig. 7. Levels were similar at the first and second baseline visits (weeks 2, P = 0.10, anova; week 0, P = 0.16, Kruskal–Wallis test, respectively). The increases in serum LH during the first 2 weeks of treatment were similar in the enclomiphene citrate groups, but transdermal testosterone significantly decreased the mean LH level (P = 0.026, t-test). The changes attributable to treatment (i.e. between week 2 and week 4) were significant for all four groups including the enclomiphene citrate 6.25 mg group; however, the serum LH for the transdermal testosterone group was the lowest and the 12.5 and enclomiphene citrate 25 mg dose groups were not significantly different. Mean serum LH values were >6 IU/L after 4 weeks of treatment with the two higher doses of enclomiphene citrate. There were no significant changes from week 2 to 4 or from week 4 to 6 for any group (P > 0.14, for any change in any group, t-test). Seven days after cessation of treatment, mean LH levels for men receiving enclomiphene citrate 25 mg were higher than those for men receiving enclomiphene citrate 6.25 mg (P = 0.001, MWW test), 12.5 mg (P = 0.001, MWW test) or transdermal testosterone (P < 0.001, MWW test). The mean LH concentration for men on transdermal testosterone increased significantly (P = 0.007, t-test) over this time period, and it was similar to baseline values; however, mean LH concentrations in men on enclomiphene citrate remained significantly higher than baseline values. The serum LH levels remained elevated over the 7 days after stopping enclomiphene citrate: there was no significant difference for the serum LH for the enclomiphene citrate doses over that time period (at least P > 0.4 for all groups, t-test). Each of the four treatments established new plateau values. Thus, 7 days after the last dose, serum LH for enclomiphene citrate 25 mg was higher than that for enclomiphene citrate 12.5 mg (P = 0.025, t-test), for enclomiphene citrate 6.25 mg (P = 0.006, t-test), and for transdermal testosterone (P < 0.001, t-test).
Fig. 7.
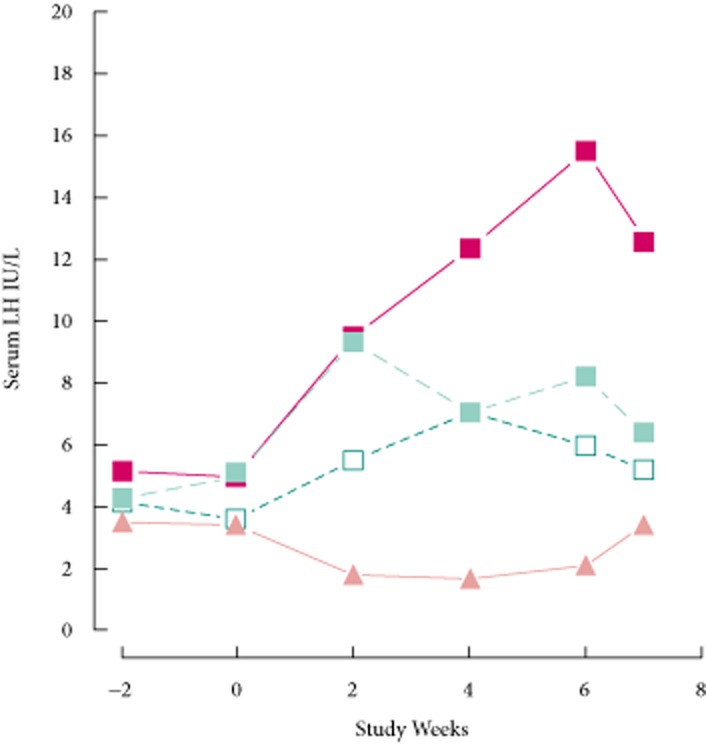
Time course of effects on LH. The levels of serum LH are shown before and after daily treatment with enclomiphene citrate 6.25 mg (open squares), enclomiphene citrate 12.5 mg (green squares), enclomiphene citrate 25 mg (red squares) or transdermal testosterone (orange triangles).
The decrease in FSH associated with transdermal testosterone was significant (P = 2 × 10−4, paired t-test). The course of the effect is shown in Fig. 8. Before initial treatment at visit 2 (week 0), subjects did not differ in terms of FSH (P = 0.32, Kruskal–Wallis test). Treatment with enclomiphene citrate increased FSH levels within 2 weeks for the 12.5 mg (P = 0.026, t-test) and the 25 mg group (P = 0.006, MWW test). Transdermal testosterone reduced serum FSH at 2, 4 and 6 weeks. FSH levels continued to rise in men in the enclomiphene citrate 25 mg group. At week 4, serum FSH levels were higher in the enclomiphene citrate 25 mg group than in the enclomiphene citrate 12.5 mg (P = 0.010, MWW test) or the enclomiphene citrate 6.25 mg groups (P = 0.004, MWW test). This pattern was maintained at week 6. The two lower doses appeared to reach a plateau which was lower than the 25 mg dose by the 4-week point. Although serum FSH at 2 weeks with the 25 mg dose of enclomiphene citrate was clearly higher, no treatment group showed a significant change in serum FSH from weeks 2 to 4 or from weeks 4 to 6 (at least P > 0.51 for all groups, t-test). The increase in FSH was significant for the 25 mg dose (P = 0.006, signed-rank test) and for the 12.5 mg dose (P = 0.022, signed-rank test) but not for the 6.25 mg dose (P = 0.15, paired t-test).
Fig. 8.
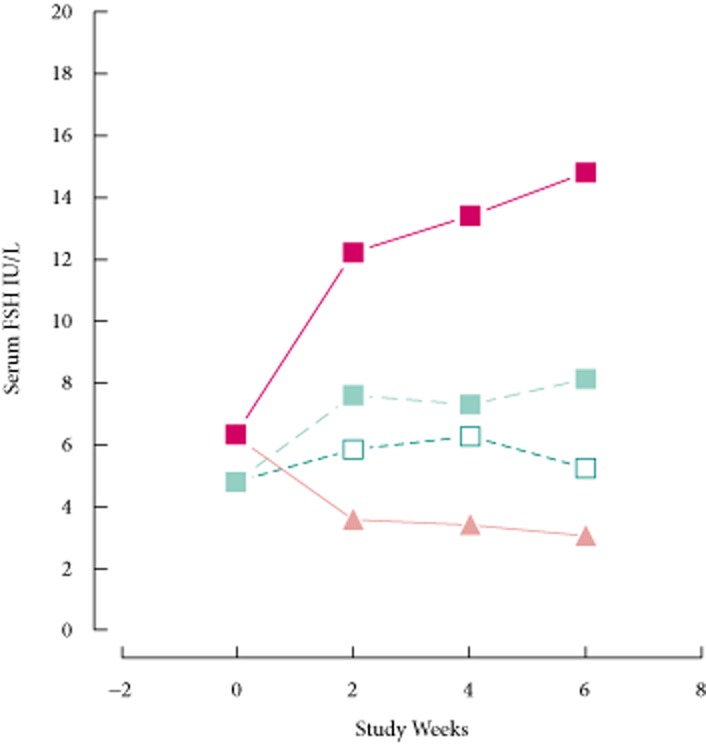
Time course of effects on FSH. The levels of serum FSH are shown before and after treatment with 6.25 mg enclomiphene citrate (open squares), 12.5 mg enclomiphene citrate (grey squares), enclomiphene citrate 25 mg (black squares) or transdermal testosterone (grey triangles).
We also investigated the effects of enclomiphene citrate on adenocorticotropic hormone (ACTH), cortisol, TSH, IGF-1 and lipids, by comparing these before treatment (baseline) and after 6 weeks of dosing. As shown in Table 4, there were no significant differences at baseline among the treatment groups except with regard to the bone marker carboxy-terminal collagen, which was unaccountably higher in the transdermal testosterone group.
Table 4.
Hormones and clinical chemistry values influenced by enclomiphene citrate in the ITT population
| Baseline | 6 Weeks of treatment | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Enclomiphene citrate 6.25 mg | Enclomiphene citrate 12.5 mg | Enclomiphene citrate 25 mg | Transdermal testosterone | Difference among groups*, P | Enclomiphene citrate 6.25 mg | Enclomiphene citrate 12.5 mg | Enclomiphene citrate 25 mg | Transdermal testosterone | Difference among groups*, P | |
| n = 12 | n = 10 | n = 12 | n = 13 | n = 12 | n = 10 | n = 12 | n = 13 | |||
| IGF-1, ug/L | 101 ± 43 | 94 ± 47 | 96 ± 45 | 103 ± 46 | 0.94 | 54 ± 30 | 50 ± 24 | 62 ± 42 | 90 ± 34 | 0.03 |
| ACTH, pmol/L | 5.4 ± 3.4 | 5.7 ± 3.9 | 4.7 ± 3.3 | 6.2 ± 5.6 | 0.91 | 6.4 ± 3.6 | 6.6 ± 3.7 | 3.8 ± 3.2 | 7.0 ± 7.4 | 0.17* |
| Cortisol, pg/mL | 8.7 ± 2.8 | 9.0 ± 3.2 | 8.4 ± 3.8 | 8.7 ± 2.4 | 0.98 | 10.3 ± 4.9 | 8.8 ± 3.0 | 12.2 ± 4.6 | 7.4 ± 3.1 | 0.03 |
| TSH, IU/L | 1.79 ± 0.90 | 1.60 ± 0.80 | 1.89 ± 1.61 | 1.42 ± 0.79 | 0.76 | 1.69 ± 0.76 | 2.66 ± 1.52 | 1.97 ± 0.75 | 1.45 ± 0.95 | 0.043 |
| Triglyceride, mg/dL | 454 ± 516 | 278 ± 197 | 231 ± 167 | 267 ± 187 | 0.53 | 451 ± 791 | 214 ± 121 | 275 ± 305 | 228 ± 169 | 0.96* |
| Cholesterol, mg/dL | 231 ± 70 | 205 ± 33 | 186 ± 33 | 198 ± 53 | 0.18 | 240 ± 142 | 198 ± 29 | 183 ± 29 | 200 ± 51 | 0.31* |
| Low density lipoprotein cholesterol, mg/dL | 106 ± 34 | 108 ± 33 | 99.3 ± 32 | 97.2 ± 42 | 0.70 | 103 ± 29 | 106 ± 31 | 99 ± 17.5 | 108 ± 43 | 0.99* |
| High density lipoprotein cholesterol, mg/dL | 51.4 ± 15.5 | 46.8 ± 10.6 | 54.2 ± 20.7 | 47.5 ± 9.6 | 0.87 | 50.7 ± 14.8 | 44.0 ± 10.7 | 55.5 ± 21.6 | 46.3 ± 11.0 | 0.78 |
| Carboxy-terminal collagen, pg/mL | 214 ± 148 | 159 ± 100 | 237 ± 80 | 320 ± 171 | 0.038 | 181 ± 99 | 139 ± 89 | 217 ± 70 | 267 ± 133 | 0.028 |
| Osteocalcin, ng/mL | 11.2 ± 7.6 | 7.3 ± 1.7 | 11.8 ± 3.6 | 12.5 ± 6.3 | 0.18 | 9.9 ± 6.2 | 7.0 ± 2.7 | 10.0 ± 3.2 | 14.8 ± 6.4 | 0.14 |
Values are mean ± sd.
Among groups, anova or Kruskal–Wallis.
We were unable to assess serum human growth hormone (hGH) in our subjects for technical reasons but we were able to measure its surrogate marker, IGF-1. Each treatment lowered the level of IGF-1 in the serum of treated men compared with the baseline values. As seen in Fig. 9, all enclomiphene treatments lowered serum IGF-1 to approximately the same extent and each was lower and significantly different from the transdermal testosterone treatment arm (for 6.25 mg, P = 0.008, for 12.5 mg, P = 0.004; for 25 mg, P = 0.008; MWW test).
Fig. 9.
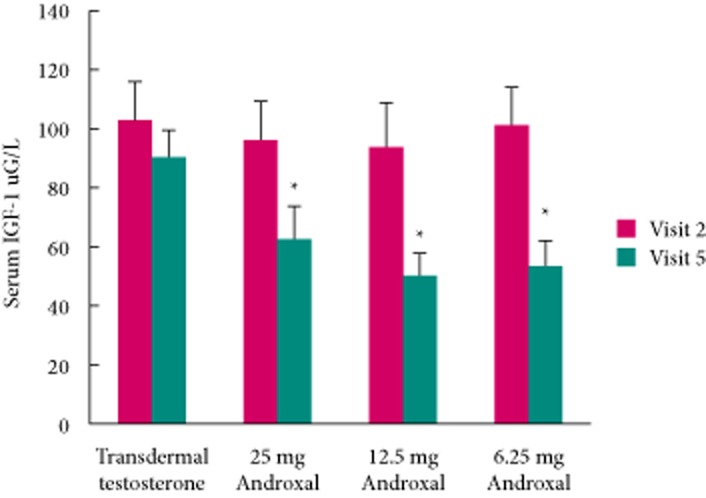
Effect of 6 weeks of treatment on serum IGF-1. Red bars represent pretreatment values, and green bars indicate levels after 6 weeks of treatment.
Discussion
In the present study, we characterized the LH and testosterone levels at baseline and after 6 weeks dosing of enclomiphene citrate 6.25 mg/day, 12.5 mg/day and 25 mg/day and after daily application of transdermal testosterone gel 5 g/day. Each dose of enclomiphene citrate increased the mean concentration of LH (CLHmean) and the CTTmean at 6 weeks. While transdermal testosterone increased the CTTmean, it suppressed the CLHmean at 6 weeks. We have shown that a single, morning total testosterone measurement is an accurate surrogate for determining the effect of enclomiphene citrate on concentration of testosterone in men with secondary hypogonadism. A single morning assessment of total testosterone, CTT0h, predicts 24-h serum total testosterone values CTTmean, as well as CTTmax and CTTmin and can, therefore, be reliably used in monitoring the response of an individual to treatment and for dose escalation for enclomiphene citrate. This also ensures that a single morning value can predict the lack of supraphysiological levels of total testosterone.
The greatest increase in LH was exhibited when doses were escalated from 12.5 to 25 mg, but the 24-h average of total testosterone only increased by 27%, suggesting a dose-limiting effect on the testosterone-producing Leydig cells of the testes, and the inability of enclomiphene citrate to stimulate the production of supernormal levels of testosterone. Additionally, there was also an increase in FSH as has been shown previously for clomiphene in healthy men [24]. Transdermal testosterone, by contrast, significantly suppressed LH after as little as 2 weeks of administration. The relatively higher levels of FSH, compared with those of LH, suggest that sperm count was being stimulated. We have demonstrated this in another study [23].
Comparison of testosterone levels stimulated by enclomiphene citrate with those obtained by application of a transdermal androgen reveals several significant differences. Using data obtained by 24-h sampling at week 6 of treatment, transdermal testosterone caused greater variability in serum testosterone levels (Fig. 1). A higher percentage of samples had total testosterone values <300 ng/dL with transdermal testosterone (50/305 [16.4%]) vs (131/287 [46%]) in the enclomiphene citrate 6.25 mg group, (42/162 [26%]) in the enclomiphene citrate 12.5 mg group and (0/272 [0%]) in the enclomiphene citrate 25 mg group. Second, a higher percentage of samples in the transdermal testosterone group had testosterone levels >1000 ng/dL (17/305 [6%]) vs 0/287 [0%]) in the enclomiphene citrate 6.25 mg group, (0/162 [0%]) in the enclomiphene citrate 12.5 mg group and (2/272 [1%]) in the enclomiphene citrate 25 mg group. Finally, the CTTrange was greater with transdermal testosterone (590 ± 465 ng/dL) than for the enclomiphene citrate 12.5 mg and 25 mg groups (246 ± 106 and 314 ± 65 ng/dL, respectively; P < 0.001). We conclude that the use of enclomiphene citrate, especially at 25 mg, has many fewer outlier values.
The natural daily pattern of total testosterone in young men would be a high morning level, a mid-day trough and a night-time rise. This has not been observed in men >40 years of age [25]. In the case of transdermal testosterone, the increase in total testosterone was evident between the pharmacodynamic studies parts 1 and 2 (Fig. 1A). This increase was also seen for the enclomiphene citrate groups, and most strongly for the 25 mg group (Fig. 1D). The relationship between total testosterone and the time after dosing was variable and there was a clear linear increase in total testosterone over time, especially in the naïve men where transdermal testosterone appeared to work early. As noted, that pattern was variable among subjects. Although all subjects took the study drug in the morning, the patterns seen for enclomiphene citrate were best characterized by a mid-day trough with relatively higher levels in the morning and evening for each individual. All subjects in the enclomiphene citrate arms (Fig. 1B–D) appeared to show the natural daily pattern of fluctuation as seen in young men. The enclomiphene citrate pattern was a poor fit for a linear regression analysis, but fit a second order polynomial regression better for the 6.25 mg (R2 = 0.86), 12.5 mg (R2 = 0.76) and the 25 mg enclomiphene citrate doses (R2 = 0.63). The trough total testosterone for all enclomiphene citrate doses occurred between 8 and 14 h after dosing. It should be noted that this treatment was not dose-dependent, therefore, this observation is interesting, but not conclusive. The transdermal testosterone pattern was a poor fit to either a linear regression or a second order polynomial (R2 = 0.14). The daily pattern for transdermal testosterone was different from that of the enclomiphene citrate treatment. It would be premature to speculate that enclomiphene citrate treatment restores the daily testosterone rhythm that is typical of younger men. A much larger study would be required to explore the phenomenon we see here.
We investigated other pituitary hormones including ACTH, TSH and IGF-I as surrogates for hGH. We also investigated other hormones, lipids and chemistries for safety. The results are shown in Table 4 and summarized above. In general, enclomiphene citrate had few changes in these hormones and markers, with the exception of IGF-1. IGF-1 is secreted by the liver and is regulated in part by hGH levels; however circulating levels also are dependent on the proteins that bind IGF-1 in the circulation. IGF-1 levels were decreased in the men in the enclomiphene citrate groups, but not in the transdermal testosterone group, but the levels remained within the normal physiological ranges. We are uncertain as to the significance of this observation. Testosterone treatment of men with testosterone deficiency usually increases serum estradiol levels, and may increase hGH and IGF-1 levels. Estrogen is known to potentiate secretion of hGH and IGF-1 levels. Enclomiphene citrate increases serum estradiol levels. We suspect that the anti-estrogen effects of enclomiphene citrate are working at either the hypothalamic-pituitary level or possibly on the liver to reduce IGF-1 levels. Unfortunately, technical issues prevented the measurement of hGH levels in the serum samples from these men. It would also be relevant to know if enclomiphene citrate treatment affects the IGF-binding proteins.
A review of the response to the three doses of enclomiphene citrate tested in the present study show that the 12.5 mg dose produces minimal improvement in LH and testosterone compared with the 6.25 mg dose, although the response seems more reliable. The responder analysis suggests that the 12.5 mg dose is superior to the 6.25 mg dose in terms of bringing men into the mid-normal range. The 25 mg dose produces significantly greater levels of LH than either of the two lower doses, which we infer to be the primary result of antagonism of the central oestrogen receptors by enclomiphene citrate. This dose-dependent increase between the 12.5 and 25 mg doses is also evidenced in the significant increase in the maximum concentration and AUC when the dose was increased from 12.5 to 25 mg (data not shown). For these reasons we posit that the 12.5- and 25-mg doses of enclomiphene citrate warrant further clinical development.
Perhaps, the most significant observation in the present study was the restoration of normal levels and pattern of total testosterone plus normal or elevated levels of LH, unlike that seen with the exogenous testosterone treatment. The persistence of serum total testosterone after discontinuation of enclomiphene citrate 25 mg was in sharp contrast to those men who had taken transdermal testosterone. In addition, the effect seen in men does not appear to be the result of increasing serum testosterone, as transdermal testosterone raised total testosterone to approximately the same extent as the highest doses of enclomiphene citrate. The effects were most likely attributable to the effects initiated through the oestrogen antagonist properties of enclomiphene citrate or its metabolite. Since this persistence or ‘legacy’ effect, cannot be attributable to the accumulation of enclomiphene citrate, which is known to have a serum half-life of ∼7 h [26], we believe that it may be the effect of the anti-hormone on a third factor, perhaps on one or more elements of the hypothalamic-pituitary-gonadal axis. This persistence of total testosterone after a drug holiday could be a practical advantage for men who took enclomiphene citrate to increase total testosterone because they would have some confidence that missing one or two dosing days would not mean that their total testosterone would drop precipitously. This effect is distinct from other known testosterone replacement therapies and could lead to an eventual intermittent dosing regimen that could also address the development of tachyphylaxis.
This legacy effect was also found for FSH and LH. It is tempting to speculate that the effect of daily administration of an anti-oestrogen such as enclomiphene citrate results in the restoration of the normal underlying LH and FSH patern that has been lost in men with secondary hypogonadism.
In conclusion, enclomiphene citrate reverses the two hallmarks of secondary hypogonadism, namely, low serum total testosterone and low or inappropriately normal LH. There is also an elevation of serum FSH. Enclomiphene citrate treatment leads to a persistence of its total testosterone elevating activity for at least 1 week after discontinuation by maintaining LH. Transdermal testosterone raised total testosterone but suppressed serum levels of LH and FSH. The elevations of LH, FSH and testosterone in men taking enclomiphene citrate provide mechanisms for the positive effects we have seen on sperm counts, and it contrasts with the suppressive effects of exogenous testosterone delivery systems on sperm counts. The pharmacokinetics of enclomiphene citrate over 24 h differ from the LH and testosterone patterns, emphasizing the likelihood that enclomiphene citrate affects biological mechanisms that persist after serum levels of enclomiphene citrate fall. There appear to be few effects of enclomiphene citrate on other hormones, lipids, or bone variables other than a decrease in IGF-1, suggesting that hGH is influenced but not the other pituitary hormones assessed in the present study. The cause of this latter effect is uncertain, but could be related to a decrease in hGH levels or to anti-oestrogen effects on the liver. These studies indicate that enclomiphene citrate restores LH and testosterone levels, while exogenous testosterone replacement can achieve testosterone levels within the normal range, but it suppresses LH and FSH levels and spermatogenesis.
Acknowledgments
The authors would like to recognize the contributions of Joy Gargis, Jose Guzman, Payton Kehn, Jennifer Nydell, Alexa Weimar and Karen Wong, who monitored the study, Jenna Encina and Nancy Torrence, who interacted with subjects at the study sites, John Shultz of Cetero Labs (Miami Gardens, FL, USA) and Don Tredway who advised on the manuscript.
Glossary
- FDA
Federal Drug Administration
- ITT
intent-to treat
- PP
per protocol
- MWW
Mann–Whitney–Wilcoxin
- CTTmean
mean total testosterone concentration
- CTTmax
maximum total testosterone concentration
- CTTmax
minimum total testosterone concentration
- CTT0h
testosterone concentration at time 0 of each 24-h sampling period
- CTTrange
range of total testosterone concentration
- AUC
area under the curve
- BMI
body mass index
- TSH
thyroid-stimulating hormone
- ACTH
adenocorticotropic hormone
- hGH
human growth hormone
- CLHmean
mean concentration of LH
Conflict of Interest
None declared.
References
- 1.Hill S, Arutchelvam AV, Quinton R. Enclomiphene, an estrogen receptor antagonist for the treatment of testosterone deficiency in men. IDrugs. 2009;12:109–119. [PubMed] [Google Scholar]
- 2.Whitten SJ, Nangia AK, Kolettis PN. Select patients with hypogonadal hypogonadism may respond to treatment with clomiphene citrate. Fertil Steril. 2006;86:1664–1668. doi: 10.1016/j.fertnstert.2006.05.042. [DOI] [PubMed] [Google Scholar]
- 3.Kaminetsky J, Hemani ML. Clomiphene citrate and enclomiphene for treatment of hypogonadal androgen deficiency. Expert Opin Investig Drugs. 2009;18:1–9. doi: 10.1517/13543780903405608. [DOI] [PubMed] [Google Scholar]
- 4.Pitteloud N, Mootha VK, Dwyer AA, et al. Relationship between testosterone levels, insulin sensitivity, and mitochondrial function in men. Diabetes Care. 2005;28:1636–1642. doi: 10.2337/diacare.28.7.1636. [DOI] [PubMed] [Google Scholar]
- 5.Pitteloud N, Hardin M, Dwyer AA, et al. Increasing insulin resistance is associated with a decrease in Leydig cell testosterone secretion in men. J Clin Endocrinol Metab. 2005;90:2636–2641. doi: 10.1210/jc.2004-2190. [DOI] [PubMed] [Google Scholar]
- 6.Lunenfeld B. Testosterone deficiency and the metabolic syndrome. Ageing Male. 2007;10:53–56. doi: 10.1080/13685530701390800. [DOI] [PubMed] [Google Scholar]
- 7.Kalyani RR, Dobbs AS. Androgen deficiency, diabetes, and the metabolic syndrome. Curr Opin Endocrinol Diabetes Obes. 2007;14:226–234. doi: 10.1097/MED.0b013e32814db856. [DOI] [PubMed] [Google Scholar]
- 8.Guay AT, Jacobson J. The relationship between testosterone levels, the metabolic syndrome (by two criteria), and insulin resistance in a population of men with organic erectile dysfunction. J Sex Med. 2007;4(4 Pt 1):1046–1055. doi: 10.1111/j.1743-6109.2007.00530.x. [DOI] [PubMed] [Google Scholar]
- 9.Laughlin GA, Barrett-Connor E, Bergstrom J. Low serum testosterone and mortality in older men. J Clin Endocrinol Metab. 2008;93:68–73. doi: 10.1210/jc.2007-1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haffner SM, Karhapaa P, Mykkanen L, Lasakso M. Insulin resistence, body fat distribution, and sex hormones in men. Diabetes. 1994;43:212–219. doi: 10.2337/diab.43.2.212. [DOI] [PubMed] [Google Scholar]
- 11.Tajar A, Forti G, O'Neill TW, et al. Characterisitcs of secondary, primary, and compensated hypogonadism in ageing men; evidence from the European Male Ageing Study. J Clin Endocrinol Metab. 2010;95:1810–1818. doi: 10.1210/jc.2009-1796. [DOI] [PubMed] [Google Scholar]
- 12.Hoffman AR, Crowley WF., Jr Induction of puberty in men by long-term pulsatile administration of low-dose gonadotropin-releasing hormone. N Engl J Med. 1982;307:1237–1241. doi: 10.1056/NEJM198211113072003. [DOI] [PubMed] [Google Scholar]
- 13.Pitteloud N, Dwyer AA, DeCruz S, et al. Ihibition of luteinizing hormone secretion by testosterone in men requires aromatization for its pituitary but not its hypothalamic effects: evidence from the tandem study of normal and gonadotropin-releasing hormone-deficient men. J Clin Endocrinol Metab. 2008;93:784–791. doi: 10.1210/jc.2007-2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pitteloud N, Dwyer AA, DeCruz S, et al. The relative role of gonadal sex steroids and gonadotropin-releasing hormone pulse frequency in the regulation of follicle-stimulating hormone secretion in men. J Clin Endocrinol Metab. 2008;93:2686–2692. doi: 10.1210/jc.2007-2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sykiotis GP, Hoang XH, Avbell M, et al. Congenital idiopathic hypogonadotropic hypogonadism: evidence of defects in the hypothalamus, pituitary, and testes. J Clin Endocrinol Metab. 2010;95:3019–3027. doi: 10.1210/jc.2009-2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gan EH, Quinton R. Physiological significance of the rhythmic secretion of hypothalamic and pituitary hormones. Prog Brain Res. 2010;181:111–126. doi: 10.1016/S0079-6123(08)81007-2. [DOI] [PubMed] [Google Scholar]
- 17.Bremner WJ, Vitiello MV, Prinz PN. Loss of circadian rhythmicity in blood testosterone levels with aging in normal men. J Clin Endocrinol Metab. 1983;56:1278–1281. doi: 10.1210/jcem-56-6-1278. [DOI] [PubMed] [Google Scholar]
- 18.Keenan DM, Veldhuis JD. Age-dependent regression analysis of male gonadal axis. Am J Physiol Integr Comp Physiol. 2009;297:R1215–1227. doi: 10.1152/ajpregu.90800.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caronia LM, Dwyer AA, Hayden D, Amati F, Pitteloud N, Hayes FJ. Abrupt decrease in serum testosterone levels after an oral glucose load in men: implications for screening for hypogonadism. Clin Endocrinol (Oxf) 2013;78:291–296. doi: 10.1111/j.1365-2265.2012.04486.x. [DOI] [PubMed] [Google Scholar]
- 20.Hayes FJ, Seminara SB, DeCruz S, Boepple PA, Crowley WF., Jr Aromatase inhibition in the human male reveals a hypothalamic site of estrogen feedback. J Clin Endocrinol Metab. 2000;85:3027–3035. doi: 10.1210/jcem.85.9.6795. [DOI] [PubMed] [Google Scholar]
- 21.van As A, Wiehle RD. Enclomiphene citrate (Androxal™) decreases serum glucose in men with idiopathic hypogonadotrophic hypogonadism. The 90th Annual meeting of the Endocrine Society, June 17, 2008, San Francisco, CA.
- 22.Anonymous. 2000. AndroGel 1% (testosterone gel) package insert from Unimed Pharmaceuticals, Inc., Deerfield IL, d 9/2000.
- 23.Kaminetsky J, Werner M, Fontenot G, Wiehle RD. Oral enclomiphene citrate stimulates the endogenous production of testosterone and sperm counts in men with low testosterone: comparison with testosterone gel. J Sex Med. 2013;10:1628–1635. doi: 10.1111/jsm.12116. [DOI] [PubMed] [Google Scholar]
- 24.Tenover JS, Dahl KD, Hsieh AJ, Lim P, Matsumoto AM, Bremner WJ. Serum bioactive and immunoreactive follicle-stimulating hormone levels and the response to clomiphene citrate in healthy young and elderly men. J Clin Endocrinol Metab. 1987;634:1103–1108. doi: 10.1210/jcem-64-6-1103. [DOI] [PubMed] [Google Scholar]
- 25.Luboshitzky R, Shen-Orr X, Herer P. Middle-aged men secrete less testosterone at night than young healthy men. J Clin Endocrinol Metab. 2003;88:3160–3166. doi: 10.1210/jc.2002-021920. [DOI] [PubMed] [Google Scholar]
- 26.Mikkelson TJ, Kroboth PD, Cameron WJ, Dittert LW, Manberg PJ. Single dose pharamacokinetics of clomiphene citrate in normal volunteers. Fertil Steril. 1986;46:392–396. doi: 10.1016/s0015-0282(16)49574-9. [DOI] [PubMed] [Google Scholar]