Abstract
Shiga toxin-producing E. coli O157:H7 and non-O157 have been implicated in many foodborne illnesses caused by the consumption of contaminated fresh produce. However, data on their persistence in soils are limited due to the complexity in datasets generated from different environmental variables and bacterial taxa. There is a continuing need to distinguish the various environmental variables and different bacterial groups to understand the relationships among these factors and the pathogen survival. Using an approach called Topological Data Analysis (TDA); we reconstructed the relationship structure of E. coli O157 and non-O157 survival in 32 soils (16 organic and 16 conventionally managed soils) from California (CA) and Arizona (AZ) with a multi-resolution output. In our study, we took a community approach based on total soil microbiome to study community level survival and examining the network of the community as a whole and the relationship between its topology and biological processes. TDA produces a geometric representation of complex data sets. Network analysis showed that Shiga toxin negative strain E. coli O157:H7 4554 survived significantly longer in comparison to E. coli O157:H7 EDL 933, while the survival time of E. coli O157:NM was comparable to that of E. coli O157:H7 EDL 933 in all of the tested soils. Two non-O157 strains, E. coli O26:H11 and E. coli O103:H2 survived much longer than E. coli O91:H21 and the three strains of E. coli O157. We show that there are complex interactions between E. coli strain survival, microbial community structures, and soil parameters.
Keywords: Shiga toxin, contamination, survival time, fresh produce, organic, conventional
Introduction
Food-borne outbreaks associated with contaminated produce have heightened concerns about the adequacy of control measures for the safe production of fresh fruits and vegetables. In the past decade, there have been over 70 fresh produce-related outbreaks in the United States, and the risk and burden is continuous (Brandl, 2006; Lynch et al., 2009). These vegetables have been implicated in approximately 20 outbreaks resulting in approximately 700 illnesses and 20 deaths between 1996 and 2006 (Doyle and Erickson, 2008; Allerberger and Sessitsch, 2009). Although there are leafy green vegetable associated outbreaks caused by Salmonella and Cyclospora, a majority of them have been due to food contamination with Escherichia coli O157:H7 (Sivapalasingam et al., 2004). The most likely mechanisms of E. coli O157: H7 contaminations include contamination from soil amendments (i.e., manure, compost and compost teas), water (irrigation or flooding/runoff from adjacent land), wildlife, and airborne deposition from off-farm activities such as cattle/dairy and manure/composting operations (Franz et al., 2008, 2011; Fremaux et al., 2008; van Elsas et al., 2011). One of the worst incidents to date was a multistate Escherichia coli O157:H7 outbreak in August and September 2006, which was associated with consumption of fresh, bagged spinach that was traced to a field in California (California Food Emergency Response Team, 2007a,b; Cooley et al., 2007; Jay et al., 2007). During this outbreak, the CDC reported over 200 illnesses, 104 hospitalizations and 3 deaths.
Although E. coli O157:H7 is reported to be the predominant STEC serotype in the United States, more than 200 non-O157 STEC serotypes have been identified in animals or foods (Karch et al., 2005). Approximately, 60 of these serotypes have been incriminated in human diseases. Recent epidemiological studies have recognized additional non-O157 serotypes, including O26, O45, O91, O103, O104, O111, O113, O121, and O145, among STEC strains that were linked to severe human disease in the United States, Europe and parts of Latin America (Brooks et al., 2005; Caprioli et al., 2005; Bettelheim, 2007; Mathusa et al., 2010; Beutin and Martin, 2012).
The mechanisms by which the pathogen is introduced into the produce are not fully understood; however, it is hypothesized that plants become contaminated when grown in fields fertilized with improperly treated manure (Beuchat, 1999) or flood irrigation with water contaminated with cattle feces or contact with contaminated surface runoff (Hillborn et al., 1999; Ibekwe et al., 2004). Depending on the soil properties and environmental factors, the survival time of E. coli O157:H7 in soils varies from 1 week to 6 months, and even longer in some extreme cases (Maule, 2000; Mubiru et al., 2000; Jiang et al., 2002; Ibekwe et al., 2007, 2011; Franz et al., 2008; Semenov et al., 2008; Ibekwe and Ma, 2011; Ma et al., 2011).
In this study, we integrated environmental data with microbial community to assess relationships among these factors and the pathogen survival. To this end, we propose a systematic evaluation of the relative effectiveness of current and potential new intervention strategies to reduce or prevent contamination of produce by employing a new analysis method called topological data analysis (TDA) (Carlsson, 2009; Lum et al., 2013), to uncover environmental variables that are correlated with survival of E. coli O157. TDA is based on an area of mathematics called topology and its implementation allows topological techniques to be used to discover subtle signals or “shape” in complex data such as this dataset. This approach has been used in the past to discover hard-to-identify signal in other complex datasets around viral evolution, breast cancer, diabetes and effects on the metagenome due to environmental stress (Nicolau et al., 2011; Chan et al., 2013; Probst et al., 2014; Sarikonda et al., 2014). We used TDA to reconstruct the relationship structure of E. coli O157:H7 and non-O157 survival in 32 soils (16 organic, 16 conventional) from California (CA) and Arizona (AZ) with a multi-resolution output. We show that differential survivability of various E. coli strains are dependent on microbial community structures and soil parameters.
Materials and methods
Datasets and bacterial strains for the dataset
Environmental and metagenomic data were obtained from three studies of the survival pattern of E. coli O157:H7 and non-O157 from produce growing region of California and Arizona. The first study (Ma et al., 2012) examined the effects of environmental variables on the survival of E. coli O157:H7 EDL 933. The second study (Ma et al., 2013) examined the effects of 454 FLX-derived sequences from the same soils on survival of E. coli O157:H7 EDL933. The third study (Ma et al., 2014) examined the effects of environmental variables on the survival of E. coli O157:H7 and non-O157. All of the E. coli strains used in this study are described in Table 1. All soil properties are as reported by Ma et al. (2012).
Table 1.
E. coli O157 and non-O157 strains used for the study.
| Strain* | Source | stx1 | stx2 | eae | hylA | References |
|---|---|---|---|---|---|---|
| E. coli O26:H11 | cow, Ontario, Canada | − | + | + | + | Louie et al., 1998 |
| E. coli O103:H2 | cow, Ontario, Canada | + | + | + | + | Louie et al., 1998 |
| E. coli O91:H21 | Human, OH, USA | + | − | − | + | Ito et al., 1990 |
| E. coli O157 NM | –, AL, USA | + | + | + | + | Fields et al., 1997 |
| E. coli O157:H7 4554 | cow, Japan | − | − | + | + | Feng et al., 2001 |
| E. coli O157:H7 EDL933 | human, USA | + | + | + | + | Perna et al., 2001 |
stx1, a gene coding for Shiga toxin1, stx2, a gene coding for Shiga toxin2, eae, a gene coding for intimin, and hylA, a gene coding for hemolysin. “+” and “−” indicate a gene was identified and not identified in a given E. coli strain, respectively. E. coli O157:H7 4554 is therefore stx negative.
−Indicates the source was not identified.
Adapted from Ma et al. (2014).
Collection, characterization, inoculation of soils samples, and survival
Soil samples were collected from three major fresh produce growing areas: Salinas Valley California, Imperial Valley, southern California, and Yuma, Arizona (Ma et al., 2012). E. coli O157:H7 culture, a 1.0 ml aliquot was transferred into a 250 ml flask containing 100 ml LB (Luria-Bertani) broth, and incubated at 37°C for 18 h to achieve early stationary phase. The cells were harvested by centrifugation at 3500 g (Beckman, Brea, CA), washed three times using 10 mM phosphate buffer (10 mM, pH 7.2), and finally resuspended in deionized water, and cells were added in soils to a final density of about 0.5 × 107 CFU per gram soil dry weight (gdw−1) according to a method slightly adapted from Franz et al. (2008). About 500 g of the inoculated soil was transferred to a plastic bag which was closed but which had some holes at the top to allow air exchange for survival studies. The inoculated soils were sampled (1 g) at days 0, 3, 6, 10, 14, 20, 27, 34, 40, and 48 to determine the survival of E. coli O157 and non-O157 over time. Details of the experimental procedure had previously been described (Ma et al., 2012).
Soil DNA extraction, pyrosequencing and sequence data analysis
Community DNA was extracted from 32 leafy green-producing soils using a Power Soil Extraction Kit (MO BIO Laboratories, CA) with the bead-beating protocol supplied by the manufacturer. The quality and concentration of the soil DNA were assessed using a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies, DE). The overall size of the soil DNA was checked by running an aliquot of soil DNA on a 1.0% agarose gel. The soil DNA samples (15.0 μl) were then submitted to Research and Testing Laboratories (Lubbock, TX) for PCR optimization and pyrosequencing analysis. Bacterial tag-encoded FLX amplicon pyrosequencing were carried out as previously described (Acosta-Martinez et al., 2008; Acosta-Martínez et al., 2010). The 16S universal Eubacterial primers 530F (5′-GTG CCA GCM GCN GCG G) and 1100R (5′-GGG TTN CGN TCG TTG) were used for amplifying the ~600 bp region of 16S rRNA genes. Primer and PCR optimizations were done at the Research and Testing Laboratories (Lubbock, TX) according to the protocol described previously (Acosta-Martínez et al., 2010; Gontcharova et al., 2010; Nonnenmann et al., 2010). All FLX related procedures were performed following Genome Sequencer FLX System manufacturers instructions (Roche, NJ, USA). Bacterial pyrosequencing population data were further analyzed by performing multiple sequence alignment techniques using the dist.seqs function in MOTHUR, version 1.9.1 (Schloss et al., 2009).
Data analysis
All data were analyzed using the Ayasdi software (http://www.ayasdi.com). The Ayasdi software uses TDA as a framework for a large repertoire of statistical and machine learning methods. The description of the implementation of TDA as a software is described in detail in the following publication (Lum et al., 2013). Briefly, the output consists of a topological network with nodes and edges, where nodes are collections of data points and an edge connects any two nodes that have one or more common data points. In this analysis, the mathematical functions (called “lenses” in the software) used are principal metric SVD 1 and 2. Principal metric SVD lenses are used when the distance metric used is non-Euclidean. Statistical test used to look at significance between sub-networks or groups is the non-parametric Kolmogorov-Smirnov test (KS score). Variables used in the analysis are the following: chemical (Sodium (Na), iron (Fe), potassium (K), electrical conductivity or salinity (EC), copper (Cu), assimilable organic carbon (AOC), total nitrogen (TN), calcium (Ca), Nickel (N), organic carbon (OC), microbial biomass carbon (MBC), sulfate (SO4), water holding capacity (WHC), magnesium (Mg), zinc (Zn), phosphate (PO4), molybdenum (Mo), physical (sand, clay, silt, and bulk density) and biological (time till detection for E. coli O157:H7 EDL933 [ttd(d)], time till detection for E. coli O157:H7 strain 4555 [ttd (d) O157-4554], time till detection for E. coli O157:H7 non-motile strain 4555 [ttd (d) O157NM], time till detection for E. coli O91 [ttd (d) O91], time till detection for E. coli O26 [ttd (d) O26], operation taxonomic units (OTUs), Nitrospira, diversity index (H′), Proteobacteria, Alphaproteobacteria, Chloroflexi, Bacteroidetes, Acidobacteria, Actinobacteria, Gemmatimonadetes, Firmicutes, Verrucomicrobia, Deltaproteobacteria, Gammaproteobacteria, Planctomycetes, Betaproteobacteria).
Results
Soil sample site similarities and management network
Using the properties of physical, chemical, and biological characteristic of these soil samples as variables, we clustered the soil samples using TDA. The resulting network represents the soil samples clustering into sub-networks. Figure 1A shows 4 sub-networks A, B, C and D with B and C connecting to form a larger sub- network. There is also a singleton (1 node comprising of 2 soil samples from Salinas that stood apart from everything else). The network can also be colored by various factors and characteristics such as location and soil management type for visualization (Figure 1). In addition, we can also apply statistics to probe what factors distinguished our soils into sub-networks. We found that “location” was one of the key differences between the sub-networks (Kolmogorov-Smirnov test PV < 0.0003). In order to visualize the effect of “location” on the soil samples, Figure 1 is colored by “location.” We show that soil samples from the Salinas areas (A) completely formed a separate sub-network from soil samples from the Imperial and Yuma areas (B, C, and D) as indicated by the color. This indicates that physical, chemical, and biological characteristic of these soil samples collectively are quite different from location to location, especially the soil samples from Salinas, which formed a distinct sub-network (A). Soil samples from Yuma and Imperial are closer to each, forming a sub-network that looks like a dumb bell, with some samples from Imperial clustering at left side of dumb bell (B) and the rest of the network comprised of a mixture between samples from Yuma and Imperial. Interestingly, physical, chemical, and biological properties measured of these soil samples did not differentiate between conventional and organic soil management as seen from the non-enrichment of any one type of soil management in the network (also see Table 2, where the P-value for soil management as a differentiating factor between those sub-networks was 0.4126). To further investigate sub-network D and the singleton, another network analysis was performed using the same distance metric and mathematical lenses but at a lower resolution (20 instead of 30). Sub-network D, which comprised of samples from Yuma and Imperial, became part of sub-network C (Figure 1C). The singleton however remained a singleton, indicating that these samples are fundamentally different from the rest of the samples due to unknown reasons including quality of the samples.
Figure 1.
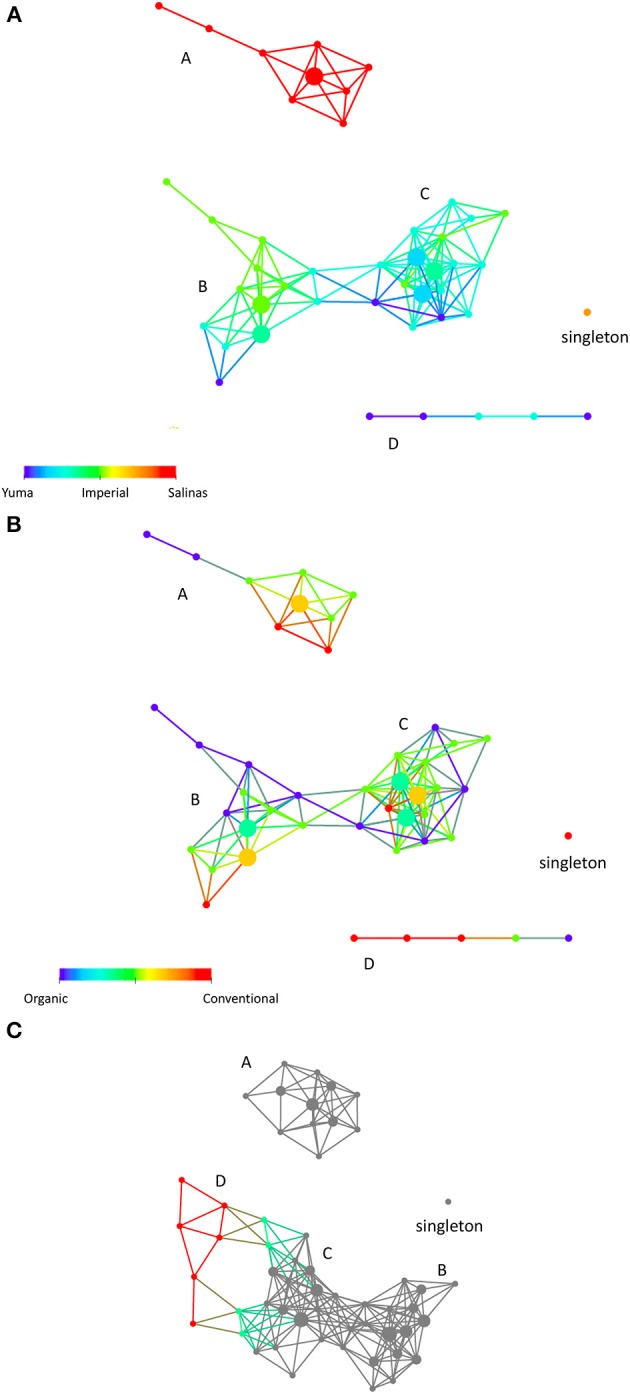
(A) Sample-sample relationships in a topological network. Using physical, chemical, and biological characteristics of the samples, we obtained a network that comprised of 4 sub-networks (A–D) and a singleton (single node comprising of 2 samples). The coloring here is by location, where each location is given a color (Salinas is red, Imperial Valley is green and Yuma is blue). Nodes that have a mixture of soils have colors in between as depicted in the color bar. Sub-networks structure indicates that physical, chemical, and biological characteristics primarily segregate the samples according to location, with Salinas being most different from soils from Yuma and Imperial Valley, (B). The same network is colored by soilmanagement type (organic (represented by 0) vs. conventional (represented by 1). The red nodes represent samples with conventional soil management while the blue nodes represented the organic soil management. The green and orange colored nodes represented mixed organic and conventional soil management with varying percent of mixture of the two types of management. (C) Another network is built using the same parameters except for resolution. The soil samples are analyzed at a lower resolution to ask if structure (D) and the singleton will merge with any part of the sub-networks. Sub-network (D), which comprised of samples from Yuma and Imperial, became part of sub-network (B) (colored nodes). Samples from sub-network (D) are not part of the gray nodes. The singleton however remained a singleton. The size of each node reflects the number of data points contained in the node. For (A,B), the distance metric and filters were Person correlation and Principal Metric SVD and secondary metric SVD. Metric, Norm Correlation; Lens, Principal Metric SVD value (Resolution 30, Gain 4.0x, Equalized) Secondary Metric SVD Value (Resolution 30, Gain 4.0x, Equalized). For (C), all analysis parameters remained the same except for resolution (20 instead of 30).
Table 2.
Kolmogorov-Smirnov test and t-test to identify soil and biological properties that best differentiate between Salinas Valley and Imperial/Yuma Valley locations.
| Column name* | Signed KS-score | KS-score | t-test p-value |
|---|---|---|---|
| Clay | −0.8571 | 0.8571 | 0.0012 |
| Na+ | −1 | 1 | 1.05E–07 |
| Fe | 0.7321 | 0.7321 | 0.0299 |
| ttd(d) | 0.8125 | 0.8125 | 8.18E–05 |
| K+ | −0.5446 | 0.5446 | 0.0848 |
| EC | −1 | 1 | 9.07E–06 |
| Nitrospira | 0.75 | 0.75 | 0.0014 |
| Cu | −0.3660 | 0.3660 | 0.0607 |
| tdd(d)_O1574554 | −0.6666 | 0.6666 | 0.4823 |
| Diversity index (H′) | 0.7321 | 0.7321 | 0.0026 |
| Molybdenum | −0.8125 | 0.8125 | 3.62E–04 |
| Proteobacteria | −0.8125 | 0.8125 | 1.63E–04 |
| WSOC | 0.6696 | 0.6696 | 0.0259 |
| T-N | 0.375 | 0.375 | 0.1693 |
| Ca++ | −0.875 | 0.875 | 5.08E–04 |
| Ni | 0.4375 | 0.4375 | 0.1660 |
| Alphaproteobacteria | 0.6875 | 0.6875 | 0.0047 |
| OC | −0.6517 | 0.6517 | 0.2614 |
| MBC | −0.3839 | 0.3839 | 0.3520 |
| SO4– | −0.9375 | 0.9375 | 4.55E–05 |
| pH | −0.5714 | 0.5714 | 0.0259 |
| Chloroflexi | 0.5446 | 0.5446 | 0.03441 |
| tdd(d)_O157NM | 0.6666 | 0.6666 | 0.0995 |
| Sand | 0.7321 | 0.7321 | 0.0027 |
| Bulk density | −0.5446 | 0.5446 | 0.0162 |
| Bacteroidetes | 0.7321 | 0.7321 | 0.0042 |
| WHC | −0.5267 | 0.5267 | 0.2032 |
| tdd(d)_O91 | 0.875 | 0.875 | 4.12E–06 |
| Acidobacteria | 1 | 1 | 0.0197 |
| Actinobacteria | 0.6071 | 0.6071 | 0.0240 |
| Mg++ | −0.7142 | 0.7142 | 0.0012 |
| tdd(d)_O26 | 1 | 1 | 0.0796 |
| Gemmatimonadetes | 0.75 | 0.75 | 0.0012 |
| Silt | −0.4821 | 0.4821 | 0.0403 |
| Firmicutes | 0.4821 | 0.4821 | 0.1989 |
| Verrucomicrobia | 0.4375 | 0.4375 | 0.3981 |
| Deltaproteobacteria | 0.9375 | 0.9375 | 8.55E–06 |
| Gammaproteobacteria | −0.8125 | 0.8125 | 6.92E–05 |
| Zn | −0.5892 | 0.5892 | 0.0056 |
| Planctomycetes | 0.8571 | 0.8571 | 0.0044 |
| PO4— | 1 | 1 | 0.0399 |
| tdd(d)_O103 | 0.875 | 0.875 | 2.84E–06 |
| Betaproteobacteria | 0.75 | 0.75 | 1.95E–05 |
| OTUs | −0.75 | 0.75 | 0.0015 |
| Location | 0.875 | 0.875 | 3.04E–06 |
| Management | −0.258 | 0.258 | 0.4128 |
Meaning of abbreviations under column names: Na, Sodium; Fe, iron; ttd(d), time till detection for E. coli O157:H7 strain 933; K, potassium; EC, electrical conductivity; Cu, copper; ttd (d) O157–4554, time till detection for E. coli O157:H7 strain 4555; AOC, assimilable organic carbon; TN, total nitrogen; Ca, calcium; N, Nickel; OC, organic carbon; MBC, microbial biomass carbon; SO4, sulfate; ttd (d) O157NM, time till detection for E. coli O157:H7 non-motile strain 4555; WHC, water holding capacity; ttd (d) O91,time till detection for E. coli O91; Mg, magnesium; ttd (d) O26, time till detection for E. coli O26; Zn, zinc; PO4, phosphate; OTUs, operation taxonomic units. Signed KS score: the minus sign indicates that the attribute indicated in the column name is on average smaller in value in Salinas Valley compared to Imperial/Yuma Valley locations.
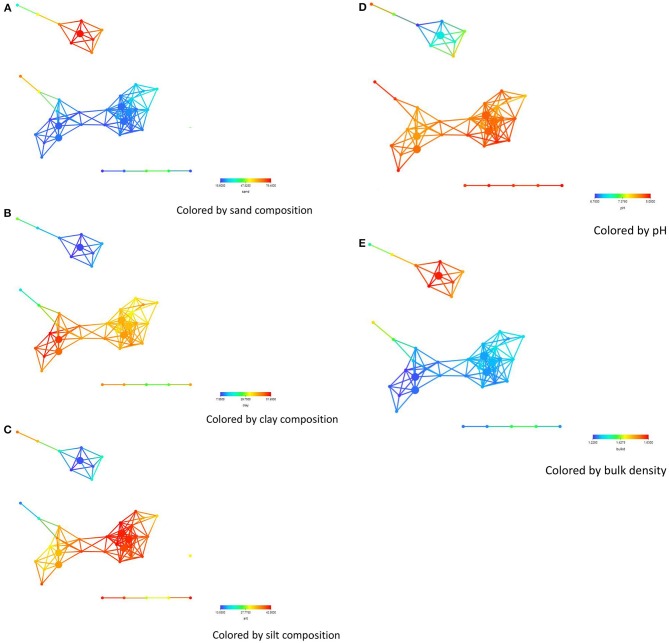
Statistical analysis to identify key distinguishers of these sub-networks were performed on all numerical columns on all data points (Table 2) including detection times, biodiversity measures, management, location, sand, silk, clay, soil pH, bulk density, assimilable organic carbon (AOC), organic carbon (OC), microbial biomass carbon (MBC), electrical conductivity EC), chemical compound (Na+, K+, Ca2+, Mg2+ etc.) and bacterial phyla. Soil sand content was significantly higher (P = 0.0027) for soils from the Salinas Valley area (Figure 2A), whereas silt and clay contents were significantly higher (P = 0.0403 for silk and 0.0012 for clay) in soils from the Imperial and Yuma Valley areas (Figures 2B,C). Soil pH was between 6.7 and 8.0, with significantly higher pH (P = 0.025) occurring in the Yuma/Imperial Valley areas (Figure 2D). Soil bulk density values ranged between 1.22 and 1.63 mg, with soils from the Salinas Valley having significantly higher bulk densities (P = 0.0162; Figure 2E). Statistical tests indicated that total iron, PO4 and calcium were significantly higher (P = 0.0299; 0.0399; 5.08E−4, respectively) in Salinas Valley samples than samples from Yuma and Imperial Valleys. On the other hand, sodium and sulfate were significantly higher (1.05E−07; −05, respectively) in Yuma and Imperial Valley samples. No differences were observed among the locations in soil contents of total nitrogen (TN).
Figure 2.
Network colored by soil physical property concentrations-(A) sand, (B) clay, (C) silt, (D) pH, (E) bulk density. The legend below the figures shows ranges in concentrations of some of the soil physical properties. The distance metric and filters are as shown in the legend of Figure 1.
Survival behavior of E. coli O157:H7 in soils
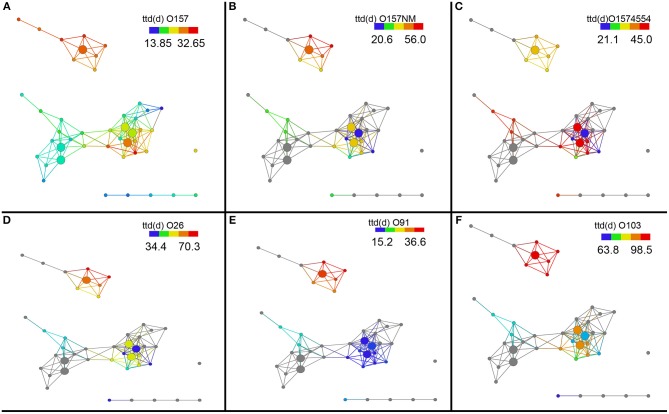
Next, we investigated survival of different E. coli strains in these different soil sub-networks. The network remains the same but we can now probe the network to see if any survival variables show any significant trends between these sub-networks. To do this we colored the same network by the length of survival of E. coli O157:H7 EDL933, E. coli O157:NM, and E. coli O157 strain 4554 (stx-) across the topological network to observe if differences exist in the soil networks. The shortest survival time (ttd) was observed for E. coli O157:H7 EDL933 (13.8–32.6 days) while the longest was observed for E. coli O157NM (20.6–56.0 days) and E. coli O157:H7 strain 4554 as intermediate at 21.1–45.0 days (Figures 3A–C). Figure 3 is colored by survival time of the indicated strain for all the soils. We also performed statistical test on the survival times and show that the survival time of E. coli O157:H7 EDL933 was significantly longer in soils from the Salinas Valley area (8.18E–05), whereas the survival time of E. coli O157:NM and the stx- E. coli O157:H7 strain 4554 were not significantly different in soils from the Salinas Valley area (0.0995 and 0.4823, respectively) and in soils from the Yuma and Imperial Valley region (Table 2). Furthermore, the coloring pattern indicates no differences in survival (ttd) between organic and conventional soils from Imperial Valley and Salinas. Survival time was much shorter in the organic soil than the conventional soils with E. coli O157:NM. This can be observed by the deep blue color (Figure 3B).
Figure 3.
Topological network data analysis of survival of E. coli O157:H7 and non-O157:H7 across the sub-networks identified in Figure 1A. Survival of E. coli O157:H7 EDL933, E. coli O157:NM, and E. coli O157 strain 4554 (stx-) and shown in (A–C). Survival of non-O157 strains, E. coli O26:H21, E. coli O103:H2, and E. coli O91:H21are shown in (D–F). Gray nodes represent missing ttd (d) measurements for E. coli O157:NM, E. coli O157 strain 4554, E. coli O26:H21, E. coli O103:H2, and E. coli O91:H21. The distance metric and filters are as shown in Figure 1.
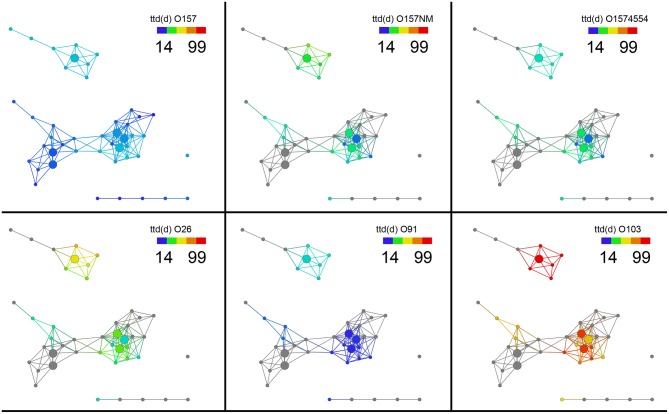
Survival of non-O157 in soils was longer that E. coli O157:H7 except E. coli O91.H21. It was found that two non-O157 strains, E. coli O26:H21 and E. coli O103:H2 survived much longer that E. coli O91:H21. The three non-O157 strains survived significantly longer (E. coli O91.H21: 4.12E–06, O26:H21:0.079, and 0103: 2.84E–06) in soils from the Salinas Valley region than in soils from the Yuma and Imperial Valleys (Table 2). There were no differences of survival between organic and conventionally managed soils with the non-O157 strains. In the current study no isogenic strains (with and without stx) were used. When the six E. coli O157 and non-O157 strains were grouped together on the same scale it was shown that E. coli O103:H2 survived the longest in all the soils, followed by E. coli O26:H21 (Figure 4).
Figure 4.
Survival of six E. coli O157 and non-O157 strains grouped together on a normalized scale showed that E. coli O103:H2 survived the longest in all the soils, followed by E. coli O26:H21. Gray nodes are as explained in Figure 3. The distance metric and filters are as shown in Figure 1.
Bacterial abundance and distribution as revealed by 454 pyrosequencing
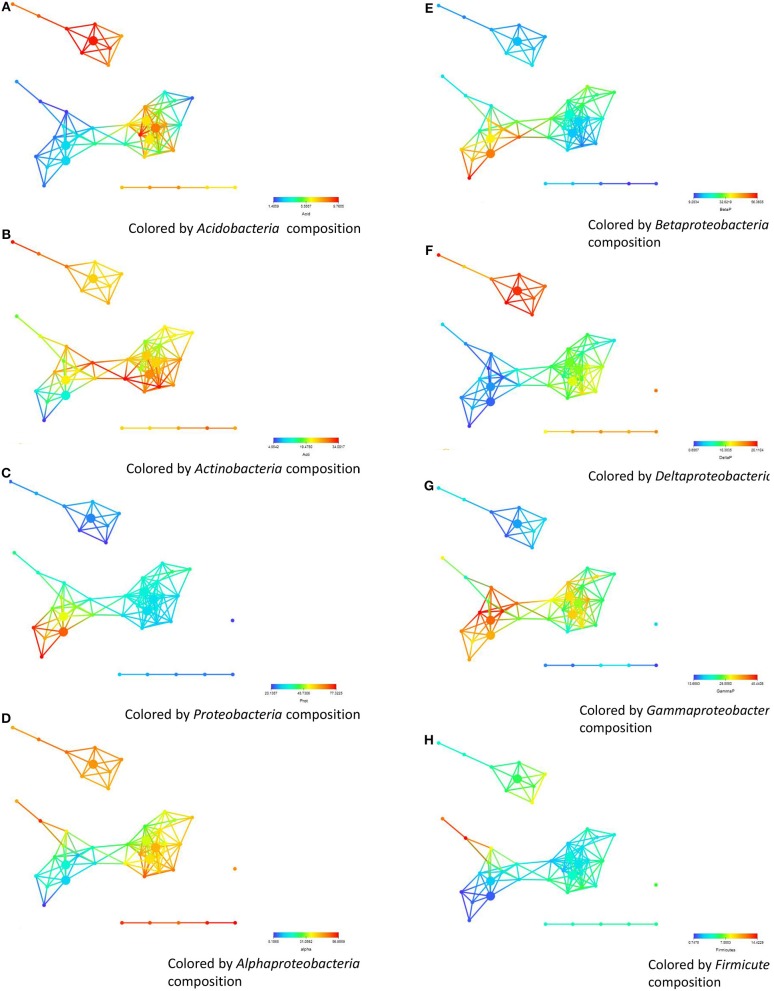
We then analyzed how the abundance and distribution of different bacterial phyla based on pyrosequencing from the 32 soils collected from the three regions associated with the different regions clustered in the networks. By coloring the same networks with now the abundance of the different bacterial phyla, we show that there are marked differences between the distributions of the different bacterial phyla (Figure 5). As shown in Figure 5A the nodes that are colored red indicate significantly higher (P = 0.097) percentage of Acidobacteria (see Table 2 for details of the phyla) in soils from the Salinas Valley area than soils from Yuma and Imperial Valleys. These soils also contained a higher percentage of Deltaproteobacteria (P = 8.55E–06), Alphaproteobacteria (P = 0.0047) (Figures 5D,F) as seen by the color scheme. Significant differences in beta (P = 1.95E–05) and Gammaproteobacteria (P = 6.92E–05) were also observed in soils from Yuma/Imperial Valleys and soils collected from Salinas Valley area (Figures 5E,G). Significant differences were also found in Proteobacteria (P = 1.63E–04) (Figure 5C), Actinobacteria (P = 0.024) (Figure 5B), and no significant differences in Firmicutes (P = 0.1989) (Figure 5H) from the three regions. Further analysis showed that Actinobacteria, Proteobacteria, Acidobacteria, and Bacteroidetes were the dominant phyla among the bacterial communities in soils, and these four phyla accounted for about 75% of the total bacterial composition based on pyrosequencing (Figure S1). The current analysis has produced the same trends as the results obtained with our previous analysis that was based on correlation between survival time and dominant bacterial communities (Ma et al., 2013). In this earlier related study, stepwise multiple regression analysis was conducted and the results showed that EC, TN, and AOC were the most important factors impacting the survival of E. coli O157:H7 in all soils tested (Figure S2), with EC showing the most negative effect (P < 0.001) on survival and TN and AOC showing positive effects (P < 0.01) (Ma et al., 2012).
Figure 5.
The abundance and distribution of different bacterial phyla based on pyrosequencing and their correlation with survival. (A) soils from Salinas Valley region, sub-networks (B,C) Yuma and the Imperial region. (A–H) represent the coloring of the abundance of Actinobacteria, Acidobacteria, Firmicutes, Proteobacteria, Bacteroidetes, Alpha-, Beta-, Delta-, and Gammaproteobacteria, respectively. The distance metric and filters are as shown in Figure 1.
Discussion
Analysis, interpretation, and visualization of complex data are major tasks confronting researchers today. Most data are presented in tabular formats after traditional statistical analysis. To better understanding the influence of selected soil properties and their impact on bacteria growth, we used TDA as implemented by the Ayasdi software. TDA can analyze disparate datasets in one setting, as well as presents topological networks as an informative visualization for understanding and interpretation. The TDA approach is sensitive to both large and small scale patterns that often fail to be detected by other analysis methods, such as principal component analysis, (PCA), multidimensional scaling, (MDS), and cluster analysis (Carlsson, 2009; Lum et al., 2013). In addition, we note that PCA and MDS produce 2-D scatterplots that are often hard to separate more subtle signal from noise. In addition, clustering methods produce distinct, unrelated groups that may obscure signal that is better captured using TDA, which is inherently suited to look for continuity in signal.
The three key concepts of topological analysis methods include coordinate freeness, which means that topology has the capability to measure properties of intrinsic shapes of data which is independent of the coordinate system. Coordinate free representations are vital when one is studying data collected with different technologies such as pyrosequencing or from survival data as we have used in this study or from different laboratories when the methodologies cannot be standardized (Lum et al., 2013). This is very critical to a study such as ours where the data collected are not from one uniform platform. As mentioned earlier, TDA has also been applied to various different studies to uncover complex signals (Nicolau et al., 2011; Chan et al., 2013; Lum et al., 2013; Romano et al., 2014; Sarikonda et al., 2014).
We have demonstrated that location is an important factor that we found to be associated with high survival of certain bacteria strains. Recent studies of metabolic network topologies across the bacterial tree of life revealed marked variation in network cluster and identified several genetic and environmental determinants affecting metabolic clustering (Parter et al., 2007). These authors showed that reduced metabolic cluster in single-species networks is associated with organisms inhabiting less variable environments. Our analysis, however, presents a unique characterization of microbial community-level cluster and demonstrates consistent differences that are associated with survival of E. coli O157:H7 from different locations. It should be noted that the correlation of certain bacterial phyla (Actinobacteria and Acidobacteria) with higher survival of E. coli O157:H7 does not necessarily mean causation of higher survival, and therefore, should be extrapolated very carefully. As discussed by Greenblum et al. (2012), in silico models of microbial communities are currently still scarce (Oberhardt et al., 2009) and mostly focus on simulated communities comprising a handful of species and on pair-wise interactions among community members (Stolyar et al., 2007; Freilich et al., 2010; Klitgord and Segrè, 2010; Wintermute and Silver, 2010). Experimental validation at the species or gene level of model components and parameters may be necessary for a successful and accurate understanding of individual species effects on survival. In essence, this study represents an important step in the development of a metagenomic systems biology approach. Such an approach can potentially advance metagenomic research in the same way systems biology advanced genomics, appreciating not only the parts list of a system but the complex interactions among parts and the impact of these interactions on function and dynamics.
In summary, the TDA networks identified various environmental factors that correlate with increased or decreased in survival of E. coli O157 in the three regions. In particular, we have identified a group of environmental factors such as EC, TN, AOC, etc. that consistently may enhance or inhibit survival of this pathogen from the three regions, and these factors were in agreement with some of our earlier studies from the same locations (Ma et al., 2012, 2013). We note that the effects of different environmental factors and bacterial community were easily detected by TDA because of the inherent ability of the analysis environment that allows analysis of all these factors simultaneously. Often times classical clustering approaches by themselves will miss these subtle signals because of the need to place data points into one cluster or another. This could end up highlighting only the most obvious signals while breaking up the more subtle ones.
As we move toward better understanding of how E. coli O157:H7 contamination could occur in the food chain, we believe a more holistic approach such as looking at all possible available factors together is important. However, because this creates complexity, there is a need to apply different approaches. We used here an approach to allow not only the mathematical analysis needed to uncover small signal but also the ability to visualize these complex relationships.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This research was supported by CSREES NIFA Agreement No., 2008-35201-18709 and the 214 Manure and Byproduct Utilization Project of the USDA-ARS. We thank Drs Jorge Fonseca of the University of Arizona Yuma, Mark Trent, UC-Davis, Imperial Agricultural Experiment Station, and James McCreight of USDA-ARS Salinas CA for providing soil samples for this study. We also thank Damon Baptista for technical help. Mention of trademark or propriety products in this manuscript does not constitute a guarantee or warranty of the property by the USDA and does not imply its approval to the exclusion of other products that may also be suitable.
Supplementary material
The Supplementary Material for this article can be found online at: http://www.frontiersin.org/journal/10.3389/fcimb.2014.00122/abstract
References
- Acosta-Martinez V., Dowd S., Sun Y., Allen V. (2008). Tag-encoded pyrosequencing analysis of bacterial diversity in a single soil type as affected by management and land use. Soil Biol. Biochem. 40, 2762–2770 10.1016/j.soilbio.2008.07.022 [DOI] [Google Scholar]
- Acosta-Martínez V., Dowd S. E., Bell C. W., Lascano R., Booker J. D., Zobeck T. M., et al. (2010). Microbial community composition as affected by dryland cropping systems and tillage in a semiarid sandy soil. Diversity 2, 910–931 10.3390/d2060910 [DOI] [Google Scholar]
- Allerberger F., Sessitsch A. (2009). Incidence and microbiology of salad-borne disease. CAB Rev. 4, 1–13 10.1079/PAVSNNR20094019 [DOI] [Google Scholar]
- Bettelheim K. A. (2007). The non-O157 Shiga-toxigenic (verocytotoxigenic) Escherichia coli; under-rated pathogens. Crit. Rev. Microbiol. 33, 67–87 10.1080/10408410601172172 [DOI] [PubMed] [Google Scholar]
- Beuchat L. R. (1999). Survival of Escherichia coli O157:H7 in bovine feces applied to lettuce and ineffectiveness of chlorinated water as a disinfectant. J. Food Prot. 62, 845–849 [DOI] [PubMed] [Google Scholar]
- Beutin L., Martin A. (2012). Outbreak of Shiga toxin-producing Escherichia coli (STEC) O104:H4 infection in Germany causes a paradigm shift with regard to human pathogenicity of STEC strains. J. Food Prot. 75, 408–418 10.4315/0362-028X.JFP-11-452 [DOI] [PubMed] [Google Scholar]
- Brandl M. T. (2006). Fitness of human enteric pathogens on plants and implications for food safety. Annu. Rev. Phytopathol. 44, 367–392 10.1146/annurev.phyto.44.070505.143359 [DOI] [PubMed] [Google Scholar]
- Brooks J. T., Sowers E. G., Wells J. G., Greene K. D., Griffin P. M., et al. (2005). Non-O157 Shiga toxin-producing Escherichia coli infections in the United States, 1983-2002. J. Infect. Dis. 192, 1422–1429 10.1086/466536 [DOI] [PubMed] [Google Scholar]
- California Food Emergency Response Team. (2007a). Investigationofan Escherichia Coli O157:H7 Outbreak Associated with Dole Pre-Packaged Spinach. Sacramento, CA: California Department of Public Health Food and Drug Branch [Google Scholar]
- California Food Emergency Response Team. (2007b). Environmental Investigation of Escherichia Coli O157:H7 Outbreak Associated with Taco Bell Restaurants in Northeastern States. Sacramento, CA: California Department of Public Health Food and Drug Branch [CALFERT] [Google Scholar]
- Caprioli A., Morabito S., Brugère H., Oswald E. (2005). Enterohaemorrhagic Escherichia coli: emerging issues on virulence and modes of transmission. Vet. Res. 36, 289–311 10.1051/vetres:2005002 [DOI] [PubMed] [Google Scholar]
- Carlsson G. (2009). Topology and data. Bull. Amer. Math. Soc. 46, 255–308 10.1090/S0273-0979-09-01249-X [DOI] [Google Scholar]
- Chan J. M., Carlsson G., Rabadan R. (2013). Topology of viral evolution. Proc. Natl. Acad. Sci. U.S.A. 110, 18566–18571 10.1073/pnas.1313480110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooley M., Carychao D., Crawford-Miksza L., Jay M. T., Myers C., Rose C., et al. (2007). Incidence and tracking of Escherichia coli O157:H7 in a major produce production region in California. PLoS ONE 2:e1159 10.1371/journal.pone.0001159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle M. P., Erickson M. C. (2008). Summer meeting 2007 – the problems with fresh produce: an overview. J. Appl. Microbiol. 105, 317–330 10.1111/j.1365-2672.2008.03746.x [DOI] [PubMed] [Google Scholar]
- Feng P., Dey M., Abe A., Takeda T. (2001). Isogenic strain of Escherichia coli O157:H7 that has lost both Shiga toxin 1 and 2 genes. Clin. Diagn. Lab. Immunol. 8, 711–717 10.1128/CDLI.8.4.711-717.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields P. I., Blom K., Hughes H. J., Helsel L. O., Feng P., Swaminathan B. (1997). Molecular characterization of the gene encoding H antigen in Escherichia coli and development of a PCR-restriction fragment length polymorphism test for identification of E. coli O157:H7 and O157:NM. J. Clin. Microbiol. 35, 1066–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz E., Semenov A. V., Termorshuizen A. J., de Vos O. J., Bokhorst J. G., van Bruggen A. H. C. (2008). Manure-amended soil characteristics affecting the survival of E. coli O157:H7 in 36 Dutch soils. Environ. Microbiol. 10, 313–327 10.1111/j.1462-2920.2007.01453.x [DOI] [PubMed] [Google Scholar]
- Franz E., van Hoek A. H. A. M., Bouw E., Aarts H. J. M. (2011). Variability of Escherichia coli O157 strain survival in manure-amended soil in relation to strain origin, virulence profile, and carbon nutrition profile. Appl. Environ. Microbiol. 77, 8088–8096 10.1128/AEM.00745-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freilich S., Kreimer A., Meilijson I., Gophna U., Sharan R., Ruppin E. (2010). The large-scale organization of the bacterial network of ecological co-occurrence interactions. Nucleic Acids Res. 38, 3857–3868 10.1093/nar/gkq118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fremaux B., Prigent-Combaret C., Delignette-Muller M. L., Mallen B., Dothal M., Gleizal A., et al. (2008). Persistence of Shiga toxin-producing Escherichia coli O26 in various manure-amended soil types. J. Appl. Microbiol. 104, 296–304 10.1111/j.1365-2672.2007.03532.x [DOI] [PubMed] [Google Scholar]
- Gontcharova V., Young E., Sun Y., Wolcott R. D., Dowd S. E. (2010). A comparison of bacterial composition in diabetic ulcers and contralateral intact skin. Open Microbiol. J. 4, 8–19 10.2174/1874285801004010008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenblum S., Turnbaugh P. J., Borenstein E. (2012). Metagenomic systems biology of the human gut microbiome reveals topological shifts associated with obesity and inflammatory bowel disease. Proc. Natl. Acad. Sci. U.S.A. 109, 594–599 10.1073/pnas.1116053109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillborn E. D., Mermin J. H., Mshar P. A., Hadler J. L., Voetsch A., Wojtkunski C., et al. (1999). A multistate outbreak of Escherichia coli O157:H7 infections associated with consumption of mesclun lettuce. Arch. Internal Med. 159, 1758–1764 10.1001/archinte.159.15.1758 [DOI] [PubMed] [Google Scholar]
- Ibekwe A. M., Grieve C. M., Yang C.-H. (2007). Survival of Escherichia coli O157:H7 in soil and on lettuce after soil fumigation. Can. J. Microbiol. 53, 623–635 10.1139/W07-003 [DOI] [PubMed] [Google Scholar]
- Ibekwe A. M., Ma J. (2011). Effects of fumigants on microbial diversity and persistence of E. coli O15:H7 in contrasting soil microcosms. Sci. Total Environ. 409, 3740–3748 10.1016/j.scitotenv.2011.06.033 [DOI] [PubMed] [Google Scholar]
- Ibekwe A. M., Papiernik S. K., Grieve C. M., Yang C.-H. (2011). Quantification of persistence of Escherichia coli O157:H7 in contrasting soils. Int. J. Microbiol. 2011:421379 10.1155/2011/421379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibekwe A. M., Watt P. M., Shouse P. J., Grieve M. C. (2004). Fate of Escherichia coli O157:H7 in irrigation water on soil and plants as validated by culture method and real-time PCR. Can. J. Microbiol. 50, 1007–1014 10.1139/w04-097 [DOI] [PubMed] [Google Scholar]
- Ito H., Terai A., Kurazono H., Takeda Y., Nishibuchi M. (1990). Cloning and nucleotide sequencing of Vero toxin 2 variant genes from Escherichia coli O91:H21 isolated from a patient with the hemolytic uremic syndrome. Microb. Pathog. 8, 47–60 10.1016/0882-4010(90)90007-D [DOI] [PubMed] [Google Scholar]
- Jay M. T., Cooley M., Carychao D., Wiscomb G. W., Sweitzer R. A., Crawford-Miksza L., et al. (2007). Escherichia coli O157:H7 in feral swine near spinach fields and cattle, Central California Coast. Emerg. Infect. Dis. 13, 1908–1911 10.3201/eid1312.070763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X., Morgan J., Doyle M. P. (2002). Fate of Escherichia coli O157:H7 in manure-amended soil. Appl. Environ. Microbiol. 68, 2605–2609 10.1128/AEM.68.5.2605-2609.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karch H., Tarr P. I., Bielaszewska M. (2005). Enterohaemorrhagic Escherichia coli in human medicine. Int. J. Med. Microbiol. 295, 405–418 10.1016/j.ijmm.2005.06.009 [DOI] [PubMed] [Google Scholar]
- Klitgord N., Segrè D. (2010). Environments that induce synthetic microbial ecosystems. PLoS Comput. Biol. 6:e1001002 10.1371/journal.pcbi.1001002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louie M., Read S., Simor A. E., Holland J., Louie L., Ziebell K., et al. (1998). Application of multiplex PCR for detection of non-O157 verocytotoxin-producing Escherichia coli in bloody stools: identification of serogroups O26 and O111. J. Clin. Microbiol. 36, 3375–3377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lum P. Y., Singh G., Lehman A., Ishkanov T., Vejdemo-Johansson M., Alagappan M., et al. (2013). Extracting insights from the shape of complex data using topology. Sci. Rep. 3:1236 10.1038/srep01236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch M. F., Tauxe R. V., Hedberg C. W. (2009). The growing burden of foodborne outbreaks due to contaminated fresh produce: risks and opportunities. Epidemiol. Infect. 137, 307–315 10.1017/S0950268808001969 [DOI] [PubMed] [Google Scholar]
- Ma J., Ibekwe A. M., Crowley D. E., Yang C.-H. (2012). Survival of Escherichia coli O157:H7 in major leafy green producing soils. Environ. Sci. Tech. 46, 12154-12161 10.1021/es302738z [DOI] [PubMed] [Google Scholar]
- Ma J., Ibekwe A. M., Crowley D. E., Yang C.-H. (2014). Persistence of Escherichia coli O157 and non-O157 strains in agricultural soils. Sci. Total Environ. 490, 822–829 10.1016/j.scitotenv.2014.05.069 [DOI] [PubMed] [Google Scholar]
- Ma J., Ibekwe A. M., Yang C.-H., Crowley D. E. (2013). Influence of bacterial communities based on 454 pyrosequencing on the survival of Escherichia coli O157:H7 in soils. FEMS Microbiol. Ecol. 84, 542–554 10.1111/1574-6941.12083 [DOI] [PubMed] [Google Scholar]
- Ma J., Ibekwe A. M., Yi X., Wang H., Yamazaki A., Crowley D. E., et al. (2011). Persistence of Escherichia coli O157:H7 and Its Mutants in Soils. PLoS ONE 6:e23191 10.1371/journal.pone.0023191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathusa E. C., Chen Y., Enache E., Hontz L. (2010). Non-O157 Shiga toxin-producing Escherichia coli in foods. J. Food Prot. 73, 1721–1736 [DOI] [PubMed] [Google Scholar]
- Maule A. (2000). Survival of verocytotoxigenic Escherichia coli O157 in soil, water and on surfaces. Symp. Ser. Soc. Appl. Microbiol. 29, 71S–78S 10.1111/j.1365-2672.2000.tb05334.x [DOI] [PubMed] [Google Scholar]
- Mubiru D. N., Coyne M. S., Grove J. H. (2000). Mortality of Escherichia coli O157:H7 in two soils with different physical and chemical properties. J. Environ. Qual. 29, 1821–1825 10.2134/jeq2000.00472425002900060012x [DOI] [Google Scholar]
- Nicolau M., Levine A. J., Carlsson G. (2011). Topology based data analysis identifies a subgroup of breast cancers with a unique mutational profile and excellent survival. Proc. Natl. Acad. Sci. U.S.A. 108, 7265–7270 10.1073/pnas.1102826108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonnenmann M. W., Bextine B., Dowd S. E., Gilmore K., Levin J. L. (2010). Culture-independent characterization of bacteria and fungi in a poultry bioaerosol using pyrosequencing: a new approach. J. Occup. Environ. Hyg. 7, 693–399 10.1080/15459624.2010.526893 [DOI] [PubMed] [Google Scholar]
- Oberhardt M. A., Palsson B. Ø., Papin J. A. (2009). Applications of genome-scale metabolic reconstructions. Mol. Syst. Biol. 5, 320 10.1038/msb.2009.77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parter M., Kashtan N., Alon U. (2007). Environmental variability and modularity of bacterial metabolic networks. BMC Evol. Biol. 7:169 10.1186/1471-2148-7-169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perna N. T., Plunkett G., 3rd., Burland V., Mau B., Glasner J. D., Rose D. J., et al. (2001). Genome sequence of enterohaemorrhagic Escherichia coli O157:H7. Nature 409, 529–533 10.1038/35054089 [DOI] [PubMed] [Google Scholar]
- Probst A. J., Lum P. Y., John B., Dubinsky E. A., Piceno Y. M., Tom L. M., et al. (2014). Microarray of 16S rRNA gene probes for quantifying population differences across microbiome samples, in Microarrays: Current Technology, Innovations and Applications. Chapter 5, ed He Z. (Norman, OK: University of Oklahoma; ), 99–119 [Google Scholar]
- Romano D., Nicolau M., Quintin E. M., Mazaika P. K., Lightbody A. A., Cody Hazlett H., et al. (2014). Topological methods reveal high and low functioning neuro-phenotypes within fragile X syndrome. Hum. Brain Mapp. 35, 4904–4915 10.1002/hbm.22521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarikonda G., Pettus J., Phatak S., Sachithanantham S., Miller J. F., Wesley J. D., et al. (2014). CD8 T-cell reactivity to islet antigens is unique to type 1 while CD4 T-cell reactivity exists in both type 1 and type 2 diabetes. J. Autoimmun. 50, 77–82 10.1016/j.jaut.2013.12.003 [DOI] [PubMed] [Google Scholar]
- Schloss P. D., Westcott S. L., Ryabin T., Hall J. R., Hartmann M., Hollister E. B., et al. (2009). Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 75, 7537–7541 10.1128/AEM.01541-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenov A. V., Franz E., van Overbeek L., Termorshuizen A. J., van Bruggen A. H. C. (2008). Estimating the stability of Escherichia coli O157:H7 survival in manure amended soils with different management histories. Environ. Microbiol. 10, 1450–1459 10.1111/j.1462-2920.2007.01558.x [DOI] [PubMed] [Google Scholar]
- Sivapalasingam S., Friedman C. R., Cohen L., Tauxe R. V. (2004). Fresh produce: a growing cause of outbreaks of foodborne illness in the United States, 1973 through 1997. J. Food Protect. 67, 2342–2353 [DOI] [PubMed] [Google Scholar]
- Stolyar S., Van Dien S., Hillesland K. L., Pinel N., Lie T. L., Leigh J. A., et al. (2007). Metabolic modeling of a mutualistic microbial community. Mol Syst Biol 3:92 10.1038/msb4100131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Elsas J. D., Semenov A. V., Costa R., Trevors J. T. (2011). Survival of Escherichia coli in the environment: fundamental and public health aspects. ISME J. 5, 173–183 10.1038/ismej.2010.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wintermute E. H., Silver P. A. (2010). Emergent cooperation in microbial metabolism. Mol. Syst. Biol. 6, 407 10.1038/msb.2010.66 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.