Abstract
The antibacterial activities of the cathelicidin peptides LL-37 and an 18-residue C-terminal fragment of prophenin, corresponding to positions 62 to 79 of native prophenin (PF-18), were analyzed in the presence of a modified surfactant preparation isolated from minced porcine lungs. At low micromolar concentrations, both LL-37 and PF-18 showed significant activities against different serotypes of group B streptococci, with LL-37 being more active on a molar basis. The surfactant preparation at a concentration of 10 mg/ml partly blocked the antibacterial activity of 9 μM LL-37 and completely blocked the antibacterial activity of 9 μM PF-18. However, 10 mg of the surfactant preparation per ml had only minor inhibitory effects on LL-37 and PF-18 at 90 μM. Addition of up to 900 μM PF-18 did not affect the surface properties of the surfactant preparation. These data suggest that surfactant preparations containing antimicrobial peptides could be useful for the local treatment of pulmonary infections.
The lung comprises a large epithelial surface, which is exposed to the external environment. To keep the alveolar surface sterile and to protect it against invading microorganisms, both innate and adaptive immune responses are required. Polypeptides with broad-spectrum microbicidal activities, e.g., β-defensins and the cathelicidin LL-37, play important roles in the innate defense response in the human lung (21). LL-37 is expressed in epithelial cells of the human lung (3), and immunohistochemistry has demonstrated that, in addition to bronchial epithelial cells, LL-37 is located in alveolar macrophages and bronchial glands (2). In vitro studies have shown that combinations of lysozyme with β-defensins or LL-37 have additive effects (16), which suggests that lung defenses might be enhanced by interactions of multiple microbicidal factors. LL-37 belongs to the cathelicidins, a family of antimicrobial polypeptides that have a conserved N-terminal proregion followed by a highly variable C-terminal domain, which after proteolysis constitutes the active peptides (20). So far LL-37 is the only known human cathelicidin, and it has potent activities against both gram-positive and gram-negative bacteria (5). LL-37 is also cytotoxic toward eukaryotic cells (10). An LL-37 binding protein (apolipoprotein A-I) has been purified from human plasma and most likely works as a scavenger for LL-37 (18). In addition, LL-37 can bind to low-density and very-low-density lipoprotein (LDL and VLDL, respectively) particles in plasma (17).
The surfactant preparations used for the treatment of respiratory distress syndrome (RDS) in premature infants are obtained from organic extracts of lung tissues or lavage fluids. Hydrophilic lung defense factors such as defensins, lysozyme, as well as the surfactant proteins A and D (SP-A and SP-D, respectively) are excluded by organic extraction. In premature neonates, group B streptococcal (GBS) pneumonia often coexists with RDS (1), and GBS-infected neonates benefit from surfactant treatment (7). Thus, it appears to be important to investigate and possibly modify the effects of surfactant preparations on bacterial growth. Previous studies have shown that surfactant and surfactant-associated proteins exhibit effects on bacterial growth. A porcine surfactant preparation (Curosurf; Chiesi Pharmaceuticals, Parma, Italy) reduced bacterial proliferation in premature ventilated rabbits with experimental GBS pneumonia (8). A recent in vitro study showed that at ≥10 mg/ml this porcine surfactant preparation significantly inhibits GBS growth (14).
The porcine cathelicidin prophenin (named after its high contents of Pro [53%] and Phe [19%]) was originally characterized in porcine leukocytes (6). Recently, prophenin and a C-terminal 18-residue fragment thereof, encompassing residues 62 to 79 (PF-18), were isolated from porcine lung tissue; and they were also found in Curosurf (19). This suggests that the antibacterial properties of Curosurf are mediated by prophenin and/or fragments thereof. Moreover, an anionic antimicrobial peptide has been purified from ovine surfactant (4), and a synthetic analogue of SP-B (residues 1 to 78) has been shown to inhibit the growth of Escherichia coli in vitro (11).
In this study, the bactericidal activities of synthetic PF-18 and LL-37 against GBS were analyzed in the presence of a modified natural porcine surfactant preparation, and the surface activity of PF-18-surfactant mixtures was studied.
MATERIALS AND METHODS
Bacteria and peptides.
GBS reference strain 090 la Colindale was separated by gradient centrifugation into low-density (LD) encapsulated and high-density (HD) nonencapsulated phase variants that were used in the antibacterial assay. In addition, a type Ib GBS strain and a type III GBS strain (14) were tested. Serotypes Ia, Ib, and III account for more than 75% of all neonatal GBS infections.
The bacteria were grown in standard I nutrient broth (Merck) and spread on agar plates (Columbia agar; Merck) with 5% sheep blood. The synthetic peptide PF-18 (NH2-FPPPPPFRPPPFGPPRFP-NH2), which corresponds to the 18-residue C-terminal fragment of prophenin (prophenin residues 62 to 79), was purchased from Interactiva, Ulm, Germany. LL-37 was synthesized as described previously (10). The correct covalent structures of both peptides were confirmed by electrospray and matrix-assisted laser desorption ionization mass spectrometry. The activities of LL-37 against E. coli D21 and Bacillus megaterium were checked as described previously (10). Curosurf (Chiesi Pharmaceuticals) is a modified surfactant preparation obtained from minced porcine lungs by organic extraction and liquid-gel chromatography in organic solvents (15). It is composed of 98% (wt/wt) phospholipids and 1 to 2% (wt/wt) SP-B and SP-C (15) and contains prophenin and PF-18 (19).
Antibacterial assay.
Bacteria were cultured in 11.5 ml of standard I nutrient broth at 37°C for 16 h, and then 7.5 ml of this bacterial suspension was transferred to 50 ml of standard I nutrient broth and incubated for another 3 h at 37°C in order to shift bacterial growth to the mid-logarithmic phase. The bacterial suspension was then centrifuged at 2,000 × g for 10 min, and the bacterial pellet was washed and resuspended in normal saline. A total of 105 or 107 CFU of GBS per ml was obtained by dilution guided by the optical density at 595 nm, as described previously (14). PF-18 is less active against GBS than LL-37, so the starting inoculum was reduced from 107 to 105 CFU/ml. For each experiment, six samples were prepared in triplicate; one aliquot of bacteria was mixed with PF-18 or LL-37 at 90 and 9 μM, respectively; one aliquot was mixed with 10 mg of surfactant per ml; one aliquot was mixed with a mixture of PF-18 or LL-37 and surfactant; and one aliquot was mixed with only bacteria in saline as a control. The samples were then incubated at 37°C under gentle agitation; and aliquots were obtained at three time points (0, 2, and 4 h), serially diluted with normal saline, and spread on blood agar plates. After incubation at 37°C for 24 h, the colonies were counted. The number of CFU (mean + standard deviation [SD]) for each sample and dilution was determined from the average colony counts for two plates. Statistical comparisons between the groups (analysis of variance and Dunnett's multiple-comparison test) were done with Graph Pad (San Diego, Calif.) software. P values <0.05 were considered statistically significant.
Surface activity measurements.
The influence of PF-18 on the surface activity of Curosurf was tested in a pulsating bubble surfactometer (Electronetics Corporation, Buffalo, N.Y.). In short, a bubble is created in a sample chamber filled with surface-active material. The size of the bubble is changed periodically between a maximum and a minimum diameter, thereby mimicking the cyclic area compression during respiration in the alveolus. The pressure in the bubble is constantly recorded, and as the diameter at the end point is preset, the surface tension at the minimum bubble size (γmin) can be calculated by using the law of Laplace (9). Under physiological circumstances a surface tension close to 0 is needed to prevent alveolar collapse at the end of expiration.
In all samples the concentration of surfactant was 2.5 mg/ml, which is close to the estimated critical concentration of surfactant in fetal lung liquid at birth (12). PF-18 was dissolved in 154 mM NaCl-1.5 mM CaCl2 and was added to Curosurf to achieve final concentrations of 9, 90, and 900 μM. Equal volumes of solvent were added to control samples. The samples were incubated at 37°C for 30 min, after which measurements were made at 37°C during 50% cyclic area compression (rate, 20 cycles per min). γmin was recorded for 5 min of pulsation, and data from five repeated measurements are presented as means ± SDs.
RESULTS
Activities of LL-37 against GBS Ia LD and GBS Ia HD phase variants with and without surfactant.
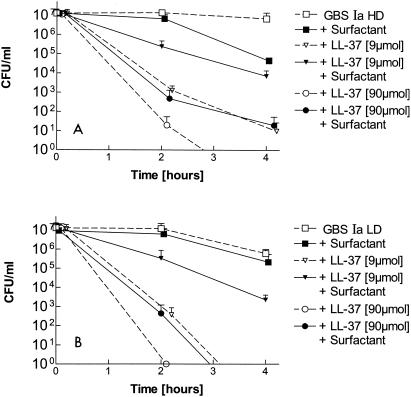
LL-37 exhibited strong activities against both the GBS Ia LD phase variant, which contains a polysaccharide capsule, and the GBS Ia HD phase variant, which lacks a polysaccharide capsule. As shown in Fig. 1, starting with 107 CFU of bacteria per ml, less than 0.01% viable GBS Ia LD or GBS Ia HD phase variants compared to the number of viable controls were detected after 4 h in the presence of 9 μM LL-37. LL-37 at 90 μM decreased the number of viable GBS below the detection limit. The antibacterial activities of LL-37 at 9 and 90 μM were partly blocked in the presence of 10 mg surfactant per ml (Fig. 1).
FIG. 1.
Activities of 9 and 90 μM LL-37 against nonencapsulated GBS (Ia HD; A) and encapsulated GBS (Ia LD; B) in the presence or absence of 10 mg of a modified porcine surfactant preparation (Curosurf) per ml. Mean + SD values from three measurements are shown. The results for all LL-37 groups are significantly different (P < 0.05) at 4 h from those for the controls of GBS Ia HD and LD phase variants incubated in normal saline.
Activities of PF-18 against GBS Ia LD and GBS Ia HD phase variants and GBS Ib and GBS III with and without surfactant.
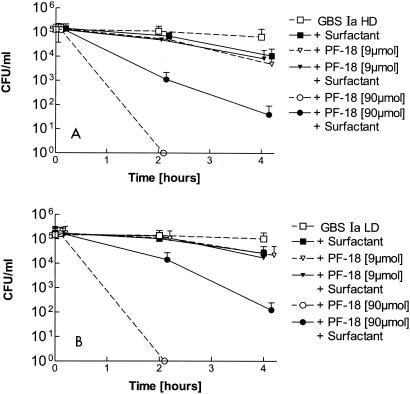
Compared to LL-37, PF-18 was less active against GBS Ia LD and GBS Ia HD. Starting with 107 CFU of bacteria per ml, 9 μM PF-18 resulted in 72% viable GBS Ia LD phase variants and 61% viable GBS Ia HD phase variants compared to the numbers of viable controls after 2 h of incubation (data not shown). The antibacterial activity of PF-18 became more evident with lower initial bacterial concentrations. Starting with 105 CFU/ml, 9 μM PF-18 resulted in a slight reduction in bacterial growth (Fig. 2) and PF-18 concentrations down to 4.5 μM inhibited bacterial growth (data not shown). With 90 μM PF-18, a substantial reduction in bacterial growth was observed after 2 h (Fig. 2). The antibacterial activity of 90 μM PF-18 was partly blocked in the presence of 10 mg of surfactant per ml (Fig. 2). The sensitivity to PF-18 was somewhat higher for the GBS Ib and GBS type III serotypes than for the GBS Ia serotype (Fig. 2 and 3).
FIG. 2.
Activities of 9 and 90 μM PF-18 against nonencapsulated GBS (Ia HD; A) and encapsulated GBS (Ia LD; B) in the presence or absence of 10 mg of a modified porcine surfactant preparation (Curosurf) per ml. Mean + SD values from three measurements are shown. Values for 9 μM PF-18 are not significantly different from those for the GBS Ia LD and HD phase variant controls. With 90 μM PF-18, a significant growth reduction was observed after 4 h, both in the presence and in the absence of surfactant (P < 0.05 versus the results for the GBS Ia LD and HD phase variants). Like for LL-37 (Fig. 1), some of the bactericidal effects of the PF-18 were blocked in the presence of surfactant.
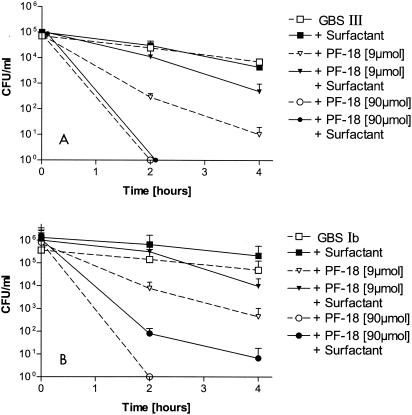
FIG. 3.
Activities of 9 and 90 μM PF-18 against different GBS serotypes III (A) and Ib (B) in the presence or absence of 10 mg of a modified porcine surfactant preparation (Curosurf) per ml. The overall patterns of antibacterial activity of PF-18 against the different GBS subtypes were similar (see Fig. 2 for the results for GBS serotype Ia).
Surface activity of surfactant-PF-18 mixtures.
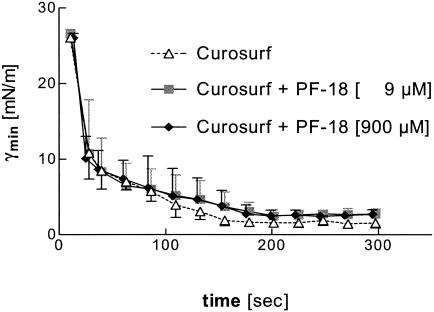
The γmin of surfactant (2.5 mg/ml) in the presence or the absence of PF-18 is shown in Fig. 4. Addition of 9, 90 (data not shown), or 900 μM PF-18 had no effects on γmin. Following 5 min of pulsation, both the adsorption rate and the end points were unchanged compared to those obtained with Curosurf without peptide, indicating that the surface properties of the surfactant preparation used are not affected by the antibacterial peptide at micromolar concentrations. Also, the surface tension at the maximum bubble size (γmax) was unaffected by the presence of PF-18 (data not shown).
FIG. 4.
γmin, measured in a pulsating bubble surfactometer, of a modified surfactant preparation obtained from porcine lungs (Curosurf; phospholipid concentration, 2.5 mg/ml) in the presence of 0, 9, or 900 μM PF-18.
DISCUSSION
The antibacterial peptide prophenin and a fragment encompassing prophenin residues 62 to 79 (PF-18) have been isolated from the porcine surfactant preparation Curosurf (19). The total concentration of prophenin in this surfactant is about 0.5% (wt/wt) relative to the total phospholipid content (19), or about 0.4 mg/ml at the surfactant concentration used for the treatment of RDS. If it is assumed that the mass ratio of PF-18 to full-length prophenin is 1:1, this corresponds to about 100 μM PF-18. In this study, we found that Curosurf inhibited the antibacterial activity of 9 μM PF-18. However, by increasing the PF-18 concentration to 90 μM, 10 mg of surfactant per ml demonstrated weak inhibitory effects. This strongly suggests that the previously observed bactericidal activity of the porcine surfactant preparation (8, 14) is, at least partly, a consequence of the presence of prophenin and/or PF-18. PF-18 is hydrophobic and associates with phospholipids, as demonstrated by its presence in organic extracts of porcine lung tissue (19) and granulocytes (Y. Wang, B. Agerberth, and J. Johanson, unpublished observations). A possible explanation for the partial inhibition of the antibacterial activity of PF-18 by Curosurf is, therefore, that PF-18 associates with surfactant phospholipids, and thereby, less free peptide is available for the interaction with bacteria. On the other hand, addition of up to 900 μM PF-18 to 2.5 mg of surfactant per ml, a surfactant concentration which is highly susceptible to inhibitory components (9), did not reduce the surface activity. This strongly suggests that surfactant preparations tolerate the presence of antibacterial, lipid-interacting peptides without negative effects on surface activity, especially at the high phospholipid concentrations (up to 80 mg/ml) used for the treatment of RDS.
The polysaccharide capsule surrounding the bacterial cell wall has been shown to protect GBS to some extent against phagocytosis mediated by opsonizing antibodies and the direct effects of the surfactant lipids (14). However, in our study the bactericidal effects of LL-37 and PF-18 on the encapsulated GBS LD phase variant and the nonencapsulated GBS HD strain were similar.
LL-37 is a highly active antibacterial peptide, and it adopts an amphipathic α-helical structure with an N-terminal hydrophobic part (10). LL-37 is both antimicrobial and cytotoxic, which has been suggested to be related to the fact that LL-37 can interact with both zwitterionic and negatively charged phopholipids in target membranes (13). In this study, the activity of LL-37 was partly blocked by the surfactant preparation. Like for PF-18, this is likely caused by interactions taking place between LL-37 and phospholipids, the main component of surfactant, which then compete with interactions between LL-37 and bacterial membranes. This conclusion is supported by a previous study, which demonstrated that in human plasma, LL-37 binds to VLDL and LDL particles (17), the outer membranes of which are mainly composed of phospholipids. LL-37 has been suggested to participate in lung defense (2, 3, 16). As surfactant can partly block the activity of LL-37, it needs to be present at high concentrations in the alveoli in order to compensate for surfactant inhibition. It is likely that LL-37 also plays a role in pulmonary innate immunity in the upper airways, which are largely devoid of surfactant.
In conclusion, LL-37 and PF-18 are active in a dose-dependent manner against various serotypes of GBS. Surfactant significantly impairs the activities of both peptides, but this blockage is overcome at peptide concentrations which do not affect the surface activity of a modified natural surfactant preparation. These findings motivate further studies to evaluate the possibility that surfactant can be used as a vehicle for antimicrobial peptides.
Acknowledgments
We are grateful to Guido Stichtenoth, Göttingen, Germany, for help with the statistical analysis. The surfactant used for the studies was a kind gift from Nycomed Germany (Munich, Germany).
This work was supported by grants from the Swedish Research Council (projects 10371 and 11217) and the Deutsche Forschungsgemeinschaft (grant DFG He 2072-2) and a collaborative project of the Swedish Institute and the German Academic Exchange Service (DAAD-313/S-PPP).
REFERENCES
- 1.Ablow, R. C., S. G. Driscoll, E. L. Effmann, I. Gross, C. J. Jolles, R. Uauy, and J. B. Warshaw. 1976. A comparison of early-onset group B streptococcal neonatal infection and the respiratory-distress syndrome of the newborn. N. Engl. J. Med. 294:65-70. [DOI] [PubMed] [Google Scholar]
- 2.Agerberth, B., J. Grunewald, E. Castanos-Velez, B. Olsson, H. Jörnvall, H. Wigzell, A. Eklund, and G. H. Gudmundsson. 1999. Antibacterial components in bronchoalveolar lavage fluid from healthy individuals and sarcoidosis patients. Am. J. Respir. Crit. Care Med. 160:283-290. [DOI] [PubMed] [Google Scholar]
- 3.Bals, R., X. Wang, M. Zasloff, and J. M. Wilson. 1998. The peptide antibiotic LL-37/hCAP-18 is expressed in epithelia of the human lung where it has broad antimicrobial activity at the airway surface. Proc. Natl. Acad. Sci. USA 95:9541-9546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brogden, K. A., A. J. De Lucca, J. Bland, and S. Elliott. 1996. Isolation of an ovine pulmonary surfactant-associated anionic peptide bactericidal for Pasteurella haemolytica. Proc. Natl. Acad. Sci. USA 93:412-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gudmundsson, G. H., B. Agerberth, J. Odeberg, T. Bergman, B. Olsson, and R. Salcedo. 1996. The human gene FALL39 and processing of the cathelin precursor to the antibacterial peptide LL-37 in granulocytes. Eur. J. Biochem. 238:325-332. [DOI] [PubMed] [Google Scholar]
- 6.Harwig, S. S., V. N. Kokryakov, K. M. Swiderek, G. M. Aleshina, C. Zhao, and R. I. Lehrer. 1995. Prophenin-1, an exceptionally proline-rich antimicrobial peptide from porcine leukocytes. FEBS Lett. 362:65-69. [DOI] [PubMed] [Google Scholar]
- 7.Herting, E., O. Gefeller, M. Land, L. van Sonderen, K. Harms, and B. Robertson. 2000. Surfactant treatment of neonates with respiratory failure and group B streptococcal infection. Pediatrics 106:957-964. [DOI] [PubMed] [Google Scholar]
- 8.Herting, E., C. Jarstrand, O. Rasool, T. Curstedt, B. Sun, and B. Robertson. 1994. Experimental neonatal group B streptococcal pneumonia: effect of a modified porcine surfactant on bacterial proliferation in ventilated near-term rabbits. Pediatr. Res. 36:784-791. [DOI] [PubMed] [Google Scholar]
- 9.Herting, E., P. Rauprich, G. Stichtenoth, G. Walter, J. Johansson, and B. Robertson. 2001. Resistance of different surfactant preparations to inactivation by meconium. Pediatr. Res. 50:44-49. [DOI] [PubMed] [Google Scholar]
- 10.Johansson, J., G. H. Gudmundsson, M. E. Rottenberg, K. D. Berndt, and B. Agerberth. 1998. Conformation-dependent antibacterial activity of the naturally occurring human peptide LL-37. J. Biol. Chem. 273:3718-3724. [DOI] [PubMed] [Google Scholar]
- 11.Kaser, M. R., and G. G. Skouteris. 1997. Inhibition of bacterial growth by synthetic SP-B 1-78 peptides. Peptides 18:1441-1444. [DOI] [PubMed] [Google Scholar]
- 12.Kobayashi, T., A. Shido, K. Nitta, S. Inui, M. Ganzuka, and B. Robertson. 1990. The critical concentration of surfactant in fetal lung liquid at birth. Respir. Physiol. 80:181-192. [DOI] [PubMed] [Google Scholar]
- 13.Oren, Z., J. C. Lerman, G. H. Gudmundsson, B. Agerberth, and Y. Shai. 1999. Structure and organization of the human antimicrobial peptide LL-37 in phospholipid membranes: relevance to the molecular basis for its non-cell-selective activity. Biochem. J. 341:501-513. [PMC free article] [PubMed] [Google Scholar]
- 14.Rauprich, P., O. Möller, G. Walter, E. Herting, and B. Robertson. 2000. Influence of modified natural or synthetic surfactant preparations on growth of bacteria causing infections in the neonatal period. Clin. Diagn. Lab. Immunol. 7:817-822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robertson, B., T. Curstedt, J. Johansson, H. Jörnvall, and T. Kobayashi. 1990. Structure and functional characterization of porcine surfactant isolated by liquid-gel chromatography. Prog. Respir. Res. 25:234-246. [Google Scholar]
- 16.Singh, P. K., B. F. Tack, P. B. McCray, and M. J. Welsh. 2000. Synergistic and additive killing by antimicrobial factors found in human airway surface liquid. Am. J. Physiol. Lung Cell. Mol. Physiol. 279:L799-L805. [DOI] [PubMed] [Google Scholar]
- 17.Sørensen, O., T. Bratt, A. H. Johnsen, M. T. Madsen, and N. Borregaard. 1999. The human antibacterial cathelicidin, hCAP-18, is bound to lipoproteins in plasma. J. Biol. Chem. 274:22445-22451. [DOI] [PubMed] [Google Scholar]
- 18.Wang, Y., B. Agerberth, A. Löthgren, A. Almstedt, and J. Johansson. 1998. Apolipoprotein A-I binds and inhibits the human antibacterial/cytotoxic peptide LL-37. J. Biol. Chem. 273:33115-33118. [DOI] [PubMed] [Google Scholar]
- 19.Wang, Y., W. J. Griffiths, T. Curstedt, and J. Johansson. 1999. Porcine pulmonary surfactant preparations contain the antibacterial peptide prophenin and a C-terminal 18-residue fragment thereof. FEBS Lett. 460:257-262. [DOI] [PubMed] [Google Scholar]
- 20.Zanetti, M., R. Gennaro, and D. Romeo. 1995. Cathelicidins: a novel protein family with a common proregion and a variable C-terminal antimicrobial domain. FEBS Lett. 374:1-5. [DOI] [PubMed] [Google Scholar]
- 21.Zhang, P., W. R. Summer, G. J. Bagby, and S. Nelson. 2000. Innate immunity and pulmonary host defense. Immunol. Rev. 173:39-51. [DOI] [PubMed] [Google Scholar]