Abstract
The increased temperature associated with climate change may have important effects on body size and predator–prey interactions. The consequences of these effects for food web structure are unclear because the relationships between temperature and aspects of food web structure such as predator–prey body-size relationships are unknown. Here, we use the largest reported dataset for marine predator–prey interactions to assess how temperature affects predator–prey body-size relationships among different habitats ranging from the tropics to the poles. We found that prey size selection depends on predator body size, temperature and the interaction between the two. Our results indicate that (i) predator–prey body-size ratios decrease with predator size at below-average temperatures and increase with predator size at above-average temperatures, and (ii) that the effect of temperature on predator–prey body-size structure will be stronger at small and large body sizes and relatively weak at intermediate sizes. This systematic interaction may help to simplify forecasting the potentially complex consequences of warming on interaction strengths and food web stability.
Keywords: global warming, temperature, food web structure, body-size ratios, temperature size rule
1. Introduction
Body size is a fundamental trait influencing multiple aspects of species ecology, including landscape use and locomotion [1], energetic requirements [2] and prey selection [3]. Larger organisms tend to eat larger prey, a pattern that holds across ecosystems and taxa [3–5]. The ratio of predator body size to prey body size affects predator–prey dynamics [6,7], interaction strengths [8,9], trophic position [8,10] and the size structure and function of food webs [11]. Because of this, body size is increasingly recognized as a factor influencing species persistence and the stability of complex food webs [8,12,13].
In addition, body size often declines with rearing temperature, a pattern known as the temperature-size rule (TSR) [14,15]. The TSR is widespread [14] and could potentially affect the way species interact because smaller organisms tend to eat smaller prey [3]. It has recently been proposed that increasing temperature will decrease average body size in food webs, leading to a reduction in the number of trophic levels and overall food web connectivity [16,17]. Hence, temperature could have important consequences for food web stability and species persistence. Because of increased global average temperatures due to human-related activities [18], the challenge now is to fully uncover the relationship between body size, temperature and food web body-size structure in order to predict and respond to warming-induced changes in ecological systems. To this end, we ask whether temperature alters the relationship between predator and prey body size using the largest known dataset compiled for aquatic food webs [19].
2. Material and methods
(a). Dataset
We used EcoData Retriever to download and prepare the dataset [20]. The data consist of 34 941 observations of predator–prey interactions from 27 locations, including shoreline to open ocean ecosystems from the poles to the tropics with different mean annual temperatures measured at sea level [19,21]. The data include 93 different types of vertebrate and invertebrate predators ranging from 10−4 kg to 415 kg, and 174 different types of vertebrate and invertebrate prey from 10−15 kg to 5 kg. In some cases, the original dataset had mass estimates derived from body length measurements [3,19]. Temperatures were included as average temperature by location measured at sea level [19].
(b). Data analysis
Because a previous study analysing this same dataset failed to find an effect of temperature [21], in order to assess the effect of temperature on the relationship between predator body mass and prey body mass, we compared three different linear mixed effects models aimed at controlling for the hierarchical structure of the data (package lme4 in R [22]). We log-transformed both predator and prey body sizes before analysis. The first model included prey body size as the response variable and predator body size as the predictor variable, with habitat type as a random intercept and predator identity (species) as a random slope. This also helped control for the error associated with the allometric estimates of predator body mass. The second model also considered the additive effect of temperature, with random effects as in the first model. The third model considered the interactive effect of predator body mass and temperature, with random effects as before. We selected the most plausible model using Akaike's information theoretical criteria [23]. Finally, we compared the relationship between predator body mass and prey body mass with simple ordinary least squares and reduced major axis (RMA) regression. RMA regression allows for error in the x-axis variable, so this comparison would allow us to determine whether accounting for error in predator mass estimates would qualitatively change our results. Since it did not, we report only the results from the linear mixed models.
3. Results
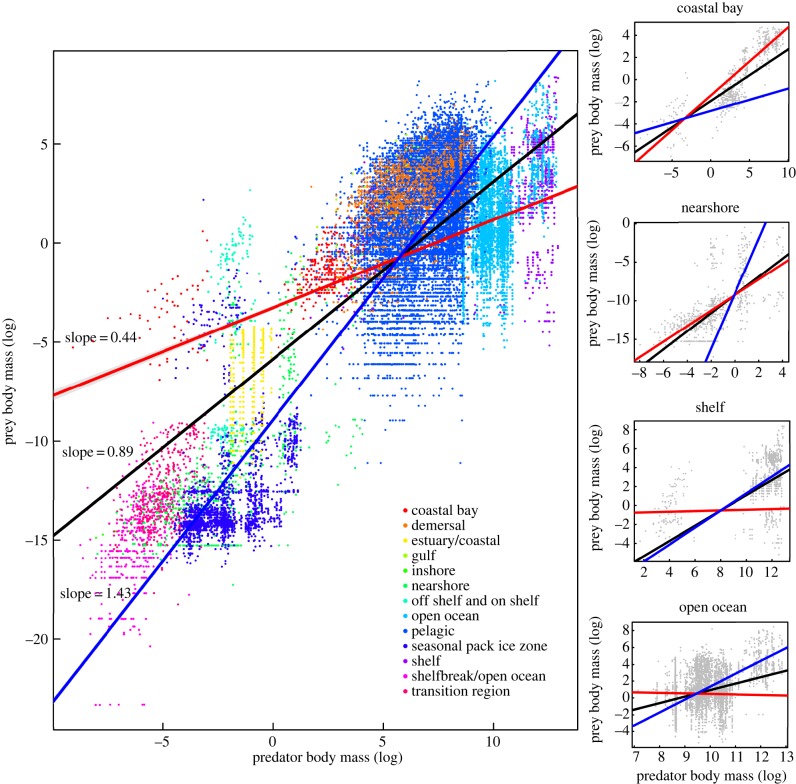
The best model suggests that prey size increased with predator size, and that effect is temperature dependent (intercept = −10.66 ± 1.43 s.e., slope = 0.43 to 1.43 ± 0.16 s.e., table 1). In short, prey size increases with predator size and temperature increases the intercept of the relationship (+0.33 ± 0.03 s.e. per °C) but decreases its slope (−0.04 ± 0.01 per °C). Hence, smaller predators tend to eat relatively larger prey at warmer temperatures than at lower temperatures, while the reverse was true for larger predators (figure 1). Note that a slope close to one implies that body-size ratios remain constant across the entire range of predator masses. By contrast, a slope < 1 indicates an increase in the ratios, and a slope > 1 indicates a decrease. Thus, our best model indicated that prey size depended on the interaction between temperature and predator body size (table 1 and figure 1). The cut-off at which the effect of temperature gets reversed is somewhere between a predator mass of 10 and 150 g.
Table 1.
Model selection for the mixed effects linear models.
| model | K | AICc | Δ AICc | AICc weights |
|---|---|---|---|---|
| log(prey mass) ∼ log(predator mass) × temperature | 8 | 153682.8 | 0.00 | 1 |
| log(prey mass) ∼ log(predator mass) + temperature | 7 | 153839.2 | 156.40 | 0 |
| log(prey mass) ∼ log(predator mass) | 6 | 153859.1 | 176.32 | 0 |
Figure 1.
Left: prey body size (log) against predator body size (log) across marine habitats. Red (T = 29°C), black (T = 15°C) and blue lines (T = −1.3°C) represent predicted curves from the best model. 95% confidence intervals are displayed in grey. Right: same as in left for a subset of the habitats studied (coastal bay is not significant). (Online version in colour.)
4. Discussion
Consistent with previous studies, our results show that prey size increases with predator size [3–5]. Unlike previous studies [21], however, we show that this relationship depends on the interaction between temperature and predator body size, as the slope of the curve becomes shallower and the intercept gets larger as temperature increases (figure 1). The difference between our results and previous analyses with this dataset [21] may simply be due to the fact that the previous analysis only controlled for the effect of location and not for the hierarchical structure of the data in terms of temperature across sites. We do not believe our results contradict their main conclusions, but rather they add an extra layer of understanding as to how predator body size and temperature can interact to yield particular body-size ratios in any given location. The magnitude of the temperature effect changes with habitat, but the direction of the effect does not, indicating some generality across sites (figure 1). Although there is error in the estimates of body size for both predator and prey, and we were only able to consider average temperatures, our broad-scale analysis clearly reveals that body size and temperature can have strong interactive effects on food web body-size structure.
There are three important consequences of this change in body-size structure. First, the range of prey body sizes is narrower in warm habitats than in cold habitats (figure 1). Second, because trophic level increases with body size [10,11] and temperature affects body size through the TSR [16,24], the trophic level of some species may vary across temperatures. In warmer habitats, larger species may have down-shifted trophic levels, whereas smaller species may have raised trophic levels, potentially decreasing the total number of trophic levels in warmed food webs (see also [17]). Finally, species at intermediate trophic levels, which are those of intermediate body size, would be the least affected by this body size–temperature interaction. Importantly, warming affects the size of predators and their prey. Thus, to actually change the body-size structure of food webs, warming must have a differential effect on predator and prey size, with predators becoming smaller at a faster pace than their prey. There is yet to be any experimental evidence suggesting that this can happen in nature, although this pattern can be obtained through a differential effect of warming in predator and prey mobility [1], which has been in turn shown to greatly affect food web network structure [25,26].
The effect of temperature on the predator–prey body-size scaling may also influence interaction strengths and food web stability. Interaction strengths are relatively large at higher trophic levels because they increase with body mass, which increases with trophic level [8,10,11]. Our results suggest that, with warming, larger species at higher trophic levels may eat relatively smaller prey, so these prey could experience larger interaction strengths than they would at colder temperatures. The opposite may be true for smaller predators. It has also been shown that the effect of temperature on interaction strengths depends upon asymmetries in the underlying parameters of the predator–prey interaction [27], which are often controlled by body size [15]. Although there are many ways in which temperature may affect interaction strengths, and the temperature variation we report reflects spatial variation rather than warming, our results suggest that the potential effects of warming upon trophic interaction strengths may be trophic-level dependent.
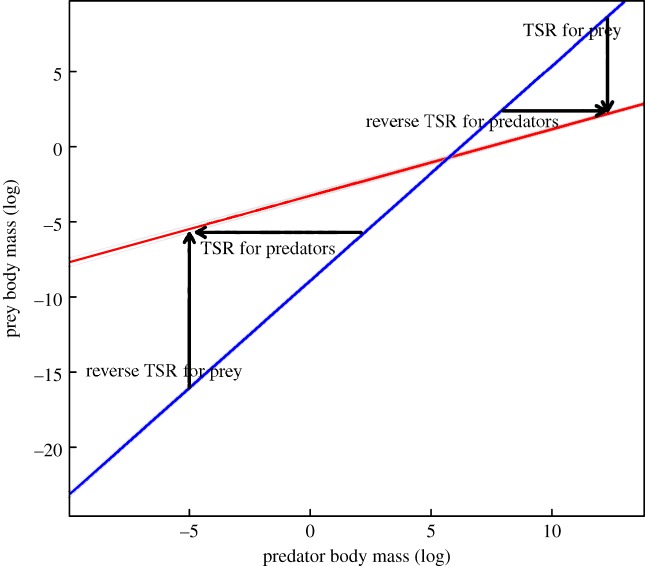
The link between temperature and body-size structure might be related to species identity across habitats, to differences in the way prey selection occurs between species of different habitats [3] or to range shifts with temperature [1,26]. Finally, it can also be due to body size changes of species occurring in different habitats due to differences in environmental temperatures [14,16,24]. If this is the case, smaller predators might be getting smaller with temperature, displaying the typical TSR pattern (figure 2). Large predators, however, might be getting larger with temperature (figure 2). Alternatively, smaller prey might be getting larger with temperature and larger prey might be getting smaller (figure 2). More focused analyses on body size and species identity across food webs at different temperatures are needed to tease this apart.
Figure 2.
The effect of temperature on prey and predator body size. Red and blue lines represent the slope of the predator–prey body size relationship for warm (red) and cold (blue) temperatures. Black arrows represent body-size changes with temperature.
It is not clear why predator–prey body sizes scale the way they do in any system. In aquatic ecosystems, such as the ones analysed here, gape-limitation may play an important role constraining food web body-size structure [28]. If this is a driving mechanism, our results suggest that gape-limitation may be less important in warmer temperatures, as the slopes of the curves are shallower. Our results also suggest the possibility that there are limits to the slopes of these relationships, as the range of slopes observed across temperatures in this study matches the range observed across taxa, which varies from 0.5 for protists [29] to 1.5 for mammalian terrestrial carnivores [30], and habitats, where it varies from 0.7 in stream food webs to about 2 in terrestrial food webs [10].
Overall, our results suggest that temperature has an interactive effect upon predator–prey body-size relationships, where smaller predators tend to eat larger prey at warmer temperatures and smaller prey at colder temperatures, while larger predators will do the opposite. This might lead to food webs with larger interaction strengths but fewer trophic levels in warm temperatures, whereas smaller interaction strengths and more trophic levels could be expected in colder food webs. Thus, we have shown that temperature has strong consequences for food web body-size structure, and very likely stability as well, which in turn has important implications for species persistence in the context of global warming.
Acknowledgements
We are indebted to Chad Brassil, Diana Pilson, Stefano Allesina, Dominique Gravel and an anonymous reviewer for valuable feedback on earlier versions of this manuscript.
Funding statement
J.P.G. was supported through an Othmer Fellowship and the School of Biological Sciences Special Funds.
References
- 1.Lurgi M, López BC, Montoya JM. 2012. Climate change impacts on body size and food web structure on mountain ecosystems. Phil. Trans. R. Soc. B 367, 3050–3057. ( 10.1098/rstb.2012.0239) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown JH, Gillooly JF, Allen AP, Savage VM, West GB. 2004. Toward a metabolic theory of ecology. Ecology 85, 1771–1789. ( 10.1890/03-9000) [DOI] [Google Scholar]
- 3.Brose U, et al. 2006. Consumer–resource body-size relationships in natural food webs. Ecology 87, 2411–2417. ( 10.1890/0012-9658(2006)87[2411:CBRINF]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 4.Memmott J, Martinez ND, Cohen JE. 2000. Predators, parasitoids and pathogens: species richness, trophic generality and body sizes in a natural food web. J. Anim. Ecol. 69, 1–15. ( 10.1046/j.1365-2656.2000.00367.x) [DOI] [Google Scholar]
- 5.Jonsson T, Cohen JE, Carpenter SR. 2005. Food webs, body size, and species abundance in ecological community description. Adv. Ecol. Res. 36, 1–84. ( 10.1016/S0065-2504(05)36001-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yodzis P, Innes S. 1992. Body size and consumer–resource dynamics. Am. Nat. 139, 1151–1175. ( 10.1086/285380) [DOI] [Google Scholar]
- 7.Kalinkat G, Schneider FD, Digel C, Guill C, Rall BC, Brose U. 2013. Body masses, functional responses and predator–prey stability. Ecol. Lett. 16, 1126–1134. ( 10.1111/ele.12147) [DOI] [PubMed] [Google Scholar]
- 8.Berlow EL, Dunne JA, Martinez ND, Stark PB, Williams RJ, Brose U. 2009. Simple prediction of interaction strengths in complex food webs. Proc. Natl Acad. Sci. USA 106, 187–191. ( 10.1073/pnas.0806823106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vucic-Pestic O, Rall BC, Kalinkat G, Brose U. 2010. Allometric functional response model: body masses constrain interaction strengths. J. Anim. Ecol. 79, 249–256. ( 10.1111/j.1365-2656.2009.01622.x) [DOI] [PubMed] [Google Scholar]
- 10.Riede JO, Brose U, Ebenman B, Jacob U, Thompson R, Townsend CR, Jonsson T. 2011. Stepping in Elton's footprints: a general scaling model for body masses and trophic levels across ecosystems. Ecol. Lett. 14, 169–178. ( 10.1111/j.1461-0248.2010.01568.x) [DOI] [PubMed] [Google Scholar]
- 11.Cohen JE, Jonsson T, Carpenter SR. 2003. Ecological community description using the food web, species abundance, and body size. Proc. Natl Acad. Sci. USA 100, 1781–1786. ( 10.1073/pnas.232715699) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yvon-Durocher G, Reiss J, Blanchard J, Ebenman B, Perkins DM, Reuman DC, Thierry A, Woodward G, Petchey OL. 2011. Across ecosystem comparisons of size structure: methods, approaches and prospects. Oikos 120, 550–563. ( 10.1111/j.1600-0706.2010.18863.x) [DOI] [Google Scholar]
- 13.DeLong JP. 2014. The body-size dependence of mutual interference. Biol. Lett. 10, 20140261 ( 10.1098/rsbl.2014.0261) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Atkinson D. 1994. Temperature and organism size-a law for ectotherms? Adv. Ecol. Res. 25, 1–58. ( 10.1016/S0065-2504(08)60212-3) [DOI] [Google Scholar]
- 15.DeLong JP. 2012. Experimental demonstration of a ‘rate–size’ trade-off governing body size optimization. Evol. Ecol. Res. 14, 343–352. [Google Scholar]
- 16.Daufresne M, Lengfellner K, Sommer U. 2009. Global warming benefits the small. Proc. Natl Acad. Sci. USA 106, 12 788–12 793. ( 10.1073/pnas.0902080106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brose U, Dunne JA, Montoya JM, Petchey OL, Schneider FD, Jacob U. 2012. Climate change in size-structured ecosystems. Phil. Trans. R. Soc. B 367, 2903–2912. ( 10.1098/rstb.2012.0232) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Houghton JT, Meira Filho LG, Callander BA, Harris N, Kattenberg A. 1996. Climate Change 1995: the science of climate change. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 19.Barnes C, et al. 2008. Predator and prey body size in marine food webs. Ecology 89, 2007–2008. ( 10.1890/07-1551.1) [DOI] [Google Scholar]
- 20.Morris BD, White EP. 2013. The EcoData retriever: improving access to existing ecological data. PLoS ONE 8, e65848 ( 10.1371/journal.pone.0065848) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barnes C, Maxwell D, Reuman DC, Jennings S. 2010. Global patterns in predator–prey size relationships reveal size dependency of trophic transfer efficiency. Ecology 91, 222–232. ( 10.1890/08-2061.1) [DOI] [PubMed] [Google Scholar]
- 22.Bates D, Martin M, Bolker BM. 2011. lme4: Linear mixed-effects models using S4 classes. R Packag. version 0.999375–42 See http//CRAN.R-project.org/package=lme4.
- 23.Burnham KP, Anderson DR. 2002. Model selection and multimodel inference: A practical information-theoretic approach, 2nd edn New York, NY: Springer Science. [Google Scholar]
- 24.Gardner JL, Peters A, Kearney MR, Joseph L, Heinsohn R. 2011. Declining body size: a third universal response to warming? Trends Ecol. Evol. 26, 285–291. ( 10.1016/j.tree.2011.03.005) [DOI] [PubMed] [Google Scholar]
- 25.Gravel D, Poisot T, Albouy C, Velez L, Mouillot D. 2013. Inferring food web structure from predator–prey body size relationships. Methods Ecol. Evol. 4, 1083–1090. ( 10.1111/2041-210X.12103) [DOI] [Google Scholar]
- 26.Albouy C, Velez L, Coll M, Colloca F, Le Loc'h F, Mouillot D, Gravel D. 2014. From projected species distribution to food-web structure under climate change. Glob. Change Biol. 20, 730–741. ( 10.1111/gcb.12467) [DOI] [PubMed] [Google Scholar]
- 27.Gilbert B, et al. 2014. A bioenergetic framework for the temperature dependence of trophic interactions. Ecol. Lett. 17, 902–914. ( 10.1111/ele.12307) [DOI] [PubMed] [Google Scholar]
- 28.Arim M, Bozinovic F, Marquet PA. 2007. On the relationship between trophic position, body mass and temperature: reformulating the energy limitation hypothesis. Oikos 116, 1524–1530. ( 10.1111/j.0030-1299.2007.15768.x) [DOI] [Google Scholar]
- 29.DeLong JP, Vasseur DA. 2012. Size-density scaling in protists and the links between consumer–resource interaction parameters. J. Anim. Ecol. 81, 1193–1201. ( 10.1111/j.1365-2656.2012.02013.x) [DOI] [PubMed] [Google Scholar]
- 30.DeLong JP, Vasseur DA. 2012. A dynamic explanation of size–density scaling in carnivores. Ecology 93, 470–476. ( 10.1890/11-1138.1) [DOI] [PubMed] [Google Scholar]