Abstract
Mycobacterial infections in laboratory zebrafish (Danio rerio) are common and widespread in research colonies. Mycobacteria within free living amoebae have been shown to be transmission vectors for mycobacteriosis. Paramecium caudatum are commonly used as a first food for zebrafish, and we investigated this ciliate’s potential to serve as a vector of Mycobacterium marinum and M. chelonae. The ability of live P. caudatum to transmit these mycobacteria to larval, juvenile and adult zebrafish was evaluated. Infections were defined by histologic observation of granulomas containing acid-fast bacteria in extraintestinal locations. In both experiments, fish fed paramecia containing mycobacteria became infected at a higher incidence than controls. Larvae (exposed at 4 days post hatch) fed paramecia with M. marinum exhibited an incidence of 30% (24/80) and juveniles (exposed at 21 days post hatch) showed 31% incidence (14/45). Adult fish fed a gelatin food matrix containing mycobacteria within paramecia or mycobacteria alone for 2 wk resulted in infections when examined 8 wk after exposure as follows: M. marinum OSU 214 47% (21/45), M. marinum CH 47% (9/19), M. chelonae 38% (5/13). In contrast, fish feed mycobacteria alone in this diet did not become infected, except for 2 fish (5%) in the M. marinum OSU 214 low dose group. These results demonstrate that Paramecium caudatum can act as a vector for mycobacteria. This provides a useful animal model for evaluation of natural mycobacterial infections and demonstrates the possibility of mycobacterial transmission in zebrafish facilities via contaminated paramecia cultures.
INTRODUCTION
The use of zebrafish (Danio rerio) in biomedical research has dramatically increased in recent years (Vascotto et al. 1997, Grunwald & Eisen 2002, Amsterdam & Hopkins 2006). Similar to other laboratory animal models, underlying infections in zebrafish can compromise health as well as research because chronic subclinical diseases, such as mycobacteriosis, are of concern as they induce non-protocol induced variation in experiments (Baker 2003, Kent et al. 2011, Kent et al. 2012, Lawrence et al. 2012). Chronic disease caused by Mycobacterium spp. is the second most common microbial infection in zebrafish research colonies (http://zebrafish.org/zirc/health/diseaseManual.php). Mycobacterium chelonae is the most common species found in laboratory zebrafish, and often presents as a subclinical infection (Watral & Kent 2007, Whipps et al. 2008, Whipps et al. 2012). Mycobacterium haemophilum is more pathogenic and infections are associated with high mortalities and severe infections (Whipps et al. 2007). In contrast, Mycobacterium marinum is not frequently seen in zebrafish, but when it occurs it has been associated with acute to chronic infections and also high mortality (Broussard and Ennis 2007, Watral and Kent 2007, Ostland et al. 2008). Importantly, we have recently verified M. marinum outbreaks in two separate, large-scale research zebrafish facilities.
Mycobacteriosis in zebrafish presents many challenges to effective management and control of the disease once established within research colonies (Astrofsky et al. 2000, Kent et al. 2009). The mycobacterial species infecting laboratory zebrafish, including Mycobacterium marinum, M. haemophilum and M. chelonae, can be commonly isolated from the aquaria holding fish, water supplies and effluents (Primm et al. 2004, Whipps et al. 2007, Whipps et al. 2008). Persistence and replication of mycobacteria in the aquatic environment is aided by the bacteria’s inherent resistance to chlorine, survival in low-nutrient environments, (starvation resistance), thermoresistance and ability to form biofilms (Nyka 1974, Schulze-Robbecke and Buchholtz 1992, Schulze-Robbecke et al. 1992, Taylor et al. 2000, Hall-Stoodley et al. 2006).
Current information regarding natural transmission and vectors of mycobacteria in laboratory fishes, particularly zebrafish, is not substantive. There are several known and utilized methods of mycobacterial transmission, representing non-natural experimental infections, which includes intraperitoneal and intramuscular injection, oral gavage and bath exposure (Prouty et al. 2003, Harriff et al. 2007, Watral & Kent 2007). Water bath exposure of Japanese medaka (Oryzias latipes) to very high concentrations of mycobacteria directly from cultures is ineffective and rarely resulted in infected fish, usually with low infection levels, in laboratory transmission studies (Mutoji 2011). Infection by feeding on infected fish tissues is one recognized route of transmission (Ross 1970, Hedrick et al. 1987, Mutoji 2011) and could be considered as a more natural route of infection. It has been previously shown that mycobacteria residing within granulomas have increased virulence, which provides an explanation for the ease of oral-enteric transmission from infected tissues (Volkman et al. 2004).
Another potential route of infection is by feeding on invertebrates colonized by mycobacteria. Mutoji (2011) showed that M. marinum within the gastrointestinal tract of mosquito larvae were significantly more infectious than cultured mycobacteria for infection in Japanese medaka following feeding on these larvae. Mycobacteria residing within amoebae are also more infectious than their counterparts from axenic cultures. Cirillo et al. (1997) showed that Mycobacterium avium pathogenicity is increased if it is phagocytized by Acanthamoeba castellanii and replicates within amoebic vacuoles. Free-living aquatic protozoans, such as Acanthamoeba and Dictyostelium, evolved as phagocytic cells that actively prey on bacteria (Barker & Brown 1994) and inhabit the same niches as environmental mycobacteria. Mycobacteria residing in macrophages also have enhanced virulence, and hence these amoebae act as surrogate macrophages in the environment. Harriff et al. (2007) demonstrated that per os infections of M. marinum in zebrafish were enhanced by passing the bacteria through amoebae. Whereas amoebae are not deliberately feed to zebrafish, the filter-feeding ciliate Paramecium caudatum is a common first food for larval zebrafish (Lawrence 2007, Westerfield 2007, Harper & Lawrence 2010) and similar to environmental amoebae, they are indiscriminant bacterivores. Transmission of mycobacteria from paramecia to zebrafish has not been previously demonstrated. Therefore, we conducted the following transmission experiments to determine if P. caudatum could serve as a vector and enhance natural transmission of M. marinum and M. chelonae in larval, post-larval and adult zebrafish.
MATERIALS AND METHODS
General Fish and Fish Husbandry
Larval, three week-old and five month old AB strain zebrafish were obtained from the Sinnhuber Aquatic Research Laboratory at Oregon State University. Fish were housed in a biosafety-level 2 (BSL-2) room. Larval fish were held in static containers, whereas 3 wk and adult fish held in flow-through 2.8 liter tanks in a modular zebrafish rack system (Aquaneering™, San Diego, CA). Incoming municipal water source was filtered, dechlorinated and heated. All fish were held at 28° C with a 14/10 light/dark photoperiod.
Mycobacterium spp
We chose two strains of Mycobacterium marinum and one strain of Mycobacterium chelonae for this study. The M. marinum OSU 214 strain was initially isolated from hybrid striped bass (Morone saxatilis×Morone chrysops) (Watral & Kent 2007, Ostland et al. 2008) where it was a significant cause of mortality, whereas the M. marinum isolate from a recent outbreak (herein referred to as “M. marinum CH”) was cultured from a laboratory colony of albino zebrafish experiencing a chronic and ongoing mortality event. The M. chelonae H1/E2 isolate originated from a Tübingen (TU) strain zebrafish, as part of a laboratory colony where there was ongoing chronic morbidity and low mortality levels (Whipps et al. 2008).
Mycobacteria were prepared for inocula from 10 day old stock cultures grown on Middlebrook 7H10 agar plates by making a 5 ml suspension in sterile distilled water with an estimated optical density of 1 and 3 (McFarland standard), respectively, which approximated the low and high dose values. The specified dosages were confirmed by serial dilution plating (Tables 1 and 2). The total dose/fish was calculated based upon serial dilution plating on CNA (colistin/nalidixic acid and blood) and Middlebrook 7H10 agar plates (Remel™, Lenexa, KS 66215 USA) with a 100 µl aliquot of the gelatin diet (minus the gelatin) including the 5 ml mycobacteria inoculum, with or without paramecia. We include CNA medium as our previous studies have shown that this medium is particularly useful for culture of M. marinum OSU 214 (Ostland et al. 2008).
Table 1.
Danio rerio. Larval and juvenile zebrafish exposed by feeding Paramecium caudatum containing mycobacteria for 7 d. Larval and juvenile fish were examined at 24 d and 8 wk post initial feeding, respectively.
| Mycobacteria | Dose/Fish/Day | No. Fish | Total Fish Infected1 (%) |
|---|---|---|---|
| Larval M. marinum (OSU 214) | 4×105 | 80 | 24(30) |
| Larval M. chelonae (H1/E2) | 3.8×105 | 112 | 44(39) |
| Larval Control | 0 | 16 | 0(0) |
| Juvenile M. marinum (OSU 214) | 3.2×105 | 45 | 14(31) |
| Juvenile M. chelonae (H1/E2) | 3.3×105 | 70 | 2(3) |
| Juvenile Control | 0 | 35 | 0(0) |
Total fish infected are numbers expressed (without parenthesis) and relative percentages infected (within parenthesis)
Table 2.
Danio rerio. Adult zebrafish fed gelatin food matrix containing mycobacteria or Paramecium caudatum containing mycobacteria
| Mycobacteria | Dose/Fish/Day | Days Fed |
Duration (Post-Exposure) |
No. Fish Examined1 |
Total Fish Infected (%) |
Intestinal Acid-Fast Bacilli (%) |
|---|---|---|---|---|---|---|
| M. marinum (OSU 214) | ||||||
| Paramecia | 3.4×105 | 14 | 8 wk | 45 | 21(47)2 | 12(34)3 |
| High Dose | 6.1×105 | 14 | 8 wk | 56 | 0(0) | 20(41) |
| Low Dose | 3.6×104 | 14 | 8 wk | 42 | 2(5) | 18(47) |
| Control | 0 | 14 | 8 wk | 60 | 0(0) | 31(51) |
| M. marinum (CH) | ||||||
| Paramecia | 3.6×105 | 14 | 8 wk | 19 | 9(47) | 8(42) |
| High Dose | 4.6×107 | 14 | 8 wk | 22 | 0(0) | 14(64) |
| Low Dose | 3.8×106 | 14 | 8 wk | 21 | 0(0) | 10(48) |
| Control | 0 | 14 | 8 wk | 20 | 0(0) | 18(90) |
| M. chelonae (H1/E2) | ||||||
| Paramecia | 3.4×105 | 14 | 8 wk | 13 | 5(38) | 12(92) |
| High Dose | 8.3×107 | 14 | 8 wk | 14 | 0(0) | 11(78) |
| Low Dose | 3.5×106 | 14 | 8 wk | 16 | 0(0) | 11(69) |
| Control | 0 | 14 | 8 wk | 11 | 0(0) | 5(45) |
| Paramecia | 3.4×105 | 14 | 16 wk | 14 | 3(21) | 11(79) |
| High Dose | 8.3×107 | 14 | 16 wk | 16 | 0(0) | 12(75) |
| Low Dose | 3.5×106 | 14 | 16 wk | 15 | 0(0) | 6(40) |
| Control | 0 | 14 | 16 wk | 12 | 0(0) | 9(75) |
No. Fish Examined includes mortalities within M. marinum (OSU 214) experimental and control groups occurring 1 week prior to conclusion of experiment
Total fish infected are numbers expressed (without parenthesis) and relative percentages infected (within parenthesis)
Total fish positive for intestinal acid-fast bacilli are numbers expressed (without parenthesis) and relative percentages infected (within parenthesis)
Paramecium culture and infections
Paramecia were cultured using standard methods (http://zfin.org/zf_info/zfbook/chapt3/3.3.html), which included maintaining a sustainable infusoria for the paramecia by adding sterile (autoclaved) wheat berries plus yeast extract to sterile aquarium water. To “infect” paramecia with mycobacteria, cultures were incubated for 8 h with either M. marinum or M. chelonae (optical density of 1–3 on the McFarland scale). They were then were collected in 50 ml screw-cap conical tubes (Becton Dickinson, Franklin Lakes, NJ 07417 USA) and spun on a microcentrifuge (Eppendorf, Hauppauge, NY 11788 USA) at 4,400 g for 5 minutes to concentrate the paramecia. The semi-concentrated paramecia were re-suspended in 5 ml of sterile aquarium water. This step was repeated twice for a total of three rinses, to reduce the amount of exogenous mycobacteria in planktonic suspension. The final rinse constituted the paramecia suspension to be added to a gelatin suspension. Paramecia were visually evaluated using a stereo microscope to confirm viability prior to adding them to a gelatin food matrix. Mycobacterial doses in the paramecia were determined by counting of individual bacteria within food vacuoles. To enumerate the total number of mycobacteria per paramecium, 30 replicates of 10 µl (larval zebrafish) and 25 µl (juvenile and adult zebrafish) aliquots of the mycobacteria- loaded paramecia were placed onto clean glass slides, air-dried for 30 minutes, fixed in 100% methanol for 5 minutes, air-dried and then acid-fast stained by Kinyoun’s cold acid-fast staining method (Kent Lab protocol). On average, there were 8 paramecia/10 µl for the larval zebrafish exposure groups and 18 paramecia/25 µl for each of the juvenile and adult zebrafish exposure groups (M. marinum OSU 214, M. chelonae and M. marinum CH), with each individual paramecium containing 70 to 125 mycobacteria in food vacuoles. In the adult zebrafish exposure groups, there were approximately 720 paramecia/ml, or between 69,000 and 71,000 mycobacteria/ml in the prepared inoculum containing paramecia. Paramecia not exposed to mycobacteria were negative by both bacterial cultures on Middlebrook 7H10 agar plates and acid-fast staining, which ensured that there was no background contamination of mycobacteria in stock paramecia cultures.
Gelatin delivery vehicle
The base gelatin feed formulation was comprised of 16.25 g of unflavored gelatin powder (HyTop Gelatin™, Arlington Heights, IL 60005 USA), 12.5 g of Otohimie “A” Marine Fish Larval and Weaning Feed™, (Marubeni Nisshin Feed Co., Tokyo, Japan), 6.25 ml of cod liver oil (Twinlab, American Fork, UT 84003 USA) and 120 ml of sterile aquarium water. The mixture was gently heated to 60°C while agitated until the gelatin went into solution, removed from the heat, allowed to cool to 40°C then 5 ml of either the mycobacteria-loaded paramecia cultures or mycobacteria in sterile aquarium water were added. Control gelatin tablets were prepared in an identical fashion and contained no mycobacteria. The total stock feed volume was doubled and approximately sixteen 4 g tablets were prepared in chilled metal molds and allowed to completely solidify, after which the tablets were refrigerated. Prepared diets in the gelatin matrix were stored by refrigeration at 5°C for two weeks. The diet was minced to pieces approximately 2 mm in diameter prior to feeding.
Survival of mycobacteria at refrigerated temperatures
We evaluated the stability of the mycobacteria under refrigerated temperatures as the gelatin diet was prepared and held at 5°C for the 2 wk feeding regime. The feed mixture minus gelatin was inoculated with 3×104 M. marinum OSU 214 and M. chelonae, and then refrigerated at 5°C to simulate refrigerated storage conditions of the gelatin diet. The feed mixture was cultured after 1 and 3 weeks, respectively, to determine if mycobacteria in the feed continued to grow, decline or remained static.
Larval fish exposure
For the larval zebrafish feeding trial, 208 AB strain zebrafish were divided into control, M. marinum OSU 214 and M. chelonae groups. Four days post-fertilization (dpf), larvae were transferred to sterile 12 well cell culture plates (COSTAR2122, Corning Incorporated, Corning, NY 14831 USA flat bottom cell culture cluster with lid). One larval fish was added to each well, which was filled with 3 ml of sterile aquarium water. Every 8 hours, 2.5 ml of well water was replaced with fresh sterile aquarium water. Larval zebrafish were fed 10 µl of free swimming paramecia with mycobacteria suspension twice daily until 12 dpf, when they were switched over to “Yellow” micronized feed (a blend of 50 g each of Zeigler AP100™ 100, 150 to 250 micron and 25 g Golden Pearl™ 200 to 300 micron, Aquatic Eco Systems and Artemia International) from 12 dpf to 24 dpf, then fish were euthanized by MS-222 overdose and evaluated. Mortalities and larvae surviving to the conclusion of the feeding trial were placed into 15 ml conical tubes containing 14 ml of 10% neutral buffered formalin.
3 wk-old fish exposure
Three week-old zebrafish (juveniles) were exposed to either M. marinum OSU 214 or M. chelonae in flow through tanks. Fish were fed 25 µl paramecia containing mycobacteria twice daily for 7 d, and the experiment ended at 56 d post exposure (pe) (Table 1). Control fish were fed paramecia not containing mycobacteria throughout the experiment. Exposed and control fish were held in 3 replicate flow-through 2.8 L tanks for each treatment, with 25 fish/tank.
Adult fish exposure
For the gelatin food matrix inoculated with either M. marinum OSU 214 strain, M. marinum CH strain or M. chelonae H1/E2, five month-old AB strain, mixed sex zebrafish were divided into 4 replicate 2.8 L tanks/exposure regimen (i.e., 4 tanks/exposure regimen). Exposure groups were comprised of fish fed both high and low dose mycobacteria in the gelatin food matrix, mycobacteria and paramecia in gelatin food matrix, and gelatin food matrix only for control groups. Each tank in the study was fed 1 g of the gelatin food matrix daily for 14 d. Fish were then switched to a standard zebrafish commercial diet mixture for another 6 wk until the end of the experimental exposure time. In the M. chelonae treatment groups, fish were examined at two time points, 8 and 16 wk pe (Table 2).
Histology
Moribund and surviving fish were euthanized with an overdose of tricaine methanesulfonate (Finquel™, Argent Chemical Laboratories, Redmond, WA 98052 USA) and processed as follows. All zebrafish in the transmission experiments, with the exception of the larval zebrafish, were processed for histology in an identical manner. Briefly, immediately after death, the coelomic cavity of each fish was opened and half of each operculum removed to facilitate fixation and mitigate post-mortem autolysis. The fish were then placed in 10X volume/fish of 10% neutral buffered formalin in 50 ml conical tubes and allowed to fix overnight on a rocker plate. After initial fixation, the fish were put into 10X volume/fish of CalExII™ (Fisher Scientific, Fair Lawn, NJ 07410 USA) for 48 h of decalcification, then rinsed in distilled water for 30 minutes prior to being sagittally sectioned, placed into standard tissue cassettes and processed for routine histology. Larval zebrafish were processed identically except for the decalcification and rinsing step, and then placed into gelatin molds prior to processing and embedding. The standard hematoxylin and eosin stain and Ziehl-Neelsen or Kinyoun’s aid-fast stains were performed for all fish.
Statistics
All statistical analyses were conducted with the program R, version 2.7.2 (R Development Core 315 Team). Significance was set at p ≤ 0.05 and p-values are 2-tailed. For each experiment, differences in infection prevalence between tanks of each treatment group were tested by the Pearson’s Chi-square test for count data in order to determine whether data from different tanks could be pooled for group comparisons. The Monte Carlo simulation method using 20,000 replications was used to estimate p-values for this test. Fisher’s exact tests were used to compare infection prevalence between treatment groups in each experiment. Because M. chelonae is less virulent than M. marinum and can occur in a chronic state, we were also interested in comparing overall infection prevalence of this species between experimental groups, regardless of the time point that was sampled. We used Fisher’s exact tests for this comparison after data from different time points had been assessed for permissible pooling by comparing infection prevalences with Fisher’s exact tests.
RESULTS
Larval fish
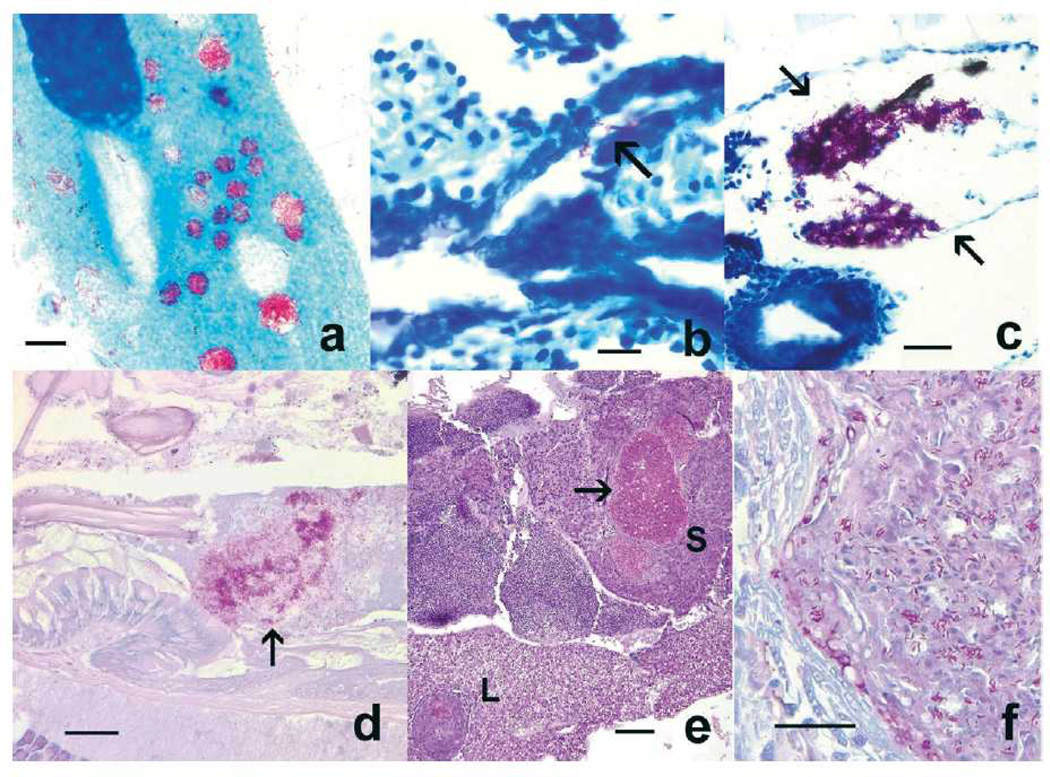
This experiment entailed feeding fry zebrafish with paramecia that had been incubated for 8 h in a culture of M. marinum OSU 214 or M. chelonae. Several larval fish that were fed paramecia containing mycobacteria showed the presence of mycobacterial aggregates within the intestinal lumen, and a few moribund fish that were examined between 3– 14 d post exposure exhibited infections of the swim bladder when (Table 1; Fig 1b,c). In the M. marinum group, acid-fast bacteria were observed in the intestinal lumens of 30% (24/80) of exposed infected fish. Within the M. chelonae group, acid-fast bacteria were observed in the intestines of 39% (44/112) of infected fish, while 2% of infections occurred in the swim bladder. In the fish with swim bladder infections, small aggregates of mycobacteria (M. chelonae) were also observed within the pneumatic duct (Fig. 1b). Mycobacteria infections observed in the larval zebrafish groups, regardless of whether infected by M. marinum or M. chelonae, were comprised of mycobacteria aggregates in close association with epithelial surfaces, with no intracellular mycobacteria and minimal to no inflammatory cell infiltrates or granuloma formation (Fig.1c). All control larval zebrafish were negative (0/16) for mycobacteria infection.
Figure 1.
. Danio rerio. Histological presentations in zebrafish exposed to Mycobacterium marinum and Mycobacterium chelonae residing within paramecia food vacuoles. (a) Paramecium caudatum cultured with M. marinum OSU 214 for 8 h, with numerous acid-fast bacteria in food vacuoles. Kinyoun’s acid-fast. Scale bar = 10 µm. (b) Mycobacteria (arrow) in pneumatic duct of larval fish exposed to M. chelonae. Ziehl-Neelsen acid-fast. Scale bar = 10 µm. (c) M. chelonae aggregate in larval fish swim bladder (arrows). Ziehl-Neelsen acid-fast. Scale bar = 25 µm. (d) Mycobacteria (arrow) in the intestinal lumen of an adult fish. Kinyoun’s acid-fast. Scale bar = 25 µm. (e) Liver (L) and spleen (S) granulomas in a juvenile fish fed paramecia containing M. marinum OSU 214. Arrow = granuloma demonstrated in Fig. 1f. Hematoxylin and eosin. Scale bar = 100 µm. (f) High magnification of spleen granuloma with numerous mycobacteria. Kinyoun’s acid-fast. Scale bar = 25 µm.
Juveniles
In three week-old zebrafish fed infected paramecium, about one third of the fish exposed to M. marinum became infected, while fish that were exposed to M. chelonae showed only 3% prevalence of infections. Infections for both mycobacterial species were confirmed by identifying granulomas containing acid-fast bacilli, which were primarily localized to kidney, spleen (Fig.1e,f) and liver. Rarely, there was involvement of the choroid rete, brain, and gonad (ovary, and less often testis). These infrequent infections were confined to fish in the M. marinum group.
Adult fish
This experiment entailed feeding M. chelonae and two strains of M. marinum to adult zebrafish incorporated into a gelatin food matrix, either by placing bacteria directly into the diet from cultures or by feeding mycobacteria to paramecia, which were subsequently added to gelatin food matrix. Surviving fish were euthanized by MS-222 overdose and evaluated by histology at 8 wk pe for the M. marinum groups, and at 8 and 16 wk for the M. chelonae groups. Each group contained 15 fish/tank (60 fish/exposure regime) at the beginning of each experiment. Across all groups, several fish were inadvertently lost before the conclusion of the study due to the lack of an in-tank screen baffle, which resulted in failure of outflow containment. Hence, between 32 and 100% of the fish in each group were available for examination compared to initial number of the fish in each group (Table 2). However, no tank within the any of the groups was completely devoid of fish at the termination of the experiment. Many fish developed extraintestinal mycobacteriosis when exposed to all three mycobacteria strains. In contrast, all but two of 305 fish examined from either the high and low dose groups without paramecia or unexposed controls showed no extraintestinal infections. However, many of the fish in all exposures, regardless of their exposure regime had acid-fast bacteria (presumably Mycobacterium spp.) in the lumen of the intestine (Fig. 2).
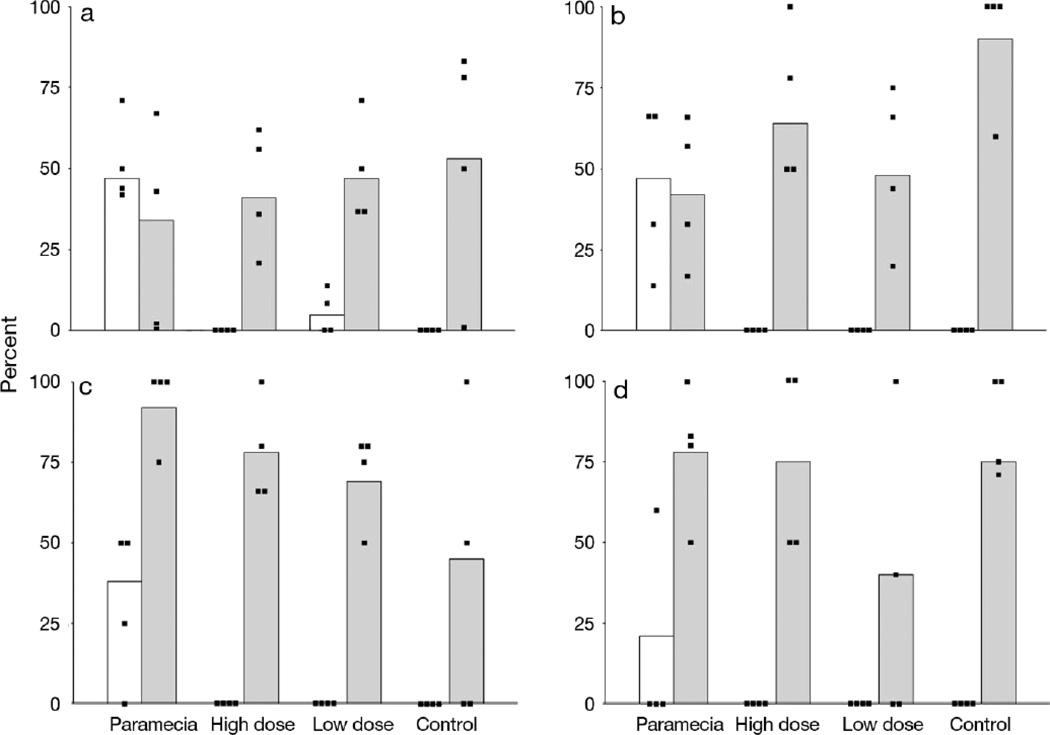
Figure 2.
Prevalence (%) of extraintestinal mycobacteriosis (white bars) and presence of mycobacteria in the intestinal lumen (gray bars). Data points indicate results from each of the for four tanks for each treatment regime. A. Mycobacterium marinum OSU 214 at 8wk pe. B. Mycobacterium marinum CH at 8 wk pe. C. Mycobacterium chelonae at 8 wk. D. M. chelonae at 16 wk pe.
M. marinum OSU 214
At 7 wk pe in this experiment in all groups there was a mortality event. This occurred across all exposure regimens including unexposed controls, and was attributed to gas supersaturation. These fish were included in the data analysis as this occurred within 1 wk of the final data collection time. Ten of these fish were from the M. marinum OSU 214 paramecia group, and 3 of these fish had severe systemic mycobacteriosis. All the other mortalities from the other groups (n = 23) did not exhibit mycobacteriosis. Fish fed mycobacteria with the paramecium vector had an approximate 50% incidence of infection, with the infections occurring in all replicate tanks (Table 2; Fig. 2a). In contrast, only 2 fish in one of the other groups (low dose mycobacteria without paramecia) demonstrated extraintestinal infections. Fish from all groups, including those not fed mycobacteria, showed acid-fast bacteria in the intestinal lumen (Fig. 2a).
Regarding statistical comparison, no difference was observed amongst tanks within each exposure regime (Chi-squared test with Monte Carlo simulation). Therefore, data from each tank within each exposure regime were pooled. Prevalence of infection in the paramecia group was significantly greater than all other groups (p < 0.001; Fisher’s exact test), whereas there was no significant difference between the low dose group compared to the high dose and control groups and the low dose infected group (< 0.001; Fisher’s exact test).
M. marinum CH
A similar pattern of infection occurred with the second strain of M. marinum. Only fish exposed to M. marinum CH via the paramecia were infected (Table 2; Fig 2b), whereas other groups had mycobacteria only in the intestinal lumen of some fish. The infected paramecia treatment group had an overall infection prevalence of 47.4%, which was statistically different than all other groups. (p < 0.001; Fisher’s exact test). Infection prevalence amongst tanks within each treatment regime was not statistically different among tanks (p = 0.532; Chi-squared test with Monte Carlo simulation), and hence pooling data across tanks was permissible.
M. chelonae
As M. chelonae infections are more chronic and less virulent than M. marinum, it was decided to examine fish as subsamples collected at 8 and 16 wk pe (Table 2; Fig. 2c,d). There were no extraintestinal infections in the low or high dose groups at either 8 or 16 wk pe. The paramecia treatment group had an infection prevalence of 38.5% at 8 wk (pe), which was statistically different from uninfected controls (p = 0.04; Fisher’s exact test).
At 16 wk pe 18.8% of the infected paramecia treatment group was infected, which did not differ from the unexposed controls (p = 0.22; Fisher’s exact test). Infection prevalence in infected paramecia treated fish did not statistically differ among tanks at 8 wk (p = 0.710; Chi-squared test with Monte Carlo simulation) or 16 weeks (p = 0.092; Chi-squared test with Monte Carlo simulation), so pooling data across tanks was permissible. There was also interest in the overall M. chelonae infection in infected paramecia treated fish, regardless of the time point sampled. There was no statistical difference between prevalence of paramecia treated fish sampled at 8 and 16 wk pe (p = 0.420; Fisher’s exact test), so data were pooled and the resulting overall infection prevalence of 29.6%, which was significantly different than uninfected controls and fish exposed via the gelatin food matrix without paramecia (p = 0.005; Fisher’s exact test).
Adult fish histology
M. marinum and M. chelonae infected fish exhibited the typical multifocally extensive pattern of mycobacteriosis, similar to the histological presentations seen in infected juvenile fish (Fig. 2), with prominent granuloma formation involving the kidney, spleen, liver, ovary, coelomic adipose tissue and less often the endomeninges, choroid rete, testis and the inner ear. Granulomas were variable in their chronicity, with the majority being well-organized and containing necrotic centers filled with cellular detritus. Fewer granulomas were recently developed and lacked necrotic centers. In all granulomas, variable numbers of acid-fast bacilli were observed, some within necrotic centers in addition to smaller intracellular aggregates within macrophages. No colonization of the swim bladder was seen in any of the infected fish, regardless of mycobacterial species. In a large percentage of fish exposed to M. marinum, M. chelonae or unexposed controls, variable numbers of mycobacterial aggregates were transiently present in the intestinal lumen, with no obvious epithelial association or colonization of the intestine (Fig. 1d).
Survival of mycobacteria at 5° C
Feed mixture containing mycobacteria, minus the gelatin, were held for up to 2 wk in a refrigerator at 5° C, and thus it was important to determine survival and growth of the mycobacteria under these conditions. In all cases, whether cultured at 1 or 3 wk after preparing the diet, CFU plate counts for both M. marinum OSU 214 and M. chelonae were initially 3×104 mycobacteria/5 ml, remained the same at 1 wk and decreased slightly to 2.8×104 at 3 wk.
DISCUSSION
Previous studies have shown that mycobacteria within amoebae, specifically the environmental amoebae Acanthamoeba castellani, Acanthamoeba polyphaga and Dictyostelium discoideum can successfully survive, replicate, may be potentiated for infectivity and spread of infection in the host (Cirillo et al. 1997, Adekambi et al. 2006, Hagedorn et al. 2007). Mycobacteria from granulomas are more infective than those from media cultures (Li et al. 2002). The enhanced infectivity of mycobacteria residing in amoeba vectors has been attributed to them acting somewhat as surrogate macrophages, with up-regulation of virulence genes when mycobacteria are within the intracellular environment (Danelishvili et al. 2004, Tenant & Bermudez 2006). Solomon et al. (2003), in concordance with this observation, found that Mycobacterium marinum effectively replicates within Dictyostelium by halting phagosomal maturation, and that mechanisms of cellular invasion elicited by M. marinum may be putatively conserved between amoebae and mammalian macrophages (Hagedorn & Soldati 2007).
This study demonstrated for the first time that a ciliate protozoan, Paramecium caudatum, like amoebae, can be a transmission vector for mycobacteria as mycobacterial infections occurred by feeding paramecia containing either M. marinum or M. chelonae. Moreover, transmission was dramatically increased with the paramecia vector compared to fish fed bacteria alone. It is likely the prevalent infections with the ciliate was due to a similar phenomenon of increased infectivity as reported with macrophages and amoebae, as fish fed mycobacteria in the paramecia actually received much lower amounts of bacteria compared to those fed the high doses of bacteria alone (Table 1).
Mycobacterial concentrations in paramecia were determined by directly counting bacteria within paramecia using acid-fast stains. It is likely that the total counts were actually slightly less for the paramecia group as non-viable mycobacteria could not be eliminated in the counts. Mycobacteria could not be counted after being placed in gelatin, but 40° C does not appreciably reduce the viability of mycobacteria (Merkal & Whipple 1980, Gao et al. 2002). We also showed that mycobacteria counts were essentially unchanged at 5 °C. The total amount of mycobacteria given to adult zebrafish within the paramecia vector over the 2 wk period using the three mycobacterial stains was about 6×106 bacteria/fish. Harriff et al. (2007) achieved 25% M. marinum infection (based on granuloma formation) in zebrafish gavage fed with amoebae containing 1.3×105 mycobacteria/fish in a single dose. This was a lower total dose compared to this study, but similar to the daily dose. These authors also achieved 67% and 35% prevalence of M. marinum infection by exposing zebrafish fish by gavage feeding at 3×107 and 8×105, respectively. These findings are quite different than those from this study using two strains of M. marinum fed without paramecia, as no fish became infected when exposed to a total dose of either 6.4×108 or 8.5×106 . One plausible explanation is that the fish in this study were exposed under more natural feeding conditions and over a 2 wk period, rather than a single bolus dose forcibly introduced to the intestine. Also, zebrafish are relatively small and agastric, so it may be possible that the intestinal epithelium was traumatically breached with the plastic gavage tube during exposure in earlier study. Another comparable transmission study was conducted by Mutoji (2011), where mosquito larvae containing M. marinum were fed to Japanese medaka. Fish receiving 4 or 7 feedings resulted in about 90% prevalence of infection, with a calculated dose of about 4 to 7×104 mycobacteria/fish, respectively.
Only two fish that were not fed paramecia developed extraintestinal infections. These occurred in the low dose M. marinum OSU 214, but the high dose group feed the same bacteria were not infected. Standardized diets fed prior to the transmission studies constitute an unlikely source of mycobacteria as these diets contain treated fish meal products. It is possible that these two fish had pre-existing background infections, as we have documented minimal background mycobacteriosis in zebrafish at the laboratory providing the fish for the present study (Kent et al. 2004, Kent et al. 2011). Mycobacterium species capable of causing disease in fish may occur in aquaria in the absence of disease (Beran et al. 2006). Indeed, all groups of the fish in this study, including controls that were not deliberately exposed to mycobacteria, exhibited the presence (perhaps colonization) of large amounts of acid-fast bacteria in the intestinal lumen. This demonstrates that the presence of mycobacteria, even if ingested, does not always result in extraintestinal infections or may represent non-virulent intestinal microflora. The latter is plausible as we frequently observed acid-fast bacteria in the intestinal lumen of control fish. As discussed above, one factor allowing for these infections may be changes in gene expression as may occur whether the mycobacteria are in macrophages, amoebae or invertebrate hosts.
Natural mycobacterial infections occasionally present as aerocystitis (swim bladder infection) in zebrafish (Whipps et al. 2008). We observed this presentation in larval fish, but not adults. Physostomous teleost fish, such as the zebrafish, have open communication (pneumatic duct) between the distal oropharynx and swim bladder and several mycobacterial species are known to colonize the swim bladder and cause aerocystitis (Matthews 2004, Whipps et al. 2008, Kent et al. 2011). In larval zebrafish, it may be anatomically favorable for non-motile mycobacteria to colonize the swim bladder, as the total distance traveled from the oropharynx to pneumatic duct and swim bladder is considerably shorter than in juvenile and adult zebrafish, and this may provide one explanation for the predilection of mycobacteria to localize in the larval swim bladder. Another possible explanation for the affinity of mycobacteria to the zebrafish swim bladder may be a correlation between the higher cholesterol content of swim bladder surfactant lipid, when compared to mammalian lung surfactant, and the requirement of mycobacterial species to import and catabolize cholesterol to maintain persistent infection in the host (Orgeig et al. 2003, Pandey & Sassetti 2008). Two of the present authors (M.K. and T.P.) have provided histologic interpretations for numerous diagnostic cases submitted to the Zebrafish International Resource Center diagnostic program, Eugene, Oregon. As reported byWhipps et al. (2008), there have been several confirmed cases of mycobacterial aerocystitis in zebrafish with mycobacteriosis from many laboratories both in the United States and internationally. Perhaps these represent fish with very chronic infections that were established as larvae or juvenile fish. The teleost swim bladder shares many conserved anatomical features with the mammalian lung, from comparative ontogeny (Perry and Sander. 2004) to conserved gene expression and transcriptomics in molecular developmental pathways (Winata et al. 2009, Zheng et al. 2011), and thus the model that we developed with larval zebrafish may be a useful surrogate for investigating certain aspects of human pulmonary tuberculosis.
Since amoebae and ciliates occurring naturally in the aquatic environment can be vectors for mycobacteria, these organisms are likely an important route of transmission and subsequent infection in zebrafish. Moreover, live foods such as aquatic protozoans, worms and crustacean larvae are deliberately included as a dietary staple of larval, juvenile and adult broodstock zebrafish (Watanabe et al. 1983, Lawrence 2007, Best et al. 2010). Commonly, these live feeds consist of natural prey items such as paramecia, rotifers, water fleas (Daphnia spp.), Artemia spp. and chironomid insect larvae. Water fleas in particular have been implicated as a source of mycobacteria within established aquaculture systems (Grange 1985, Conroy & Conroy 1999, Somsiri et al. 2005). Considering that paramecia are a common first feed for larval zebrafish, the results shown in this study demonstrate one specific potential source of infection. Therefore, facility staff involved in propagating zooplankton, such as paramecia, as a food source for larval zebrafish should exercise caution to avoid exposing these cultures to potential sources of mycobacteria. Future mycobacteria transmission studies with live feeds commonly used in laboratory zebrafish larviculture, including rotifers and Artemia spp., are warranted to determine whether these microinvertebrates can also act as vectors for mycobacterial infection within zebrafish colonies. Given that a wide variety of ciliate species occur in the aquatic environment and undoubtedly encounter mycobacteria, further studies are warranted to compare and contrast the inter-relationship of paramecia with mycobacteria to the amoebae models.
Acknowledgments
The authors thank the Oregon State University Veterinary Diagnostic Laboratory, especially Kay Fisher, Misty Corbus and Renee Norred for their excellent histotechnical assistance with slide preparation as well as Colleen Paquette and Justin Sanders for additional technical assistance. This work was supported by NIH/NCRR grants T32 RR023917, R24 RR017886 and NIEHS Center grant P30 ES000210.
LITERATURE CITED
- Adekambi T, Ben Salah S, Khlif M, Raoult D, Drancourt M. Survival of environmental mycobacteria in Acanthamoeba polyphaga. App Environ Microbiol. 2006;72:5974–5981. doi: 10.1128/AEM.03075-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amsterdam A, Hopkins N. Mutagenesis strategies in zebrafish for identifying genes involved in development and disease. Trends Genet. 2006;22:473–478. doi: 10.1016/j.tig.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Astrofsky KM, Schrenzel MD, Bullis RA, Smolowitz RM, Fox JG. Diagnosis and management of atypical Mycobacterium spp. infections in established laboratory zebrafish (Brachydanio rerio) facilities. Comp Med. 2000;50:666–672. [PubMed] [Google Scholar]
- Baker D. Natural pathogens of laboratory animals: Their effects on research. Herdon, VA: ASM Press; 2003. [Google Scholar]
- Barker J, Brown MRW. Trojan horses of the microbial world: protozoa and the survival of bacterial pathogens in the environment. Microbiol. 1994;140:1253–1259. doi: 10.1099/00221287-140-6-1253. [DOI] [PubMed] [Google Scholar]
- Beran V, Matlova L, Dvorska L, Svastova P, Pavlik I. Distribution of mycobacteria in clinically healthy ornamental fish and their aquarium environment. J Fish Dis. 2006;29:383–393. doi: 10.1111/j.1365-2761.2006.00729.x. [DOI] [PubMed] [Google Scholar]
- Best J, Adatto I, Cockington J, James A, Lawrence C. A novel method for rearing first-feeding larval zebrafish: Polyculture with Type L saltwater rotifers (Brachionus plicatilis) Zebrafish. 2010;7:289–295. doi: 10.1089/zeb.2010.0667. [DOI] [PubMed] [Google Scholar]
- Broussard GW, Ennis DG. Mycobacterium marinum produces long-term chronic infections in medaka: A new animal model for studying human tuberculosis. Comp Biochem Physiol C Toxicol Pharmacol. 2007;145:145–154. doi: 10.1016/j.cbpc.2006.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirillo JD, Falkow S, Tompkins LS, Bermudez LE. Interaction of Mycobacterium avium with environmental amoebae enhances virulence. Infect Immun. 1997;65:3759–3767. doi: 10.1128/iai.65.9.3759-3767.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conroy G, Conroy DA. Acid-fast bacterial infection and its control in guppies (Lebistes reticulates) reared on an ornamental fish farm in Venezuela. Vet Rec. 1999;144:177–178. doi: 10.1136/vr.144.7.177. [DOI] [PubMed] [Google Scholar]
- Danelishvili L, Poort MJ, Bermudez LE. Identification of Mycobacterium avium genes up-regulated in cultured macrophages and in mice. FEMS Microbiol Lett. 2004;239:41–49. doi: 10.1016/j.femsle.2004.08.014. [DOI] [PubMed] [Google Scholar]
- Gao A, Mutharia L, Chen S, Rahn K, Odumero J. Effect of pasteurization on survival of Mycobacterium paratuberculosis in milk. J Dairy Sci. 2002;85:3198–3205. doi: 10.3168/jds.S0022-0302(02)74408-1. [DOI] [PubMed] [Google Scholar]
- Grange JM. Enfermedades Micobacterianas. Mexico: DF Editorial Cientifica PLM; 1985. pp. 1–52. [Google Scholar]
- Grunwald DJ, Eisen JS. Headwaters of the zebrafish – emergence of a new model vertebrate. Nature Rev Genetics. 2002;3:714–724. doi: 10.1038/nrg892. [DOI] [PubMed] [Google Scholar]
- Hagedorn M, Soldati T. Flotillin and RacH modulate the intracellular immunity of Dictyostelium to Mycobacterium marinum infection. Cell Microbiol. 2007;9:2716–2733. doi: 10.1111/j.1462-5822.2007.00993.x. [DOI] [PubMed] [Google Scholar]
- Hall-Stoodley L, Brun OS, Polshyna G, Barker LP. Mycobacterium marinum biofilm formation reveals cording morphology. FEMS Microbiol Lett. 2006;257:43–49. doi: 10.1111/j.1574-6968.2006.00143.x. [DOI] [PubMed] [Google Scholar]
- Harper C, Lawrence C. The Laboratory Zebrafish. Boca Raton, FL: CRC Press; 2010. [Google Scholar]
- Harriff MJ, Bermudez LE, Kent ML. Experimental exposure of zebrafish, Danio rerio (Hamilton), to Mycobacterium marinum and Mycobacterium peregrinum reveals the gastrointestinal tract as the primary route of infection: a potential model for environmental mycobacterial infection. J Fish Dis. 2007;30:587–600. doi: 10.1111/j.1365-2761.2007.00839.x. [DOI] [PubMed] [Google Scholar]
- Hedrick RP, McDowell T, Groff J. Mycobacteriosis in cultured striped bass from California. J Wildlife Dis. 1987;23(3):391–395. doi: 10.7589/0090-3558-23.3.391. [DOI] [PubMed] [Google Scholar]
- Kent ML, Whipps CM, Matthews JL, Florio D, Watral V, Bishop-Stewart JK, Poort M, Bermudez LE. Mycobacteriosis in zebrafish (Danio rerio) research facilities. Comp Biochem and Physiol Part C. 2004;138:383–390. doi: 10.1016/j.cca.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Kent ML, Feist SW, Harper C, Hoogstraten-Miller S, Law JM, Sanchez-Morgado JM, Tanguay RL, Sanders GE, Spitsbergen JM, Whipps CM. Recommendations for control of pathogens and infectious diseases in fish research facilities. Comp Biochem Physiol Part C. 2009;149:240–248. doi: 10.1016/j.cbpc.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent ML, Buchner C, Watral VG, Sanders JL, LaDu J, Peterson TS, Tanguay RL. Development and maintenance of a specific pathogen-free (SPF) zebrafish research facility for Pseudoloma neurophilia. Dis Aquat Org. 2011;95:73–79. doi: 10.3354/dao02333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent ML, Harper C, Wolf JC. Documented and Potential Research Impacts of Subclinical Diseases in Zebrafish. ILAR J. 2012;53:126–134. doi: 10.1093/ilar.53.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence C. The husbandry of zebrafish (Danio rerio): A review. Aquaculture. 2007;269:1–20. [Google Scholar]
- Lawrence C, Ennis DG, Harper C, Kent ML, Murray K, Sanders GE. The challenges of implementing pathogen control strategies for fishes used in biomedical research. Comp Biochem Physiol, Part C. 2012;155:160–166. doi: 10.1016/j.cbpc.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YJ, Petrofsky M, Bermudez LE. Mycobacterium tuberculosis uptake by recipient host macrophages is influenced by environmental conditions in the granuloma of the infectious individual and is associated with impaired production of interleukin-12 and tumor necrosis factor alpha. Infect Immun. 2002;70:6223–6230. doi: 10.1128/IAI.70.11.6223-6230.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews JL. Common diseases of the laboratory zebrafish. Method Cell Biol. 2004;77:617–643. doi: 10.1016/s0091-679x(04)77033-8. [DOI] [PubMed] [Google Scholar]
- Merkal R, Whipple DL. Inactivation of Mycobacterium bovis in meat products. Appl Environ Microbiol. 1980;40:282–284. doi: 10.1128/aem.40.2.282-284.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miltner EC, Bermudez LE. Mycobacterium avium grown in Acanthamoeba castellanii is protected from the effects of antimicrobials. Antimicrob Agents Chemother. 2000;44:1990–1994. doi: 10.1128/aac.44.7.1990-1994.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutoji KN. PhD Thesis. University of Louisiana-Lafayette; 2011. Investigation into mechanisms of mycobacterial transmission between fish. [Google Scholar]
- Nyka W. Studies on the effect of starvation of mycobacteria. Infect Immun. 1974;9:843–850. doi: 10.1128/iai.9.5.843-850.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orgeig S, Daniels CB, Johnston SD, Sullivan LC. The pattern of surfactant cholesterol during vertebrate evolution and development: does ontogeny recapitulate phylogeny? Reprod Fetil Dev. 2003;15:55–73. doi: 10.1071/rd02087. [DOI] [PubMed] [Google Scholar]
- Ostland VE, Watral V, Whipps CM, Austin FW, St-Hilaire S, Westerman ME, Kent ML. Biochemical, molecular, and virulence characteristics of select Mycobacterium marinum isolates in hybrid striped bass Morone chrysops x M. saxatilis and zebrafish Danio rerio. Dis Aquat Org. 2008;79:107–118. doi: 10.3354/dao01891. [DOI] [PubMed] [Google Scholar]
- Pandey AK, Sassetti CM. Mycobacterial persistence requires the utilization of host cholesterol. PNAS. 2008;105:4376–4380. doi: 10.1073/pnas.0711159105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry SF, Sander M. Reconstructing the evolution of the respiratory apparatus in tetrapods. Respir Physiol Neurobiol. 2004;144:125–139. doi: 10.1016/j.resp.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Primm TP, Lucero CA, Falkinham JO., III Health impacts of environmental mycobacteria. Clin Microbiol Rev. 2004;17:98–106. doi: 10.1128/CMR.17.1.98-106.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prouty MG, Correa NE, Barker LP, Jagadeeswaran P, Klose KE. Zebrafish- Mycobacterium marinum model for mycobacterial pathogenesis. FEMS Microbiol Lett. 2003;225:177–182. doi: 10.1016/S0378-1097(03)00446-4. [DOI] [PubMed] [Google Scholar]
- Ross AJ. Mycobacteriosis among Pacific salmonid fishes. In: Sniesko SF, editor. A Symposium on Diseases of Fishes and Shellfishes. Washington, DC: AFS; 1970. pp. 279–283. [Google Scholar]
- Schulze-Robbecke R, Buchholtz K. Heat susceptibility of aquatic mycobacteria. Appl Environ Microbiol. 1992;58:1869–1873. doi: 10.1128/aem.58.6.1869-1873.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze-Robbecke R, Janning B, Fischeder R. Occurrence of mycobacteria in biofilm samples. Tuber Lung Dis. 1992;73:141–144. doi: 10.1016/0962-8479(92)90147-C. [DOI] [PubMed] [Google Scholar]
- Solomon JM, Leung GS, Isberg RR. Intracellular replication of Mycobacterium marinum within Dictyostelium discoideum: Efficient replication in the absence of host coronin. Infect Immun. 2003;71:3578–3586. doi: 10.1128/IAI.71.6.3578-3586.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somsiri T, Puttinaowarat S, Soontornwit S, Lacharoje S. Contamination of Mycobacterium spp. in live feeds. In: Walker P, Lester R, Bondad-Reantaso MG, editors. Diseases in Asian Aquaculture V. Manila, Philippines: Fish Health Section, Asian Fisheries Society; 2005. pp. 227–235. [Google Scholar]
- Taylor RH, Falkinham III JO, Norton D, LeChevallier MW. Chlorine, chloramine, chlorine dioxide, and ozone susceptibility of Mycobacterium avium. Appl Environ Microbiol. 2000;66:1702–1705. doi: 10.1128/aem.66.4.1702-1705.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenant R, Bermudez LE. Mycobacterium avium genes upregulated upon infection of Acanthamoeba castellani demonstrate a common response to the intracellular environment. Curr Microbiol. 2006;52:128–133. doi: 10.1007/s00284-005-0218-4. [DOI] [PubMed] [Google Scholar]
- Thomas V, McDonnell G. Relationship between mycobacteria and amoebae: ecological and epidemiological concerns. Lett App Microbiol. 2007;45:349–357. doi: 10.1111/j.1472-765X.2007.02206.x. [DOI] [PubMed] [Google Scholar]
- Vascotto SG, Beckham Y, Kelly GM. The zebrafish’s swim to fame as an experimental model in biology. Biochem Cell Biol. 1997;75:479–485. [PubMed] [Google Scholar]
- Volkman HE, Clay H, Beery D, Chang JCW, Sherman DR, Ramakrishnan L. Tuberculous granuloma formation is enhanced by a mycobacterium virulence determinant. PLoS Biology. 2004;2:1946–1956. doi: 10.1371/journal.pbio.0020367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wantanabe T, Kitajima C, Fujita S. Nutritional values of live organisms used in Japan for mass production of fish: A review. Aquaculture. 1983;34:115–145. [Google Scholar]
- Watral V, Kent ML. Pathogenesis of Mycobacterium spp. in zebrafish (Danio rerio) from research facilities. Comp Biochem and Physiol Part C. 2007;145:55–60. doi: 10.1016/j.cbpc.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Westerfield M. A guide for the laboratory use of zebrafish (Danio rerio) 5th ed. Eugene: Univ. of Oregon Press; 2007. The zebrafish book. [Google Scholar]
- Whipps CM, Dougan ST, Kent ML. Mycobacterium haemophilum infections of zebrafish (Danio rerio) in research facilities. FEMS Microbiol Lett. 2007;270:21–26. doi: 10.1111/j.1574-6968.2007.00671.x. [DOI] [PubMed] [Google Scholar]
- Whipps CM, Matthews JL, Kent ML. Distribution and genetic characterization of Mycobacterium chelonae in laboratory zebrafish Danio rerio. Dis Aquatic Org. 2008;82:45–54. doi: 10.3354/dao01967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whipps CM, Lieggi C, Wagner R. Mycobacteriosis in zebrafish colonies. ILAR J. 2012;53:95–105. doi: 10.1093/ilar.53.2.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winata CL, Korzh S, Kondrychyn I, Zheng W, Korzh V, et al. Development of zebrafish swimbladder: The requirements of Hedgehog signaling in specification and organization of the three tissue layers. Dev Biol. 2009;331:222–226. doi: 10.1016/j.ydbio.2009.04.035. [DOI] [PubMed] [Google Scholar]
- Zheng W, Wang Z, Collins JE, Andrews RM, Stemple D, Gong Z. Comparative transcriptome analyses indicate molecular homology of zebrafish swimbladder and mammalian lung. PLoS One. 2011;6:1–13. doi: 10.1371/journal.pone.0024019. [DOI] [PMC free article] [PubMed] [Google Scholar]