Abstract
Zebrafish (Danio rerio) are important models for biomedical research, and thus there is an increased concern about diseases afflicting them. Here we describe infections by Pleistophora hyphessobryconis (Microsporidia) in zebrafish from three laboratories. As reported in other aquarium fishes, affected zebrafish exhibited massive infections in the skeletal muscle, with no involvement of smooth or cardiac muscle. In addition, numerous spores within macrophages were observed in the visceral organs, including the ovaries. Transmission studies and ribosomal RNA (rRNA) gene sequence comparisons confirmed that the parasite from zebrafish was P. hyphessobryconis as described from neon tetra Paracheirodon innesi. Ten 15-day-old zebrafish were exposed to P. hyphessobryconis collected from one infected neon tetra, and 7 of 10 fish became infected. Comparison of P. hyphessobryconis small subunit rRNA gene sequence from neon tetra with that obtained from zebrafish was nearly identical, with < 1% difference. Given the severity of infections, P. hyphessobryconis should be added to the list of pathogens that should be avoided in zebrafish research facilities, and it would be prudent to not mix zebrafish used in research with other aquarium fishes.
Introduction
The zebrafish, Danio rerio, is an important research model for the study of infectious disease (Dooley & Zon 2000), developmental and genetic biology (Grunwald & Eisen 1999, Ackermann 2003), cancer (Amatruda et al 2002), immunology (Yoder et al 2002, Trede et al 2004), toxicology (Hill et al 2005), and drug discovery (Zon & Peterson 2005). Their small size, relative ease of husbandry, large research community, and ex vivo development of transparent embryos makes them an amenable model for such studies. As a result, numerous laboratory colonies have been established containing wild-type, mutant, and transgenic strains with a wide variety of genetic backgrounds.
Unfortunately, as is the case with any animal model the zebrafish can be afflicted with a number of diseases, potentially confounding experimental results and causing growing concern among investigators. The most common infectious diseases found in laboratory zebrafish are mycobacteriosis (Kent et al 2004) and microsporidiosis caused by the microsporidian parasite, Pseudoloma neurophilia. As the name implies, the microsporidium infects neural tissue in the brain and spinal cord of zebrafish (Matthews et al 2001).
We recently detected another microsporidium in zebrafish: Pleistophora hyphessobryconis. This is the causative agent of “neon tetra disease,” which targets the skeletal muscle of many aquarium fishes. The primary and type host is the neon tetra Paracheirodon innesi (Characiformes: Characidae). However, this microsporidium shows broad host specificity, and has been reported from many species of aquarium fishes in several families (Characidae, Cyprinidae, Cyprinodontidae, Poecilidae, Cichlidae), including D. rerio and D. nigrofasciatus (Steffens 1962). Some host range reports of this parasite were derived from cross transmission studies (Canning et al 1986) but most reports regarding the host range were from observations of naturally infected fishes. Therefore, it is conceivable that some of these infections may have been caused by other related, undescribed species that are morphologically indistinguishable.
We detected severe muscle infections of P hyphessobryconis (Lom & Dyková 1992, Shaw & Kent 1999) in zebrafish from three separate research facilities. We report here on the case histories, including macroscopic and histological changes associated with the infection in laboratory zebrafish. We demonstrate that the parasite recently found in zebrafish from research facilities was P. hyphessobryconis by conducting cross transmission experiments and rRNA gene sequence comparisons with P. hyphessobryconis obtained from the type host, neon tetra.
Materials and Methods
Case Histories
Zebrafish from three research facilities were examined by histology either as part of routine health screening or because fish exhibited clinical disease. The index case (at Lab 1) was evaluated by two of us (D.N. and J.B.) and the specimens from the other two facilities were submitted to the Zebrafish International Resource Center Diagnostic Service (http://zebrafish.org/zirc/health/index.phpwebsite). Information regarding fish husbandry and quarantine procedures was provided by the submitting client.
Histology
Fish were preserved in either 10% buffered formalin (Lab 1) or Dietrich’s fixative (Labs 2 and 3) and processed for routine histology. Transverse sections were made of the fish from Lab 1; sagittal sections were prepared of the fish from Labs 2 and 3. All sections were stained with hematoxylin and eosin. Additional tissue sections of the one infected fish from Lab 1 were stained with Lillie-Twort Gram stain (Culling 1974) and sections from several infected fish from Lab 3 were also stained with Accustain™, (Sigma-Aldrich, St. Louis, MO, USA), a commercial Brown-Hopps stain.
rRNA Gene Sequencing
After diagnosis of the infection by histology in several fish from Lab 3, additional live fish were euthanized and skeletal muscle was examined by wet mount. Infected muscle tissues from three fish were processed for sequencing. Approximately 25 mg of muscle tissue was extracted using the QIAgen Blood and Tissue kit (QIAgen, Valencia, CA) following the manufacturers protocol for extraction from tissue with an initial proteinase K digestion at 56°C for 3 hr. Approximately 50 juvenile neon tetras with a suspected history of the infection were obtained from a private retail fish store in the Corvallis, OR area. Muscle tissue from one infected fish was frozen and processed for sequencing as above.
PCR was performed using the general microsporidian small subunit rRNA gene primers V1F (5′-CACCAGGTTGATTCTGCCTGAC-3′) and 1492R (5′-GTTACCTTGTTACGACTT-3′). Amplifications were performed on a Peltier 200 thermocycler (MJ Research, Watertown, MA, USA) with an initial denaturation at 94°C for 2 min, 35 cycles at 94°C for 1 min, 55°C for 1 min, and 68°C for 1 min with a final extension at 68°C for 7 min. PCR products were cloned into TOPO TA Cloning vectors (Invitrogen, Carlsbad, CA, USA) and sequenced in both directions using primers flanking the inserted sequence. In order to exclude concomitant Pseudoloma neurophilia infection, a reverse primer was designed using the Primer-BLAST program available online from the NCBI (http://www3.ncbi.nlm.nih.gov/). The novel primer, PleistR (5′-TCTCGCTTGTTCGCGCCTGA-3′), was used with the forward primer, V1F to perform the PCR on samples from Lab 3 using the same thermocycling conditions as described above. PCR products from these samples were sequenced directly. All DNA analyzed in the study was sequenced on an ABI Prism®3730 Genetic Analyzer with the BigDye® Terminator v. 3.1 Cycle Sequencing kit (Applied Biosystems, Foster City, California).
Phylogenetic Analysis
The 16S rRNA gene sequence of P. hyphessobryconis was aligned with several Pleistophora sequences and other closely related genera returned by BLAST query of the GenBank database (Altschul 1990) using the Clustalw2 software (Larkin et al 2007). Pseudoloma neurophilia and Glugea anomala (Microsporidia) were selected as outgroup species. The jModelTest software (Posada & Buckley 2004) was used to determine the most likely model of sequence evolution for the dataset and phylogenetic analyses were performed using Bayesian inference as implemented in MrBayes3.1.2 (Huelsenbeck et al 2001) and maximum likelihood algorithms (Guindon et al 2009) as implemented in the PHYML webserver (http://www.atgc-montpellier.fr/phyml/). MrBayes was run using the General Time Reversible (GTR) model of nucleotide substitution with γ-distributed rate variation across sites and a proportion of invariable sites (GTR+G+I) for 1,000,000 generations. PHYML was run using the GTR model of nucleotide substitution and bootstrap support was based on 100 replicates.
The following sequences were obtained from GenBank and used for alignment and subsequent phylogenetic analysis: Pleistophora ovariae (AJ252955.1), P. mirandellae (AJ252954.1), Ovipleistophora mirandellae (AF356223.1), Heterosporis anguillarum (AF387331.1), P. mulleri (AJ438985.1), P. hippoglossoideos (AJ252953.1), Pleistophora sp. (AJ252957.1), P. typicalis (AJ252956.1), P. anguillarum (U47052.1), Pleistophora sp. 2 (AF044389.1), Trachipleistophora (AJ002605.1), Vavraia culicis (AJ252961.1), Pleistophora sp. 3 (AF044390.1), G. anomala (AF056016.1), G. stephani (AF056015.1), G. atherinae (U15987.1), Loma acerinae (AJ252951.1), Pleistophora sp. 1 (AF044394.1), and Pseudoloma neurophilia (AF322654.1).
Transmission studies
All fish were maintained and treated humanely, and the study protocol was conducted with approval from the Oregon State University Institutional Animal Care and Use Committee (ACUP# 3652).
Two groups of 15-day-old AB zebrafish (10 fish/group) were obtained from the Sinnhuber Aquatic Research Laboratory, Oregon State University and each group was placed into a 1.5 L tank. Spore counts were obtained by hemocytometer. Homogenized muscle tissue containing 50,000 spores from a heavily infected neon tetra was added to the tank of one group. The other group was held as an unexposed control. Tanks remained static for 12 hr postexposure after which water flow was slowly applied. Fish were held in a flow through system supplied by approximately 100 mL per hr of dechlorinated tap water heated to 28°C and fed Zeigler® Larval Diet (Zeigler, Gardners, PA, USA) twice daily. After 30 days, all fish were euthanized by an overdose of tricaine methanesulfate (MS-222) and processed for histology. In addition, two moribund fish from the parasite exposed group were collected at 20 days postexposure and examined by wet mount.
Results
Case History: Lab 1
Zebrafish at this facility had been maintained as a closed colony for several years. In an effort to add genetic diversity to this colony, a group of approximately 400 adult zebrafish was purchased in June 2006 from a commercial wholesale vendor that primarily supplies pet stores with various species of tropical fish. The new fish were held in quarantine for 90 days and no disease problems were noted during this time. While in quarantine, the new fish were segregated from the other fish in a circular 85 gal tank with a continuous flow-through of fresh, non-recycled water. The water source was the same as that used for the established research colony: a mixture of reverse-osmosis filtered municipal water and water from a deep on-site well. The fish were provided with the same food as that given to the adult fish in the established colony, consisting of dry flake food (TetraMin tropical fish flake food) twice daily and live brine shrimp once daily. At the end of the quarantine period, a female fish from the recently purchased group was mated with a male fish from the long-established colony. The eggs/developing embryos were bleach disinfected by immersion in a water bath containing 30 ppm sodium hypochlorite for 2 minutes after which the embryos were transferred to 10-gal aquariums where they developed into fry. These aquariums all shared the same recirculating water system. The food provided to the fry consisted of live Tetrahymena on days 1–4 post hatching, live microworms on days 3–21, and live brine shrimp on days 6–21. After day 21, the fish were fed flake food and brine shrimp as described above for adult zebrafish. All live invertebrates fed to the fish were from cultures maintained at Lab 1.
At approximately 1 month of age, the young fish were transferred from the 10-gal aquariums to a single 85-gal circular tank with a continuous flow-through of fresh, non-recycled water. In October 2007 (i.e., at 13 months of age), eight apparently healthy fish from this group were selected at random for routine health assessment and one of these fish was found through histologic examination to be infected with microsporidian parasites morphologically consistent with P. hyphessobryconis. Over several weeks after the detection of the infected fish, the remaining 135 fish of this sibling cohort group were euthanized and examined grossly (98 fish) and/or histologically (37 fish). No other infected fish were detected in this group and there have not been any subsequent cases detected in Lab 1.
Case History: Lab 2
A microsporidian infection consistent with P. hyphessobryconis was detected in 1 of 30 moribund fish submitted to the diagnostic laboratory of the Zebrafish International Resource Center (ZIRC), University of Oregon, Eugene, OR. The fish was a wild-type strain purchased from a commercial tropical fish vendor and was being held in quarantine at the time it became ill. The fish were hatched Sept 2008 and submitted to the diagnostic laboratory March 2009.
Case History Lab 3
The infection was initially detected in 2 of 13 moribund fish submitted to the ZIRC diagnostic service in June of 2009. The infected animals were 18-month-old fish from the CG1 strain: A clonal, homozygous strain of fish used exclusively for tissue transplantation studies and developed using AB fish obtained from a laboratory colony and a brass mutant line obtained from a pet store (Mizgireuv & Revskoy 2006).
The “founding” CG1 fish in Lab 3 (i.e., grandparents of the infected fish) were originally imported from another institution into Lab 3’s quarantine facility as surface-disinfected eggs. This quarantine facility is physically separated from the main fish holding room, with isolated recirculating water systems, ultraviolet treatment of effluent post-filtration, restricted access, and dedicated equipment. Fish are moved from quarantine into the main facility as eggs after being disinfected in 30 ppm sodium hypochlorite for 2 min and transfered to sterile water.
After this discovery, more fish in this facility were screened for the parasite. First, 10 live fish from the same stock in which the initial infection was detected were shipped live to Oregon State University for histology. All of these animals, many of which presented obvious clinical signs of infection, tested positive for the parasite by histology. An additional 13 individuals from the subsequent (F3) generation of CG1 in this facility were examined and confirmed as positive for the infection by histology. Finally, infections were detected in three more fish in August 2009, two more from the F3 generation of the CG1 strain and another fish from a separate population of WIK strain zebrafish. All three of these animals had been exposed to sublethal doses of gamma radiation to suppress the immune system before tissue transplantation (Traver et al 2004). The WIK fish in this facility were imported as described for the CGI line and had been maintained internally for many generations, with no known history of exposure to fish outside of the colony.
In October 2009, 46 AB strain fish from Lab 3 were submitted to ZIRC for histological analysis as part of the parent institution’s health sentinel monitoring program. These fish had been directly imported into the facility from ZIRC as surface disinfected eggs and reared in small groups on each of the facility’s recirculating systems. At 6 months, all of these fish were euthanized and examined by histology. A single fish from this group was positive for P. hyphessobryconis and it had been housed in the same rack as the infected CG1 fish.
Macroscopic changes and clinical signs
The one infected fish from Lab 1 showed no obvious clinical or macroscopic changes. The one infected fish from Laboratory 2 was sluggish, appeared bloated, and exhibited a white area in the integument below the dorsal fin that was obvious in the swimming fish when examined from above.
In Lab 3, large, depigmented regions in the central dorsal fin area were observed in infected fish while still swimming in tanks. Some fish also displayed spinal curvatures. Examination of infected fish with a dissecting microscope revealed multifocal to coalescing white-gray, slightly raised regions where the skin appeared depigmented (Fig 1). Removal of the skin showed that the underlying skeletal muscle was white, soft, and edematous (Fig 1). The one positive AB fish from the Lab 3 sentinel group appeared normal when euthanized.
Figure 1.
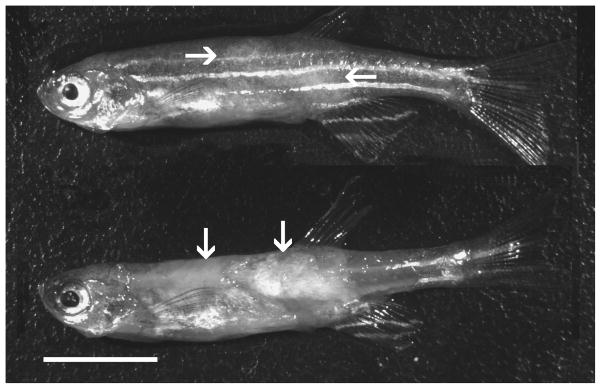
Zebrafish infected with P. hyphessobryconis. Upper image shows mottled appearance with light areas (arrows) on flanks. Lower image is same fish with skin removed, exhibiting opaque regions in muscle (arrows) representing massive infection. Bar = 500 μm.
Microscopic Examinations
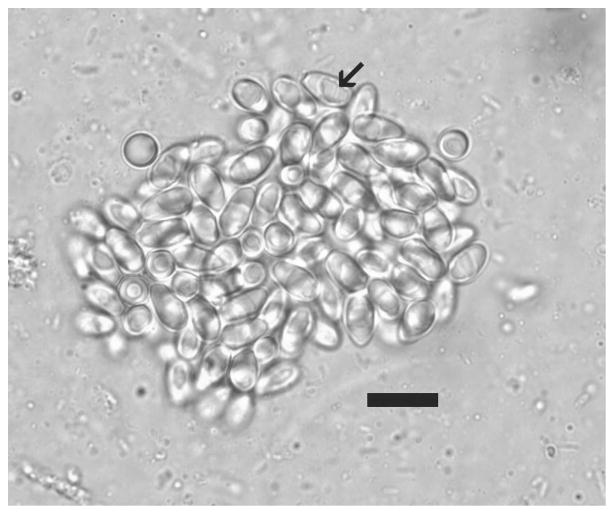
Wet-mount preparations of skeletal muscle from infected fish revealed numerous sporophorous vesicles, many which contained fully developed spores. Spores in wet mounts were 7 μm by 4 μm (n=20), and contained a prominent posterior vacuole measuring approximately 3 μm by 3 μm (Fig 2). Histopathologic changes were consistent from all three laboratories and thus are described together. As previously described by other investigators (Canning et al 1986, Dyková & Lom 1980, Schäperclaus 1941), intramuscular infection by P. hyphessobryconis microsporidian organisms caused disruption of skeletal muscle comprising myomeric units. However, in several of the fish examined as a part of this study, the severity of infection was much more pronounced and was associated with severe chronic inflammation. In these fish, on average, 50 to 80% of affected skeletal muscle myofibers displayed extensive liquefactive necrosis and marked expansion by the intramuscular microsporidial parasite. Skeletal muscle degeneration, denoted by loss of cross-striations as well as fragmented, hypereosinophilic myofibers and centralized nuclei, was also observed among the affected myofibers. In sections, muscle myofibers contained from 2 to 30-plus P. hyphessobryconis organisms at varying developmental stages, ranging from rounded-up multinucleated meronts to sporophorous vesicles containing sporoblast mother cells and vesicles with mature spores (Fig. 3). The affected skeletal muscle myofibers were frequently surrounded by large numbers of intermixed histiocytes and lymphocytes with fewer eosinophilic granular cells, which tracked along the endomysial connective tissue and were accompanied by marked endomysial edema that widely separated myofibers. Regenerating myofibers characterized by enhanced sarcoplasmic basophilia and nuclear rowing were frequently adjacent to many of the necrotic myofibers (data not shown). Numerous liberated mature spores within the endomysial spaces were surrounded by dense aggregates of histiocytes and lymphocytes (Fig. 3b). Phagocytized mature spores were occasionally seen within activated histiocytes.
Figure 2.
Wet mount of P. hyphessobryconis spores. Note prominent posterior vacuole (arrow). Bar = 10 μm.
Figure 3.
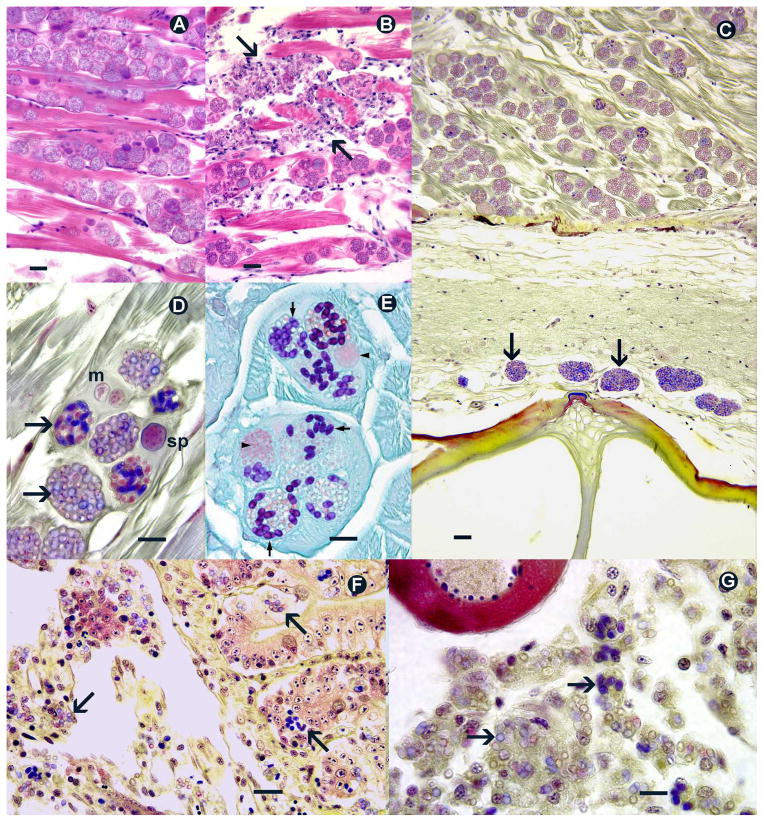
Histological sections of zebrafish infected with P. hyphessobryconis. A. Numerous sporophorous vesicles with spores and developmental stages within myocytes. Hematoxylin and eosin (H&E). Bar = 20 μm B. Spores in phagocytes associated with chronic infections and myolysis (demarked by arrows). H&E. Bar = 20 μm. C. Mixed infection with P. hyphessobryconis in muscle and xenomas of Pseudoloma neurophilia in spinal cord (arrows). Accustain Gram. Bar = 20 μm. D. Meronts (m), and developing and mature spores (sp) in sporophorous vesicles. Some fully developed spores stain deep blue (arrows). Accustain Gram. Bar = 10 μm. E. Mature spores stain deep blue to purple (arrows), developmental stages are light orange (arrow heads). Lillie Twort Gram. Bar = 10 μm. F. Numerous spores (arrows) throughout all layers of the intestine and mesenteries. Accustain Gram. Bar = 20 μm.
G. Masses of spores in phagocytes (arrows) in ovaries. Accustain Gram. Bar = 10 μm.
The parasitic infection in the single fish from Lab 1 was limited to the skeletal muscles; however in the fish from the other two laboratories, spores within phagocytes were also detected in various other organs, including the kidney interstitium, spleen, ovaries, intestine, and mesenteries. Massive numbers of spores within phagocytes were frequently observed in the connective tissues of the ovary, but never within ova (Fig. 3g). Aggregates of spores were observed in all layers of the intestine, extending through the serosal surface and into mesenteric connective tissue. Smooth and cardiac muscle were not affected in any of the fish examined. Both Gram stain methods were effective for distinguishing spores. With the Accustain method, mature spores stained deep blue while immature spores appeared to take up less of the stain (Fig. 3d). With the Lillie-Twort method, fully formed spores stained deep blue to purple (Fig 3e).
All seven of the fish from Lab 3 examined by histology exhibited a mixed infection with Pseudoloma neurophilia (Fig 3c). This microsporidium was easily distinguished from P. hyphessobryconis by its location in the central nervous system and lack of a sporophorous vesicle with a prominent wall.
Ribosomal DNA sequence
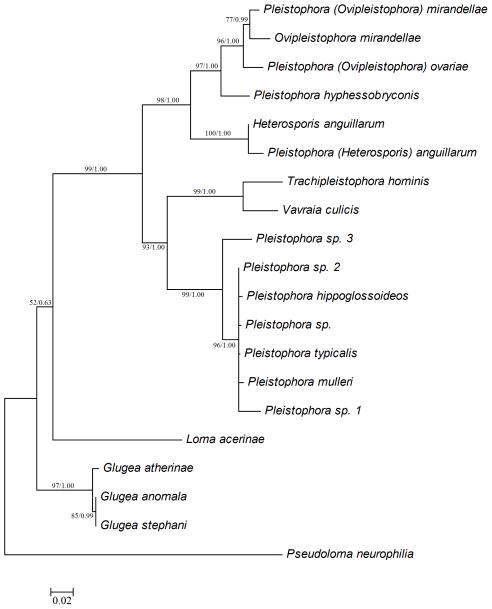
1361 bp of the small subunit rRNA gene was sequenced from P. hyphessobryconis obtained from an infected neon tetra and is available in the GenBank database under accession number GU126672. The maximum likelihood and Bayesian analyses produced phylogenetic trees with identical topology (Figure 4). The topology is also consistent with other phylogenetic analyses of fish microsporidian parasites (Moran et al 1999). P. hyphessobryconis was placed in the clade containing Ovipleistophora ovariae and Ovipleistophora mirandellae as well as Heterosporis anguillarum.
Figure 4.
Phylogenetic tree of Pleistophora hyphessobryconis obtained from neon tetra and related microsporidia based on small subunit rRNA gene sequences. 1361 bp small subunit rRNA gene sequences from 20 microsporidia infecting fish were used to reconstruct phylogeny. Maximum likelihood tree is shown. Branch numbers are maximum likelihood bootstrap support based on 100 replicates/Bayesian posterior probabilities. Genus names shown are as recorded in GenBank with new genus designations in parentheses. The microsporidian parasite, Pseudoloma neurophilia was selected as an outgroup taxa.
A total of 1224 bp (position 1 to 1224) of the small subunit rRNA gene was sequenced from P. hyphessobryconis obtained from three zebrafish submitted by Lab 3. All three of these sequences were identical, with no insertions/deletions nor transitions/transversions. Comparisons of the 1224 bp subset of the sequence obtained from the neon tetra and the three sequences obtained from zebrafish showed no insertions/deletions and two transitions: One at site 149 (T to C) and at site 180 (G to A) for an overall paired distance of 0.002.
Transmission
Both moribund zebrafish collected from the group exposed to infected neon tetra tissue exhibited massive muscle infections based on wet mount observations at 20 days postexposure. Histological examination of the remaining fish collected at 30 days showed infections in five of eight fish. These fish had light infections exhibiting various stages of development.
Discussion
Identification and taxonomy
P. hyphessobryconis infections in aquarium fishes have been documented for many decades after the original description in 1941 by Schäperclaus (Schäperclaus 1941). Unusual for microsporidia, the parasite shows remarkably broad host specificity, infecting some 20 species of freshwater fishes in four orders (Lom & Dyková 1992, Schäperclaus 1991, Steffens 1962). Among those are zebrafish (Opitz 1942) and the dwarf danio, D. nigrofasciatus (Spence et al 2008). Here we found the infection in three separate zebrafish research colonies with no known movement of fish between the facilities. Confirmation of host ranges of parasites with limited morphological characters (such as Microsporidia) often requires cross transmission studies or sequence comparisons. There are reports of experimental transmission experiments of P. hyphessobryconis amongst various fishes (Canning et al 1986). Here we confirmed that the infection in zebrafish was P. hyphessobryconis by histology, rRNA gene sequence comparisons, and cross transmission studies.
We found only < 1% (0.002) difference in small subunit rRNA gene sequence between parasites from the type host (the neon tetra) and from zebrafish. This is consistent with intraspecific variation in this region of the small subunit rRNA for other Pleistophora spp. and related microporidia. The intraspecific pairwise distance between other available sequences from multiple individuals in GenBank are as follows: P. typicalis, 0.013; Heterosporis (syn Pleistophora) anguillarum, 0.02; Ovipleistophora (syn, Pleistophora) mirandellae, 0.069; and P. mulleri., 0.007. Further, we found an average pairwise interspecific distance of 0.198 with all members of the genera Pleistophora, Ovipleistophora, and Heterosporis with sequences published in GenBank. The minor, but consistent, differences seen between the three zebrafish sequences compared to that of neon tetra might suggest different strains of the parasite. Regardless, the parasite from neon tetra was easily transmitted to zebrafish. Our findings agree with previous transmission studies, confirming the broad host specificity of this microsporidium (Canning et al 1986).
It was noted previously that the genus Pleistophora does not form a monophyletic clade (Nilsen et al 1998). In fact, ultrastructural characterization and molecular analyses of the small subunit rRNA gene sequences have resulted in the movement of P. mirandellae (Pekkarinen 2002) and P. anguillarum (Lom et al 2000) to new genera (Ovipleistophora and Heterosporis, respectively). Prior ultrastructural descriptions of P. hyphessobryconis (Lom & Corliss 1967) confirm the placement of this species in the genus Pleistophora as redescribed by Canning and Nicholas (Canning & Nicholas 1980). However, phylogenetic analyses of P. hyphessobryconis place it more closely to both Ovipleistophora mirandellae and Heterosporis anguillarum than P. typicalis, the type species described for the genus (Figure 4). Interestingly, unlike the members of Ovipleistophora, this species does not infect oocytes but rather myocytes as does the type species of this genus, P. typicalis. Comprehensive analysis of the genus is beyond the scope of this study but further investigation appears to be needed.
Transmission
The three facilities had no history of sharing fish and are located in different areas of the United States. Thus we presume that the infections arose in three independent instances by exposure to other species of infected aquarium fishes. Indeed, the CG1 line was actually derived from zebrafish purchased from a pet store (Mizgireuv & Revskoy 2006). The affected fish from Lab 2 had also been purchased from a pet store and the maternal parent of the infected fish at Lab 1 had been purchased from a vendor that supplied fish to the commercial pet trade. As stated above, transmission of P. hyphessobryconis, ostensibly per os, was achieved by placing large numbers of spores in water with fish, a method that has been used by others (Canning et al 1986). As with other microsporidia, it is assumed that infection is initiated by ingestion of spores. Spores released from dead fish are a likely source of infection, but could also be released from the intestines. Schäperclaus (1941) suggested that spores may be released from the skin or urinary tract of infected fish.
Zebrafish spawn frequently within aquaria, and tank mates quickly eat available eggs (Lawrence 2007, Spence et al 2008). Although spores were not found within eggs, the massive numbers seen within ovaries of some infected zebrafish suggest that infectious spores could be released during spawning and thus would be available to fish feeding on eggs or to infect the next generation of fish. Maternal transmission, including true vertical transmission within eggs, has been verified or implicated for other microsporidia of fishes (Docker et al 1997, Kent & Bishop-Stewart 2003, Phelps & Goodwin 2008). Schäperclaus (1941) suggested the possibility of maternal transmission as he found infections in 8-day-old neon tetras derived from infected parents. Once established in zebrafish, it is conceivable that the infection could be maternally transferred to the next generation. This could even occur with spores outside the egg as microsporidian spores are very resistant to chlorine (Ferguson et al 2007). This provides one explanation for the occurrence of the infection in fish derived from surface-disinfected eggs. Alternatively, these fish may have become infected by an unrecognized breach in biosecurity protocols.
The infected fish from Lab 1 was the 13-month-old offspring of a female fish that had been purchased from an outside vendor and brought into the laboratory. If this fish was infected as an embryo, this would mean that the fish was subclinically infected for more than 1 year. All of the fish purchased from the vendor had been removed from Lab 1 more than 7 months before the infected fish was detected. Even if the fish had not been infected as an embryo but instead at a later time through accidental cross-contamination with an infected fish from the group purchased from the vendor, it indicates that this fish was subclinically infected for more than 7 months.
As with other microsporidian infections, it is likely that immune status influences susceptibility of zebrafish to P. hyphessobryconis. Recently, Ramsay et al.(Ramsay et al 2009) showed that stress enhances infections of P. neurophilia in zebrafish. Infections by P. hyphessobryconis were widespread in only one strain (CG1) in one of the labs with the infection suggesting that this line may be particularly susceptible to the parasite. The microsporidium was also found in fish (CG1 and WIK) that were irradiated and thus were immune compromised. Although immune status likely affects the susceptibility and progression of the disease in zebrafish, apparently immunocompetent fish can become infected as demonstrated by reports of P. hyphessobryconis in zebrafish from aquaria (Opitz 1942, Steffens 1962) and experimental infections in presumably healthy zebrafish are reported here. The latter were exposed at 15-days old, a time at which fish have innate immunity but adaptive immunity has not fully developed (Lam et al 2004, Trede et al 2004).
Pathology
The infection in zebrafish was consistent with reports from neon tetras and other species (Dyková & Lom 1980, Canning et al 1986, Schäperclaus 1991). The most remarkable pathological feature of P. hyphessobryconis is the severe intensity of infection in the skeletal muscle, with well over half of the myofibers containing numerous spores and developmental stages in some fish. Parasites within myocytes were not associated with inflammation; however, mature spores released from degenerate myofibers into interstitial spaces were consistently associated with inflammation, and these liberated spores were often engulfed by phagocytes. This is similar to that seen with other intramuscular parasites of fishes, such as Kudoa thyrsites of salmon (Moran et al 1999). Likewise, other microsporidia show a similar sequelae of infection in which spores elicit significant inflammation only after they are released from their intracellular environment within xenomas (Dyková & Lom 1980, Kent & Speare 2005). Microsporidian spores remain intact within phagocytes, and thus may be transported to sites beyond where they originally developed (Kent et al 1999). The occurrence of large numbers of spores of P. hyphessobryconis within phagocytes in organs other than the skeletal muscle, as seen in the present study, has been previously observed (Lom J, Dyková 1992). Spores of Microsporidia are gram positive in histological sections (Gardiner et al 1998, Bruno et al 2006). Both Lilly-Twort and Accustain Gram stains were very effective for demonstrating spores and particularly useful for visualizing individual spores within visceral organs.
Some infected zebrafish exhibited concurrent infections by Pseudoloma neurophilia, a common microsporidium parasite of zebrafish that demonstrates myelinotropic behavior and is directly associated with encephalomyelitis and polyneuritis involving the peripheral nerves and spinal nerve roots. The two microsporidian infections can be easily differentiated by histology. Skeletal muscle is the primary site of development P. hyphessobryconis, with prominent developmental stages and spores within thick-walled sporophorous vesicles. In contrast, the central and peripheral nervous systems are the primary sites of infection for P. neurophilia and finding individual spores or xenomas in extraneural tissue is uncommon. In cases of myositis attributed to P. neurophilia, few spores are found in the muscle but these are frequently associated with severe chronic inflammation (Matthews et al 2001). In the case of coinfections, the opaque, depigmented muscle lesions were clearly caused by P. hyphessobryconis. However, it is possible that P. neurophilia contributed to other clinical changes.
Given the potential for severe infections and long-term subclinical chronic infections, P. hyphessobryconis should be added to the list of pathogens that should be avoided in zebrafish research facilities. As suggested for Pseudoloma neurophilia (Kent et al 2009), the most feasible strategy would be to hold brood fish in quarantine and screen them and their progeny for the infection using a PCR test specific to the parasite. Sentinel fish programs and sampling of moribund fish are also recommended as a means of surveillance of colony health. Notably, two of these cases were from fish that had been in contact with commercial pet store fish. It would be prudent to not mix zebrafish used in research with other aquarium fishes. Further studies on the role of maternal transmission, susceptibility of spores to disinfectants, and the role of age and fish strain in severity of disease are all warranted.
Acknowledgments
This study was supported by grants from the National Institutes of Health (NIH NCRR 5R24RR017386-02 and NIH NCRR P40 RR12546-03S1). Mention of a brand name does not imply endorsement of the product by the U.S. Federal Government. Opinions, interpretations, conclusions, and recommendations stated are those of the authors and are not necessarily endorsed by the U.S. Army. We thank Dr. G. Sanders, University of Washington for manuscript review, Dr. M. Schuster for assistance in translation of German references, and K. Berkenkamp for histology slide preparation and technical support.
References
- Ackermann G. Zebrafish: a genetic model for vertebrate organogenesis and human disorders. Front Biosci. 2003;8:d1227–d1253. doi: 10.2741/1092. [DOI] [PubMed] [Google Scholar]
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Amatruda JF, Shepard JL, Stern HM, Zon LI. Zebrafish as a cancer model system. Cancer Cell. 2002;1:229–231. doi: 10.1016/s1535-6108(02)00052-1. [DOI] [PubMed] [Google Scholar]
- Bruno DW, Nowak B, Elliott DG. Guide to the identification of fish protozoan and metazoan parasites in stained tissue sections. Dis Aquat Org. 2006;70:1–36. doi: 10.3354/dao070001. [DOI] [PubMed] [Google Scholar]
- Canning EU, Lom J, Dyková I. The Microsporidia of Vertebrates. London: Academic Press; 1986. Description of species infecting fish: Pleistophora hyphessobryconis Schäperclaus, 1941; pp. 101–107. [Google Scholar]
- Canning E, Nicholas JP. Genus Pleistophora (Phylum Microspora): redescription of the type species, Pleistophora typicalis Gurley, 1893 and ultrastructural characterization of the genus. J Fish Dis. 1980;3:317–338. [Google Scholar]
- Culling CFA. Handbook of Histopathological and Histochemical Techniques. Butterworths; London: 1974. Microorganisms; pp. 391–403. [Google Scholar]
- Docker M, Devlin R, Richard J, Khattra J, Kent M. Sensitive and specific polymerase chain reaction assay for detection of Loma salmonae (Microsporea) Dis Aquat Org. 1997;29:41–48. [Google Scholar]
- Dooley K, Zon LI. Zebrafish: a model system for the study of human disease. Curr Opin Genet Dev. 2000;10:252–256. doi: 10.1016/s0959-437x(00)00074-5. [DOI] [PubMed] [Google Scholar]
- Dyková I, Lom J. Tissue reactions to microsporidian infections in fish. J Fish Dis. 1980;3:265–283. [Google Scholar]
- Ferguson J, Watral V, Schwindt A, Kent ML. Spores of two fish Microsporidia (Pseudoloma neurophilia and Glugea anomola) are highly resistant to chlorine. Dis Aquat Org. 2007;76:205–214. doi: 10.3354/dao076205. [DOI] [PubMed] [Google Scholar]
- Gardiner CH, Fayer R, Dubey JP. An Atlas of Protozoan Parasites in Animal Tissues. Armed Forces Institute of Pathology; Washington, DC: 1998. Microspora; pp. 12–13. [Google Scholar]
- Grunwald DJ, Eisen JS. Headwaters of the zebrafish—emergence of a new model vertebrate. Nat Rev Genet. 1999;3:717–724. doi: 10.1038/nrg892. [DOI] [PubMed] [Google Scholar]
- Guindon S, Delsuc F, Dufayard J, Gascuel O. Estimating maximum likelihood phylogenies with PhyML. Methods Mol Biol. 2009;537:113–37. doi: 10.1007/978-1-59745-251-9_6. [DOI] [PubMed] [Google Scholar]
- Hill AJ, Teraoka H, Heideman W, Peterson RE. Zebrafish as a model vertebrate for investigating chemical toxicity. Toxicol Sci. 2005;86:6–19. doi: 10.1093/toxsci/kfi110. [DOI] [PubMed] [Google Scholar]
- Huelsenbeck JP, Ronquist F, Nielsen R, Bollback JP. Bayesian inference of phylogeny and its impact on evolutionary biology. Science. 2001;294:2310–2314. doi: 10.1126/science.1065889. [DOI] [PubMed] [Google Scholar]
- Kent ML, Bishop-Stewart JK. Transmission and tissue distribution of Pseudoloma neurophilia (Microsporidia) of zebrafish, Danio rerio (Hamilton) J Fish Dis. 2003;26:423–426. doi: 10.1046/j.1365-2761.2003.00467.x. [DOI] [PubMed] [Google Scholar]
- Kent ML, Dawe SC, Speare DJ. Resistance to reinfection in chinook salmon Oncorhynchus tshawytscha to Loma salmonae (Microsporidia) Dis Aquat Org. 1999;37:205–208. doi: 10.3354/dao037205. [DOI] [PubMed] [Google Scholar]
- Kent ML, Speare DJ. Review of the sequential development of Loma salmonae (Microsporidia) based on experimental infections of rainbow trout (Oncorhynchus mykiss) and Chinook salmon (O. tshawytscha) Folia Parasitol. 2005;52:63–68. doi: 10.14411/fp.2005.009. [DOI] [PubMed] [Google Scholar]
- Kent ML, Feist SW, Harper C, Hoogstraten-Miller S, Law JM, Sánchez-Morgado JM, Tanguay RL, Sanders GE, Spitsbergen JM, Whipps CM. Recommendations for control of pathogens and infectious diseases in fish research facilities. Comp Biochem Physiol, C. 2009;149:240–248. doi: 10.1016/j.cbpc.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent ML, Whipps CM, Matthews JL, Florio D, Watral V, Bishop-Stewart JK, Poort M, Bermudez L. Mycobacteriosis in zebrafish (Danio rerio) research facilities. Comp Biochem Physiol, C. 2004;138:383–390. doi: 10.1016/j.cca.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Lam SH, Chua HL, Gong Z, Lam TJ, Sin YM. Development and maturation of the immune system in zebrafish, Danio rerio: a gene expression profiling, in situ hybridization and immunological study. Dev Comp Immunol. 2004;28:9–28. doi: 10.1016/s0145-305x(03)00103-4. [DOI] [PubMed] [Google Scholar]
- Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R. ClustalW and Clustal X version 2.0. Bioinformatics. 2007;23:2947–8. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- Lawrence C. The husbandry of zebrafish (Danio rerio): a review. Aquaculture. 2007;269:1–20. [Google Scholar]
- Lom J, Dyková I. Protozoan Parasites of Fishes. Amsterdam, The Netherlands: Elsevier; 1992. Microsporidia (Phylum Microspora Sprague, 1977) pp. 125–157. [Google Scholar]
- Lom J, Dyková I, Wang CH, Lo CF, Kou GH. Ultrastructural justification for the transfer of Pleistophora anguillarum Hoshina, 1959 to the genus Heterosporis Schubert, 1969. Dis Aquat Org. 2000;43:225–31. doi: 10.3354/dao043225. [DOI] [PubMed] [Google Scholar]
- Lom J, Corliss JO. Ultrastructural observations on the development of the microsporidian protozoon Plistophora hyphessobryconis Schäperclaus*. J Eukaryot Microbiol. 1967;14:141–152. [Google Scholar]
- Matthews J, Brown A, Larison K, Bishop-Stewart J, Rogers P, Kent M. Pseudoloma neurophilia ng, n. sp., a new microsporidium from the central nervous system of the zebrafish (Danio rerio) J Eukaryot Microbiol. 2001;48:227–233. doi: 10.1111/j.1550-7408.2001.tb00307.x. [DOI] [PubMed] [Google Scholar]
- Mizgireuv IV, Revskoy SY. Transplantable tumor lines generated in clonal zebrafish. Cancer Res. 2006;66:3120–3125. doi: 10.1158/0008-5472.CAN-05-3800. [DOI] [PubMed] [Google Scholar]
- Moran JDW, Margolis L, Webster JM, Kent ML. Development of Kudoa thyrsites (Myxozoa: Myxosporea) in netpen-reared Atlantic salmon determined by light microscopy and a polymerase chain reaction test. Dis Aquat Org. 1999;37:185–193. doi: 10.3354/dao037185. [DOI] [PubMed] [Google Scholar]
- Nilsen F. Small subunit ribosomal DNA phylogeny of microsporidia with particular reference to genera that infect fish. J Parasitol. 2000;86:128–133. doi: 10.1645/0022-3395(2000)086[0128:SSRDPO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Nilsen F, Endresen C, Hordvik I. Molecular phylogeny of microsporidians with particular reference to species that infect the muscles of fish. J Eukaryot Microbiol. 1998;45:535–543. doi: 10.1111/j.1550-7408.1998.tb05113.x. [DOI] [PubMed] [Google Scholar]
- Opitz H. Mikrosporidienkrankheit Plistophora auch beim Hemigrammus ocellifer und Brachydanio rerio. Wochenschrift fur Aquarien-und Terrarienkunde. 1942;39:75–77. [Google Scholar]
- Pekkarinen M, Lom J, Nilsen F. Ovipleistophora gen. n., a new genus for Pleistophora mirandellae-like microsporidia. Dis Aquat. 2002;48:133–142. doi: 10.3354/dao048133. [DOI] [PubMed] [Google Scholar]
- Phelps NBD, Goodwin AE. Vertical transmission of Ovipleistophora ovariae (microspora) within the eggs of the golden shiner. J Aquat Anim Health. 2008;20:45–53. doi: 10.1577/H07-029.1. [DOI] [PubMed] [Google Scholar]
- Posada D, Buckley TR. Model selection and model averaging in phylogenetics: Advantages of akaike information criterion and bayesian approaches over likelihood ratio tests. Syst Biol. 2004;53:793–808. doi: 10.1080/10635150490522304. [DOI] [PubMed] [Google Scholar]
- Ramsay JM, Watral V, Schreck CB, Kent ML. Pseudoloma neurophilia (Microsporidia) infections in zebrafish (Danio rerio): Effects of stress on survival, growth and reproduction. Dis Aquat Org. 2009;297:157–162. doi: 10.3354/dao02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäperclaus W. Eine neue Mikrosporidien-krankheit beim Neonfisch und seinen Verwandten. Wochenschrift für Aquarienund Terrarienkunde. 1941;39/40:381–384. [Google Scholar]
- Schäperclaus W. Plistophora disease of neon and other aquarium fish (Pleistophorosis) In: Schäperclaus W, Kulow H, Schreckenbach, editors. Fish Diseases. 5. Vol. 2. New Dehli: Amerind Publishing Co; 1991. [Google Scholar]
- Shaw R, Kent M. Fish microsporidia. In: Wittner M, Weiss L, editors. The Microsporidia and Microsporidiosis. Washington DC: ASM Press; 1999. pp. 418–444. [Google Scholar]
- Spence R, Gerlach G, Lawrence C, Smith C. The behaviour and ecology of the zebrafish, Danio rerio. Biol Rev Camb Philos Soc. 2008;83:13. doi: 10.1111/j.1469-185X.2007.00030.x. [DOI] [PubMed] [Google Scholar]
- Steffens W. Der heutige stand der verbreitung von Plistophora hyphessobryconis Schäperclaus 1941 (Sporozoa, Microsporidia) Parasitol Res. 1962;21:535–541. doi: 10.1007/BF00260258. [DOI] [PubMed] [Google Scholar]
- Traver D, Winzeler A, Stern HM, Mayhall EA, Langenau DM, Kutok JL, Look AT, Zon LI. Effects of lethal irradiation in zebrafish and rescue by hematopoietic cell transplantation. Blood. 2004;104:1298–1305. doi: 10.1182/blood-2004-01-0100. [DOI] [PubMed] [Google Scholar]
- Trede NS, Langenau DM, Traver D, Look A, Zon LI. The use of zebrafish to understand immunity. Immunity. 2004;20:367–379. doi: 10.1016/s1074-7613(04)00084-6. [DOI] [PubMed] [Google Scholar]
- Yoder JA, Nielsen ME, Amemiya CT, Litman GW. Zebrafish as an immunological model system. Microbes Infect. 2002;4:1469–1478. doi: 10.1016/s1286-4579(02)00029-1. [DOI] [PubMed] [Google Scholar]
- Zon LI, Peterson RT. In vivo drug discovery in the zebrafish. Nat Rev Drug Discov. 2005;4:35–44. doi: 10.1038/nrd1606. [DOI] [PubMed] [Google Scholar]