Abstract
Objective
To explore linear growth, puberty, and predictors of linear growth impairment among pubertal liver transplant recipients.
Study design
Review of data collected prospectively through the Studies of Pediatric Liver Transplantation registry. Thirty-one variables were tested as risk factors for linear growth impairment, and factors significant at P < .1 were included in a logistic regression model. Risk factor analysis was limited to 512 patients who had complete demographic and medical data.
Results
A total of 892 patients surviving their first liver transplant by >1 year, with ≥1 height recorded, who were between 8 and 18 years old between the years 2005 and 2009 were included. Median follow-up was 70.2 ± 38.6 months, mean age was 12.9 ± 3.3 years, and mean height z-score (zH) was –0.5 ±1.4 SD. Twenty percent had linear growth impairment at last follow-up. Of 353 subjects with Tanner stage data, 39% of girls and 42% of boys ages 16-18 years were not yet Tanner 5. Growth impairment rates were higher among boys than girls (30% vs 7%, P < .05) at Tanner stage 4, and occurred in 8/72 (11 %) of Tanner 5 subjects. Among patients with parental height data, zH were lower than calculated mid-parental zH (P< .005). Independent predictors of growth impairment included linear growth impairment at transplant (OR 11.53, P ≤ .0001), re-transplantation (OR 4.37, P = .001), non-white race (P = .0026), and primary diagnosis other than biliary atresia (P = .0105).
Conclusions
Linear growth impairment and delayed puberty are common in pubertal liver transplant recipients, with pre-transplant growth impairment identified as a potentially modifiable risk factor. Catch-up growth by the end of puberty may be incomplete.
Physical growth is an important indicator of overall health in children with chronic disease states, including those with liver disease who require transplantation. Prior to transplant, factors that contribute to linear growth impairment include increased resting energy expenditure, decreased intake, nutrient malabsorption, abnormal nitrogen balance, and alterations of the growth hormone axis.1-3 Linear growth is expected to improve after replacement of a diseased liver as growth hormone and insulin-like growth factor 1 levels return to normal and nutritional status improves.4 The beneficial effects of restored hepatic function, however, may be offset by immunosuppressive medication, particularly high dose glucocorticoids.5,6 Previous studies have demonstrated that catch-up growth following transplant occurs but may be incomplete, and puberty is often delayed.7,8
In an analysis of prepubertal children after liver transplant (n = 1143), risk factors for poor linear growth were identified as prolonged steroid exposure, lower weight percentiles at time of transplant, linear growth impairment prior to transplant, and metabolic disease as the primary diagnoses.9 Analyses of factors impacting linear growth after transplant in the pubertal age group are limited by relatively small sample sizes and a wide distribution of age at transplant, primary disease, and outcome status. The aims of this study were to describe the linear growth and pubertal trends of older children included in the Studies of Pediatric Liver Transplantation (SPLIT) registry and identify potentially modifiable predictors of linear growth impairment in this large, prospective, multi-center cohort.
Methods
Initiated in 1995, the SPLIT registry is a multi-center data repository for pediatric liver transplant candidates and recipients and has included 44 centers in Canada and the US. All SPLIT centers have individual institutional review board approval, and individual informed consent is obtained from the parents or guardians prior to patient enrollment. Coded information is submitted to the SPLIT data coordinating center via a standardized Web-based data entry system beginning at the time of listing for transplantation. Data collection includes detailed information regarding clinical status, laboratory values, medical and operative therapies, and patient complications and outcomes.
The patient sample used in this analysis included children with data entered in the registry between ages 8 and 18 years who had undergone first liver transplantation while included in the registry, survived at least 1 year post-transplant, and had at least 1 recorded height between August 1, 2005 and May 31, 2009. The lower age limit of 8 years was selected to improve capture of all subjects who might undergo puberty in the study period. We chose August 2005 as the start date because that marked the introduction of Tanner staging as an element of SPLIT data collection.
Data Collection and Analysis
Linear growth data were obtained by wall-mounted stadiometer for ambulatory children. Heights were measured prior to transplant, at time of transplant, at 6, 12, 18, and 24 months following transplant, and annually thereafter. Some patients may have had multiple measurements over sequential years included if they continued to meet inclusion criteria (range 1-4 measurements per subject). Parental heights were self-reported for the majority of patients for whom data were collected, with 3 centers contributing 82% of the results. Height SD scores (height z-score [zH]) for patients and mid-parental height targets (MPH) were calculated using age-and sex-specific references for the general population provided by the Centers for Disease Control and Prevention growth charts.10 Seven patients received recombinant human growth hormone (rhGH) therapy and were excluded from the study. Tanner stage (pubic hair for boys and breast development for girls) was assessed by the attending gastroenterologist during the course of the physical examination for 63% of patients and by self-report for the remainder, using a validated self-report form.11,12 For the purpose of this analysis, linear growth impairment was defined as a zH below –1.64 (5th percentile for age and sex). Factors analyzed as possible predictors of linear growth impairment included 5 demographic and 26 medical variables routinely collected by SPLIT (Table I; available at www.jpeds.com). These were hypothesized to either have a direct impact on linear growth or to be markers for acuity of illness at the time of transplant.
Table I. List of demographic and medical variables analyzed as possible predictors of linear growth impairment.
| Sex | EBV before last height measurement |
| Race | PTLD before last height measurement |
| Primary diagnosis | CNI level at last height measurement |
| Era of transplant | Steroid use at last height measurement |
| Age at Transplant | Tanner stage at last height measurement |
| Donor type | Total bilirubin at transplant (mg/dL) |
| Patient status | Total bilirubin at 12 months (mg/dL) |
| Nutritional intake at listing | Total bilirubin at last height measurement (mg/dL) |
| Height failure at transplant | Albumin at transplant (g/dL) |
| Immunosuppression at transplant | Albumin at 12 months (g/dL) |
| Education prior to transplant | Albumin at last height measurement (g/dL) |
| Special education prior to transplant | Log(INR) at transplant |
| Prednisone use up to 12 months | Log(INR) at 12 months |
| CMV before last height measurement | Log(INR) at last height measurement |
| Re-transplanted | GGT at 12 months (U/L) GGT at last height measurement (U/L) |
CMV, cytomegalovirus; CNI, calcineurin inhibitor; EBV, Epstein–Barr virus; GGT, gamma glutamyl transferase; INR, international normalized ratio; PTLD, post-transplant lymphoproliferative disorder.
Statistical Analyses
Data were summarized using means and SE for continuous factors and proportions for categorical factors. Risk factors for linear growth impairment were identified using logistic regression. Univariate analyses were performed using Kruskal–Wallis test for continuous factors and χ2 test for categorical factors on 31 variables (Table I). Factors significant at the 0.10 level in the univariate analyses were included in the multivariate model, which was derived using stepwise backward elimination procedure. Model simplification continued until the reduced model yielded a significant worsening of fit according to the likelihood ratio criterion (P ≤ .05). All statistical analyses were performed using SAS for Windows, v. 9.1 (SAS Institute Inc, Cary, North Carolina).
Results
A total of 1022 children underwent primary liver transplant during the study period and 892 met the inclusion criteria. The mean age at transplant was 7.0 ± 5.1 years, the mean age at survey was 12.9 ± 3.3 years, and mean zH was – 0.5 ± 1.4 SD. The mean follow-up for all subjects was 70.2 ± 38.6 months; 43% of patients were transplanted between ages 5 and 12 and 15.8% were transplanted as infants. A complete list of patient demographic and clinical status is displayed in Table II (available at www.jpeds.com).
Table II. Comparison of demographic and clinical status of patients included in the analysis vs those excluded due to lack of height measurement.
| Having at least 1 height measurement at follow-up | |||||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| No | Yes | Total | |||||
|
|
|
|
|||||
| n | % | n | % | n | % | χ2 P value | |
| Total | 123 | 100.0 | 899 | 100.0 | 1022 | 100.0 | |
| Sex | |||||||
| Male | 61 | 49.6 | 415 | 46.2 | 476 | 46.6 | .5006 |
| Female | 62 | 50.4 | 484 | 53.8 | 546 | 53.4 | |
| Race | |||||||
| Missing | 1 | 0.8 | 5 | 0.6 | 6 | 0.6 | .2194 |
| White | 86 | 69.9 | 543 | 60.4 | 629 | 61.5 | |
| Black | 15 | 12.2 | 148 | 16.5 | 163 | 15.9 | |
| Hispanic | 11 | 8.9 | 114 | 12.7 | 125 | 12.2 | |
| Other | 10 | 8.1 | 89 | 9.9 | 99 | 9.7 | |
| Primary diagnosis | |||||||
| Biliary atresia | 49 | 39.8 | 289 | 32.1 | 338 | 33.1 | .3545 |
| Other cholestasis | 21 | 17.1 | 143 | 15.9 | 164 | 16.0 | |
| Fulminant liver failure | 15 | 12.2 | 142 | 15.8 | 157 | 15.4 | |
| Metabolic disease | 22 | 17.9 | 165 | 18.4 | 187 | 18.3 | |
| Cirrhosis | 9 | 7.3 | 65 | 7.2 | 74 | 7.2 | |
| Other | 7 | 5.7 | 95 | 10.6 | 102 | 10.0 | |
| Age at transplant | |||||||
| <6 mo | 12 | 9.8 | 39 | 4.3 | 51 | 5.0 | .0001 |
| 6-11 mo | 28 | 22.8 | 103 | 11.5 | 131 | 12.8 | |
| 1-4 y | 21 | 17.1 | 230 | 25.6 | 251 | 24.6 | |
| 5-12 y | 40 | 32.5 | 386 | 42.9 | 426 | 41.7 | |
| 13+ y | 22 | 17.9 | 141 | 15.7 | 163 | 15.9 | |
| Length of follow-up | |||||||
| 12 mo | 7 | 5.7 | 99 | 11.0 | 106 | 10.4 | .0411 |
| 24 mo | 9 | 7.3 | 76 | 8.5 | 85 | 8.3 | |
| 36 mo | 21 | 17.1 | 84 | 9.3 | 105 | 10.3 | |
| 48 mo | 8 | 6.5 | 70 | 7.8 | 78 | 7.6 | |
| 60 mo | 6 | 4.9 | 76 | 8.5 | 82 | 8.0 | |
| 72 mo | 11 | 8.9 | 71 | 7.9 | 82 | 8.0 | |
| 84 mo | 5 | 4.1 | 93 | 10.3 | 98 | 9.6 | |
| 96 mo | 22 | 17.9 | 107 | 11.9 | 129 | 12.6 | |
| 108 mo | 17 | 13.8 | 90 | 10.0 | 107 | 10.5 | |
| 120 mo | 6 | 4.9 | 49 | 5.5 | 55 | 5.4 | |
| 132 mo | 8 | 6.5 | 49 | 5.5 | 57 | 5.6 | |
| 144 mo | 3 | 2.4 | 31 | 3.4 | 34 | 3.3 | |
| 156 mo | 0 | 0.0 | 4 | 0.4 | 4 | 0.4 | |
| Re-transplant | |||||||
| No | 111 | 90.2 | 818 | 91.0 | 929 | 90.9 | .7397 |
| Yes | 12 | 9.8 | 81 | 9.0 | 93 | 9.1 | |
| Received growth hormone therapy | |||||||
| Missing | 78 | 63.4 | 363 | 40.4 | 441 | 43.2 | .4774 |
| No | 44 | 35.8 | 529 | 58.8 | 573 | 56.1 | |
| Yes | 1 | 0.8 | 7 | 0.8 | 8 | 0.8 | |
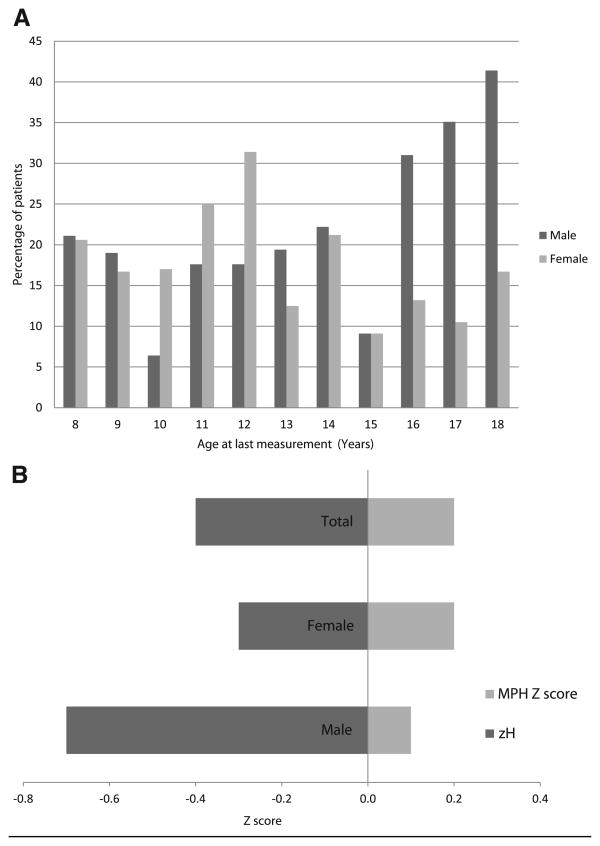
Linear growth impairment defined as a height less than the 5th percentile at last follow-up was observed in 174 children (19.5%). Figure 1, A displays the percentage of subjects with a zH below the 5th percentile by sex at 1-year age intervals. Linear growth impairment exceeded the expected prevalence of the general population (5%) for both sexes at all ages. A greater proportion of girls displayed growth impairment in the 10-12 age group after which the proportion of boys increased.
Figure 1.
Prevalence of linear growth impairment at last follow up visit. A, By age compared with the general population (n = 892). The percentage of males is greater than females in the late teens. B, By mean zH compared with calculated mean MPH zH (n = 138). P < .005 for the 3 groups.
Data on parental height were collected for 137 patients (15.3%); 82% of these data were collected at 3 centers that collected it on 63% of their overall population. There were no significant differences between this group and the overall cohort by sex, race, diagnosis, and re-transplantation rate. At transplant, this group had more infants and fewer children age 1-5 years (P < .005), and a greater proportion had their most recent height measurement within the last 24 months (36.2% vs 18.0%) compared with the overall cohort (data not shown). Although still within the normal range, the mean zH of both males and females were significantly lower than their calculated MPH z-scores with boys faring worse (P < .005) (Figure 1, B).
Figure 2, A and B display the height and weight zH at yearly intervals from transplant to 60 months post-transplant for the group and by sex. Mean group standardized height scores increased from –0.83 at time of transplant to –0.67 at 60 months, with the most rapid growth occurring in the first 12 months. There were no significant differences between males and females. Weight zH did not change significantly over the first 60 months. Figure 2, C stratifies zH by primary diagnosis and demonstrates the poorer outcomes of patients with Alagille syndrome and those with other cholestatic diseases.
Figure 2.
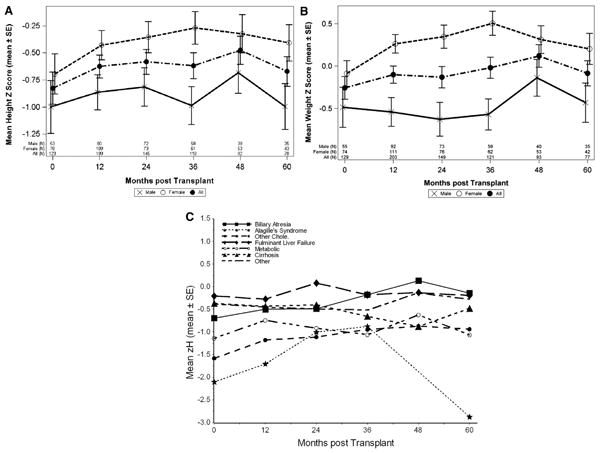
A, Height; B, weight z-score for study cohort at yearly intervals from transplant by sex (mean ± SE) showing modest improvement over 5 years; C, zH at yearly intervals by primary diagnosis revealing lower zH of patients with Alagille and other cholestatic diseases at time of transplant.
Pre-transplant growth impairment, previously identified in 262/781(33.5%) patients, resolved in 156/262 (60%) who no longer had growth impairment at last follow-up. Eight percent (42/519) of patients whose height had been greater than the 5th percentile pre-transplant subsequently developed linear growth impairment after transplantation.
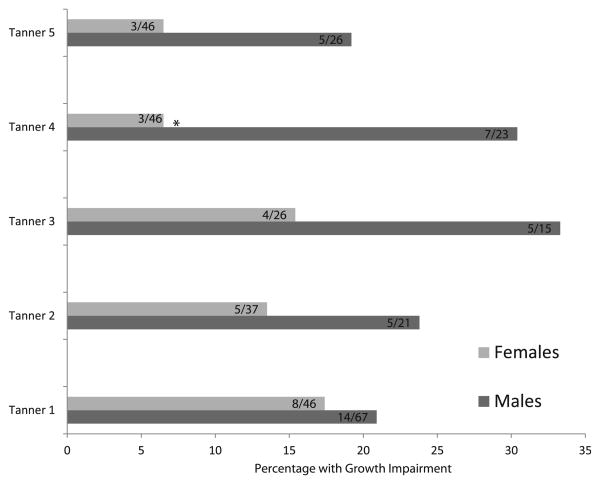
Tanner stage data were available on 42% of female and 37% of male subjects, two-thirds of which was collected by six centers. These centers collected data between 43% and 100% (pooled mean of 70%) of all their transplant recipients; 61% of girls and 58% of boys age 16-18 years were Tanner 5 compared with 100% of a normative population. At all Tanner stages, a greater proportion of male patients had height impairment compared with their female counterparts, though the difference reached statistical significance (P < .05) only for Tanner stage 4 (27% boys vs 7% girls) (Figures 3 and 4; available at www.jpeds.com). Growth impairment was present in 8/72 (11%) of Tanner 5 subjects.
Figure 3.
Proportion of patients with linear growth impairment by sex and Tanner stage (n = 353). A higher percentage of males are growth impaired at each tanner stage, which reaches significance at Tanner 4; *P < .05.
Figure 4.
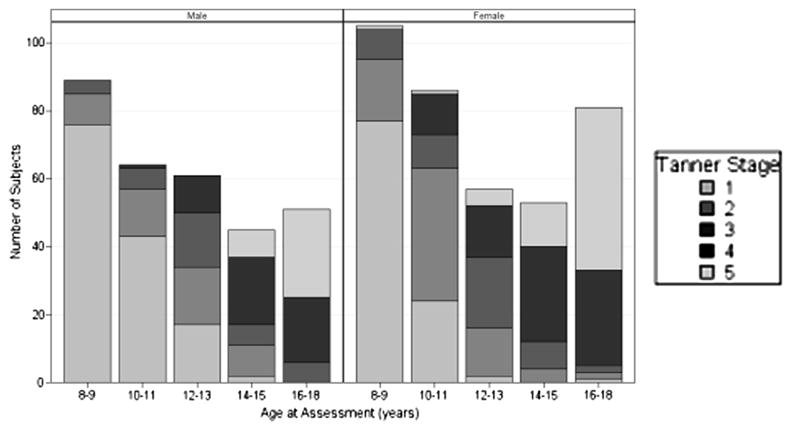
Tanner stage of patients by sex and age at assessment. A large proportion of both male and female patients have not attained Tanner 5 by age 16-18 years.
Modeling Growth Impairment
Race, primary diagnosis, patient status, nutritional intake at listing, height impairment at transplant, immunosuppression at transplant, requiring special education prior to transplant, re-transplantation, steroid use at last height measurement, albumin at 12 months (g/dL), Log (international normalized ratio) at transplant, and gamma glutamyltranspeptidase (GGTP) at 12 months (U/L) were identified as factors predictive of linear growth impairment in univariate analyses (Tables III and IV; Table IV available at www.jpeds.com).
Table III. List of significant factors (P < 0.1) in univariate logistic regression for linear growth impairment at last follow-up visit.
| Factor | Comparison group | Reference group | OR | CI | P value | Overall P value |
|---|---|---|---|---|---|---|
| Race | Black | White | 1.27 | (0.79, 2.02) | .3213 | .0009 |
| Hispanic | 1.79 | (1.11, 2.90) | .0172 | |||
| Other | 2.60 | (1.57, 4.29) | .0002 | |||
| Primary diagnosis | Other cholestatic | Biliary atresia | 3.84 | (2.42, 6.11) | <.0001 | <.0001 |
| Fulminant liver failure | 0.38 | (0.18, 0.80) | .0105 | |||
| Metabolic disease | 1.68 | (1.03, 2.73) | .0359 | |||
| Cirrhosis | 1.45 | (0.73, 2.88) | .2929 | |||
| Other | 0.84 | (0.42, 1.66) | .6072 | |||
| Patient status at transplant | ICU | Not hospitalized | 0.59 | (0.38, 0.92) | .0209 | .0488 |
| Hospitalized, not in ICU | 0.73 | (0.44, 1.21) | .2198 | |||
| Nutrition intake at listing | Intravenous | Oral | 0.72 | (0.39, 1.34) | .2999 | .0102 |
| Enteral (nasogastric tube) | 1.87 | (1.18, 2.96) | .0072 | |||
| Height failure at transplant | Yes | No | 7.72 | (5.17, 11.52) | <.0001 | <.0001 |
| Special education prior to transplant | Yes | No | 2.69 | (1.49, 4.86) | .0011 | .0031 |
| No education | 1.06 | (0.73, 1.53) | .7661 | |||
| Re-transplanted | Yes | No | 2.35 | (1.43, 3.87) | .0008 | .0008 |
| Steroids at last height measurement | Yes | No | 1.81 | (1.24, 2.64) | .0022 | .0022 |
| Albumin at 12 mo (g/dL) | Continuous | 0.70 | (0.50, 0.98) | - | .0392 | |
| Log(INR) at transplant | Continuous | 0.59 | (0.39, 0.89) | - | .0126 | |
| GGTat 12 mo (U/L) | Continuous | 1.001 | 1.000, 1.002 | - | .0031 | |
ICU, intensive care unit.
Bold values indicate significant < .05.
Table IV. Complete univariate logistic regression for linear growth impairment at last follow-up visit.
| Factor | Comparison group | Reference group | OR | CI | P value | Overall P value |
|---|---|---|---|---|---|---|
| Sex | Female | Male | 0.82 | (0.59, 1.14) | .2342 | .2342 |
| Race | Black | White | 1.27 | (0.79, 2.02) | .3213 | .0009 |
| Hispanic | 1.79 | (1.11, 2.90) | .0172 | |||
| Other | 2.60 | (1.57, 4.29) | .0002 | |||
| Primary diagnosis | Other cholestatic | Biliary atresia | 3.84 | (2.42, 6.11) | <.0001 | <.0001 |
| Fulminant liver failure | 0.38 | (0.18, 0.80) | .0105 | |||
| Metabolic disease | 1.68 | (1.03, 2.73) | .0359 | |||
| Cirrhosis | 1.45 | (0.73, 2.88) | .2929 | |||
| Other | 0.84 | (0.42,1.66) | .6072 | |||
| Era of transplant | ≥2002 | 1995-2001 | 1.13 | (0.81, 1.58) | .4558 | .4558 |
| Age at transplant | <6 mo | 13+ y | 0.42 | (0.14, 1.28) | .1287 | .2118 |
| 6-11 mo | 0.89 | (0.47, 1.68) | .7224 | |||
| 1-4 y | 0.69 | (0.41, 1.19) | .1828 | |||
| 5-12 y | 1.05 | (0.65, 1.68) | .8499 | |||
| Donor type | Live | Cadaveric whole | 0.77 | (0.46, 1.28) | .3153 | .1709 |
| Cadaveric reduced | 0.60 | (0.36, 1.00) | .0505 | |||
| Cadaveric split | 0.68 | (0.34, 1.33) | .2552 | |||
| Patient status at transplant | ICU | Not hospitalized | 0.59 | (0.38, 0.92) | .0209 | .0488 |
| Hospitalized, not in ICU | 0.73 | (0.44, 1.21) | .2198 | |||
| Nutrition intake at listing | Intravenous | Oral | 0.72 | (0.39, 1.34) | .2999 | .0102 |
| Enteral | 1.87 | (1.18, 2.96) | .0072 | |||
| Height failure at transplant | Yes | No | 7.72 | (5.17, 11.52) | <.0001 | <.0001 |
| Immunosuppression at transplant | Cyclosporin base | Tacrolimus base | 1.02 | (0.68, 1.54) | .9178 | .7735 |
| Other | 0.77 | (0.37, 1.61) | .4908 | |||
| Education prior to transplant | Part time | Full time | 1.36 | (0.61, 3.01) | .4535 | .3590 |
| Home schooling | 1.57 | (0.72, 3.39) | .2563 | |||
| No education | 1.74 | (0.77, 3.97) | .1849 | |||
| Not of school age | 0.85 | (0.59, 1.23) | .3921 | |||
| In college/completed HS/GED | 0.00 | (0.00, 1) | .9857 | |||
| Special education prior to transplant | Yes | No | 2.69 | (1.49, 4.86) | .0011 | .0031 |
| No education | 1.06 | (0.73, 1.53) | .7661 | |||
| Prednisone use up to 12 mo | #6 mo | No | 0.51 | (0.20, 1.32) | .1656 | .2894 |
| >6 mo | 0.65 | (0.27, 1.59) | .3458 | |||
| CMV before last height measurement | Yes | No | 1.09 | (0.66, 1.83) | .7293 | .7293 |
| EBV before last height measurement | Yes | No | 1.03 | (0.56, 1.91) | .9129 | .9129 |
| PTLD before last height measurement | Yes | No | 1.09 | (0.40, 2.96) | .8680 | .8680 |
| Re-transplanted | Yes | No | 2.35 | (1.43, 3.87) | .0008 | .0008 |
| CNI level at last height measurement | Low | Very low | 0.71 | (0.44, 1.13) | .1510 | .1045 |
| Medium | 0.82 | (0.48, 1.41) | .4671 | |||
| High | 1.36 | (0.73, 2.52) | .3295 | |||
| Tanner stage at last height measurement | 1 | 5 | 1.85 | (0.77, 4.43) | .1696 | .5055 |
| 2 | 1.67 | (0.61, 4.54) | .3178 | |||
| 3 | 2.25 | (0.79, 6.38) | .1274 | |||
| 4 | 1.22 | (0.44, 3.37) | .7009 | |||
| Steroids at transplant | Yes | No | 1.81 | (1.24, 2.64) | .0022 | .0022 |
| Total bilirubin at transplant (mg/dL) | Continuous | 0.997 | (0.981, 1.014) | - | .7461 | |
| Total bilirubin at 12 mo (mg/dL) | Continuous | 1.003 | (0.964, 1.045) | - | .8695 | |
| Total bilirubin at last height measurement (mg/dL) | Continuous | 1.027 | (0.958, 1.101) | - | .4555 | |
| Albumin at transplant (g/dL) | Continuous | 1.10 | (0.88, 1.37) | - | .4129 | |
| Albumin at 12 mo (g/dL) | Continuous | 0.70 | (0.50, 0.98) | - | .0392 | |
| Albumin at last height measurement (g/dL) | Continuous | 0.72 | (0.50, 1.03) | - | .0726 | |
| Log(INR) at transplant | Continuous | 0.59 | (0.39, 0.89) | - | .0126 | |
| Log(INR) at 12 mo | Continuous | 3.75 | (0.43, 33.04) | - | .2341 | |
| Log(INR) at last height measurement | Continuous | 2.29 | (0.72, 7.29) | - | .1594 | |
| GGT at 12 mo (U/L) | Continuous | 1.001 | 1.000, 1.002 | - | .0031 | |
| GGT at last height measurement (U/L) | Continuous | 1.001 | (1.000, 1.002) | - | .1272 | |
HS/GED, high school/general educational development.
Bold values indicate significant < .05.
Multivariate analyses included 512 patients with complete data for all variables selected by the univariate analyses. Characteristics of the included population compared with the overall group are provided in Table V (available at www.jpeds.com), with only age at transplant reaching a statistically significant difference. Patients who required re-transplantation and those with height impairment at time of transplant were the most likely to have linear growth impairment at last follow-up (Table VI). Patients who had a metabolic disease as their indication for transplant were twice (OR 2.61) as likely to have linear growth impairment than children with biliary atresia. The group ‘other cholestatic,’ which included 142 patients (34 with Alagille syndrome), were also more likely to have linear growth impairment (Table VII; available at www.jpeds.com)
Table V. Comparison of baseline characteristics for patients with complete data vs patients with incomplete data for the multivariate analysis on linear growth impairment.
| Patients included in multivariate mode | |||||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Total | No | Yes | |||||
|
|
|
|
|||||
| n | % | n | % | n | % | χ2 P value | |
| Total | 892 | 100.0 | 380 | 100.0 | 512 | 100.0 | |
| Sex | |||||||
| Male | 410 | 46.0 | 165 | 43.4 | 245 | 47.9 | .1972 |
| Female | 482 | 54.0 | 215 | 56.6 | 267 | 52.1 | |
| Race | |||||||
| Missing | 5 | 0.6 | 5 | 1.3 | 0 | 0.0 | .1231 |
| White | 539 | 60.4 | 222 | 58.4 | 317 | 61.9 | |
| Black | 148 | 16.6 | 55 | 14.5 | 93 | 18.2 | |
| Hispanic | 113 | 12.7 | 57 | 15.0 | 56 | 10.9 | |
| Other | 87 | 9.8 | 41 | 10.8 | 46 | 9.0 | |
| Primary diagnosis | |||||||
| Biliary atresia | 289 | 32.4 | 133 | 35.0 | 156 | 30.5 | .4937 |
| Other cholestatic | 142 | 15.9 | 52 | 13.7 | 90 | 17.6 | |
| Fulminant liver failure | 142 | 15.9 | 59 | 15.5 | 83 | 16.2 | |
| Metabolic disease | 164 | 18.4 | 70 | 18.4 | 94 | 18.4 | |
| Cirrhosis | 63 | 7.1 | 24 | 6.3 | 39 | 7.6 | |
| Other | 92 | 10.3 | 42 | 11.1 | 50 | 9.8 | |
| Age at transplant | |||||||
| <6 mo | 39 | 4.4 | 19 | 5.0 | 20 | 3.9 | <.0001 |
| 6-11 mo | 103 | 11.5 | 46 | 12.1 | 57 | 11.1 | |
| 1-4 y | 228 | 25.6 | 123 | 32.4 | 105 | 20.5 | |
| 5-12 y | 381 | 42.7 | 148 | 38.9 | 233 | 45.5 | |
| 13+ y | 141 | 15.8 | 44 | 11.6 | 97 | 18.9 | |
| Donor type | |||||||
| Missing | 14 | 1.6 | 10 | 2.6 | 4 | 0.8 | .0645 |
| Live | 120 | 13.5 | 50 | 13.2 | 70 | 13.7 | |
| Cadaveric whole | 541 | 60.7 | 212 | 55.8 | 329 | 64.3 | |
| Cadaveric reduced | 147 | 16.5 | 72 | 18.9 | 75 | 14.6 | |
| Cadaveric split | 70 | 7.8 | 36 | 9.5 | 34 | 6.6 | |
| Patient status at transplant | |||||||
| Missing | 5 | 0.6 | 5 | 1.3 | 0 | 0.0 | .7720 |
| ICU | 197 | 22.1 | 79 | 20.8 | 118 | 23.0 | |
| Hospitalized, not in ICU | 124 | 13.9 | 54 | 14.2 | 70 | 13.7 | |
| Not hospitalized | 566 | 63.5 | 242 | 63.7 | 324 | 63.3 | |
| Height failure at transplant | |||||||
| Missing | 111 | 12.4 | 111 | 29.2 | 0 | 0.0 | .2024 |
| No | 519 | 58.2 | 187 | 49.2 | 332 | 64.8 | |
| Yes | 262 | 29.4 | 82 | 21.6 | 180 | 35.2 | |
| Re-transplanted | |||||||
| No | 813 | 91.1 | 341 | 89.7 | 472 | 92.2 | .2334 |
| Yes | 79 | 8.9 | 39 | 10.3 | 40 | 7.8 | |
Table VI. Multivariate logistic regression of linear growth impairment at last follow-up visit (n = 512).
| Factor | Comparison group | Reference group | OR | CI | P value | OverallP value |
|---|---|---|---|---|---|---|
| Race | Black | White | 1.70 | (0.80, 3.63) | .1686 | .0042 |
| Hispanic | 2.09 | (0.92, 4.76) | .0788 | |||
| Other | 4.65 | (1.94, 11.12) | .0006 | |||
| Primary diagnosis | Other cholestatic | Biliary atresia | 2.59 | (1.24, 5.41) | .0116 | .0144 |
| Fulminant liver failure | 0.68 2.61 | (0.22, 2.13) | .5048 | |||
| Metabolic disease | 2.09 0.74 | (1.15, 5.92) | .0223 | |||
| Cirrhosis | (0.69, 6.34) | .1928 | ||||
| Other | (0.23, 2.35) | .6031 | ||||
| Height failure at transplant | Yes | No | 12.16 | (6.54, 22.61) | - | <.0001 |
| Re-transplanted | Yes | No | 4.14 | (1.72, 9.94) | - | .0015 |
Table VII. Patients with primary diagnosis of other cholestatic by linear growth impairment at last follow-up visit.
| Linear growth failure at last visit | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| Total | No | Yes | ||||
|
|
|
|
||||
| Other cholestatic | n | % | n | % | n | % |
| Total | 142 | 100.0 | 84 | 100.0 | 58 | 100.0 |
| Alagille syndrome | 34 | 23.9 | 19 | 22.6 | 15 | 25.9 |
| Byler disease and Familial cholestasis/cirrhosis | 19 | 13.4 | 11 | 13.1 | 8 | 13.8 |
| Idiopathic cholestasis/cirrhosis | 14 | 9.9 | 6 | 7.1 | 8 | 13.8 |
| Total parenteral nutrition induced | 5 | 3.5 | 1 | 1.2 | 4 | 6.9 |
| Primary sclerosing cholangitis | 41 | 28.9 | 27 | 14 | 32.1 | 24.1 |
| Biliary strictures | 2 | 1.4 | 2 | 2.4 | 0 | 0.0 |
| Neonatal hepatitis | 7 | 4.9 | 5 | 6.0 | 2 | 3.4 |
| Other cholestatic | 20 | 14.1 | 13 | 15.5 | 7 | 12.1 |
Discussion
We confirm earlier single center studies indicating that a significant number of children continue to have linear growth impairment and delayed puberty after transplant.8 These data advance the understanding of risk factors for linear growth impairment for use in developing guidelines to improve long-term linear growth and health status outcomes in this population.
Lower zH at transplant was the factor most strongly associated with linear growth impairment. This is similar to previous findings, wherein prepubertal patients with linear growth impairment at transplant were at risk for growth impairment up to 5 years post-transplant.9 Granting that children with more severe growth arrest prior to transplant have the most to recover after a successful liver transplant, they are also less likely to achieve normal growth percentiles post-transplant, underscoring the importance of appropriate pre-transplant growth and nutrition. Greater attention towards optimizing nutrition and preserving muscle mass may have a positive impact on post-transplant growth.13
Children with biliary atresia were less likely to have growth impairment compared with those with metabolic diseases. Patients receiving steroids at the most recent follow up were more likely to have growth impairment, which was expected based on previous reports.8,9,14,15 Albumin at 12 months and Log(international normalized ratio) at transplant were protective. GGTP at 12 months following transplant was statistically significant, but considering the associated OR, it had less clinical impact.
The prevalence of linear growth impairment decreased after liver transplant (19.5% vs 33.5%), but remained above the expected for a normal population (5%). Linear growth impairment was common in both sexes; however, the proportion of boys affected increased in the 16-18 years age range. The reasons for this are unclear as sex did not reach significance as a factor in our analysis. A possible explanation is that puberty occurs earlier in girls and we may have more girls who are Tanner 5 and have reached their adult heights, as evidenced by fewer girls with growth impairment who are Tanner 5 and who are 16-18 years old. Boys may have the potential to catch up once they complete puberty, in their late teens or early twenties.
zH at the time of transplant have not increased demon-strably for liver transplant recipients over the last 20 years.8,16 Pre-transplant zH vary between a median of –1.3 to –1.77 and a mean of – 1.55 from the SPLIT group.7,9,17 In contrast, a 2-decade review of the North American Pediatric Renal Transplant Cooperative Study data revealed increased zH at transplant and final adult height with the greatest increase occurring in pre-transplant zH. This improvement may be due to the older age of kidney transplant recipients, the use of rhGH treatment, or due to the fact that a temporizing measure in the form of dialysis is available. zH at renal transplant in 2009 for all ages was –1.23, an improvement from –2.43 in 1987. Patients aged 12 years and older had a baseline zH of –1.38 and 6 years post-transplant were –1.58, and the youngest patients had the greatest growth impairment and demonstrated some catch-up growth.18 rhGH has been used in these patients and has increased zH by 0.5 SD over a 5-ear period.18
Reports of the use of rhGH in liver transplant recipients in Europe have been promising. Although zH in patients treated with rhGH remained negative, there was a significant improvement without any reported side-effects.19,20 However, because of the hypothesized risk of rejection, further study is needed on whether the ultimate gain in linear growth is worth the added risk. After an initial rapid improvement in linear growth zH, there is a plateauing as time from transplant increases. The mean zH in our group post-transplant remained negative but were within the normal range, were comparable with previous reports, and were better than other solid organ transplant recipients.7, 17,18,21 Patients of both sexes had significantly lower zH compared with calculated MPH zH but these were also within the normal range.
Pubertal trends are an important part of overall growth and development, and pubertal delay has a negative effect on attainment of final adult height.22,23 Puberty was delayed across both sexes and at all ages with 60% of 16-18 year olds having attained Tanner 5 status compared with 100% of a normative population.24 Viner previously reported delayed puberty by 3-5 years in liver transplant recipients.8 In kidney transplant recipients, Fine et al reported a mean age of 15.8 and 17.7 years for Tanner stage 4 and 5 in boys compared with a mean age of 13.5-14 years for normal boys; girls also had delayed puberty.18 A Finnish study reported normal puberty in girls and delayed puberty in 22% of boys following renal transplantation.25
The association between prolonged steroid use and growth impairment has been documented previously.9 Current immunosuppression protocols call for the elimination of steroids between 6 and 18 months post-transplant, and there has been progress in decreasing post-transplant steroid exposure despite its continued requirement in the treatment of rejection or auto-immune liver disease26 Our findings support the use of more aggressive steroid-withdrawal protocols or steroid-free regimens in pediatric liver transplantation that have been shown to improve linear growth.27,28 The deleterious effects of corticosteroids on bone formation and growth hormone release may be modified by reducing the dose or changing to an alternate day schedule; alternatively, induction with interleukin 2 receptor antibodies, such as Basilixmab, may also be helpful.
Many children receiving prolonged steroids may also have chronic graft dysfunction contributing to impaired linear growth. Lower albumin and elevated GGTP were included as markers of post-transplant graft function and were significantly associated with impaired growth in the univariate analysis at 1 year post-transplant but lost significance at later follow-up. GGTP is a sensitive but nonspecific marker of bile duct injury; our data were not detailed enough to discriminate between patients with transient and chronic graft injury and the rationale for chronic steroid use. Although this may have masked the impact of certain types of chronic graft injury, our findings do further the idea that even low-level chronic injury may impair linear growth.
Liver transplant survivors have lower quality of life scores, particularly with respect to emotional and school functioning, compared with healthy controls.29 Mood, feelings of well-being, and self-esteem improve in children with short stature who are successfully treated with growth hormone therapy.30 Improvements in final adult height may improve the health status of liver transplant recipients and may be an important determinant in enhancing long-term outcomes. Additionally, the emphasis on growth impairment in organ allocation policies must be determined both by its impact on wait list mortality as well as on post-transplant growth potential and long-term outcomes.
This analysis shares limitations common to many large registry studies. Although data are gathered in a standardized, prospective fashion, compliance with data collection and entry is not complete. All data elements were not routinely collected at all centers, reducing the number of subjects included in the multivariate model. Data on Tanner staging were often missing; however, the 7 centers that contributed the most data on this variable collected it on 70% of their patients, reducing the potential for bias. Over one-third of Tanner stage data were self-reported, and although not as accurate as physician assessments, there are data that support significant agreement with physician measures.31-34 There is no uniform protocol for the management of patients following liver transplant. Centers that aggressively wean steroids and provide a strong focus on nutrition may have improved growth outcomes. Comparisons of patients included in the model and those excluded suggested the primary difference was age at transplant. However, as this was not significant in the univariate or multivariate analysis, it is unlikely that it confounded our results.
In summary, analysis of this large multi-center cohort of pubertal children revealed that linear growth impairment remains prevalent post-transplant and survivors are likely to be shorter adults than their parents. Height impairment at time of transplant, long-term steroid use, re-transplant, and metabolic disease independently predicted worse height outcomes. Delay in pubertal development is common, with boys more affected than girls. The zH post-transplant, although negative, was still within the normal range. Concerted nutritional support while awaiting transplant, organ allocation to infants prior to marked linear growth impairment, and early weaning of steroids post-transplant are important strategies to avoid linear growth impairment.
Glossary
- GGTP
Gamma glutamyltranspeptidase
- MPH
Mid-parental height targets
- rhGH
Recombinant human growth hormone
- SPLIT
Studies of Pediatric Liver Transplantation
- zH
Height z-score
Appendix
Members of the SPLIT Research Consortium.
| Transplant Center | Principal Investigator |
|---|---|
| University of California, Los Angeles | Sue McDiarmid |
| Stanford University, Stanford, California | William Berquist |
| Children's Hospital of Cincinnati Medical Center, Cincinnati, Ohio | John Bucuvalas |
| Children's Hospital of Colorado, Aurora, Colorado | Michael Narkewicz |
| University of Chicago, Chicago, Illinois | J. Michael Millis |
| Sainte-Justine Hospital, Montreal, Quebec, Canada | Steven Martin |
| Children's Medical Center, Dallas, Texas | Naveen Mittal |
| Children's Hospital, Western Ontario, London, Ontario, Canada | Paul Atkison |
| Hospital for Sick Children, Toronto, Ontario, Canada | Annie Fecteau |
| University of Nebraska, Omaha, Nebraska | Alan Langnas |
| Mayo Medical School, Rochester, Minnesota | Deborah Freese |
| Mount Sinai Medical Center, New York, New York | Nanda Kerkar |
| University of Alberta, Edmonton, Alberta, Canada | Susan Gilmour |
| Medical College of Virginia, Richmond, Virginia | Robert Fisher |
| University of Wisconsin, Madison, Wisconsin | Anthony D'Alessandro |
| LeBonheur Children's Medical Center, Memphis, Tennessee | James Eason |
| Cardinal Glennon Children's Hospital, St Louis, Missouri | Robert Kane |
| Lurie Children's Hospital, Chicago, Illinois | Estella Alonso |
| Children's Hospital of Philadelphia, Philadelphia, Pennsylvania | Elizabeth Rand |
| University of Miami/Jackson Memorial Hospital, Miami, Florida | Andreas Tzakis |
| University of California, San Francisco, San Francisco, California | Phillip Rosenthal |
| Children's Healthcare of Atlanta, Atlanta, Georgia | Thomas Heffron |
| Johns Hopkins Hospital, Baltimore, Maryland | Kathleen Schwarz |
| Children's Mercy Hospital, Kansas City, Missouri | Walter Andrews |
| University of Michigan, Ann Arbor, Michigan | James Lopez |
| University of Rochester, Rochester, New York | Adel Bozorgzadeh |
| St Louis Children's Hospital, St Louis, Missouri | Jeffrey Lowell |
| Texas Children's Hospital, Houston, Texas | Saul Karpen |
| University of Minnesota, Minneapolis, Minnesota | Abhi Humar |
| University of Florida, Gainesville, Florida | Regino Gonzalez-Peralta |
| Children's Hospital of Pittsburgh, Pittsburgh, Pennsylvania | George Mazariegos |
| University of California, San Diego, San Diego, California | Joel Lavine |
| Alfred I. DuPont Hospital for Children, Wilmington, Delaware | Stephen Dunn |
| Boston Children's Hospital, Boston, Massachusetts | Maureen Jonas |
| New York Presbyterian Hospital, New York, New York | Steven Lobritto |
| University of Texas, San Antonio, San Antonio, Texas | Glenn Halff |
| Children's Hospital of Wisconsin, Milwaukee, Wisconsin | Grzegorz Telega |
| Primary Children's Medical Center, Salt Lake City, Utah | Linda Book |
| University of Washington, Seattle, Washington | Simon Horslen |
| Indiana University, Indianapolis, Indiana | A. Joseph Tector |
| Duke University Medical Center, Durham, North Carolina | Elizabeth Tuttle-Newhall |
Footnotes
The authors declare no conflicts of interest.
Portions of this study were presented at the International Conference on Nutrition and Growth, March 1-3, 2012, Paris, France, March 2012, and presented as a poster at the Liver Meeting, November 9-13, 2012, Boston, MA.
References
- 1.Bucuvalas JC, Cutfield W, Horn J, Sperling MA, Heubi JE, Campaigne B, et al. Resistance to the growth-promoting and metabolic effects of growth hormone in children with chronic liver disease. J Ped. 1990;117:397–402. doi: 10.1016/s0022-3476(05)81079-0. [DOI] [PubMed] [Google Scholar]
- 2.Chin SE, Shepherd RW, Thomas BJ, Cleghorn GJ, Patrick MK, Wilcox JA, et al. Nutritional support in children with end-stage liver disease: a randomized crossover trial of a branched-chain amino acid supplement. Am J Clin Nutr. 1992;56:158–63. doi: 10.1093/ajcn/56.1.158. [DOI] [PubMed] [Google Scholar]
- 3.Quirk P, Owens P, Moyse K, Chin S, Wall C, Ballard J, et al. Insulin-like growth factors I and II are reduced in plasma from growth retarded children with chronic liver disease. Growth Reg. 1994;4:35–8. [PubMed] [Google Scholar]
- 4.Sarna S, Laine J, Sipila I, Koistinen R, Holmberg C. Differences in linear growth and cortisol production between liver and renal transplant recipients on similar immunosuppression. Transplantation. 1995;60:656–61. doi: 10.1097/00007890-199510150-00007. [DOI] [PubMed] [Google Scholar]
- 5.Cohen A, Shane E. Osteoporosis after solid organ and bone marrow transplantation. Osteoporos Int. 2003;14:617–30. doi: 10.1007/s00198-003-1426-z. [DOI] [PubMed] [Google Scholar]
- 6.Weinstein RS, Jilka RL, Parfitt AM, Manolagas SC. Inhibition of osteoblastogenesis and promotion of apoptosis of osteoblasts and osteocytes by glucocorticoids. Potential mechanisms of their deleterious effects on bone. J Clin Investig. 1998;102:274–82. doi: 10.1172/JCI2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scheenstra R, Gerver WJ, Odink RJ, van Soest H, Peeters PM, Verkade HJ, et al. Growth and final height after liver transplantation during childhood. J Ped Gastroenterol Nutr. 2008;47:165–71. doi: 10.1097/MPG.0b013e3181623279. [DOI] [PubMed] [Google Scholar]
- 8.Viner RM, Forton JT, Cole TJ, Clark IH, Noble-Jamieson G, Barnes ND. Growth of long-term survivors of liver transplantation. Arch Dis Childhood. 1999;80:235–40. doi: 10.1136/adc.80.3.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alonso EM, Shepherd R, Martz KL, Yin W, Anand R. Linear growth patterns in prepubertal children following liver transplantation. Am J Transplant. 2009;9:1389–97. doi: 10.1111/j.1600-6143.2009.02634.x. [DOI] [PubMed] [Google Scholar]
- 10.Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, et al. 2000 CDC growth charts for the United States: methods and development. Vital Health Stat. 2002;11:1–190. [PubMed] [Google Scholar]
- 11.Tanner J. Growth at adolescence. 2nd ed. Oxford: Blackwell Scientific Pub; 1962. pp. 30–6. [Google Scholar]
- 12.Morris N, Udry JR. Validation of a self-administered instrument to assess stage of adolescent development. J Youth Adolesc. 1980;9:271–80. doi: 10.1007/BF02088471. [DOI] [PubMed] [Google Scholar]
- 13.Holt RI, Miell JP, Jones JS, Mieli-Vergani G, Baker AJ. Nasogastric feeding enhances nutritional status in paediatric liver disease but does not alter circulating levels of IGF-I and IGF binding proteins. Clin Endocrinol (Oxf) 2000;52:217–24. doi: 10.1046/j.1365-2265.2000.00934.x. [DOI] [PubMed] [Google Scholar]
- 14.Bartosh SM, Thomas SE, Sutton MM, Brady LM, Whitington PF. Linear growth after pediatric liver transplantation. J Ped. 1999;135:624–31. doi: 10.1016/s0022-3476(99)70062-4. [DOI] [PubMed] [Google Scholar]
- 15.Codoner-Franch P, Bernard O, Alvarez F. Long-term follow-up of growth in height after successful liver transplantation. J Ped. 1994;124:368–73. doi: 10.1016/s0022-3476(94)70357-4. [DOI] [PubMed] [Google Scholar]
- 16.Urbach AH, Gartner JC, Malatack JJ, Zitelli BJ, Iwatsuki S, Shaw BW, et al. Linear growth following pediatric liver transplantation. Am J Dis Child. 1987;141:547–9. doi: 10.1001/archpedi.1987.04460050089037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sarna S, Sipila I, Vihervuori E, Koistinen R, Holmberg C. Growth delay after liver transplantation in childhood: studies of underlying mechanisms. Pediatr Res. 1995;38:366–72. doi: 10.1203/00006450-199509000-00016. [DOI] [PubMed] [Google Scholar]
- 18.Fine RN, Martz K, Stablein D. What have 20 years of data from the North American Pediatric Renal Transplant Cooperative Study taught us about growth following renal transplantation in infants, children, and adolescents with end-stage renal disease? Pediatr Nephrol (Berlin) 2010;25:739–46. doi: 10.1007/s00467-009-1387-3. [DOI] [PubMed] [Google Scholar]
- 19.Puustinen L, Jalanko H, Holmberg C, Merenmies J. Recombinant human growth hormone treatment after liver transplantation in childhood: the 5-year outcome. Transplantation. 2005;79:1241–6. doi: 10.1097/01.tp.0000161668.09170.f4. [DOI] [PubMed] [Google Scholar]
- 20.Rodeck B, Kardorff R, Melter M, Ehrich JH. Improvement of growth after growth hormone treatment in children who undergo liver transplantation. J Ped Gastroenterol Nutr. 2000;31:286–90. doi: 10.1097/00005176-200009000-00016. [DOI] [PubMed] [Google Scholar]
- 21.Peterson RE, Perens GS, Alejos JC, Wetzel GT, Chang RK. Growth and weight gain of prepubertal children after cardiac transplantation. Pediat Transplant. 2008;12:436–41. doi: 10.1111/j.1399-3046.2007.00826.x. [DOI] [PubMed] [Google Scholar]
- 22.Crowne EC, Shalet SM, Wallace WH, Eminson DM, Price DA. Final height in boys with untreated constitutional delay in growth and puberty. Arch Dis childhood. 1990;65:1109–12. doi: 10.1136/adc.65.10.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wehkalampi K, Pakkila K, Laine T, Dunkel L. Adult height in girls with delayed pubertal growth. Horm Res Paediatr. 2011;76:130–5. doi: 10.1159/000328055. [DOI] [PubMed] [Google Scholar]
- 24.Susman EJ, Houts RM, Steinberg L, Belsky J, Cauffman E, Dehart G, et al. Longitudinal development of secondary sexual characteristics in girls and boys between ages 9-1/2 and 15-1/2 years. Arch Pediatr Adolesc Med. 2010;164:166–73. doi: 10.1001/archpediatrics.2009.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tainio J, Qvist E, Vehmas R, Jahnukainen K, Holtta T, Valta H, et al. Pubertal development is normal in adolescents after renal transplantation in childhood. Transplantation. 2011;92:404–9. doi: 10.1097/TP.0b013e3182247bd5. [DOI] [PubMed] [Google Scholar]
- 26.Shapiro R, Young JB, Milford EL, Trotter JF, Bustami RT, Leichtman AB. Immunosuppression: evolution in practice and trends, 1993-2003. Am J Transplant. 2005;5:874–86. doi: 10.1111/j.1600-6135.2005.00833.x. [DOI] [PubMed] [Google Scholar]
- 27.Gras JM, Gerkens S, Beguin C, Janssen M, Smets F, Otte JB, et al. Steroid-free, tacrolimus-basiliximab immunosuppression in pediatric liver transplantation: clinical and pharmacoeconomic study in 50 children. Liver Transpl. 2008;14:469–77. doi: 10.1002/lt.21397. [DOI] [PubMed] [Google Scholar]
- 28.Reding R, Gras J, Sokal E, Otte JB, Davies HF. Steroid-free liver transplantation in children. Lancet. 2003;362:2068–70. doi: 10.1016/S0140-6736(03)15104-5. [DOI] [PubMed] [Google Scholar]
- 29.Ng VL, Alonso EM, Bucuvalas JC, Cohen G, Limbers CA, Varni JW, et al. Health status of children alive 10 years after pediatric liver transplantation performed in the US and Canada: report of the studies of pediatric liver transplantation experience. J Pediatr. 2012;160:820–6. e3. doi: 10.1016/j.jpeds.2011.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chaplin JE, Kristrom B, Jonsson B, Hagglof B, Tuvemo T, Aronson AS, et al. Improvements in behavior and self-esteem following growth hormone treatment in short prepubertal children. Horm Res Paediatr. 2011;75:291–303. doi: 10.1159/000322937. [DOI] [PubMed] [Google Scholar]
- 31.Taylor SJ, Whincup PH, Hindmarsh PC, Lampe F, Odoki K, Cook DG. Performance of a new pubertal self-assessment questionnaire: a preliminary study. Paediatr Perinat Epidemiol. 2001;15:88–94. doi: 10.1046/j.1365-3016.2001.00317.x. [DOI] [PubMed] [Google Scholar]
- 32.Schmitz KE, Hovell MF, Nichols JF, Irvin VL, Keating K, Simon GM, et al. A validation study of early adolescents' pubertal self-assessments. J Early Adolesc. 2004;24:357–84. [Google Scholar]
- 33.Coleman L, Coleman J. The measurement of puberty: a review. J Adolesc. 2002;25:535–50. doi: 10.1006/jado.2002.0494. [DOI] [PubMed] [Google Scholar]
- 34.Schall JI, Semeao EJ, Stallings VA, Zemel BS. Self-assessment of sexual maturity status in children with Crohn's disease. J Pediatr. 2002;141:223–9. doi: 10.1067/mpd.2002.125907. [DOI] [PubMed] [Google Scholar]