Abstract
Purpose
Ampullary carcinoma is a rare malignancy. Despite radical resection, survival rates remain low with high rates of local failure. We performed a single institution outcomes analysis to define the role of concurrent chemoradiotherapy (CRT) in addition to surgery.
Methods
A retrospective analysis was performed of all patients undergoing potentially curative pancreaticoduodenectomy for adenocarcinoma of the ampulla of Vater at Duke University Hospitals between 1976 and 2009. Time to event analysis was performed comparing all patients who underwent surgery alone to the cohort of patients receiving CRT in addition to surgery. Local control (LC), overall survival (OS), disease-free survival (DFS), and metastases-free survival (MFS) were estimated using the Kaplan-Meier Method.
Results
One hundred thirty-seven patients with ampullary carcinoma underwent Whipple procedure. Sixty-one patients undergoing resection received adjuvant (n= 43) or neoadjuvant (n=18) CRT. Patients receiving chemoradiotherapy were more likely to have poorly differentiated tumors (p=0.03). Of 18 patients receiving neoadjuvant therapy, 67% were downstaged on final pathology with 28% achieving pathologic complete response (pCR). With a median follow up of 8.8 years, three-year local control was improved in patients receiving CRT (88% vs. 55%, p= 0.001) with trend toward 3-year DFS (66% vs 48%, p=0.09) and OS (62% vs. 46%, p=0.074) benefit in patients receiving CRT.
Conclusions
Long term survival rates are low and local failure rates high following radical resection alone. Given patterns of relapse with surgery alone and local control benefit in patients receiving CRT, the use of chemoradiotherapy in selected patients should be considered.
Introduction
Adenocarcinoma of the ampulla of Vater is a rare malignancy, estimated to account for less than one percent of all gastrointestinal malignancies[1]. Periampullary carcinomas can arise from the ampulla of Vater, duodenum, distal common bile duct, or pancreas. This anatomic heterogeneity makes pathological classification difficult but represents an important distinction as ampullary carcinomas portend a better prognosis compared to pancreatic malignancies[2]. This may be due to the presenting symptom of painless jaundice that is typical of ampullary tumor location, potentially resulting in earlier evaluation and detection. In addition, there may be underlying differences in biology and behavior of ampullary and pancreatic carcinomas. In contrast to pancreatic cancers, at least 80% of patients with ampullary carcinoma present with potentially resectable disease[3]. While surgery remains the only curative option for patients, five-year survival rates following resection range from 20–50%[4,5]. Factors shown to adversely impact prognosis include nodal involvement, adjacent organ involvement, tumor size, positive surgical margins, poor differentiation and perineural invasion[6–8].
For patients undergoing resection with curative intent, local-regional failure is common and impacts survival. Reported rates of local failure vary widely but rates as high as 50–75% have been described[6,9,10]. Although adjuvant therapy may be beneficial in light of patterns of failure and poor survival, the role of CRT in resected patients remains poorly defined. This is largely due to the rarity of this malignancy and limited data examining outcomes of patients who undergo adjuvant therapy. This study reports the largest single institution series of patients undergoing pancreaticoduodenectomy for ampullary carcinoma and evaluates outcomes for patients treated with or without chemoradiotherapy.
Materials and Methods
Following Institutional Review Board approval, the medical records of all patients seen at Duke University Hospitals diagnosed with ampullary carcinoma between 1976–2009 were reviewed. Patients with carcinoma of the duodenum, minor papillae, pancreas, and bile duct were excluded. Patients with metastatic disease, who underwent ampullectomy, had disease progression during neoadjuvant CRT, or received adjuvant chemotherapy alone were also excluded from this analysis.
Surgery
All patients underwent potentially curative pancreaticoduodenectomy. Surgical specimens were staged according to the American Joint Committee on Cancer Guidelines, 2006. All pathological specimen assessments were performed or reviewed at Duke University Hospitals. Pathological data pertaining to grade, perineural invasion, lymphovascular invasion, and margin status were collected. In more recent years, patients undergoing neoadjuvant therapy were clinically staged by computerized tomography (CT) scan and endoscopic ultrasound (EUS) to determine tumor stage (T) and nodal status (N).
Chemoradiotherapy
After 1990 the use of adjuvant CRT in addition to surgery became more common practice. As data for benefits of preoperative therapy emerged in other GI sites, our institution moved toward a preference to delivery of neoadjuvant CRT with the first patient with ampullary carcinoma treated neoadjuvantly in 1997. The decision to deliver neoadjuvant therapy was multifactorial, but primarily advised in situations of locally advanced tumors where there was concern of achieving adequate (R0) margins and/or in the setting of evaluation in a multidisciplinary setting, where there was a bias towards neoadjuvant therapy by the multidisciplinary team. Multi-field external beam techniques were used to treat the tumor bed (or primary tumor) and local-regional lymph node basins (including porta hepatis, pancreaticoduodenal, celiac and superior mesenteric artery). Patients were treated at 1.8–2 Gy per fraction, 5 days per week. Field arrangements were primarily anterior–posterior/posterior-anterior with opposed lateral fields. Beam energies included 4 MV, 6 MV and 15 MV photons. Beginning in 1997, all patients underwent three-dimensional treatment planning. The concurrent chemotherapy regimen was determined by the treating medical oncologist.
Outcome Measures/Statistical Analysis
The primary objective of this analysis was to compare local control (LC), disease-free survival (DFS), overall survival (OS) and metastasis-free survival (MFS) outcomes between patients undergoing surgery alone versus surgery and CRT. Secondary objectives included determination of pathologic features, either alone or in combination, that predict for LC, DFS, OS, and MFS. Patterns of failure were analyzed by follow-up clinical examination, radiographic imaging, endoscopy, biopsy, and autopsy data. Disease-related endpoints were estimated using the Kaplan-Meier method with 95% confidence intervals (CI). All endpoints were calculated from date of diagnosis. Local failures were defined as recurrent disease in tumor bed or local nodal regions (porta, pancreaticodudodenal, celiac, superior mesenteric artery). Disease outside these regions was defined as a distant failure. Patients who received CRT were generally followed every three months with physical examination and CT following treatment completion primarily by treating medical and radiation oncologist. Patients who received surgery alone were followed by surgeon initially with care subsequently transferred to their local physician
Patients were grouped into two categories for analysis: those who underwent pancreaticoduodenectomy alone and patients who underwent pancreaticoduodenectomy with either preoperative or postoperative CRT. Kaplan-Meier curves were compared using the log-rank test. LC was defined as time to local failure and patients censored at last follow up or death for those without local failure. OS was defined as time to death and censored at last follow up for those patients still alive. DFS was defined as time to either local or distant failure and censored at last follow up or death for those patients without disease failure. MFS was defined as time to distant failure with patients censored at last follow up or death for those without distant failure. Wilcoxon rank-sums test was used to compare central tendency of age in the two groups. Association of CRT with various pathological factors, including, tumor grade, lymphovascular invasion, perineural invasion, margin status, T and N stage, were analyzed using Pearson’s Chi-squared test of proportions, excluding patients with unknown pathologic data. Both univariate and multivariate Cox Proportional-Hazards Regression models were performed for LC, DFS, and OS to evaluate the predictability of various pathologic factors on outcomes. Factors analyzed included age, tumor and nodal stage, stage group, tumor grade, year of surgery and radiotherapy use. Statistical analyses were performed using SAS Version 9.2 (Cary, NC) and TIBCO Spotfire S-plus.
Results
One hundred thirty-seven patients underwent pancreaticoduodenectomy for non-metastatic adenocarcinoma of the ampulla of Vater at Duke University Hospitals from 1976–2009. Median age was 66 years (range 35–86). The median follow up for all patients is 8.8 years (range 0.02 – 34). Median follow-up for the surgery alone and the surgery plus CRT groups are 10.5 (range 0.19 – 34) and 5.8 (range 0.02–21) years, respectively (p<0.001). Median follow-up for survivors is 9.4 years for surgery alone and 2.8 years for CRT group.
Patient characteristics are shown in Table 1. Ninety-five percent of entire cohort had a margin negative resection. Patients receiving chemoradiation were more likely to have poorly differentiated histology (38% vs. 19%, p= 0.03). This subgroup received concurrent chemotherapy with a median radiotherapy dose of 50 Gy. Most (91%) received concurrent fluoropyrimidine-based treatment, either by infusion or oral preparations (capecitabine). Two patients (3%) received mitomycin concurrent with 5-fluorouracil, and four (6.5%) patients received concurrent gemcitabine. Ninety-five percent of patients completed a full course of radiotherapy.
Table 1.
Patient/Tumor Characteristics
| Surgery (n=76) | Surgery + CRT (n=61) | p-value | |
|---|---|---|---|
|
| |||
| Age Median (Range) | 66 (35–86) | 65 (42–84) | 0.15 |
|
| |||
| Grade | 0.03 | ||
| Well | 14 (18%) | 5 (8%) | |
| Moderate | 46 (61%) | 29 (48%) | |
| Poor | 14 (18%) | 21 (34%) | |
| Unknown | 2 (3%) | 6 (10%) | |
|
| |||
| Margin | 0.46 | ||
| Positive | 1 (1%) | 2 (3%) | |
| Negative | 71 (93%) | 59 (97%) | |
| Unknown | 4 (5%) | 0 (0%) | |
|
| |||
| T stage | 0.28 | ||
| ≤ 2 | 42 (55%) | 28 (46%) | |
| ≥ 3 | 34 (45%) | 33 (54%) | |
|
| |||
| Nodal Positivity | 0.21 | ||
| Positive | 22 (29%) | 24 (39%) | |
| Negative | 51 (67%) | 35 (57%) | |
| Unknown | 3 (4%) | 2 (3%) | |
|
| |||
| LVI | 0.60 | ||
| Positive | 19 (25%) | 19 (31%) | |
| Negative | 9 (12%) | 12 (20%) | |
| Unknown | 48 (63%) | 30 (49%) | |
|
| |||
| PNI | 0.86 | ||
| Positive | 15 (20%) | 17 (28%) | |
| Negative | 5 (7%) | 5 (9%) | |
| Unknown | 56 (74%) | 39 (64%) | |
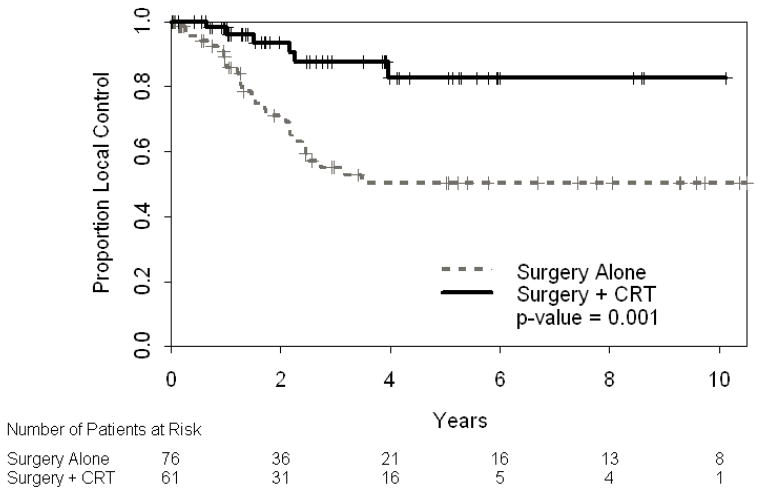
Three-year local control was significantly improved in the surgery plus CRT cohort compared to surgery alone: 88% [95% CI: 78–99%] vs. 55% [95% CI: 43–70%], p= 0.001 (Figure 1). Local failures were defined as recurrent disease in tumor bed, anastomotic sites or nodal regions (porta, pancreaticodudodenal, celiac, or superior mesenteric artery). Disease outside these regions was defined as a distant failure. Thirty-three patients (24%) experience a local failure, 27 patients who underwent surgery alone and 6 patients who received CRT. Twenty-two of these patients had components of both local and distant failure, 18 patients who underwent surgery alone and 4 patients who received CRT.
Figure 1.
Local control
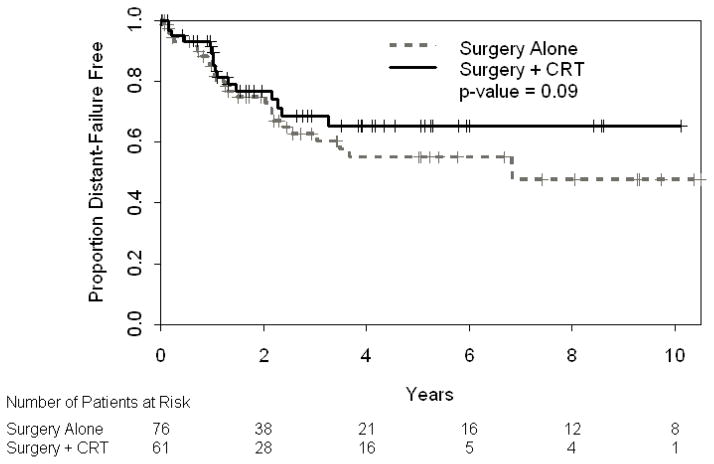
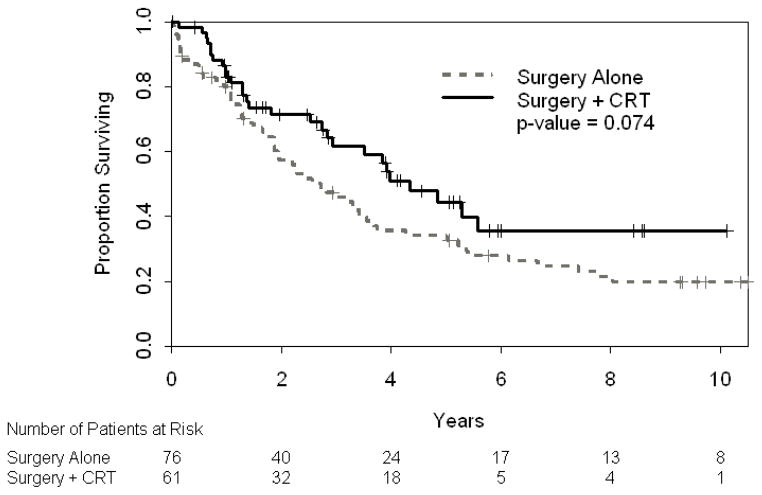
Although there was not a statistically significant difference in 3-year DFS (66% [95% CI: 54–81%] vs. 48% [95%CI: 36–62], Figure 2) or 3-year OS (62% [95% CI: 49–77] vs. 46% [95% CI: 36–59], Figure 3), a strong trend favors surgery in combination with CRT. No difference was seen in 3-year MFS (69% [95% CI: 56–84] vs. 63% [95% CI: 51–77%], p=0.34). Higher grade, older age, advanced T stage, and stage grouping were independently associated with worse OS on univariate analysis (Table 2A). T stage and stage grouping were important univariate predictors of DFS. In multivariate analysis, grade, age, stage and CRT administration were statistically significant predictors of OS when controlling for year of surgery, which was not found to be significant (Table 2B). Multivariate analysis for DFS was not possible given the two significant factors in univariate analyses (T stage and stage grouping) were correlated with one another.
Figure 2.
Disease-Free Survival
Figure 3.
Overall Survival
Table 2.
Univariate and Multivariate Analysis. A. Univariate Analysis of LC, DFS, and OS. B. Multivariate Analysis of OS
| A. | ||||||
|---|---|---|---|---|---|---|
| LC | DFS | OS | ||||
| Characteristic | p value | Hazard ratio | p value | Hazard ratio | p value | Hazard Ratio |
|
Grade Well/Moderate Poor |
0.713 | 1.20 | 0.838 | 0.93 | 0.015 | 0.55 |
|
Age, y <65 ≥65 |
0.754 | 1.12 | 0.182 | 1.44 | 0.031 | 1.62 |
|
T stage T1/T2 T3/T4 |
0.207 | 1.55 | 0.005 | 2.14 | 0.024 | 1.64 |
|
Nodal Stage N0/unknown N1 |
0.766 | 0.89 | 0.287 | 1.35 | 0.069 | 1.52 |
|
Stage grouping IA-IIA ≥ IIB |
0.622 | 1.21 | 0.005 | 2.21 | 0.011 | 1.77 |
| B. | ||
|---|---|---|
| Characteristic | p value | Hazard ratio |
|
Grade Well/Moderate Poor |
0.0009 | 0.39 |
|
Age, y <65 ≥65 |
0.016 | 1.81 |
|
Stage grouping IA-IIA ≥ IIB |
0.026 | 1.7 |
|
RT Yes No |
0.0053 | 0.41 |
| Surgery Year | 0.64 | 0.99 |
Eighteen patients underwent preoperative CRT. These patients were staged with pretreatment CT and EUS. When possible, abnormal appearing lymph nodes were sampled by fine needle aspiration. After neoadjuvant CRT, 12 patients (67%) were downstaged on final pathology and 5 (28%) had a pathologic complete response (pCR). Although 4 patients had clinically node positive disease at presentation, no patients receiving preoperative therapy had involved nodes at resection.
Discussion
Carcinoma of the ampulla Vater is an uncommon malignancy. In contrast to pancreatic cancer, at least 80% of patients presenting with ampullary adenocarcinoma are candidates for potentially curative resection[3]. For patients with localized disease, standard therapy is surgical resection, consisting of either pancreaticoduodenectomy, or ampullectomy in patients where radical resection is not feasible. Select series report 5-year survival rates ranging from 0–61%, with a collective review of nearly 1000 patients undergoing surgery alone between 1975–1993 showing a 5-year survival of 35% [5]. Survival in patients with curative resection correlates with depth of penetration into adjacent organs as well as nodal involvement, which is present in 30–50% of patients with ampullary carcinoma[5,11,12]. Other prognostic factors include completeness of resection, tumor differentiation, and margin status.
Reports describing patterns of failure following surgery for ampullary carcinoma are limited. Available data suggest that local recurrence (LR) is common and adversely impacts OS. Historic series of surgery alone indicate that local recurrence occurs in up to 75% of patients following pancreaticoduodenectomy[6,10]. However, in more contemporary institutional series, 5-year LR rates with surgery alone range from 12–40%, with our series demonstrating an even higher incidence of 50%[13–15]. These high rates of failure may be explained by the presence of subclinical nodal and regional disease outside the resection bed. In many series (including the present), local failure rates may be underestimated given not all patients undergo thorough post treatment imaging, pathologic assessment, or autopsy[3]. Additionally, regional failure may be overlooked once patients develop metastatic disease. The role persistent loco-regional disease plays in subsequent development of distant metastasis remains uncertain. Given patterns of failure following radical resection and morbidity and mortality associated with LR, the LC benefit of chemoradiotherapy may lead to improvements in OS and quality of life.
Few reports have examined the role of adjuvant therapy in resectable ampullary adenocarcinoma. A prospective, randomized European study evaluating the role of adjuvant chemoradiotherapy in pancreatic and periampullary tumors showed no survival benefit. This study, however, did not distinguish ampullary carcinoma from other periampullary tumors, and has been criticized for high rates of treatment noncompliance and split-course radiotherapy technique[16]. Several contemporary single institution studies have evaluated the role of adjuvant chemoradiotherapy in the treatment of resected ampullary cancers (Table 3). In published series, present series included, patients considered for radiation therapy often have adverse prognostic factors (i.e. higher stage, poor differentiation, involved margins, etc.) relative to patients treated with surgery alone. This often results in “negative-selection” bias in irradiated patients, with correspondingly worse outcomes[8,17]. One recent report concluded adjuvant chemoradiotherapy did not improve outcomes in patients with resected ampullary cancers, despite irradiated patients having significantly more high risk pathologic features (higher T stage, N stage) relative to patients receiving surgery alone[3]. Conversely, patients undergoing surgery could experience postoperative complications or decline in functional status, potentially foregoing adjuvant therapies. In our series there was no significant difference in T or N stage between the surgery alone and surgery plus CRT arms. Despite overall low rates of margin positivity, patients who received CRT were more likely to have poorly differentiated histology and a LC benefit was still seen with a trend toward improved DFS and OS in this subset.
Table 3.
5 year LC and OS for surgery and surgery plus CRT from selected series
| # Patients/# CRT | 5 yr LC Surgery alone | 5 yr LC CRT | 5 yr OS Surgery alone | 5 yr OS CRT | |
|---|---|---|---|---|---|
| Mayo Clinic [14]a | 125/29 | 72% | - | 47% | - |
| MD Anderson [13] | 96/54 | 83% | 73% | 69% | 60% |
| Seoul [15] | 118/41 | 80% | 80% | 67% | 53% |
| Johns Hopkins[18] | 111/50 | - | - | 38% | 35% |
| Current Series b | 137/61 | 50% | 80% | 36% | 45% |
5 yr LC and OS for entire cohort
5 yr estimates
In other gastrointestinal malignancies (i.e. rectum, esophagus), the use of neoadjuvant CRT has become standard practice. The role of preoperative CRT for periampullary and pancreatic disease remains less well defined. Potential advantages of preoperative therapy include undisrupted tumor vasculature allowing for delivery of chemotherapy and tumor sensitizing oxygenation. Downstaging may occur potentially allowing advanced lesions to be more easily resected and sterilization of operative region reducing risk of spread during surgical manipulation. Preoperative treatment avoids delay in therapy delivery due to postoperative recovery and radical resection in patients with rapidly progressive disease. Potential disadvantages of a neoadjuvant approach include possible overtreatment of patients with very early stage disease, decline in functional status during neoadjuvant therapy precluding surgery or disease progression despite neoadjuvant therapy. Our institution has adopted a preference toward the administration of preoperative CRT in the majority of gastrointestinal malignancies. In our series, 18 patients received neoadjuvant CRT for ampullary carcinoma. Sixty-seven percent of patients were downstaged with 28% pCR, suggesting that ampullary carcinomas may be more CRT sensitive than pancreatic cancer, highlighting the potential clinical value of neoadjuvant radiation for the management of this malignancy.
The present study has a number of limitations inherent to retrospective series. Selection bias was evident by the disproportionately high number of patients with poorly differentiated histology who received CRT. As other studies have shown, patients with more adverse pathologic features (i.e. more advanced tumor stage, nodal disease, positive margins, etc.) are generally referred for CRT. In addition, many patients experiencing perioperative complications or mortality may not have received CRT. Without prospective, thorough data collection, the true local failure rate is likely to be underestimated as once distant disease is detected there is less vigorous assessment of the primary and regional disease sites. Given the rarity of ampullary carcinoma, patients included in this analysis were treated over a 33 year time period. During this time significant advances in radiotherapy planning and treatment delivery occurred, including implementation of 3D conformal techniques. In our series, the LC and OS rates with surgery alone are lower compared to other retrospective series. This may be due to institutional variation in surgical technique, referral bias of more advanced lesions, or purely a reflection of the retrospective nature of this study.
Despite these limitations, given the rarity of this tumor, randomized, level I evidence is lacking and large institutional experiences help guide practice patterns and treatment recommendations. Our experience suggests, given patterns of relapse after surgery alone, CRT (either neoadjuvant or adjuvant) provides a local control benefit with trends towards improved DFS and OS and should be considered in treatment of patients with ampullary carcinoma.
Synopsis.
This series represents the largest single institution experience of ampullary carcinoma comparing outcomes with surgery alone to surgery and chemoradiation. Given patterns of relapse after surgery alone, chemoradiotherapy enhances local control with trends towards improved DFS and OS and should be considered in treatment of patients with ampullary carcinoma.
Acknowledgments
Jonathan Jenkins for editorial assistance.
Funding: None
Footnotes
Conflict of Interest: None related to the subject matter of this manuscript
Presentations: Poster Presentation at 2011 Gastrointestinal Cancers Symposium
References
- 1.Chareton B, Coiffic J, Landen S, Bardaxoglou E, Campion JP, Launois B. Diagnosis and therapy for ampullary tumors: 63 cases. World J Surg. 1996;20:707–712. doi: 10.1007/s002689900108. [DOI] [PubMed] [Google Scholar]
- 2.Mehta V, Fisher GA, Ford JM, et al. Adjuvant chemoradiotherapy for “Unfavorable” Carcinoma of the ampulla of vater. Arch Surg. 2001;136:65–69. doi: 10.1001/archsurg.136.1.65. [DOI] [PubMed] [Google Scholar]
- 3.Sikora SS, Balachandran P, Dimri K, Rastogi N, Kumar A, Saxena R, Kapoor VK. Adjuvant chemo-radiotherapy in ampullary cancers. Eur J Surg Oncol. 2005;31:158–163. doi: 10.1016/j.ejso.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 4.Sarmiento JM, Nagomey DM, Sarr MG, Farnell MB. Periampullary cancers: Are there differences? Surg Clin North Am. 2001;81:543–555. doi: 10.1016/s0039-6109(05)70142-0. [DOI] [PubMed] [Google Scholar]
- 5.Allema JH, Reinders ME, van Gulik TM, van Leeuwen DJ, Verbeek PC, de Wit LT, Gouma DJ. Results of pancreaticoduodenectomy for ampullary carcinoma and analysis of prognostic factors for survival. Surgery. 1995;117:247–253. doi: 10.1016/s0039-6060(05)80197-7. [DOI] [PubMed] [Google Scholar]
- 6.Willett CG, Warshaw AL, Convery K, Compton CC. Patterns of failure after pancreaticoduodenectomy for ampullary carcinoma. Surg Gynecol Obstet. 1993;176:33–38. [PubMed] [Google Scholar]
- 7.Duffy JP, Hines OJ, Liu JH, Ko CY, Cortina G, Isacoff WH, Nguyen H, Leonardi M, Tompkins RK, Reber HA. Improved survival for adenocarcinoma of the ampulla of vater: Fifty-five consecutive resections. Arch Surg. 2003;138:941–948. doi: 10.1001/archsurg.138.9.941. discussion 948–950. [DOI] [PubMed] [Google Scholar]
- 8.Lee JH, Whittington R, Williams NN, Berry MF, Vaughn DJ, Haller DG, Rosato EF. Outcome of pancreaticoduodenectomy and impact of adjuvant therapy for ampullary carcinomas. Int J Radiat Oncol Biol Phys. 2000;47:945–953. doi: 10.1016/s0360-3016(00)00537-x. [DOI] [PubMed] [Google Scholar]
- 9.Barton RM, Copeland EM., 3rd Carcinoma of the ampulla of vater. Surg Gynecol Obstet. 1983;156:297–301. [PubMed] [Google Scholar]
- 10.Kopelson G, Galdabini J, Warshaw AL, Gunderson LL. Patterns of failure after curative surgery for extra-hepatic biliary tract carcinoma: Implications for adjuvant therapy. Int J Radiat Oncol Biol Phys. 1981;7:413–417. doi: 10.1016/0360-3016(81)90118-8. [DOI] [PubMed] [Google Scholar]
- 11.Monson JR, Donohue JH, McEntee GP, McIlrath DC, van Heerden JA, Shorter RG, Nagorney DM, Ilstrup DM. Radical resection for carcinoma of the ampulla of vater. Arch Surg. 1991;126:353–357. doi: 10.1001/archsurg.1991.01410270099016. [DOI] [PubMed] [Google Scholar]
- 12.Todoroki T, Koike N, Morishita Y, Kawamoto T, Ohkohchi N, Shoda J, Fukuda Y, Takahashi H. Patterns and predictors of failure after curative resections of carcinoma of the ampulla of vater. Ann Surg Oncol. 2003;10:1176–1183. doi: 10.1245/aso.2003.07.512. [DOI] [PubMed] [Google Scholar]
- 13.Krishnan S, Rana V, Evans DB, Varadhachary G, Das P, Bhatia S, Delclos ME, Janjan NA, Wolff RA, Crane CH, Pisters PW. Role of adjuvant chemoradiation therapy in adenocarcinomas of the ampulla of vater. International Journal of Radiation Oncology, Biology, Physics. 2008;70:735–743. doi: 10.1016/j.ijrobp.2007.07.2327. [DOI] [PubMed] [Google Scholar]
- 14.Bhatia S, Miller RC, Haddock MG, Donohue JH, Krishnan S. Adjuvant therapy for ampullary carcinomas: The mayo clinic experience. Int J Radiat Oncol Biol Phys. 2006;66:514–519. doi: 10.1016/j.ijrobp.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 15.Kim K, Chie EK, Jang JY, Kim SW, Oh DY, Im SA, Kim TY, Bang YJ, Ha SW. Role of adjuvant chemoradiotherapy for ampulla of vater cancer. Int J Radiat Oncol Biol Phys. 2009;75:436–441. doi: 10.1016/j.ijrobp.2008.11.067. [DOI] [PubMed] [Google Scholar]
- 16.Klinkenbijl J, Jeekel J, Sahmoud T, et al. Adjuvant radiotherapy and 5-fluorouracil after curative resection of cancer of the pancreas and periampullary region. Ann Surg. 1999;230:776–784. doi: 10.1097/00000658-199912000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alden ME, Waterman FM, Topham AK, Barbot DJ, Shapiro MJ, Mohiuddin M. Cholangiocarcinoma: Clinical significance of tumor location along the extrahepatic bile duct. Radiology. 1995;197:511–516. doi: 10.1148/radiology.197.2.7480704. [DOI] [PubMed] [Google Scholar]
- 18.Zhou J, Hsu CC, Winter JM, Pawlik TM, Laheru D, Hughes MA, Donehower R, Wolfgang C, Akbar U, Schulick R, Cameron J, Herman JM. Adjuvant chemoradiation versus surgery alone for adenocarcinoma of the ampulla of vater. Radiother Oncol. 2009;92:244–248. doi: 10.1016/j.radonc.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]