Abstract
Maladaptation to stress is associated with psychopathology. However, our understanding of the underlying neural circuitry involved in adaptations to stress is limited. Previous work from our lab indicated the paraventricular hypothalamic neuropeptides orexins/hypocretins regulate behavioral and neuroendocrine responses to stress. To further elucidate the role of orexins in adaptation to stress, we employed optogenetic techniques to specifically examine the effects of orexin cell activation on behavior in the social interaction test and in the home cage as well as orexin receptor 1 internalization and ERK phosphorylation in brain regions receiving orexin inputs. In the social interaction test, optogenetic stimulation of orexin neurons decreased time spent in the interaction zone while increasing the frequency of entries into the interaction zone. In addition, optogenetic stimulation of orexin neurons increased the total distance traveled in the social interaction arena but had no effect on their home cage behavior. Together, these results suggest that orexin release increases anxiety in the social interaction test while increasing the salience of novel but not familiar environmental stimuli. Consistent with activation of orexin neurons, optogenetic stimulation increased orexin receptor1 internalization and ERK phosphorylation in the paraventricular thalamus (PVT) and locus coeruleus (LC), two regions heavily innervated by orexin neurons. Together these results show for the first time that elevation of orexin activity, possibly in the PVT and LC, is associated with increased anxiety, activity, and arousal in a context-specific manner.
Keywords: Optogenetics, Orexins, Hypocretins, Social interaction, Anxiety, Stress
1. Introduction
Orexins (also called hypocretins) are neuropeptides exclusively produced in cells of the lateral and posterior hypothalamus [7,28]. Orexins are synthesized from the precursor molecule pre-pro-orexin that is cleaved into two structurally related and highly conserved peptides, orexinA and orexinB [7,28] that bind to two G-protein coupled receptors, orexin1 (orexin1R) and orexin2 receptors (orexin2R). Stimulation of orexin receptors promotes arousal or a heightened responsiveness to sensory inputs and increased wakefulness [29]. An obvious extension of the role of orexins in initiating arousal is increasing the salience of novel potentially threatening environmental cues. Previous work from our lab has indicated a role for orexins acting in the posterior paraventricular thalamus (PVT) in regulating the stress response and increasing anxiety-related behavior in repeatedly stressed rats [13,19]. Other work has reported that acute intraventricular (ICV) or systemic administration of orexins can also increase anxiety-related behavior in the open field test [14,33], depolarize cells [13], and increase CRH and AVP mRNA in the paraventricular hypothalamus (PVN), the hypothalamic arm of the hypothalamic pituitary adrenal (HPA) axis [6,31]. These increases in CRH and AVP are also consistent with orexin-induced increases in plasma ACTH and corticosterone [4,17,31]. However, it is unclear whether endogenous orexins induce anxiety in all contexts. Further, little is known about the possible neural substrates for these actions. In order to further elucidate the role of orexins in anxiety related behavior we employed optogenetic techniques to specifically activate orexin cells. This technique allows for specific temporal resolution in activating the orexin system and produces the release of orexins within the physiologic capabilities of the cell [1].
Effects of orexin cell activation on behavior in the social interaction test and home cage were examined. To determine the brain regions that could be the site of orexin actions, orexin receptor1 internalization, ERK phosphorylation, and cFos expression were quantified in three specific brain regions receiving orexin inputs following optogenetic stimulation of orexin neurons. These three brain regions were the PVT, the locus coeruleus (LC), and prefrontal cortex (PFC). When taken together, the results presented here suggest that orexins mediate context-specific actions on anxiety and arousal and that the paraventricular thalamus (PVT) and locus coeruleus (LC) are two brain regions important in mediating these effects.
2. Methods
2.1. Animals
Male Sprague–Dawley rats (Charles River, Kingston, NY) weighing between 225 and 250 g were given ad libitum access to food and water and individually housed in plastic tub cages on a 12:12 h light: dark cycle with lights on at 0600 h. A 5–7 day acclimation period was allowed prior to start of experimentation and surgery. All procedures were approved by the IACUC at the CHOP Research Institute.
2.2. Surgery
Rats were anesthetized using a cocktail containing ketamine, xylazine, and acepromizine. Using stereotaxic techniques, unilateral guide cannulae (26ga) were implanted in the lateral posterior hypothalamus, the site of orexin cell bodies, with the following coordinates (from bregma): AP: −2.8, ML: −1.8 mm, DV: −7.8 mm. The guide cannula was used for injection of the viral vector and introduction of the optic fiber.
2.3. Viral vectors
Targeting the genetic expression of channelrhodopsin-2 (ChR2) to orexin expressing neurons was achieved by use of a lentivirus carrying the 3.1-kilobase (kb) mouse prepro-hypocretin (Hcrt, which encodes orexin A and orexin B) gene promoter [29]. The Hcrt::ChR2-YFP construct and the Hcrt::YFP control construct were kindly provided by Dr. Luis DeLecea (Department of Psychiatry and Behavioral Sciences at Stanford University). A description of both constructs with the mCherry reporter instead of the YFP can be found in [1]. Briefly, the 3086-base-pair (EcoRI–SacI) mouse Hcrt promoter [29] was used to replace the CaMKIIa promoter in the CaMKIIa::ChR2-YFP lentivirus vector [36]. The Hcrt::YFP control viruses were made by swapping ChR2-YFP with YFP alone. High-titre lentiviruses were produced at the CHOP Viral vector core facility using 293 T cells that were cultured in DMEM growth media containing 10% FBS and 1x P/S antibiotics. The vectors were aliquotted and stored at −80 °C till use. Injection of 4 ul of the high titer virus (approximately 109 plaque forming units) directly into hypothalamus was performed the day following surgery. Administering the virus in this manner allowed for injection of freshly thawed virus for all animals at a single time from a single aliquot of virus, which resulted in more consistent ChR2 expression. All rats were unanesthetized and freely moving at time of virus injection. The specificity of lentivirus-mediated expression was tested by dual stain immunocyctochemistry for orexinA expression and cFos activation, described in detail below. Peak expression of ChR2 was determined, via fluorescent imaging of YFP signal in freshly sliced tissue, to occur at approximately 4 weeks following virus injection. Therefore, all experiments were begun at 4–5 weeks following injection.
2.4. Experiment 1: validation of construct
2.4.1. Determining the effect of optogenetic stimulation on sleep/wake transitions
The Chr2 construct had not previously been used in rats. In order to validate its functionality, a validation study based on previous work in mice was performed [1]. In this previous study, optogenetic stimulation of orexin neurons was conducted in sleeping mice and the latency to wakefulness was assessed. Stimulation of orexin neurons decreased this latency in these mice [1]. In the present experiment, Hcrt::YFP and Hcrt::ChR2-YFP transduced animals (n = 4 and 6, respectively) that exhibited behavioral indices of sleep were exposed to optogenetic stimulation of 20 Hz for 10 s at a time every 2 min. Sleep was assessed by behavioral measures as previous work showed little difference between assessment of sleep through EEG/EMG and high throughput behavioral screening of sleep-wake transitions in mice [26]. Optogenetic stimulation parameters were chosen based on DeLecea’s previous work showing a similar stimulation paradigm increases cFos immunoreactivity in orexin cells in Hcrt::ChR2-mCherry mice compared to the Hcrt::mCherry control animals [1]. In order to ensure that all rats were tested under similar conditions rats were given a minimum of 4 h of acclimation to the testing room and indwelling optic fiber. Following acclimation, rats were tested in their home cage starting at 1600h and continued until a minimum of 20 trials was achieved while asleep. Rats were deemed asleep following a minimum of 3 min without any movement as assessed through visual observation. Rats were monitored for movement while asleep, and the latency to first movement following photostimulation was recorded. Rats then remained undisturbed until they were asleep at which time another trial began. Because of previous literature showing effects of repeated stress to induce abnormal sleep patterns [12], we examined the impact of repeated stress on sleep/wake latency. After 20 trials on one day, all rats were then exposed to 4 days of 15 min swim stress each day. On the day following the last swim stress, rats were again monitored for movement during sleep while being exposed to photostimulation, as on the first day.
2.4.2. Examination of optogenetic stimulation effects on cellular activity
To validate the functionality of the ChR2 construct at a cellular level, cFos immunoreactivity in orexin cells was assessed following optogenetic stimulation at 20 Hz for 10 s at a time every minute for 30 min in their home cage. Brains from Hcrt::YFP and Hcrt::ChR2-YFP transduced animals (n = 10 and 15 respectively) were collected following photostimulation. Brains were sectioned at 12 μm onto slides and double immunostained for orexinA and cFos. The number of cFos positive/orexinA positive cells was counted and quantified as a percent of total orexin cells on the side of the brain being examined. Briefly, sections were fixed in 10% paraformaldehyde and successively incubated in the following: (1) a goat antiserum to orexinA (1:500, Santa Cruz, sc-8070) in PBST supplemented with 4% normal horse serum (NHS, Sigma) for 3 days at 4 °C; (2) a rabbit antiserum to cFos (1:1,000, Santa Cruz, sc-52) in PBST supplemented with 4% normal horse serum (NHS, Sigma) for 1 day at 4 °C; (3) a donkey anti goat secondary (1:2,000, Abcam, ab6950) and a donkey anti rabbit secondary (1:2000, invitrogen A-21206) both overnight at 4 °C. Finally, the slides were cover slipped with flouromount (Sigma, St Louis, MO). Slides were then visualized on a fluorescence microscope (Leica). Digital images were slightly modified to optimize for image resolution, brightness and contrast in Open Lab software (version 5.5.2, PerkinElmer). Quantification was performed on the virus-injected side and compared to the non-injected side as a control.
2.5. Experiment 2: effects of optogenetically stimulated orexins on behavior in the social interaction test
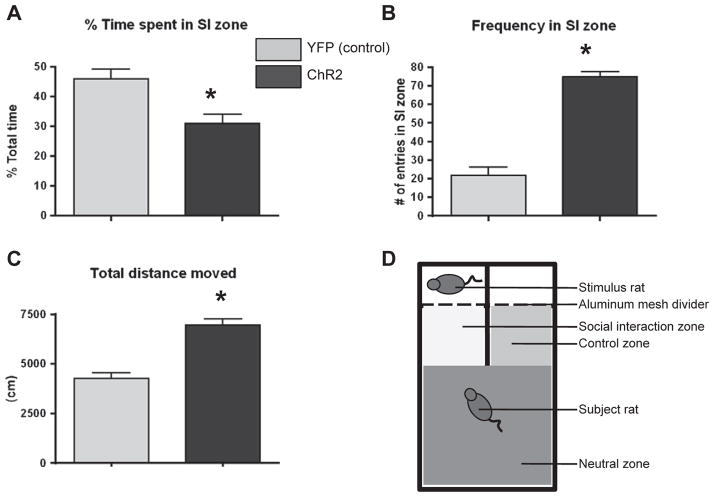
Naïve Hcrt::YFP and Hcrt::ChR2-YFP transduced animals (n = 10 and 15 respectively) were placed in a three-chamber social interaction arena for 30min with an age and sex-matched stimulus rat behind a wire mesh divider on one side (social interaction or SI zone; 8in × 10in) and an empty chamber on the other side (control zone; 8in × 10in). The third chamber was the neutral zone (16in × 19in) away from the SI and control zones but giving equal access to both zones (Fig. 2D). The apparatus was made from white opaque plexiglass with aluminum mesh barriers. Rats were initially placed in the neutral zone allowing equal access to both the SI zone and the control zone. Optogenetic stimulation occurred at a rate of 20 Hz for 10 s at a time every minute for the duration of the 30-min test. Time spent within, and entries into, the SI zone (as a function of time in all zones) were quantified as measures of anxiety-related behavior and exploratory behavior respectively [10]. Total distance traveled was measured for quantification of locomotor activity. Quantification of time spent in the specific zones and distance traveled was performed using EthovisionPro 3.1 and respectively expressed as % of total time and total cm traveled.
Fig. 2.
A) Effect of hypothalamic photostimulation on percent time spent in the social interaction zone in Hcrt::YFP control (YFP; n = 10), and Hcrt:: ChR2-YFP (ChR2; n = 15) transduced animals. * = p < 0.05 Hcrt::YFP control vs. Hcrt::ChR2-YFP. B) Effect of hypothalamic photostimulation on the number of entries into the social interaction zone of animals described above. * = p < 0.05 Hcrt::YFP control vs. Hcrt::ChR2-YFP. C) Effect of hypothalamic photostimulation on the total distance traveled during the social interaction test of animals described above. * = p < 0.05 Hcrt::YFP control vs. Hcrt::ChR2-YFP. D) Diagram of social interaction apparatus detailing the placement of the different zones, stimulus rat, and subject rat.
2.6. Experiment 3: relationship between behavior in the social interaction test and behavior during social defeat
We determined whether orexin-induced behavior in the social interaction test could predict behavior during repeated social defeat. Our previous work had indicated that the latency to exhibit the defeat posture during social defeat following exposure to a resident rat is associated with potential vulnerability or resiliency to stress, with longer latencies suggesting active coping and a more resilient phenotype compared to rats exhibiting shorter latencies [35]. Half of the rats previously exposed to the social interaction test in Experiment 2 (Hcrt::YFP; n = 5 and Hcrt::ChR2-YFP; n = 6) were exposed to 5 days of a resident–intruder model of social stress modified from the resident–intruder model originally developed by Miczek [22]. The other half of the rats, previously exposed to social interaction testing in Experiment 2, were not disturbed until home cage behavior was analyzed in Experiment 4. Socially defeated rats were exposed to resident–intruder social stress 30 min per day and with exposure to a different resident rat for each of the 5 days to minimize habituation to the resident rats [35]. Defeat was defined by the intruder assuming a supine posture that was held for a minimum of 3 s. Latency to exhibit a supine defeat posture was recorded and averaged over the five days for each individual rat.
2.7. Experiment 4: effect of optogenetically stimulated orexin activity on home cage behaviors and HPA activity
Prior to sacrifice, all rats from experiments 2 and 3 (Hcrt::YFP; n=9 and Hcrt::ChR2-YFP; n = 13, decreased number of animals resulting from broken guide cannulae or optic fibers) were exposed to optogenetic stimulation of 20 Hz for 10 s at a time every minute for 30 min in their home cage. Some of these animals were not exposed to repeated social defeat although they had been exposed to prior social interaction testing. Behavior of the animals were assessed and scored by a trained scorer blind to the specific group types using both EthovisionPro 3.1 for locomotor activity and manual counting of rearing, burying and grooming. Rats were then decapitated 30min after the last stimulation, trunk blood and brains were collected for hormone analysis and western blot/immunocytochemistry analysis respectively. Plasma ACTH and corticosterone were measured using kits from MP Biomedicals (Orangeburg, NY). The minimum levels of detection for ACTH and corticosterone were 5.7 pg/ml and 0.6 mg/dl, respectively. Intra- and inter-assay variability was less than 10%.
2.8. Experiment 5: effects of optogenetically stimulated orexin activity in potential neural substrates
To determine possible neural substrates for the actions of orexins, we examined three brain regions expressing high levels of orexin1 receptors (orexin1R). We quantified cFos immunoreactivity, orexin receptor internalization and intracellular messenger activation in the posterior paraventricular thalamus (pPVT), locus coeruleus (LC) and pre frontal cortex (PFC) [13,16,18,37,38]. Orexin1R was chosen for quantification because of previous work from our lab showing that orexin1R is the predominant receptor activated in the pPVT during repeated stress [13]. Orexin1R protein was quantified in enriched cytosolic and membrane fractions of pPVT, LC, and PFC (infralimbic and prelimbic) punch homogenates separated through centrifugation in a 30%/70% sucrose gradient (as described in [13]). Briefly, sample homogenates were placed as a top layer on a frozen 30%/70% sucrose gradient and thawed at room temp prior to spinning at 13,000 g for 90 min. The 30% sucrose layer was dyed with blue lamalar dye allowing for easier visual separation following centrifugation. Separation was performed by precise removal of the respective fractions from the bottom of the centrifuge tube. Previous studies have used similar methods for quantifying the distribution of cytosolic and membrane proteins [3,24]. Orexin1R was visualized using sc-8073 (Santa Cruz Biotechnology) at a concentration of 1:500. Orexin receptor antibody specificity was tested by comparison of specified orexin1R expressing brain regions with control tissue samples taken from motor cortex with no orexin1R expression. Effectiveness of the sucrose gradient to enrich membrane and cytosolic fractions was validated via western blot analysis of the membrane-bound protein synaptophysin, which was predominantly observed in the membrane fraction and the cytosolic protein GapDH, which was predominantly observed in the cytosolic fraction [13]. Blots were visualized on a Li-Cor Odyssey infrared imaging system. All groups were evenly represented on each gel in order to compensate for inter-gel variations. Orexin1R yielded a band at 55 kD and the orexin2R yielded a band at 48 kD which is consistent with the range seen in previously published work [15]. Receptors were quantified as a percent of total Beta-actin (A2228, Sigma, St. Louis, MO) in their respective fractions. Phosphorylated ERK was quantified in the cytosolic fractions. Incubation with primary antibodies for pERK and total ERK (Santa Cruz Biotechnology) yielded two bands at 44kD and 42kD for ERK1 and ERK2, respectively, and quantified as a percent of total ERK [11]. CFos expression was visualized using a primary antibody to cFos (Santa Cruz, sc-52) and quantified as a percent of Beta-tubulin (Sigma, T0198) expression.
2.8.1. Statistical analyses
For analysis in Experiment 1, 2-way ANOVAs were performed to test the effect of virus (Hcrt::YFP vs. Hcrt::ChR2-YFP) × stress (swim stress vs. no stress). A 2-way ANOVA was used to test for the effects of virus (Hcrt::YFP vs. Hcrt::ChR2-YFP) × injection side (injected side vs. non-injected side) for cFos expression in orexin positive cells. For experiments 2 and 4, t-tests were used to determine significant differences between Hcrt::YFP and Hcrt::ChR2-YFP transduced animals in the social interaction test and on home cage behavior. Experiment 3 used correlations to examine the predictive effect of social interaction test results on social defeat latencies. Cytosolic and membrane fractions were analyzed separately in Experiment 5 using t-tests to determine significant differences in orexin1R expression between Hcrt::YFP and Hcrt::ChR2-YFP transduced animals. T-tests were also used in comparisons western data representing ERK phosphorylation and cFos expression. For all tests performed the p value was set at p < .05.
3. Results
3.1. Experiment 1: validation of construct
3.1.1. Determining the effect of optogenetic stimulation on sleep/wake transitions
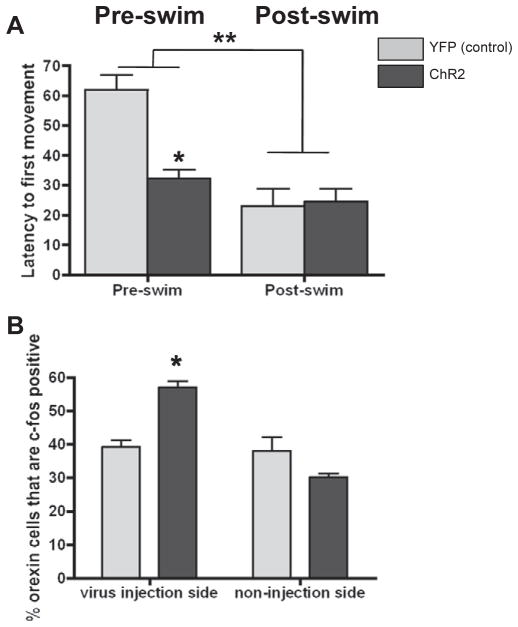
There was a significant main effect of stress with all rats exposed to repeated swim (post-swim) exhibiting lower latencies compared to non-stressed (pre-swim) rats (Fig. 1A). Furthermore, there was a significant Virus × Stress group interaction (Fig. 1A; F1,12 = 10.30, p < 0.0075). Post-hoc tests indicated that, prior to swim, photostimulation of orexin cells in Hcrt::ChR2-YFP rats decreased latency to first movement compared to the Hcrt::YFP animals (p < 0.006). However, post-swim, there was no difference between the two groups. As a result, swim stress decreased latencies in the Hcrt::YFP animals to the level of the non-stressed Hcrt::ChR2-YFP animals (p<0.003) whereas stress did not further decrease latencies in the Hcrt::Chr2 animals.
Fig. 1.
A) Effect of hypothalamic photostimulation on average latency to movement following stimulation in Hcrt::YFP control (YFP; n = 4), and Hcrt::ChR2-YFP (ChR2; n = 6) transduced animals prior to 4 days of swim stress (Pre-swim, left bars) and after 4 days of swim stress (Post-swim, right bars). * = p < 0.05 pre-swim Hcrt::YFP control vs. pre-swim Hcrt::ChR2-YFP; ** = p < 0.05 Pre-swim vs. Post-swim. B) Effect of hypothalamic photostimulation on c-cFos expression in orexin positive cells in YFP and ChR2 animals on the virus injected side (left bars) and the non-virus injected side (right bars). * = p < 0.05 Hcrt::YFP control vs. Hcrt::ChR2-YFP.
3.1.2. Examination of optogenetic stimulation effects on cellular activity
Dual staining for OrexinA and cFos revealed a significant main injection Side × Virus interaction (Fig. 1B; F1,40 = 15.83, p = 0.0003) and a significant main effect of Virus injection (F1,40 = 18.98, p < 0.0001). Post-hoc tests revealed photostimulation of orexin cells significantly increased the number of cFos positive orexin cells in the Hcrt::ChR2-YFP animals compared to the Hcrt::YFP control animals (p=0.0169) only on the virus injected side. This effect was not apparent on the non-virus injected side, indicating specific activation of ChR2 in the orexin cells of Hcrt::ChR2-YFP transduced animals.
3.2. Experiment 2: effects of optogenetically stimulated orexins on behavior in the social interaction test
There was a significant effect of photostimulation to decrease the time spent in the social interaction zone in the Hcrt::ChR2-YFP animals compared to the Hcrt::YFP control animals (Fig. 2A; p = 0.0423). There was also a significant effect of photostimulation to increase the frequency of entrances into the social interaction zone in the Hcrt:: ChR2-YFP animals compared to the Hcrt::YFP control animals (Fig. 2B; p < 0.0001). Additionally there was a significant effect of photostimulation to increase the total distance traveled in the Hcrt:: ChR2-YFP (Fig. 2C; p < 0.0001). Observation of the rats during the test showed that the YFP rats were initially slow to approach the SI zone, but when in the SI zone they spent most of their time interacting with the stimulus rat. The ChR2 rats, however, were quicker to approach the SI zone but spent less time interacting with the stimulus rat in the SI zone overall.
3.3. Experiment 3: relationship between behavior in the social interaction test and behavior during social defeat
There was no correlation observed between behavior in the social interaction test and defeat latency during 5 days of repeated social defeat stress (data not shown).
3.4. Experiment 4: effect of optogenetically stimulated orexin activity on home cage behaviors and HPA activity
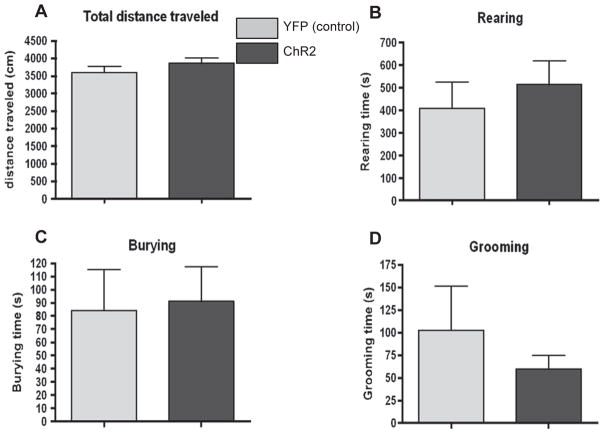
There was no significant effect of photostimulation on the total distance traveled in the home cage in the Hcrt::ChR2-YFP animals compared to the Hcrt::YFP control animals (Fig. 3A). There was also no significant effect of photostimulation on the number of occurrences (data not shown) or the time spent rearing (Fig. 3B), burying (Fig. 3C) or grooming (Fig. 3D) in the Hcrt::ChR2-YFP animals compared to the Hcrt::YFP control animals. There was no effect of optogenetic stimulation of the orexin system on blood ACTH and corticosterone levels. Blood was collected at 30min following the last photostimulation. ACTH levels were 291.61 ± 49.21 ng/ml for Hcrt::YFP control animals and 297.98 ± 32.94 ng/ml for Hcrt::ChR2-YFP animals. Corticosterone levels were 30.58 ± 1.31 ug/dl for Hcrt::YFP control animals and 28.42 ± 1.21 ug/dl for Hcrt::ChR2-YFP animals.
Fig. 3.
A) Effect of hypothalamic photostimulation on total distance traveled in Hcrt::YFP control (YFP; n = 9), and Hcrt::ChR2-YFP (ChR2; n = 13) transduced animals following hypothalamic photostimulation of 20 Hz for 10 s at a time every minute for 30 min in their home cage. B) Effect of hypothalamic photostimulation on the number of rearings in the home cage of animals described above. C) Effect of hypothalamic photostimulation on the number of rearings in the home cage of animals described above.
3.5. Experiment 5: effects of optogenetically stimulated orexin activity in potential neural substrates
3.5.1. PosteriorPVT data
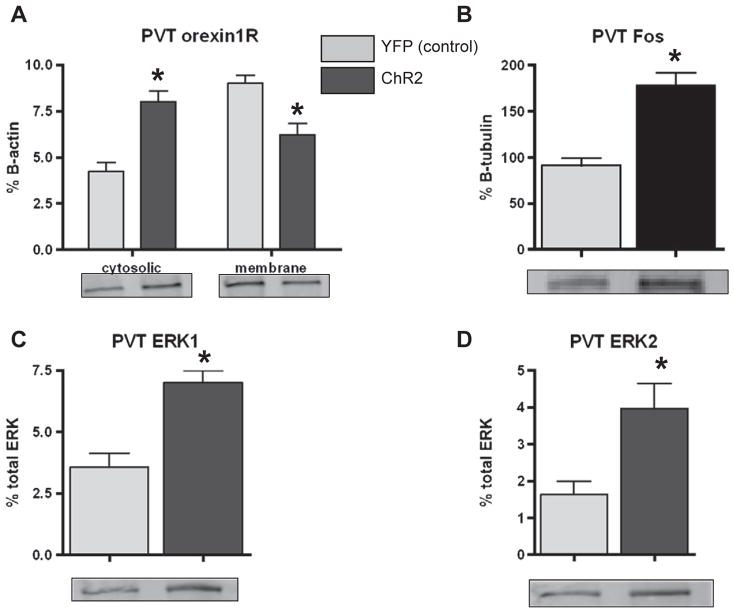
Following 30 min of photostimulation, orexin1R expression was significantly increased in the posteriorPVT cytosolic fraction of Hcrt:: ChR2-YFP animals compared to the Hcrt::YFP control animals (Fig. 4A; p < 0.0001). Consistent with this effect there was a significant decrease in the expression of orexin1R in the membrane fraction of Hcrt::ChR2-YFP animals (Fig. 4A; p = 0.0028). There was also a significant effect of photostimulation to increase posteriorPVT cFos expression in Hcrt:: ChR2-YFP animals (Fig. 4B; p < 0.0001). Photostimulation of orexin neurons significantly increased phosphorylation of ERK1 (Fig. 4C; p = 0.0002), and ERK2 (Fig. 4D; p = 0.0145) in Hcrt::ChR2-YFP animals.
Fig. 4.
A) Western blot analysis of orexin1R expression in the cytosolic (left bars) and membrane (right bars) fractions of posteriorPVT homogenates of Hcrt::YFP control (YFP; n = 9), and Hcrt::ChR2-YFP (ChR2; n = 13) transduced animals following hypothalamic photostimulation of 20 Hz for 10 s at a time every minute for 30min in their home cage. * = p < 0.05 Hcrt::YFP control vs. Hcrt::ChR2-YFP. B) Western blot analysis of cFos expression in pPVT homogenates of animals described above. * = p < 0.05 Hcrt::YFP control vs. Hcrt::ChR2-YFP. C) Western blot analysis of ERK1 phosphorylation expressed as % total ERK in pPVT homogenates of animals described above. * = p < 0.05 Hcrt::YFP control vs. Hcrt::ChR2-YFP. D) Western blot analysis of ERK2 phosphorylation expressed as % total ERK in pPVT homogenates of animals described above. * = p < 0.05 Hcrt::YFP control vs. Hcrt::ChR2-YFP. In each graph, representative blots are shown for each group.
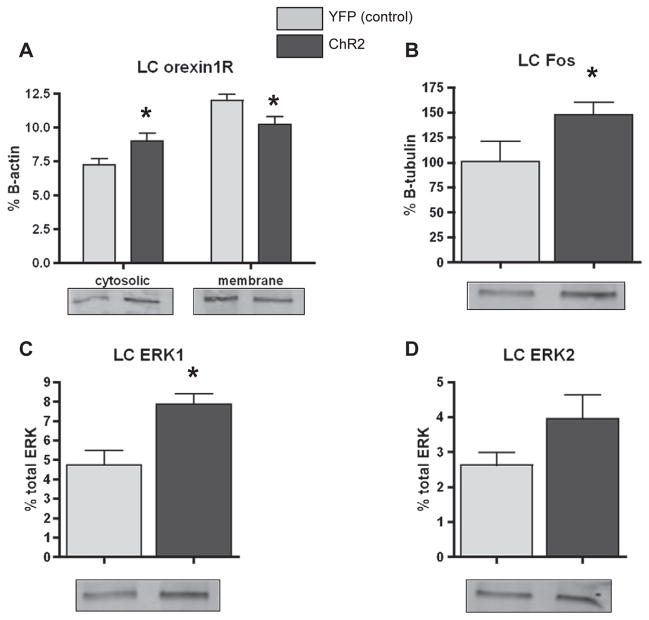
3.5.2. LC data
Orexin1R expression was significantly increased in the LC cytosolic fraction of Hcrt::ChR2-YFP animals compared to the Hcrt::YFP control animals (Fig. 5A; p = 0.0393). Consistent with this effect there was a significant decrease in the expression of orexin1R in the membrane fraction of Hcrt::ChR2-YFP animals compared to the Hcrt::YFP control animals (Fig. 5A; p = 0.0406). There was also a significant effect of photostimulation to increase cFos expression in Hcrt::ChR2-YFP animals (Fig. 5B; p = 0.0478). Photostimulation of orexin neurons significantly increased phosphorylation of ERK1 (Fig. 3C; p = 0.0015), but not ERK2 (Fig. 5D; p = 0.1452) in Hcrt::ChR2-YFP animals.
Fig. 5.
A) Western blot analysis of orexin1R expression in the cytosolic (left bars) and membrane (right bars) fractions of LC homogenates of Hcrt::YFP control (YFP; n = 9), and Hcrt:: ChR2-YFP (ChR2; n=13) transduced animals following hypothalamic photostimulation of 20Hz for 10s at a time every minute for 30min in their home cage. *=p<0.05 Hcrt::YFP control vs. Hcrt::ChR2-YFP. B) Western blot analysis of cFos expression in LC homogenates of animals described above. *=p<0.05 Hcrt::YFP control vs. Hcrt::ChR2-YFP. C) Western blot analysis of ERK1 phosphorylation expressed as % total ERK in LC homogenates of animals described above. * = p < 0.05 Hcrt::YFP control vs. Hcrt::ChR2-YFP. D) Western blot analysis of ERK2 phosphorylation expressed as % total ERK in LC homogenates of animals described above. In each graph, representative blots are shown for each group.
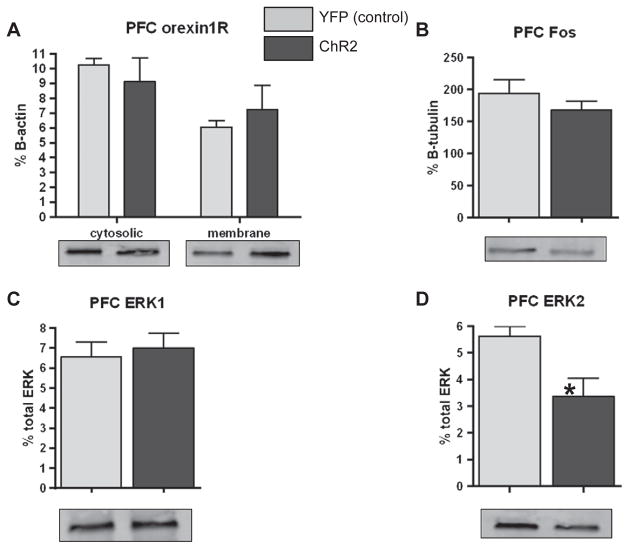
3.5.3. PFC data
30 minutes of photostimulation had no effect on orexin1R expression in either the cytosolic or membrane fractions of the infralimbic or prelimbic regions of the PFC (Fig. 6A). There was no significant effect of photostimulation on either infralimbic or prelimbic PFC cFos expression in either Hcrt::ChR2-YFP animals or Hcrt::YFP control animals (Fig. 6B). Photostimulation of orexin neurons had no effect on the phosphorylation of infralimbic PFC ERK1 (Fig. 6C), but did significantly decrease infralimbic ERK2 phosphorylation (Fig. 6D; p = 0.0171) in Hcrt::ChR2-YFP animals compared to the Hcrt::YFP control animals. Thus, there was also no effect of photostimulation on prelimbic PFC orexin1R distribution, ERK1 phosphorylation, and cFos expression.
Fig. 6.
A) Western blot analysis of orexin1R expression in the cytosolic (left bars) and membrane (right bars) fractions of infralimbic (left graph) and prelimbic (right graph) PFC homogenates of Hcrt::YFP control (YFP; n = 9), and Hcrt::ChR2-YFP (ChR2; n = 13) transduced animals following hypothalamic photostimulation of 20Hz for 10s at a time every minute for 30min in their home cage. B) Western blot analysis of c-Fos expression in PFC homogenates of animals described above. C) Western blot analysis of ERK1 phosphorylation expressed as % total ERK in PFC homogenates of animals described above. D) Western blot analysis of ERK2 phosphorylation expressed as % total ERK in PFC homogenates of animals described above. * = p < 0.05 Hcrt::YFP control vs. Hcrt::ChR2-YFP. In each graph, representative blots are shown for each group.
4. Discussion
Our results show that orexin photostimulation decreased time spent in social interaction with a concomitant increase in locomotor activity and frequency of entrances into the social interaction zone. The decreases in time spent interacting suggest that stimulation of the orexin system increases the anxiety state of the animal. The increases in locomotor activity and entrances into the social interaction zone are consistent with previously reported orexinA-induced increases in alertness and arousal [2]. However, stimulation of orexin release did not have an effect on home cage behavior, suggesting that orexin release may increase the salience of novel, but not familiar, environmental stimuli. Finally, results from western blot analysis of brain regions heavily innervated by orexin neurons indicated increased orexin 1 receptor internalization and ERK phosphorylation in the PVT and LC suggesting that these are important neural substrates for orexin actions.
Because the Hcrt::ChR2-YFP construct had not been previously used to examine orexin function in rats, we validated its functionality in rats at a behavioral and at a cellular level. We observed decreased latency to transition from a sleeping to a waking state following photostimulation. These findings are consistent with previously published work following optogenetic stimulation of orexin cells in mice [1], although different measures were used to assess the sleep state in the two studies. There was also a significant increase in the activation of orexin cells in response to photostimulation when compared to animals that received the control vector as measured by cFos/orexin dual immunocytochemistry. Dual immunostaining for orexinA and cFos was used to determine the extent of orexin cell activation following photostimulation. Because the virus was injected unilaterally, the non-injected side could be used as a control for further comparison of the level of orexin cell activation by ChR2. Photostimulation significantly increased the number of cFos positive orexin cells in the ChR2 expressing rats when compared with the YFP control rats. This effect was only present on the virus-injected side and not on the non-injected side. Photostimulation activated approximately 55% of the total orexin cells on the virus-injected side compared to approximately 35% activation of orexin cells in the YFP control animals and the non-injected side. These results are in the range of work done with this construct in mice in which approximately 65% activation of orexin cells in the animals transfected with the ChR2 containing virus were activated compared with 25% activation of orexin cells in the control animals [1]. Together, these data suggest that photostimulation specifically activates orexin cells only in the ChR2 expressing animals and support the functionality of orexin promoter-induced ChR2 in rats.
Following the initial sleep/wake transition study, the rats were exposed to repeated swim stress for 4 days and examined again for sleep/wake transitions. Repeated swim stress decreased latency to first movement following stimulation in the YFP control rats suggesting more rapid sleep to wake transitions as a result of repeated stress. The latency in the YFP rats was decreased to the level of the ChR2 expressing rats after repeated stress and latencies were unaltered by stress in ChR2 expressing rats, which were already low prior to the repeated swim stress. One interpretation of these data is that repeated swim stress had the same effect on sleep/wake transitions as did optogenetic stimulation of the orexin system, suggesting that orexin system activity may be a mechanism underlying stress-induced sleep disturbances [5,9,20,30].
Optogenetic stimulation of ChR2-expressing orexin neurons during the social interaction test uncovered a specific role for the orexin system in novel situations. The social interaction test is often used as a measure of anxiety-related behavior [10]. In addition, measures of locomotor activity and general exploratory behavior can be assessed. Optogenetic stimulation of the orexin system significantly decreased the time the ChR2 expressing rats spent in the interaction zone when compared to YFP controls. These data suggest that stimulation of the orexin system leads to increases in anxiety-related behavior. These results should be confirmed with a social interaction test that allows direct interaction between the two animals to confirm that the increased time in the interaction zone observed here is associated with interaction between the two animals. Nonetheless, these findings with endogenous orexin release are consistent with previous literature with exogenous orexin administration showing anxiogenic effects of orexinA administered both ICV [14,19,33] and directly in the paraventricular thalamus (PVT) [13]. However, concurrent with a decrease of time in the social interaction zone, optogenetic stimulation of the orexin system also significantly increased the number of entries into the social interaction zone and locomotor activity when compared with YFP controls. These increases suggest that enhanced orexin activity increases arousal and exploratory behavior. Together, these results suggest that orexins mediate the arousal that underlies anxiety by potentially increasing the salience of environmental stimuli.
In order to determine if the effects of activating the orexin system in the social interaction test were due to the novel setting of the test itself, animals were examined in their home cage during optogenetic stimulation. In addition to total distance traveled, rearing, grooming, and burying behaviors were assessed. There was no effect of photostimulation on any of these measures. This lack of effect in the home cage combined with the anxiogenic and arousal effects in the social interaction test suggests that the behavioral effects of orexins are context-specific and present primarily in novel situations. As a caveat, rats assessed in the home cage had been exposed to social defeat and previous testing which could have influenced the results. Although a small cohort of 3 naïve ChR2 expressing animals tested in the home cage under similar circumstances as those in Experiment 4 showed no behavioral differences when compared with YFP controls, additional work is necessary to confirm the context-specific effects of orexin stimulation observed here.
In order to examine which brain regions could be mediating the effects of orexins, we examined the PVT, LC and PFC because each expresses a high density of orexin receptors [21,34] and receives dense orexin inputs [16,21,25,32,34]. We examined activation of these regions via three measures. First, we assessed the distribution of orexin receptors in membrane and cytosolic fractions of brain punch homogenates. Because orexin receptors are G-protein coupled receptors, activation induces a shift of the receptor from the membrane into the cytosol. Our previous work demonstrated that orexin receptor agonists induce a shift of orexin receptors from the membrane to the cytosol and this shift is blocked by orexin receptor antagonists, validating the use of this approach in determining activation of orexin receptors [13]. Second, we assessed the relative levels of ERK phosphorylation in these particular brain regions. Previous work from our lab and others has shown that orexin receptor activation increases ERK phosphorylation as part of an intracellular messenger cascade [13,23,27]. Third we examined expression of the neuronal activity marker cFos in orexin-expressing neurons [8]. We observed that optogenetic stimulation of the orexin system increased orexin-1 receptor expression in the cytosolic fraction and decreased receptor expression in the membrane fraction of both the PVT and LC of ChR2 expressing animals compared to YFP controls. Furthermore, optogenetic stimulation increased ERK1 and ERK2 phosphorylation and cFos expression in the PVT and increased ERK1 in the LC of ChR2 expressing animals compared to YFP controls. In the PFC there was no effect on ERK1 phosphorylation and no effect on cFos expression, however there was a significant effect of photostimulation to decrease ERK2 phosphorylation in the ChR2 expressing rats compared to the YFP controls in the infralimbic PFC. While this effect is the opposite of what we expected, it is possible that other inputs to the PFC in concert with orexins reduce activation in the PFC. Another possibility is that the lack of effect in the PFC may be a product of already enhanced activity in the PFC caused by the handling associated with introducing the optic fiber into the guide cannulae. Finally, it is possible that the specific subregion of hypothalamic orexin cell bodies that was stimulated does not project to the PFC. Further work is needed to better understand these results. It must be noted that these brain structures were examined from tissue collected after optogenetic stimulation in the home cage in which no behavioral differences between ChR2 and YFP control animals were observed. Therefore, it is not possible to state that the PVT and LC specifically mediate the effects of orexin on exploratory and anxiety related behavior in the social interaction test. Nonetheless, the results indicate that the PVT and LC are likely important mediators of endogenous orexin effects, that the orexin actions in the PVT and LC occur through activation and internalization of orexin1 receptors and through ERK phosphorylation, and that orexin effects in the PFC are likely unrelated to the behaviors assessed here. Together, these results show for the first time that elevation of endogenous orexins in rats produces increased anxiety that is accompanied by increased exploration and arousal that occurs in novel but not familiar contexts.
HIGHLIGHTS.
Activation of orexin system increases anxiety-related behavior in novel context
Activation of orexin system increases exploration and arousal only in novel environment
Orexin-mediated increases in anxiety and arousal associated with orexin activity in PVT and LC
References
- 1.Adamantidis A, De Lecea L. Physiological arousal: a role for hypothalamic systems. Cell Mol Life Sci. 2008;65:1475–88. doi: 10.1007/s00018-008-7521-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borgland SL, Chang S-J, Bowers MS, Thompson J, Vittoz N, Floresco SB, et al. Orexin A/hypocretin-1 selectively promotes motivation for positive reinforcers. J Neurosci. 2009;29:11215–25. doi: 10.1523/JNEUROSCI.6096-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Castro MG, Morrison E, Perone MJ, Brown OA, Murray CA, Ahmed I, et al. Corticotropin-releasing hormone receptor type 1: generation and characterization of polyclonal antipeptide antibodies and their localization in pituitary cells and cortical neurones in vitro. J Neuroendocrinol. 1996;8:521–31. doi: 10.1046/j.1365-2826.1996.04866.x. [DOI] [PubMed] [Google Scholar]
- 4.Chang H, Saito T, Ohiwa N, Tateoka M, Deocaris CC, Fujikawa T, et al. Inhibitory effects of an orexin-2 receptor antagonist on orexin A- and stress-induced ACTH responses in conscious rats. Neurosci Res. 2007;57:462–6. doi: 10.1016/j.neures.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 5.Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, et al. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–51. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- 6.Date Y, Ueta Y, Yamashita H, Yamaguchi H, Matsukura S, Kangawa K, et al. Orexins, orexinergic hypothalamic peptides, interact with autonomic, neuroendocrine and neuroregulatory systems. Proc Natl Acad Sci. 1999;96:748–53. doi: 10.1073/pnas.96.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Lecea L, Kilduff TS, Peyron C, Gaos X-B, Foye PE, Danielson PE, et al. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A. 1998;95:322–7. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dragunow M, Faull R. The use of c-fos as a metabolic marker in neuronal pathway tracing. J Neurosci Methods. 1989;29:261–5. doi: 10.1016/0165-0270(89)90150-7. [DOI] [PubMed] [Google Scholar]
- 9.Espana RA, Baldo BA, Kelley AE, Berridge CW. Wake-promoting and sleep-suppressing actions of hypocretin (orexin): basal forebrain sites of action. Neuroscience. 2001;106:699–715. doi: 10.1016/s0306-4522(01)00319-0. [DOI] [PubMed] [Google Scholar]
- 10.File SE. Anxiolytic action of a neurokinin1 receptor antagonist in the social interaction test. Pharmacol Biochem Behav. 1997;58:747–52. doi: 10.1016/s0091-3057(97)90002-2. [DOI] [PubMed] [Google Scholar]
- 11.Grissom N, Iyer V, Bhatnagar S. Repeated post-stress blockade of the MAP-kinase signaling pathway in the basolateral amygdala (BLA) - effects on hypothalamic-pituitary-adrenal (HPA) activity. Soc Neurosci Abstr. 2006 [Google Scholar]
- 12.Hegde P, Singh K, Chaplot S, Shankaranarayana Rao BS, Chattarji S, Kutty BM, et al. Stress-induced changes in sleep and associated neuronal activity in rat hippocampus and amygdala. Neuroscience. 2008;153(1):20–30. doi: 10.1016/j.neuroscience.2008.01.042. [DOI] [PubMed] [Google Scholar]
- 13.Heydendael W, Sharma K, Iyer V, Luz S, Piel D, Beck S, et al. Orexins/Hypocretins act in the posterior paraventricular thalamic nucleus during repeated stress to regulate facilitation to novel stress. Endocrinology. 2011;152:4738–52. doi: 10.1210/en.2011-1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson PL, Truitt W, Fitz SD, Minick PE, Dietrich A, Sanghani S, et al. A key role for orexin in panic anxiety. Nat Med. 2010;16:111–5. doi: 10.1038/nm.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karteris E, Randeva HS. Orexin receptors and G-protein coupling: evidence for another “promiscuous” seven transmembrane domain receptor. J Pharmacol Sci. 2003;93:126–8. doi: 10.1254/jphs.93.126. [DOI] [PubMed] [Google Scholar]
- 16.Kirouac G, Parsons M, Li S. Orexin (hypocretin) innervation of the paraventricular nucleus of the thalamus. Brain Res. 2005;1059:179–88. doi: 10.1016/j.brainres.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 17.Kuru M, Ueta Y, Serino R, Nakazato M, Yamamoto Y, Shibuya I, et al. Centrally administered orexin/hypocretin activates HPA axis in rats. Neuroreport. 2000;11:1977–80. doi: 10.1097/00001756-200006260-00034. [DOI] [PubMed] [Google Scholar]
- 18.Li Y, Li S, Sui N, Kirouac GJ. Orexin-A acts on the paraventricular nucleus of the midline thalamus to inhibit locomotor activity in rats. Pharmacol Biochem Behav. 2009;93:506–14. doi: 10.1016/j.pbb.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 19.Li Y, Li S, Wei C, Wang H, Sui N, Kirouac G. Orexins in the paraventricular nucleus of the thalamus mediate anxiety-like responses in rats. Psychopharmacology (Berl) 2010;212:251–65. doi: 10.1007/s00213-010-1948-y. [DOI] [PubMed] [Google Scholar]
- 20.Lin L, Faraco J, Li R, Kadotani H, Rogers W, Lin X, et al. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999;98:365–76. doi: 10.1016/s0092-8674(00)81965-0. [DOI] [PubMed] [Google Scholar]
- 21.Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, et al. Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol. 2001;435:6–25. doi: 10.1002/cne.1190. [DOI] [PubMed] [Google Scholar]
- 22.Miczek KA. A new test for aggression in rats without aversive stimulation: differential effects of d-amphetamine and cocaine. Psychopharmacology (Berl) 1979;60:253–9. doi: 10.1007/BF00426664. [DOI] [PubMed] [Google Scholar]
- 23.Milasta S, Evans NA, Ormiston L, Wilson S, Lefkowitz RJ, Milligan G. The sustainability of interactions between the orexin-1 receptor and beta-arrestin-2 is defined by a single C-terminal cluster of hydroxy amino acids and modulates the kinetics of ERK MAPK regulation. Biochem J. 2005;387:573–84. doi: 10.1042/BJ20041745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Min-Huei CCC. G-protein-coupled receptor-associated A-kinase anchoring proteins AKAP5 and AKAP12: differential trafficking and distribution. Cell Signal. 2008;21:136–42. doi: 10.1016/j.cellsig.2008.09.019. [DOI] [PubMed] [Google Scholar]
- 25.Nambu T, Sakurai T, Mizukami K, Hosoya Y, Yanagisawa M, Goto K. Distribution of orexin neurons in the adult rat brain. Brain Res. 1999;827:243–60. doi: 10.1016/s0006-8993(99)01336-0. [DOI] [PubMed] [Google Scholar]
- 26.Pack AI, Galante RJ, Maislin G, Cater J, Metaxas D, Lu S, et al. Novel method for high throughput phenotyping of sleep in mice. Physiol Genomics. 2007;28(2):232–8. doi: 10.1152/physiolgenomics.00139.2006. [DOI] [PubMed] [Google Scholar]
- 27.Ramanjaneya M, Conner AC, Chen J, Stanfield PR, Randeva HS. Orexins stimulate steroidogenic acute regulatory protein expression through multiple signaling pathways in human adrenal H295R cells. Endocrinology. 2008;149:4106–15. doi: 10.1210/en.2007-1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–85. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- 29.Sakurai T, Moriguchi T, Furuya K, Kajiwara N, Nakamura T, Yanagisawa M, et al. Structure and function of human prepro-orexin gene. J Biol Chem. 1999;274:17771–6. doi: 10.1074/jbc.274.25.17771. [DOI] [PubMed] [Google Scholar]
- 30.Samson WK, Taylor MM, Ferguson AV. On-sleep effects of hypocretin/orexin. Sleep Med Rev. 2005;9:243–52. doi: 10.1016/j.smrv.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 31.Samson WK, Taylor MM, Follwell M, Ferguson AV. Orexin actions in hypothalamic paraventricular nucleus: physiological consequences and cellular correlates. Regul Pept. 2002;104:97–103. doi: 10.1016/s0167-0115(01)00353-6. [DOI] [PubMed] [Google Scholar]
- 32.Steininger TL, Kilduff TS, Behan M, Benca RM, Landry CF. Comparison of hypocretin/orexin and melanin-concentrating hormone neurons and axonal projections in the embryonic and postnatal rat brain. J Chem Neuroanat. 2004;27:165–81. doi: 10.1016/j.jchemneu.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 33.Suzuki M, Beuckmann CT, Shikata K, Ogura H, Sawai T. Orexin-A (hypocretin-1) is possibly involved in generation of anxiety-like behavior. Brain Res. 2005:1044. doi: 10.1016/j.brainres.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 34.Trivedi P, Yu H, MacNeil DJ, Van der Ploeg LH, Guan XM. Distribution of orexin receptor mRNA in the rat brain. FEBS Lett. 1998;438:71–5. doi: 10.1016/s0014-5793(98)01266-6. [DOI] [PubMed] [Google Scholar]
- 35.Wood SK, Walker HE, Valentino RJ, Bhatnagar S. Individual differences in reactivity to social stress predict susceptibility and resilience to a depressive phenotype: role of corticotropin-releasing factor. Endocrinology. 2010;151:1795–805. doi: 10.1210/en.2009-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang S, Lin L, Kaur S, Thankachan S, Blanco-Centurion C, Yanagisawa M, et al. The development of hypocretin (orexin) deficiency in hypocretin/ataxin-3 transgenic rats. Neuroscience. 2007;148:34–43. doi: 10.1016/j.neuroscience.2007.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hagan JJ, Leslie RA, Patel S, Evans ML, Wattam TA, Holmes S, et al. Orexin A activates locus coeruleus cell firing and increases arousal in the rat. Proc Natl Acad Sci U S A. 1999;96(19):10911–6. doi: 10.1073/pnas.96.19.10911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taheri S, Mahmoodi M, Opacka-Juffry J, Ghatei MA, Bloom SR. Distribution and quantification of immunoreactive orexin A in rat tissues. FEBS Lett. 1999;457(1):157–61. doi: 10.1016/s0014-5793(99)01030-3. [DOI] [PubMed] [Google Scholar]