Abstract
Parkinson’s disease was long considered a non-hereditary disorder. Despite extensive research trying to find environmental risk factors for the disease, genetic variants now stand out as the major causative factor. Since a number of genes have been implicated in the pathogenesis it seems likely that several molecular pathways and downstream effectors can affect the trophic support and/or the survival of dopamine neurons, subsequently leading to Parkinson’s disease. The present review describes how toxin-based animal models have been valuable tools in trying to find the underlying mechanisms of disease, and how identification of disease-linked genes in humans has led to the development of new transgenic rodent models. The review also describes the current status of the most common genetic susceptibility factors for Parkinson’s disease identified up to today.
Keywords: Genetic risk factors, PARK, Mutation, Animal model, Association study, Linkage study
1. Introduction
Paralysis agitans or Parkinson’s disease is well described in the ancient Indian medical treatise Ayurveda (Sanskrit: ayur, life; veda, science) with the oldest material dating from 2000–4000 B.C. and a complete treatise completed around 1000 B.C. According to Ayurveda, the disorder was referred to as Kampavata (Kampa, tremor; vata, lack of movements) and manifested symptoms such as rigidity, akinesia, tremors, depression, somnolence, “loss of mind” and mental confusion. It was treated with seeds from Mucuna pruriens, a plant in the Leguminosae family. At that time the active substance in the plants was unknown, and it was not until the 1930s that the active component L-3,4-dihydroxyphenylalanine (L-dopa) was isolated (Damodaran and Ramaswamy, 1937). However, this finding had limited impact at that time, since the involvement of dopamine in the disease had not yet been discovered.
For long, Parkinson’s disease, as we know it today (Parkinson, 1817), was considered a typical non-genetic disorder. However, the fact that Parkinson’s disease has been present since ancient times, presumably without major changes of prevalence caused by the industrial revolution and the increasing use of man-made chemicals as well as the findings of similar prevalences in different populations across the world, suggest that environmental factors play a less important role in Parkinson’s disease than previously thought.
Even though the underlying mechanisms of Parkinson’s disease remain partly unknown, several hypotheses have been put forward for its causes. Implicated mechanisms involve protein misfolding, mitochondrial and ubiquitin-proteasome dysfunction, oxidative stress, inflammation, apoptosis, exposure to and/or increased vulnerability to environmental toxins and infectious agents. It is unclear, however, how these different pathogenic events, and others yet to be discovered, cause Parkinson’s disease. The variable phenotypes observed among Parkinson patients suggest involvement of several different molecular pathways. Moreover, it remains to be resolved if the underlying causes act separately or if they converge into one or only a few final common pathways. All pathogenic events however will affect the survival and/or death of neurons in vulnerable brain areas including the substantia nigra, locus coeruleus and the dorsal motor nucleus of the vagus nerve (Braak et al., 2004).
2. Dopamine neurotoxins
The use of toxin-based animal models has given useful insights into the pathology of Parkinson’s disease. Ideally, a valuable and reliable animal model of disease should mimic one or preferably several of the specific features of the human disease. The Parkinson’s disease-like rodent models used today can mimic motor dysfunctions, dopamine neuron degeneration, olfactory loss and, albeit to a lesser degree, formation of intracellular inclusion bodies in affected neurons.
2.1. Toxin-based animal models
Two commonly used rodent models of Parkinson’s disease are based on administration of 6-hydroxy-dopamine (6-OHDA) (into the brain) or 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) (systemically) in order to rapidly and selectively destroy catecholaminergic neurons. 6-OHDA is a hydroxylated analogue of dopamine (Blum et al., 2001) which was first shown to cause noradrenergic depletion of sympathetic nerves to the heart (Porter et al., 1963, 1965) and destruction of noradrenergic nerves (Tranzer and Thoenen, 1973). In the CNS, 6-OHDA causes destruction of dopaminergic and noradrenergic neurons (Ungerstedt, 1968). The toxin is taken up by dopamine and noradrenaline membrane transporters and accumulates in the cell cytosol. Cell death is caused by formation of reactive oxygen species and mitochondrial respiratory chain deficiency (Blum et al., 2001). The drug does not cross the blood-brain barrier and hence, has to be stereotaxically injected to striatum, substantia nigra, the medial forebrain bundle, or administered directly into the ventricular system. Intrastriatal injection of 6-OHDA can result in a progressive, retrograde partial lesioning, whereas injection into the substantia nigra or medial forebrain bundle results in complete lesioning of the nigrostriatal pathway (Ungerstedt and Arbuthnott, 1970; Ungerstedt, 1971a,b; Sachs and Jonsson, 1975). The strength of the lesioning is dependent on the site of injection, the amount of 6-OHDA administered and on the species used (Betarbet et al., 2002). Both unilateral (hemiparkinsonian model, where the unlesioned hemisphere serves as an internal control) and bilateral lesioning models are used. Unilateral lesioning causes asymmetrical and quantifiable motor behaviors induced by systemic administration of dopamine receptor agonists, levodopa or dopamine-releasing drugs (Ungerstedt and Arbuthnott, 1970; Hefti et al., 1980). The bilateral model on the other hand, results in parkinsonian motor complications, but due to the need for intensive nursing care of the animals, the use of the model is less common (Cenci et al., 2002). While the 6-OHDA model is widely used in Parkinson’s disease research, the model does not recapitulate all pathological features of the disease. For instance, the animals do not develop cytoplasmic Lewy bodies. Moreover, intracerebral injection of 6-OHDA does not affect other brain areas involved in Parkinson’s disease such as locus coeruleus, the brain stem or olfactory areas (Betarbet et al., 2002) or cortex cerebri.
A link between parkinsonism and mitochondrial dysfunction, a suggested causative event for the disease, was established when the neurotoxic substance MPTP was found to cause severe and irreversible parkinsonism in a small group of drug addicts in California (Langston et al., 1983). The affected individuals displayed several clinical and neuropathological characteristics of Parkinson’s disease. MPTP is a lipophilic molecule which readily crosses the blood–brain barrier. In non-dopaminergic neurons (mostly astrocytes) MPTP is converted by monoamine oxidase B (MAO-B) to 1-methyl-4-phenyl-2,3-dihydropyridinium (MPDP) which is oxidized to 1-methyl-4-phenylpyridinium (MPP+) (Nicklas et al., 1985, 1987; Przedborski and Vila, 2003). The active metabolite MPP+ is taken up by dopamine neurons through the dopamine transporter (DAT), and acts as an inhibitor of mitochondrial complex I of the respiratory chain (Nicklas et al., 1987; Mizuno et al., 1987). MPTP functions as a potent neurotoxin in both mice and primates, although mice are less sensitive than monkeys (Nicklas et al., 1985; Blum et al., 2001).
It is well known that agricultural chemicals such as the pesticide rotenone can induce specific parkinsonian symptoms (Betarbet et al., 2000, 2002). Structurally and functionally rotenone is related to MPTP and also acts as an inhibitor of mitochondrial complex I (Perier et al., 2003). Studies of rotenone thus add to the evidence that dopamine neurons may be particularly vulnerable to mitochondrial dysfunction. However, while there are some epidemiological studies to indicate that pesticide exposure may increase the risk to develop Parkinson’s disease, the relative importance of such exposure for the prevalence of Parkinson’s disease worldwide is not clear.
Most toxin-based animal models used today are focused on the nigrostriatal system and the loss of dopamine neurons, which is a prerequisite for understanding the underlying mechanisms of the disease, and for developing symptomatic treatments. However, the animal models are limited in that they do not recapitulate the complete spectrum of symptoms seen in humans, and in particular not the slow and progressive loss of dopamine neurons, which is characteristic of Parkinson’s disease.
3. Genetics
Today, the underlying pathology of Parkinson’s disease is well explored, but we have limited understanding of the etiology. Long considered a typical non-genetic disorder, the majority of the cases are still classified as idiopathic. However, the view of Parkinson’s disease as a sporadic disorder has been subject to dramatic change in recent years. Today, genetic risk factors stand out as the major cause of the disease, possibly in combination with environmental factors. The impact of genetic risk factors has been significantly underestimated in the past and this may be attributed to the late onset of the disease. Inherited diseases with a late-in-life onset are difficult to detect because of failing memory of the patient and his/her relatives, death of relatives, and the patient’s prolonged exposure to other possibly disease-causing agents during life. Complex genetic traits and reduced penetrance further complicate the identification of risk factors. A disease phenotype may be caused by a major genetic component, but it does not necessarily imply that only one gene is involved in generating the specific disorder. A disease may be caused by inherited mutations in several genes, some of which give rise to the same phenotype but with a different mode of inheritance. Yet other mutations may convey resistance to disease. This complex situation appears to be another reason why non-genetic causes of Parkinson’s disease were in favor for so long. Moreover, it has long been known that infections (Von Economo’s disease, Encephalitis lethargica) can cause a Parkinson-like disease, as can trauma, which further adds to the complexity. It has been estimated that approximately 10–15% of all Parkinson’s disease cases can now be explained by a known genetic component (Bonifati, 2006). We assume this percentage will increase as new genetic markers are continuously being identified.
Even though the genetic involvement in Parkinson’s disease has been well established, the findings have gotten limited support from epidemiological studies (Tanner et al., 1999; Sveinbjornsdottir et al., 2000; Wirdefeldt et al., 2004). The risk ratio of disease has been reported to be increased in siblings and the offspring of affected patients, but the concordance between mono- and di-zygotic twins has been found to be lower than expected. Interestingly, positron emission tomography (PET) data from monozygotic twins has shown higher concordance for decreased L-dopa binding, indicating marked inheritance, albeit with other factors influencing the age of onset (Piccini et al., 1999). This opens the possibility that environmental and/or epigenetic factors may also play essential roles in genetically predisposed mutation carriers.
3.1. Parkinson’s disease genetics from a historical perspective
An early documented observation of a genetic component in Parkinson’s disease was made by Leroux (Leroux, 1880), who in 1880 suggested a link between heritable factors and increased disease susceptibility (Farrer, 2006). More than half a century later, Allen reported familial forms of parkinsonism inherited as a dominant trait in North Carolina, USA (Allen, 1937), and Henry Mjönes described autosomal dominantly inherited cases in Sweden (Mjönes, 1949). In 1996 a pioneering genetic finding was made by Polymeropoulos and colleagues who found genetic linkage in an Italian family with an autosomal dominant form of Parkinson’s disease (Polymeropoulos et al., 1996). The ground-breaking discovery was strengthened by identification of a missense mutation in the SNCA gene (encoding the α-synuclein protein) in the affected family members (Polymeropoulos et al., 1997), and subsequent identification of α-synuclein as one of the major components of Lewy bodies (Spillantini et al., 1997). After the identification of the first chromosomal locus linked to Parkinson’s disease (PARK1), fourteen more chromosomal loci (PARK2-15) with suggested linkage to disease have been identified, although for some of these loci a specific gene implicated in Parkinson’s disease is yet to be found (Lesage and Brice, 2009). Some of the genes linked to monogenic forms of Parkinson’s disease have also been identified as risk factors for sporadic forms of the disease. Through identification of families carrying mutations in PARK genes, the weight of evidence has now shifted towards genetic or, possibly gene-environmental interactions, as the causes of Parkinson’s disease. A genetic variant can be a mutation or a single nucleotide polymorphism (the latter is present at a frequency of >1% in the population), a deletion, an insertion, a whole gene rearrangement or a copy number variation. Disease-associated genetic variants can be present both in coding and non-coding parts of the gene. Still, the mechanism by which intronic variability predisposes to disease is obscure, although altered transcriptional regulation, mRNA stability and regulation by miRNAs, as well as alternative splicing, have been suggested (Wang et al., 2008).
3.2. Linkage studies
Thanks to today’s increased longevity, families with several affected generations can be studied, enabling easier identification of chromosomal regions carrying a disease-causing gene. Identification of candidate genes through linkage analysis is a hypothesis-free method which is based on the segregation of a genetic marker with a known genomic location through several generations in a family. Linkage analyses are most successful for chromosomal loci with high penetrance, whereas analyses of mutations with low-penetrance and diseases with complex traits are more difficult to perform. Linkage or co-segregation is presented as the logarithm of the odds (LOD) score, in which a high positive score shows evidence for linkage whereas a negative score shows evidence against (Dawn and Barrett, 2005). Calculation of LOD scores requires information about the mode of disease inheritance (i.e. dominant or recessive), allele frequencies, and a full marker map for each chromosome.
3.3. Association studies
Association studies are based on the hypothesis that a genetic variant associates with increased or decreased risk of disease. The studies are carried out using sample sets consisting of unrelated patients and controls. Specific candidate genes must be chosen, and can be identified through linkage analyses or they can be genes with a particular relevance for the dopamine system, for mitochondrial function, protein aggregation or any other function for which a hypothesis can be formulated about a possible involvement in the specific disease. Variable findings are frequently reported from association studies, sometimes due to small sample sizes. A way to increase reliability in association analyses is to investigate larger samples from genetically diverse populations. However, geographically distinct populations may differ in terms of mutation frequencies such that large samples may also mask locally significant disease-associated mutations. A single association study has limited power to detect true susceptibility genes, and has to be replicated in other populations in order to improve statistical significance. Other strategies to increase reliability are to perform retrospective meta-analyses of multiple independent studies or collaborative multi-center studies with standardized methodologies and diagnostic criteria. In the field of Parkinson’s disease, collaborative analyses have led to the identification of the promoter polymorphism NACP-Rep1 in SNCA and the inversely associated missense mutation S18Y in UCH-L1 as risk/protective susceptibility factors (Maraganore et al., 2004, 2006).
In order to deliver valid and reproducible results from an association study, standardized methodological and statistical approaches are needed. In terms of the study groups, the number of cases and controls has to be sufficient for the study to have enough power and the groups should also be matched for age and gender. Moreover, careful consideration has to be taken when diagnosing and classifying patients and controls. A correct diagnosis (in both study groups) is essential, since both positive and negative diagnostic errors impair statistical analyses. Further adding to the complexity of association studies is the presence of population-specific gene–gene interactions, genes with different modes of inheritance, population stratification and presence of phenocopies.
With a large number of polymorphisms available in public databases and the development of high-throughput techniques for genotyping, the interest in using whole-genome association studies to unravel genetic susceptibility factors is increasing. The method is based on the scanning of a large number of genetic markers across the complete genome in order to find genetic variations associated with disease. Given the large number of genes and mutations implicated in Parkinson’s disease, it appears evident that multiple methodological approaches are required for identification of susceptibility factors. Moreover, studies of both isolated and heterogeneous populations are important in order to identify pathogenic mutations in different parts of the world.
3.4. Autosomal dominant PARK genes
3.4.1. α-Synuclein (PARK1 and PARK4)
The first finding strengthening the involvement of genetic risk factors in Parkinson’s disease was the identification of the PARK1 locus on chromosome 4q21, linking to familial forms of the disease (Polymeropoulos et al., 1996). The finding of a chromosomal region linked to Parkinson’s disease was confirmed by identification of the point mutation A53T in the SNCA/α-synuclein gene in Italian and Greek families with autosomal dominant inheritance of the disease (Polymeropoulos et al., 1997). In the following years a Korean A53T-mutated Parkinson’s disease family (Ki et al., 2007) and two unrelated German and Spanish families, carrying the point mutations A30P and E46K respectively, were found (Kruger et al., 1998; Zarranz et al., 2004), as well as whole SNCA gene multiplications (PARK4) (Singleton et al., 2003; Chartier-Harlin et al., 2004; Farrer et al., 2004; Ibanez et al., 2004; Nishioka et al., 2006; Fuchs et al., 2007; Ahn et al., 2008; Ikeuchi et al., 2008; Ross et al., 2008). Parkinson patients with a gene duplication exhibit a 1.5 fold increase in α-synuclein levels whereas an SNCA triplication causes a two-fold increase in protein levels (Farrer et al., 2004), showing that the gene dose is critical in causing the disease. Polymorphisms in non-coding regions of SNCA have also been shown to contribute to the risk of sporadic Parkinson’s disease (Mueller et al., 2005; Mizuta et al., 2006; Westerlund et al., 2008), as has the SNCA promoter variability NACP-Rep1 (Maraganore et al., 2006). Mutated forms of the protein may be more likely than wild-type protein to aggregate, resulting in formation of insoluble protein inclusions.
Compelling evidence from biochemical studies and animal models has also strengthened the involvement of α-synuclein in Parkinson’s disease. The α-synuclein protein has, together with a number of other proteins, been identified as a constituent of Lewy bodies (Spillantini et al., 1997), the intracellular inclusions found in brain stem and cortical areas of Parkinson patients. α-Synuclein was originally identified as a precursor protein of the non-β-amyloid component (NAC) of Alzheimer’s disease amyloid plaques (Ueda et al., 1993). It is highly expressed in the human brain, including the cerebral cortex, hippocampus and cerebellum, where it is localized to presynaptic nerve terminals. Because of its extensive neuronal expression in the brain, abnormal aggregation or disruption of α-synuclein function may have similarly widespread consequences. However, α-synuclein is not essential for survival, since α-synuclein knockout mice are viable and fertile (Abeliovich et al., 2000). The complete function of α-synuclein is not yet understood, although the protein has been implicated in learning, synaptic vesicle mobilization, presynaptic functions and maintenance of the synaptic vesicle pool (Murphy et al., 2000; Cabin et al., 2002; Chandra et al., 2004). Moreover, the suggested mechanism by which α-synuclein causes neurodegeneration is obscure, but formation of protofibrils or fibrils has been suggested (Goedert, 2001). Interestingly, a protective role of Lewy bodies as a scavenger of misfolded proteins has also been suggested. The recent observation that embryonic dopamine neurons grafted to patients with Parkinson’s disease may develop Lewy bodies (Kordower and Brundin, 2009) adds yet another dimension to the possible roles of α-synuclein in Parkinson pathology.
3.4.2. UCH-L1 (PARK5)
Mutations in ubiquitin carboxyl-terminal esterase L1 (UCH-L1) at the PARK5 locus have been suggested to cause autosomal dominant Parkinson’s disease. Linkage to chromosome 4p14 has been established, however the finding has been questioned since it has only been found in rare cases from a single Parkinson’s disease family. An I93M mutation was found in a Parkinson’s disease family of German ancestry (Leroy et al., 1998) and this finding has later been followed by identification of the more common S18Y variant (Lincoln et al., 1999), which is associated with a reduced risk of Parkinson’s disease (Maraganore et al., 1999; Zhang et al., 2000; Wintermeyer et al., 2000; Satoh and Kuroda, 2001; Momose et al., 2002; Wang et al., 2002; Elbaz et al., 2003; Carmine et al., 2007). However, the finding of an inverse association of S18Y with Parkinson’s disease has also been questioned since contradictory results or lack of association has been reported (Mellick and Silburn, 2000; Levecque et al., 2001; Savettieri et al., 2001). UCH-L1, also known as the neuronal marker PGP9.5, constitutes a key component of the ubiquitin-proteasome system, removing abnormal and misfolded proteins, and generating free ubiquitin monomers (Wilkinson et al., 1989). The presence of variable sites in the UCH-L1 gene may result in a dysfunctional protein and subsequent aggregation of misfolded proteins. UCH-L1 shows high and specific expression in all central and peripheral neurons (Doran et al., 1983). It comprises up to 2% of the total soluble brain proteins (Wilkinson et al., 1989) and hence, it is one of the most abundant proteins in the brain. It has also been identified as one of the components of the proteinaceous inclusion bodies in the remaining neurons in substantia nigra of Parkinson patients (Lowe et al., 1990).
3.4.3. Leucine-rich repeat kinase 2 (PARK8)
A large number of genetic variants (most of them missense mutations) have been found in the 51 exon long leucine-rich repeat kinase 2 (LRRK2) gene. Among the variable sites, seven have been shown to be pathogenic, and they are all located in protein domains of high functional importance (Lesage and Brice, 2009). Together, the LRRK2 mutations constitute the most common known genetic cause of Parkinson’s disease identified to date, accounting for up to 10% of the familial Parkinson cases with autosomal dominant inheritance and 3.6% of the sporadic cases. The LRRK2 gene is located at the PARK8 locus on chromosome 12p11.2-q13.1 (Funayama et al., 2002) which is linked to autosomal dominant Parkinson’s disease (Paisan-Ruiz et al., 2004; Zimprich et al., 2004). Since the discovery of LRRK2 mutations in Basque Parkinson families, the gene and its variable sites have been extensively studied. The G2019S mutation alone is responsible for approximately 1–2% of the “sporadic” Parkinson cases and 2–6% of all familial Parkinson cases. However in certain populations, like Ashkenazi Jews and North African Arabs, mutation frequencies up to 30–40% have been reported (Lesage et al., 2006, 2008; Ozelius et al., 2006; Ishihara et al., 2007; Orr-Urtreger et al., 2007; Hulihan et al., 2008). Studies have also revealed presence of the G2019S mutation in healthy control individuals (Lesage et al., 2005, 2006; Farrer et al., 2005; Kay et al., 2005; Carmine et al., 2006; Clark et al., 2006; Ozelius et al., 2006; Change et al., 2008) as well as in other neurological disorders (Chen-Plotkin et al., 2008). The penetrance of the G2019S mutation varies with ethnicity, and it has been estimated by the International LRRK2 Consortium to be around 28% at the age of 59 and 74% at the age of 79 (Healy et al., 2008). Moreover, the G2019S mutation is believed to have evolved from a few common founders (Zabetian et al., 2006a, b). Another common LRRK2 variant, the G2385R polymorphism, has been found to be present in 10% of Asian Parkinson cases but only 4% of matched control individuals. Although a significant risk factor among Asians, this particular polymorphism is rare or even absent in most other populations (Berg et al., 2005; Di Fonzo et al., 2006; Tan et al., 2008) and previous large scale whole-genome association studies have failed to identify G2385R as a risk factor for Parkinson’s disease. Some missense mutations in LRRK2 have been associated with an increased kinase activity (West et al., 2005; Gloeckner et al., 2006) as well as with generation of inclusion bodies and cell death in vitro, whereas mutations eliminating the kinase activity, have been found to inhibit formation of aggregates (Greggio et al., 2006). Patients carrying mutations in LRRK2 exhibit typically late onset, L-dopa-responsive Parkinson’s disease, although somewhat surprisingly with varying pathology between, and even within families, for example presence or absence of Lewy bodies. The LRRK2 protein, also known as dardarin, consists of several domains including ARM (Armadillo), ANK (Ankyrin repeat), a leucine-rich repeat (LRR), Roc, COR (C-terminal of Roc), MAPKKK (mitogen-activated protein kinase kinase kinase) and WD40 repeats (Zimprich et al., 2004; Lesage and Brice, 2009). LRRK2 has been suggested to be implicated in apoptosis, regulation of neuronal survival, maintenance of neurites and protein–protein interactions. LRRK2 is localized to membranous and vesicular structures such as mitochondria, vesicles, lysosomes and endosomes (Biskup et al., 2006). Using in situ hybridization to localize transcriptional activity at the cellular level, it is striking to find that none of the many genes so far linked or associated to Parkinson’s disease is specifically expressed in dopamine neurons, as compared to other cells in the brain or elsewhere. LRRK2 is not an exception, although it does differ from other “Parkinson’s disease genes” by being markedly, but not exclusively, expressed in the striatal dopamine target area (Galter et al., 2006).
3.5. Autosomal recessive PARK genes
3.5.1. Parkin (PARK2)
The PRKN gene encoding the Parkin protein harbors a number of genetic variants including insertions, deletions and point mutations and these variable sites have been identified in populations of all ethnic origins. Mutations in the PRKN gene constitute the most common cause of early onset Parkinson’s disease, responsible for up to 50% of the cases. The number of mutations in this gene is inversely associated with disease onset and mutations are thus rare in patients with late onset Parkinson’s disease. Patients with PRKN mutations exhibit a clinical phenotype resembling sporadic disease. Interestingly, neuropathological findings from patients carrying mutations in PRKN, rarely show presence of Lewy bodies, although nigrostriatal cell loss may occur. The PRKN gene is located at the PARK2 locus, mapped to chromosome 6q25.2-q27 and causes an autosomal recessive early onset form of Parkinson’s disease (Kitada et al., 1998). The function of the ubiquitin E3 ligase encoded by the PRKN gene is to add ubiquitin onto specific substrates, thereby targeting them for proteasomal degradation. It has been suggested that loss-of-function mutations in the gene can cause abnormal accumulation of Parkin substrates, such as α-synuclein and synphilin-1. The important role of Parkin in the ubiquitin proteasome pathway strengthens the involvement of protein degradation and aggregation as a major causative event of Parkinson’s disease.
3.5.2. PINK1 (PARK6)
Mutations in the tumor suppressor PTEN induced putative kinase 1 (PINK1) gene causes autosomal recessive early onset Parkinson’s disease (<50 years) and a typical parkinsonian phenotype. PINK1 is localized to the PARK6 locus on chromosome 1p35-36, originally mapped in European Parkinson’s disease families by Valente and coworkers in 2004 (Valente et al., 2001). In addition to the familial cases carrying mutations in PINK1, mutations have also been found in rare, possibly sporadic early onset cases (Valente et al., 2004b). Findings from genetic studies have identified several mutations in PINK1, distributed throughout the gene, in populations of different geographical origins including Europe, Asia and North America. The protein encoded by the PINK1 gene is a serine-threonine kinase which has been suggested to be protective against stress induced by mitochondrial dysfunction (Valente et al., 2004a). Moreover, PINK1 has a mitochondrial targeting motif which makes it prone to accumulation in the mitochondrial inter-membranous space.
3.5.3. DJ-1 (PARK7)
Genetic variability in the DJ-1 gene, located on chromosome 1p36 at the PARK7 locus, causes autosomal recessive early onset parkinsonism (van Duijn et al., 2001). However, mutations in the gene are rare, accounting for only 1% of the early onset cases (Abou-Sleiman et al., 2003; Lockhart et al., 2004). Besides the characteristic early onset, patients carrying mutations in DJ-1 display a slowly progressing disease with good response to levodopa. A genetic link between DJ-1 and Parkinson’s disease was first presented by Bonifati and colleagues, who found a missense mutation (L166P) and a homozygous deletion of exons 1–5 in the gene (Bonifati et al., 2003). Presence of the L166P mutation causes altered protein folding properties (Olzmann et al., 2004), resulting in a less stable protein (Miller et al., 2003) along with decreased protein levels (Lockhart et al., 2004). Further genetic screening of DJ-1 has resulted in identification of several variable sites containing deletions, missense and nonsense mutations (Abou-Sleiman et al., 2003; Hague et al., 2003; Hedrich et al., 2004).
DJ-1 has been suggested to be a sensor of oxidative stress since it shifts its isoelectric point to a more acidic form following oxidative stress (Mitsumoto and Nakagawa, 2001; Bandopadhyay et al., 2004). In agreement with this, wild-type DJ-1 can reduce the motor abnormalities and dopamine neuron death caused by 6-OHDA in rats (Inden et al., 2006). Moreover, involvements of DJ-1 in apoptosis, protein folding, chaperone activity and transcriptional regulation have also been proposed. The DJ-1 gene is conserved across species and is present in nervous as well as in peripheral tissues. The protein is localized to the nucleus and cytoplasm (Nagakubo et al., 1997; Zhang et al., 2005), but it relocalizes to mitochondria under oxidizing conditions (Canet-Aviles et al., 2004; Blackinton et al., 2005).
3.5.4. ATP13A2 (PARK9)
Mutations in the ATPase type 13A2 gene on the PARK9 locus on chromosome 1p36 (Hampshire et al., 2001) have been linked to autosomal recessive parkinsonism in families with Kufor-Rakeb syndrome (Najim al-Din et al., 1994; Hampshire et al., 2001). The patients have juvenile onset atypical parkinsonism, accompanied by pyramidal cell degeneration and cognitive dysfunctions, features not commonly seen in Parkinson’s disease. More recently, genetic variants in ATP13A2 were associated with a more typical early-onset parkinsonism in Brazil and Italy (Di Fonzo et al., 2007).
3.6. Genetic risk factors
PARK genes may harbor less severe mutations that do not per se cause disease, but merely increase risk. For instance, this appears to be the case for LRRK2. GBA, glucocerebrosidase originally implicated in Gaucher’s disease, may constitute risk of both familial and sporadic Parkinson’s disease (see Lees et al., 2009). Current data suggest mutations in GBA may constitute a relatively common cause/risk factor. Located at 1q21, GBA encodes a lysosomal protein which cleaves the beta-glucosidic linkage of glycosylceramide, an intermediate formed during glycolipid metabolism. In addition, variants in a large number of other non-PARK genes have been associated with increased risk to develop Parkinson’s disease in case–control studies. These genes are too many to be dealt with individually in this review. However, the evidence from the hypothesis-driven association studies, the linkage studies and the emerging whole genome-wide studies taken together provides a strong case for genetic factors as the dominating cause of Parkinson’s disease.
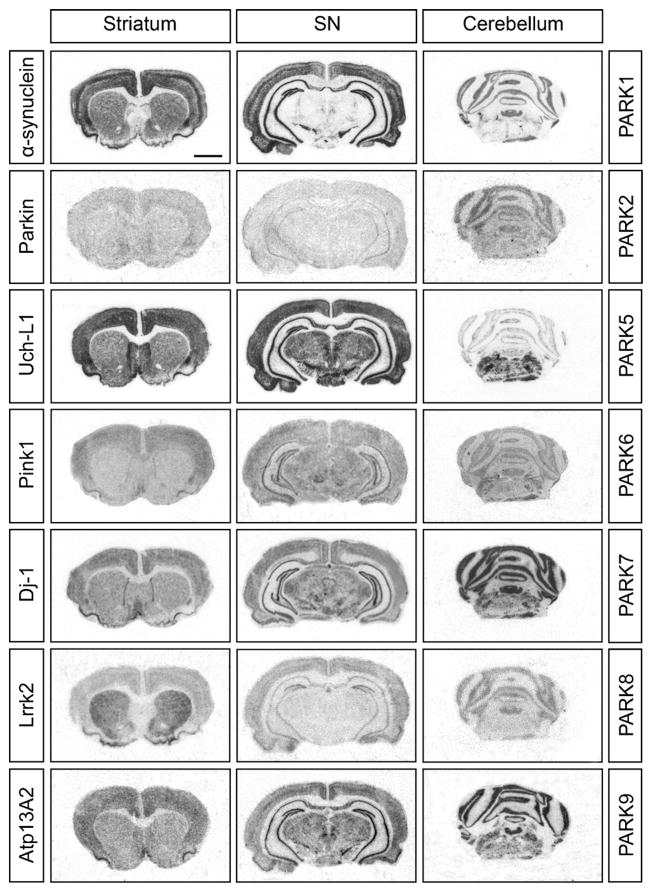
The large and increasing number of genes in which mutations have been found that increase, or sometimes decrease (e.g. UCH-L1) risk to develop Parkinson’s disease is bewildering. However, it is possible to group the implicated genes. Several are important for mitochondrial function, sometimes assumed to participate in the same metabolic pathways, other genes are important for detoxification and/or protection against oxidative stress. Yet others are involved in protein degradation, proteasome or lysosomal functions. It is striking that none of the genes implicated to date is specifically expressed in dopamine neurons (see Fig. 1). Instead, many of the implicated genes have rather general neuronal or cellular functions in and outside of the brain, suggesting that dopamine neurons are more susceptible to stress than other neurons, and, by the same token, explaining why many other neuron systems are eventually damaged in Parkinson’s disease. Clearly, from an etiologic standpoint, “Parkinson’s disease” is de facto the definition of several different types of neurodegenerative diseases.
Fig. 1.
mRNA expression patterns in the rat brain (at the level of striatum, substantia nigra/hippocampus and cerebellum) of the PARK genes α-synuclein/SNCA (PARK1), Parkin/PRKN (PARK2), Uch-L1 (PARK5), Pink1 (PARK6), Dj-1 (PARK7), Lrrk2 (PARK8) and Atp13a2 (PARK9) revealed by in situ hybridization and radioactively labeled oligo probes. All PARK genes presented in the figure have been implicated in the pathogenesis of Parkinson’s disease. Despite being involved in a common disease, they show variable levels and patterns of expression. Uch-L1 for instance is expressed by all neurons, α-synuclein/SNCA show high expression in substantia nigra, striatum and cortical areas whereas Lrrk2 shows particularly high expression in striatum, the target area of dopamine neurons. Interestingly none of the candidate genes shows a restricted expression in the dopamine system only.
3.7. Transgenic animal models
Based on the findings that mutations in human genes can cause Parkinson’s disease, a number of animal models have been developed in attempts to mimic the characteristic features of the disease (see Terzioglu and Galter, 2008). Like drug-induced models, these genetically modified animal models have contributed to the understanding of the disease. Following the identification of α-synuclein as a cause of Parkinson’s disease in the 1990s, several transgenic mouse models based on this gene have been developed, including mice over-expressing human α-synuclein, mice carrying the point mutations found in familial Parkinson’s disease (A30P and/or A53T) and mice which are null mutants for the gene. Since the α-synuclein gene dose appears critical in Parkinson’s disease, a number of α-synuclein over-expressing lines have also been generated. The mice show varying degrees of behavioral and pathological disturbances, and the most pronounced phenotypes are observed in the high expressing lines. However, only a few of the over-expressing lines show alterations in the nigrostriatal pathway that worsen with age (Masliah et al., 2000; Richfield et al., 2002; Rockenstein et al., 2002). Interestingly, α-synuclein knock-out mice have reduced striatal dopamine levels (Abeliovich et al., 2000) and they show decreased rearing and a reduced reserve vesicle pool (Cabin et al., 2002). The latter is in line with the suggested involvement of α-synuclein in maintenance of synaptic vesicles. Moreover, α-synuclein null mutant mice show resistance to MPTP exposure (Dauer et al., 2002; Schluter et al., 2003; Drolet et al., 2004).
Identification of LRRK2 mutations as the most common cause of Parkinson’s disease has lead to a high demand for LRRK2 transgenic mouse models, which are currently being developed. A desirable model would carry mutations in functionally important domains, like for instance the Roc and kinase domains, or in other sites known to be mutated in Parkinson patients.
Parkin knock-out mice have been generated by deleting different exons in the gene, resulting in a loss of Parkin function (Goldberg et al., 2003; Itier et al., 2003; von Coelln et al., 2004; Perez and Palmiter, 2005). The mouse lines have variable phenotypes with only modest behavioral effects, and none of the lines show loss of nigrostriatal neurons. However, Parkin knock-out mice do show changes in striatal dopamine release and synaptic dysfunction making them suitable for studying the early phases of Parkinson’s disease.
Studies of DJ-1 transgenic mice are useful for understanding sporadic forms of Parkinson’s disease. Targeted deletion of exon 2 or insertion of a truncating mutation in exon 1 of the DJ-1 gene, results in reduced spontaneous or drug-induced locomotor activity in mice (Kim et al., 2005; Goldberg et al., 2005). As is the case for Parkin mice, DJ-1 knock-out mice also fail to show loss of nigrostriatal dopamine neurons. Another mouse model based on the linkage of a recessively inherited PARK gene with Parkinson’s disease, is the PINK1 knock-out mouse which shows a decrease in evoked dopamine release in striatum and deficits in corticostriatal plasticity (Kitada et al., 2007).
Recently, conditional knock-out models of Parkinson’s disease were generated, in which a gene of interest is flanked by two LoxP sites for recombination. By breeding mice carrying a floxed gene on each allele, with a mouse expressing Cre-recombinase under a particular promoter, the gene of interest can be conditionally knocked out. In MitoPark mice, one such example, the mitochondrial transcription factor A (TFAM) has been selectively deleted in dopamine neurons. The animals are generated by crossing TFAM floxed mice with mice expressing Cre-recombinase under the DAT promoter (Ekstrand et al., 2007). TFAM is a nuclear encoded protein which is essential for transcription and replication of mitochondrial DNA (Kang et al., 2007), which encodes some of the subunits of the mitochondrial respiratory chain. Loss of TFAM activity in MitoPark mice thus results in impaired oxidative phosphorylation specifically in dopamine neurons. Interestingly, these mice show several parkinsonian features including a progressive loss of dopamine neurons in substantia nigra, reduced striatal dopamine levels, reduced locomotor activity and formation of intracellular aggregates, and the mice die prematurely (Ekstrand et al., 2007).
4. Concluding remarks
In recent years, genetic risk factors have become increasingly important in the search for possible causes of Parkinson’s disease. Genetic variations are present at 0.1% of the human genome, and these differences determine not only properties, but also susceptibility to disease. Mutation frequencies may vary considerably between populations. A factor found to be linked to, or associated with disease in one geographically or genetically confined family or population, can be present at another frequency or completely absent in another sample set. These population-specific differences make identification of genetic risk factors complex, and they also point at the importance of mapping genetically diverse materials. Characterization of genes and their variable sites is essential for understanding the pathways in which they are involved and also for identifying their interacting moieties. The heterogenic nature of Parkinson’s disease with regard to both age of onset, symptoms and pathology is compatible with an involvement of multiple genes and pathways rather than a single gene or mutation.
A desirable outcome of genetic studies is identification of candidate genes which can be used as biomarkers for early and reliable diagnosis. However, identification of new genetic markers may also lead to the demand for individual genetic testing of healthy relatives at risk. Genetic testing of healthy individuals is controversial and requires cautious considerations of potential risks. Another important goal of genetic studies would be identification of new drug targets for improved therapy with fewer side effects. While the pharmacological and surgical treatments used in Parkinson’s disease today are effective in improving motor complications, they have limited or no effects on depression, hallucinations, dementia or the autonomic and sleep disturbances seen in Parkinson patients. Importantly, there is currently no treatment that stops the progressive loss of dopamine neurons or modifies the rate of progression. Extended knowledge about disease-causing genes may aid in finding pathogenic mechanisms, suitable for therapeutic intervention.
Acknowledgments
Research reviewed in this article was supported by The Swedish Research Council, Swedish Brain Power, The Swedish Brain Foundation, The Swedish Parkinson Foundation, The Michael J. Fox Foundation, Karolinska Institutet, The Magnus Bergvall Foundation, and The National Institute on Drug Abuse, National Institutes of Health, USA.
Abbreviations
- ATP13A2
ATPase type 13A2
- LRRK2
leucine-rich repeat kinase 2
- PINK1
PTEN induced putative kinase 1
- SNCA
synuclein alpha
- TFAM
mitochondrial transcription factor A
- UCH-L1
ubiquitin C-terminal esterase L1
References
- Abeliovich A, Schmitz Y, Farinas I, Choi-Lundberg D, Ho WH, Castillo PE, Shinsky N, Verdugo JM, Armanini M, Ryan A, Hynes M, Phillips H, Sulzer D, Rosenthal A. Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 2000;25:239–252. doi: 10.1016/s0896-6273(00)80886-7. [DOI] [PubMed] [Google Scholar]
- Abou-Sleiman PM, Healy DG, Quinn N, Lees AJ, Wood NW. The role of pathogenic DJ-1 mutations in Parkinson’s disease. Ann Neurol. 2003;54:283–286. doi: 10.1002/ana.10675. [DOI] [PubMed] [Google Scholar]
- Ahn TB, Kim SY, Kim JY, Park SS, Lee DS, Min HJ, Kim YK, Kim SE, Kim JM, Kim HJ, Cho J, Jeon BS. alpha-Synuclein gene duplication is present in sporadic Parkinson disease. Neurology. 2008;70:43–49. doi: 10.1212/01.wnl.0000271080.53272.c7. [DOI] [PubMed] [Google Scholar]
- Allen W. Inheritance of the shaking palsy. Arch Int Med. 1937;60:424–436. [Google Scholar]
- Bandopadhyay R, Kingsbury AE, Cookson MR, Reid AR, Evans IM, Hope AD, Pittman AM, Lashley T, Canet-Aviles R, Miller DW, McLendon C, Strand C, Leonard AJ, Abou-Sleiman PM, Healy DG, Ariga H, Wood NW, de Silva R, Revesz T, Hardy JA, Lees AJ. The expression of DJ-1 (PARK7) in normal human CNS and idiopathic Parkinson’s disease. Brain. 2004;127:420–430. doi: 10.1093/brain/awh054. [DOI] [PubMed] [Google Scholar]
- Berg D, Schweitzer K, Leitner P, Zimprich A, Lichtner P, Belcredi P, Brussel T, Schulte C, Maass S, Nagele T. Type and frequency of mutations in the LRRK2 gene in familial and sporadic Parkinson’s disease. Brain. 2005;128:3000–3011. doi: 10.1093/brain/awh666. [DOI] [PubMed] [Google Scholar]
- Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT. Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nature Neurosci. 2000;3:1301–1306. doi: 10.1038/81834. [DOI] [PubMed] [Google Scholar]
- Betarbet R, Sherer TB, Greenamyre JT. Animal models of Parkinson’s disease. Bioessays. 2002;24:308–318. doi: 10.1002/bies.10067. [DOI] [PubMed] [Google Scholar]
- Biskup S, Moore DJ, Celsi F, Higashi S, West AB, Andrabi SA, Kurkinen K, Yu SW, Savitt JM, Waldvogel HJ, Faull RL, Emson PC, Torp R, Ottersen OP, Dawson TM, Dawson VL. Localization of LRRK2 to membranous and vesicular structures in mammalian brain. Ann Neurol. 2006;60:557–569. doi: 10.1002/ana.21019. [DOI] [PubMed] [Google Scholar]
- Blackinton J, Ahmad R, Miller DW, van der Brug MP, Canet-Aviles RM, Hague SM, Kaleem M, Cookson MR. Effects of DJ-1 mutations and polymorphisms on protein stability and subcellular localization. Brain Res Mol Brain Res. 2005;134:76–83. doi: 10.1016/j.molbrainres.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Blum D, Torch S, Lambeng N, Nissou M, Benabid AL, Sadoul R, Verna JM. Molecular pathways involved in the neurotoxicity of 6-OHDA, dopamine and MPTP: contribution to the apoptotic theory in Parkinson’s disease. Prog Neurobiol. 2001;65:135–172. doi: 10.1016/s0301-0082(01)00003-x. [DOI] [PubMed] [Google Scholar]
- Bonifati V. Parkinson’s disease: the LRRK2-G2019S mutation: opening a novel era in Parkinson’s disease genetics. Eur J Hum Genet. 2006;14:1061–1062. doi: 10.1038/sj.ejhg.5201695. [DOI] [PubMed] [Google Scholar]
- Bonifati V, Rizzu P, van Baren MJ, Schaap O, Breedveld GJ, Krieger E, Dekker MC, Squitieri F, Ibanez P, Joosse M, van Dongen JW, Vanacore N, van Swieten JC, Brice A, Meco G, van Duijn CM, Oostra BA, Heutink P. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science. 2003;299:256–259. doi: 10.1126/science.1077209. [DOI] [PubMed] [Google Scholar]
- Braak H, Ghebremedhin E, Rub U, Bratzke H, Del TK. Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res. 2004;318:121–134. doi: 10.1007/s00441-004-0956-9. [DOI] [PubMed] [Google Scholar]
- Cabin DE, Shimazu K, Murphy D, Cole NB, Gottschalk W, McIlwain KL, Orrison B, Chen A, Ellis CE, Paylor R, Lu B, Nussbaum RL. Synaptic vesicle depletion correlates with attenuated synaptic responses to prolonged repetitive stimulation in mice lacking alpha-synuclein. J Neurosci. 2002;22:8797–8807. doi: 10.1523/JNEUROSCI.22-20-08797.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canet-Aviles RM, Wilson MA, Miller DW, Ahmad R, McLendon C, Bandyopadhyay S, Baptista MJ, Ringe D, Petsko GA, Cookson MR. The Parkinson’s disease protein DJ-1 is neuroprotective due to cysteine-sulfinic acid-driven mitochondrial localization. Proc Natl Acad Sci USA. 2004;101:9103–9108. doi: 10.1073/pnas.0402959101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmine BA, Westerlund M, Bergman O, Nissbrandt H, Lind C, Sydow O, Galter D. S18Y in ubiquitin carboxy-terminal hydrolase L1 (UCH-L1) associated with decreased risk of Parkinson’s disease in Sweden. Parkinsonism Relat Disord. 2007;13:295–298. doi: 10.1016/j.parkreldis.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Carmine BA, Westerlund M, Sydow O, Lundströmer K, Håkansson A, Niss-brandt H, Olson L, Galter D. Leucine-rich repeat kinase 2 (LRRK2) mutations in a Swedish Parkinson cohort and a healthy nonagenarian. Mov Disord. 2006;21:1731–1734. doi: 10.1002/mds.21016. [DOI] [PubMed] [Google Scholar]
- Cenci MA, Whishaw IQ, Schallert T. Animal models of neurological deficits: how relevant is the rat? Nat Rev Neurosci. 2002;3:574–579. doi: 10.1038/nrn877. [DOI] [PubMed] [Google Scholar]
- Chandra S, Fornai F, Kwon HB, Yazdani U, Atasoy D, Liu X, Hammer RE, Battaglia G, German DC, Castillo PE, Sudhof TC. Double-knockout mice for alpha- and beta-synucleins: effect on synaptic functions. Proc Natl Acad Sci USA. 2004;101:14966–14971. doi: 10.1073/pnas.0406283101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Change N, Mercier G, Lucotte G. Genetic screening of the G2019S mutation of the LRRK2 gene in Southwest European, North African, and Sephardic Jewish subjects. Genet Test. 2008;12:333–339. doi: 10.1089/gte.2007.0098. [DOI] [PubMed] [Google Scholar]
- Chartier-Harlin MC, Kachergus J, Roumier C, Mouroux V, Douay X, Lincoln S, Levecque C, Larvor L, Andrieux J, Hulihan M, Waucquier N, Defebvre L, Amouyel P, Farrer M, Destee A. Alpha-synuclein locus duplication as a cause of familial Parkinson’s disease. Lancet. 2004;364:1167–1169. doi: 10.1016/S0140-6736(04)17103-1. [DOI] [PubMed] [Google Scholar]
- Chen-Plotkin AS, Yuan W, Anderson C, McCarty WE, Hurtig HI, Clark CM, Miller BL, Lee VM, Trojanowski JQ, Grossman M, Van DV. Corticobasal syndrome and primary progressive aphasia as manifestations of LRRK2 gene mutations. Neurology. 2008;70:521–527. doi: 10.1212/01.WNL.0000280574.17166.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark LN, Wang Y, Karlins E, Saito L, Mejia-Santana H, Harris J, Louis ED, Cote LJ, Andrews H, Fahn S, Waters C, Ford B, Frucht S, Ottman R, Marder K. Frequency of LRRK2 mutations in early- and late-onset Parkinson disease. Neurology. 2006;67:1786–1791. doi: 10.1212/01.wnl.0000244345.49809.36. [DOI] [PubMed] [Google Scholar]
- Damodaran M, Ramaswamy R. Isolation of L-3:4-dihydroxyphenylalanine from the seeds of Mucuna pruriens. Biochem J. 1937;31:2149–2152. doi: 10.1042/bj0312149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauer W, Kholodilov N, Vila M, Trillat AC, Goodchild R, Larsen KE, Staal R, Tieu K, Schmitz Y, Yuan CA, Rocha M, Jackson-Lewis V, Hersch S, Sulzer D, Przedborski S, Burke R, Hen R. Resistance of alpha-synuclein null mice to the parkinsonian neurotoxin MPTP. Proc Natl Acad Sci USA. 2002;99:14524–14529. doi: 10.1073/pnas.172514599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawn TM, Barrett JH. Genetic linkage studies. Lancet. 2005;366:1036–1044. doi: 10.1016/S0140-6736(05)67382-5. [DOI] [PubMed] [Google Scholar]
- Di Fonzo A, Chien HF, Socal M, Giraudo S, Tassorelli C, Iliceto G, Fabbrini G, Marconi R, Fincati E, Abbruzzese G, Marini P, Squitieri F, Horstink MW, Montagna P, Libera AD, Stocchi F, Goldwurm S, Ferreira JJ, Meco G, Martignoni E, Lopiano L, Jardim LB, Oostra BA, Barbosa ER, Bonifati V. ATP13A2 missense mutations in juvenile parkinsonism and young onset Parkinson disease. Neurology. 2007;68:1557–1562. doi: 10.1212/01.wnl.0000260963.08711.08. [DOI] [PubMed] [Google Scholar]
- Di Fonzo A, Tassorelli C, De MM, Chien HF, Ferreira J, Rohe CF, Riboldazzi G, Antonini A, Albani G, Mauro A, Marconi R, Abbruzzese G, Lopiano L, Fincati E, Guidi M, Marini P, Stocchi F, Onofrj M, Toni V, Tinazzi M, Fabbrini G, Lamberti P, Vanacore N, Meco G, Leitner P, Uitti RJ, Wszolek ZK, Gasser T, Simons EJ, Breedveld GJ, Goldwurm S, Pezzoli G, Sampaio C, Barbosa E, Martignoni E, Oostra BA, Bonifati V. Comprehensive analysis of the LRRK2 gene in sixty families with Parkinson’s disease. Eur J Hum Genet. 2006;14:322–331. doi: 10.1038/sj.ejhg.5201539. [DOI] [PubMed] [Google Scholar]
- Doran JF, Jackson P, Kynoch PA, Thompson RJ. Isolation of PGP 9.5, a new human neurone-specific protein detected by high-resolution two-dimensional electrophoresis. J Neurochem. 1983;40:1542–1547. doi: 10.1111/j.1471-4159.1983.tb08124.x. [DOI] [PubMed] [Google Scholar]
- Drolet RE, Behrouz B, Lookingland KJ, Goudreau JL. Mice lacking alpha-synuclein have an attenuated loss of striatal dopamine following prolonged chronic MPTP administration. Neurotoxicology. 2004;25:761–769. doi: 10.1016/j.neuro.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Ekstrand MI, Terzioglu M, Galter D, Zhu S, Hofstetter C, Lindqvist E, Thams S, Bergstrand A, Hansson FS, Trifunovic A, Hoffer B, Cullheim S, Mohammed AH, Olson L, Larsson NG. Progressive parkinsonism in mice with respiratory-chain-deficient dopamine neurons. Proc Natl Acad Sci USA. 2007;104:1325–1330. doi: 10.1073/pnas.0605208103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbaz A, Levecque C, Clavel J, Vidal JS, Richard F, Correze JR, Delemotte B, Amouyel P, Alperovitch A, Chartier-Harlin MC, Tzourio C. S18Y polymorphism in the UCH-L1 gene and Parkinson’s disease: evidence for an age-dependent relationship. Mov Disord. 2003;18:130–137. doi: 10.1002/mds.10326. [DOI] [PubMed] [Google Scholar]
- Farrer M, Kachergus J, Forno L, Lincoln S, Wang DS, Hulihan M, Maraganore D, Gwinn-Hardy K, Wszolek Z, Dickson D, Langston JW. Comparison of kindreds with parkinsonism and alpha-synuclein genomic multiplications. Ann Neurol. 2004;55:174–179. doi: 10.1002/ana.10846. [DOI] [PubMed] [Google Scholar]
- Farrer M, Stone J, Mata IF, Lincoln S, Kachergus J, Hulihan M, Strain KJ, Maraganore DM. LRRK2 mutations in Parkinson disease. Neurology. 2005;65:738–740. doi: 10.1212/01.wnl.0000169023.51764.b0. [DOI] [PubMed] [Google Scholar]
- Farrer MJ. Genetics of Parkinson disease: paradigm shifts and future prospects. Nat Rev Genet. 2006;7:306–318. doi: 10.1038/nrg1831. [DOI] [PubMed] [Google Scholar]
- Fuchs J, Nilsson C, Kachergus J, Munz M, Larsson EM, Schule B, Langston JW, Middleton FA, Ross OA, Hulihan M, Gasser T, Farrer MJ. Phenotypic variation in a large Swedish pedigree due to SNCA duplication and triplication. Neurology. 2007;68:916–922. doi: 10.1212/01.wnl.0000254458.17630.c5. [DOI] [PubMed] [Google Scholar]
- Funayama M, Hasegawa K, Kowa H, Saito M, Tsuji S, Obata F. A new locus for Parkinson’s disease (PARK8) maps to chromosome 12p11.2-q13.1. Ann Neurol. 2002;51:296–301. doi: 10.1002/ana.10113. [DOI] [PubMed] [Google Scholar]
- Galter D, Westerlund M, Carmine A, Lindqvist E, Sydow O, Olson L. LRRK2 expression linked to dopamine-innervated areas. Ann Neurol. 2006;59:714–719. doi: 10.1002/ana.20808. [DOI] [PubMed] [Google Scholar]
- Gloeckner CJ, Kinkl N, Schumacher A, Braun RJ, O’Neill E, Meitinger T, Kolch W, Prokisch H, Ueffing M. The Parkinson disease causing LRRK2 mutation I2020T is associated with increased kinase activity. Hum Mol Genet. 2006;15:223–232. doi: 10.1093/hmg/ddi439. [DOI] [PubMed] [Google Scholar]
- Goedert M. Alpha-synuclein and neurodegenerative diseases. Nat Rev Neurosci. 2001;2:492–501. doi: 10.1038/35081564. [DOI] [PubMed] [Google Scholar]
- Goldberg MS, Fleming SM, Palacino JJ, Cepeda C, Lam HA, Bhatnagar A, Meloni EG, Wu N, Ackerson LC, Klapstein GJ, Gajendiran M, Roth BL, Chesselet MF, Maidment NT, Levine MS, Shen J. Parkin-deficient mice exhibit nigrostriatal deficits but not loss of dopaminergic neurons. J Biol Chem. 2003;278:43628–43635. doi: 10.1074/jbc.M308947200. [DOI] [PubMed] [Google Scholar]
- Goldberg MS, Pisani A, Haburcak M, Vortherms TA, Kitada T, Costa C, Tong Y, Martella G, Tscherter A, Martins A, Bernardi G, Roth BL, Pothos EN, Calabresi P, Shen J. Nigrostriatal dopaminergic deficits and hypokinesia caused by inactivation of the familial parkinsonism-linked gene DJ-1. Neuron. 2005;45:489–496. doi: 10.1016/j.neuron.2005.01.041. [DOI] [PubMed] [Google Scholar]
- Greggio E, Jain S, Kingsbury A, Bandopadhyay R, Lewis P, Kaganovich A, van der Brug MP, Beilina A, Blackinton J, Thomas KJ, Ahmad R, Miller DW, Kesavapany S, Singleton A, Lees A, Harvey RJ, Harvey K, Cookson MR. Kinase activity is required for the toxic effects of mutant LRRK2/dardarin. Neurobiol Dis. 2006;23:329–341. doi: 10.1016/j.nbd.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Hague S, Rogaeva E, Hernandez D, Gulick C, Singleton A, Hanson M, Johnson J, Weiser R, Gallardo M, Ravina B, Gwinn-Hardy K, Crawley A, PHSGH, Lang AE, Heutink P, Bonifati V, Hardy J, Singleton A. Early-onset Parkinson’s disease caused by a compound heterozygous DJ-1 mutation. Ann Neurol. 2003;54:271–274. doi: 10.1002/ana.10663. [DOI] [PubMed] [Google Scholar]
- Hampshire DJ, Roberts E, Crow Y, Bond J, Mubaidin A, Wriekat AL, Al-Din A, Woods CG. Kufor-Rakeb syndrome, pallido-pyramidal degeneration with supranuclear upgaze paresis and dementia, maps to 1p36. J Med Genet. 2001;38:680–682. doi: 10.1136/jmg.38.10.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healy DG, Falchi M, O’Sullivan SS, Bonifati V, Durr A, Bressman S, Brice A, Aasly J, Zabetian CP, Goldwurm S, Ferreira JJ, Tolosa E, Kay DM, Klein C, Williams DR, Marras C, Lang AE, Wszolek ZK, Berciano J, Schapira AH, Lynch T, Bhatia KP, Gasser T, Lees AJ, Wood NW. Phenotype, genotype, and worldwide genetic penetrance of LRRK2-associated Parkinson’s disease: a case–control study. Lancet Neurol. 2008;7:583–590. doi: 10.1016/S1474-4422(08)70117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrich K, Djarmati A, Schafer N, Hering R, Wellenbrock C, Weiss PH, Hilker R, Vieregge P, Ozelius LJ, Heutink P, Bonifati V, Schwinger E, Lang AE, Noth J, Bressman SB, Pramstaller PP, Riess O, Klein C. DJ-1 (PARK7) mutations are less frequent than Parkin (PARK2) mutations in early-onset Parkinson disease. Neurology. 2004;62:389–394. doi: 10.1212/01.wnl.0000113022.51739.88. [DOI] [PubMed] [Google Scholar]
- Hefti F, Melamed E, Wurtman RJ. Partial lesions of the dopaminergic nigrostriatal system in rat brain: biochemical characterization. Brain Res. 1980;195:123–137. doi: 10.1016/0006-8993(80)90871-9. [DOI] [PubMed] [Google Scholar]
- Hulihan MM, Ishihara-Paul L, Kachergus J, Warren L, Amouri R, Elango R, Prinjha RK, Upmanyu R, Kefi M, Zouari M, Sassi SB, Yahmed SB, El Euch-Fayeche G, Matthews PM, Middleton LT, Gibson RA, Hentati F, Farrer MJ. LRRK2 Gly2019Ser penetrance in Arab-Berber patients from Tunisia: a case–control genetic study. Lancet Neurol. 2008;7:591–594. doi: 10.1016/S1474-4422(08)70116-9. [DOI] [PubMed] [Google Scholar]
- Ibanez P, Bonnet AM, Debarges B, Lohmann E, Tison F, Pollak P, Agid Y, Durr A, Brice A. Causal relation between alpha-synuclein gene duplication and familial Parkinson’s disease. Lancet. 2004;364:1169–1171. doi: 10.1016/S0140-6736(04)17104-3. [DOI] [PubMed] [Google Scholar]
- Ikeuchi T, Kakita A, Shiga A, Kasuga K, Kaneko H, Tan CF, Idezuka J, Wakabayashi K, Onodera O, Iwatsubo T, Nishizawa M, Takahashi H, Ishikawa A. Patients homozygous and heterozygous for SNCA duplication in a family with parkinsonism and dementia. Arch Neurol. 2008;65:514–519. doi: 10.1001/archneur.65.4.514. [DOI] [PubMed] [Google Scholar]
- Inden M, Taira T, Kitamura Y, Yanagida T, Tsuchiya D, Takata K, Yanagisawa D, Nishimura K, Taniguchi T, Kiso Y, Yoshimoto K, Agatsuma T, Koide-Yoshida S, Iguchi-Ariga SM, Shimohama S, Ariga H. PARK7 DJ-1 protects against degeneration of nigral dopaminergic neurons in Parkinson’s disease rat model. Neurobiol Dis. 2006;24:144–158. doi: 10.1016/j.nbd.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Ishihara L, Gibson RA, Warren L, Amouri R, Lyons K, Wielinski C, Hunter C, Swartz JE, Elango R, Akkari PA, Leppert D, Surh L, Reeves KH, Thomas S, Ragone L, Hattori N, Pahwa R, Jankovic J, Nance M, Freeman A, Gouider-Khouja N, Kefi M, Zouari M, Ben SS, Ben YS, El Euch-Fayeche G, Middleton L, Burn DJ, Watts RL, Hentati F. Screening for Lrrk2 G2019S and clinical comparison of Tunisian and North American Caucasian Parkinson’s disease families. Mov Disord. 2007;22:55–61. doi: 10.1002/mds.21180. [DOI] [PubMed] [Google Scholar]
- Itier JM, Ibanez P, Mena MA, Abbas N, Cohen-Salmon C, Bohme GA, Laville M, Pratt J, Corti O, Pradier L, Ret G, Joubert C, Periquet M, Araujo F, Negroni J, Casarejos MJ, Canals S, Solano R, Serrano A, Gallego E, Sanchez M, Denefle P, Benavides J, Tremp G, Rooney TA, Brice A, Garcia de YJ. Parkin gene inactivation alters behaviour and dopamine neurotransmission in the mouse. Hum Mol Genet. 2003;12:2277–2291. doi: 10.1093/hmg/ddg239. [DOI] [PubMed] [Google Scholar]
- Kang D, Kim SH, Hamasaki N. Mitochondrial transcription factor A (TFAM): roles in maintenance of mtDNA and cellular functions. Mitochondrion. 2007;7:39–44. doi: 10.1016/j.mito.2006.11.017. [DOI] [PubMed] [Google Scholar]
- Kay DM, Kramer P, Higgins D, Zabetian CP, Payami H. Escaping Parkinson’s disease: a neurologically healthy octogenarian with the LRRK2 G2019S mutation. Mov Disord. 2005;20:1077–1078. doi: 10.1002/mds.20618. [DOI] [PubMed] [Google Scholar]
- Ki CS, Stavrou EF, Davanos N, Lee WY, Chung EJ, Kim JY, Athanassiadou A. The Ala53Thr mutation in the alpha-synuclein gene in a Korean family with Parkinson disease. Clin Genet. 2007;71:471–473. doi: 10.1111/j.1399-0004.2007.00781.x. [DOI] [PubMed] [Google Scholar]
- Kim RH, Smith PD, Aleyasin H, Hayley S, Mount MP, Pownall S, Wakeham A, You-Ten AJ, Kalia SK, Horne P, Westaway D, Lozano AM, Anisman H, Park DS, Mak TW. Hypersensitivity of DJ-1-deficient mice to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyrindine (MPTP) and oxidative stress. Proc Natl Acad Sci USA. 2005;102:5215–5220. doi: 10.1073/pnas.0501282102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitada T, Asakawa S, Hattori N, Matsumine H, Yamamura Y, Minoshima S, Yokochi M, Mizuno Y, Shimizu N. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature. 1998;392:605–608. doi: 10.1038/33416. [DOI] [PubMed] [Google Scholar]
- Kitada T, Pisani A, Porter DR, Yamaguchi H, Tscherter A, Martella G, Bonsi P, Zhang C, Pothos EN, Shen J. Impaired dopamine release and synaptic plasticity in the striatum of PINK1-deficient mice. Proc Natl Acad Sci USA. 2007;104:11441–11446. doi: 10.1073/pnas.0702717104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordower JH, Brundin P. Lewy body pathology in long-term fetal nigral transplants: is Parkinson’s disease transmitted from one neural system to another? Neuropsychopharmacology. 2009;34:254. doi: 10.1038/npp.2008.161. [DOI] [PubMed] [Google Scholar]
- Kruger R, Kuhn W, Muller T, Woitalla D, Graeber M, Kosel S, Przuntek H, Epplen JT, Schols L, Riess O. Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson’s disease. Nat Genet. 1998;18:106–108. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- Langston JW, Ballard P, Tetrud JW, Irwin I. Chronic parkinsonism in humans due to a product of meperidine-analog synthesis. Science. 1983;219:979–980. doi: 10.1126/science.6823561. [DOI] [PubMed] [Google Scholar]
- Lees AJ, Hardy J, Revesz T. Parkinson’s disease. Lancet. 2009;373:2055–2066. doi: 10.1016/S0140-6736(09)60492-X. [DOI] [PubMed] [Google Scholar]
- Leroux PD. Thèse de Paris. Imprimeur de la Faculté de Médecine; 1880. Contribution à l’Étude des Causes de la Paralysie Agitante. [Google Scholar]
- Leroy E, Boyer R, Auburger G, Leube B, Ulm G, Mezey E, Harta G, Brownstein MJ, Jonnalagada S, Chernova T, Dehejia A, Lavedan C, Gasser T, Steinbach PJ, Wilkinson KD, Polymeropoulos MH. The ubiquitin pathway in Parkinson’s disease. Nature. 1998;395:451–452. doi: 10.1038/26652. [DOI] [PubMed] [Google Scholar]
- Lesage S, Belarbi S, Troiano A, Condroyer C, Hecham N, Pollak P, Lohman E, Benhassine T, Ysmail-Dahlouk F, Durr A, Tazir M, Brice A. Is the common LRRK2 G2019S mutation related to dyskinesias in North African Parkinson disease? Neurology. 2008;71:1550–1552. doi: 10.1212/01.wnl.0000338460.89796.06. [DOI] [PubMed] [Google Scholar]
- Lesage S, Brice A. Parkinson’s disease: from monogenic forms to genetic susceptibility factors. Hum Mol Genet. 2009;18:R48–R59. doi: 10.1093/hmg/ddp012. [DOI] [PubMed] [Google Scholar]
- Lesage S, Durr A, Tazir M, Lohmann E, Leutenegger AL, Janin S, Pollak P, Brice A. LRRK2 G2019S as a cause of Parkinson’s disease in North African Arabs. N Engl J Med. 2006;354:422–423. doi: 10.1056/NEJMc055540. [DOI] [PubMed] [Google Scholar]
- Lesage S, Ibanez P, Lohmann E, Pollak P, Tison F, Tazir M, Leutenegger AL, Guimaraes J, Bonnet AM, Agid Y, Durr A, Brice A. G2019S LRRK2 mutation in French and North African families with Parkinson’s disease. Ann Neurol. 2005;58:784–787. doi: 10.1002/ana.20636. [DOI] [PubMed] [Google Scholar]
- Levecque C, Destee A, Mouroux V, Becquet E, Defebvre L, Amouyel P, Chartier-Harlin MC. No genetic association of the ubiquitin carboxy-terminal hydrolase-L1 gene S18Y polymorphism with familial Parkinson’s disease. J Neural Transm. 2001;108:979–984. doi: 10.1007/s007020170017. [DOI] [PubMed] [Google Scholar]
- Lincoln S, Vaughan J, Wood N, Baker M, Adamson J, Gwinn-Hardy K, Lynch T, Hardy J, Farrer M. Low frequency of pathogenic mutations in the ubiquitin carboxy-terminal hydrolase gene in familial Parkinson’s disease. Neuroreport. 1999;10:427–429. doi: 10.1097/00001756-199902050-00040. [DOI] [PubMed] [Google Scholar]
- Lockhart PJ, Lincoln S, Hulihan M, Kachergus J, Wilkes K, Bisceglio G, Mash DC, Farrer MJ. DJ-1 mutations are a rare cause of recessively inherited early onset parkinsonism mediated by loss of protein function. J Med Genet. 2004;41:e22. doi: 10.1136/jmg.2003.011106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe J, McDermott H, Landon M, Mayer RJ, Wilkinson KD. Ubiquitin carboxyl-terminal hydrolase (PGP 9.5) is selectively present in ubiquitinated inclusion bodies characteristic of human neurodegenerative diseases. J Pathol. 1990;161:153–160. doi: 10.1002/path.1711610210. [DOI] [PubMed] [Google Scholar]
- Maraganore DM, de Andrade M, Elbaz A, Farrer MJ, Ioannidis JP, Kruger R, Rocca WA, Schneider NK, Lesnick TG, Lincoln SJ, Hulihan MM, Aasly JO, Ashizawa T, Chartier-Harlin MC, Checkoway H, Ferrarese C, Hadjigeorgiou G, Hattori N, Kawakami H, Lambert JC, Lynch T, Mellick GD, Papapetropoulos S, Parsian A, Quattrone A, Riess O, Tan EK, Van BC. Collaborative analysis of alpha-synuclein gene promoter variability and Parkinson disease. JAMA. 2006;296:661–670. doi: 10.1001/jama.296.6.661. [DOI] [PubMed] [Google Scholar]
- Maraganore DM, Farrer MJ, Hardy JA, Lincoln SJ, McDonnell SK, Rocca WA. Case–control study of the ubiquitin carboxy-terminal hydrolase L1 gene in Parkinson’s disease. Neurology. 1999;53:1858–1860. doi: 10.1212/wnl.53.8.1858. [DOI] [PubMed] [Google Scholar]
- Maraganore DM, Lesnick TG, Elbaz A, Chartier-Harlin MC, Gasser T, Kruger R, Hattori N, Mellick GD, Quattrone A, Satoh J, Toda T, Wang J, Ioannidis JP, de AM, Rocca WA. UCHL1 is a Parkinson’s disease susceptibility gene. Ann Neurol. 2004;55:512–521. doi: 10.1002/ana.20017. [DOI] [PubMed] [Google Scholar]
- Masliah E, Rockenstein E, Veinbergs I, Mallory M, Hashimoto M, Takeda A, Sagara Y, Sisk A, Mucke L. Dopaminergic loss and inclusion body formation in alpha-synuclein mice: implications for neurodegenerative disorders. Science. 2000;287:1265–1269. doi: 10.1126/science.287.5456.1265. [DOI] [PubMed] [Google Scholar]
- Mellick GD, Silburn PA. The ubiquitin carboxy-terminal hydrolase-L1 gene S18Y polymorphism does not confer protection against idiopathic Parkinson’s disease. Neurosci Lett. 2000;293:127–130. doi: 10.1016/s0304-3940(00)01510-x. [DOI] [PubMed] [Google Scholar]
- Miller DW, Ahmad R, Hague S, Baptista MJ, Canet-Aviles R, McLendon C, Carter DM, Zhu PP, Stadler J, Chandran J, Klinefelter GR, Blackstone C, Cookson MR. L166P mutant DJ-1, causative for recessive Parkinson’s disease, is degraded through the ubiquitin-proteasome system. J Biol Chem. 2003;278:36588–36595. doi: 10.1074/jbc.M304272200. [DOI] [PubMed] [Google Scholar]
- Mitsumoto A, Nakagawa Y. DJ-1 is an indicator for endogenous reactive oxygen species elicited by endotoxin. Free Radic Res. 2001;35:885–893. doi: 10.1080/10715760100301381. [DOI] [PubMed] [Google Scholar]
- Mizuno Y, Sone N, Saitoh T. Effects of 1-methyl-4-phenyl-1,2,3,6-tetra-hydropyridine and 1-methyl-4-phenylpyridinium ion on activities of the enzymes in the electron transport system in mouse brain. J Neurochem. 1987;48:1787–1793. doi: 10.1111/j.1471-4159.1987.tb05737.x. [DOI] [PubMed] [Google Scholar]
- Mizuta I, Satake W, Nakabayashi Y, Ito C, Suzuki S, Momose Y, Nagai Y, Oka A, Inoko H, Fukae J, Saito Y, Sawabe M, Murayama S, Yamamoto M, Hattori N, Murata M, Toda T. Multiple candidate gene analysis identifies alpha-synuclein as a susceptibility gene for sporadic Parkinson’s disease. Hum Mol Genet. 2006;15:1151–1158. doi: 10.1093/hmg/ddl030. [DOI] [PubMed] [Google Scholar]
- Mjönes H. Paralysis agitans: A clinical and genetic study. Acta Psychiatr Neurol Scand Supplement. 1949;54:1–195. [Google Scholar]
- Momose Y, Murata M, Kobayashi K, Tachikawa M, Nakabayashi Y, Kanazawa I, Toda T. Association studies of multiple candidate genes for Parkinson’s disease using single nucleotide polymorphisms. Ann Neurol. 2002;51:133–136. doi: 10.1002/ana.10079. [DOI] [PubMed] [Google Scholar]
- Mueller JC, Fuchs J, Hofer A, Zimprich A, Lichtner P, Illig T, Berg D, Wullner U, Meitinger T, Gasser T. Multiple regions of alpha-synuclein are associated with Parkinson’s disease. Ann Neurol. 2005;57:535–541. doi: 10.1002/ana.20438. [DOI] [PubMed] [Google Scholar]
- Murphy DD, Rueter SM, Trojanowski JQ, Lee VM. Synucleins are developmentally expressed, and alpha-synuclein regulates the size of the presynaptic vesicular pool in primary hippocampal neurons. J Neurosci. 2000;20:3214–3220. doi: 10.1523/JNEUROSCI.20-09-03214.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagakubo D, Taira T, Kitaura H, Ikeda M, Tamai K, Iguchi-Ariga SM, Ariga H. DJ-1, a novel oncogene which transforms mouse NIH3T3 cells in cooperation with ras. Biochem Biophys Res Commun. 1997;231:509–513. doi: 10.1006/bbrc.1997.6132. [DOI] [PubMed] [Google Scholar]
- Najim al-Din AS, Wriekat A, Mubaidin A, Dasouki M, Hiari M. Pallidopyramidal degeneration, supranuclear upgaze paresis and dementia: Kufor-Rakeb syndrome. Acta Neurol Scand. 1994;89:347–352. doi: 10.1111/j.1600-0404.1994.tb02645.x. [DOI] [PubMed] [Google Scholar]
- Nicklas WJ, Vyas I, Heikkila RE. Inhibition of NADH-linked oxidation in brain mitochondria by 1-methyl-4-phenyl-pyridine, a metabolite of the neurotoxin, 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine. Life Sci. 1985;36:2503–2508. doi: 10.1016/0024-3205(85)90146-8. [DOI] [PubMed] [Google Scholar]
- Nicklas WJ, Youngster SK, Kindt MV, Heikkila RE. MPTP, MPP+ and mitochondrial function. Life Sci. 1987;40:721–729. doi: 10.1016/0024-3205(87)90299-2. [DOI] [PubMed] [Google Scholar]
- Nishioka K, Hayashi S, Farrer MJ, Singleton AB, Yoshino H, Imai H, Kitami T, Sato K, Kuroda R, Tomiyama H, Mizoguchi K, Murata M, Toda T, Imoto I, Inazawa J, Mizuno Y, Hattori N. Clinical heterogeneity of alpha-synuclein gene duplication in Parkinson’s disease. Ann Neurol. 2006;59:298–309. doi: 10.1002/ana.20753. [DOI] [PubMed] [Google Scholar]
- Olzmann JA, Brown K, Wilkinson KD, Rees HD, Huai Q, Ke H, Levey AI, Li L, Chin LS. Familial Parkinson’s disease-associated L166P mutation disrupts DJ-1 protein folding and function. J Biol Chem. 2004;279:8506–8515. doi: 10.1074/jbc.M311017200. [DOI] [PubMed] [Google Scholar]
- Orr-Urtreger A, Shifrin C, Rozovski U, Rosner S, Bercovich D, Gurevich T, Yagev-More H, Bar-Shira A, Giladi N. The LRRK2 G2019S mutation in Ashkenazi Jews with Parkinson disease: is there a gender effect? Neurology. 2007;69:1595–1602. doi: 10.1212/01.wnl.0000277637.33328.d8. [DOI] [PubMed] [Google Scholar]
- Ozelius LJ, Senthil G, Saunders-Pullman R, Ohmann E, Deligtisch A, Tagliati M, Hunt AL, Klein C, Henick B, Hailpern SM, Lipton RB, Soto-Valencia J, Risch N, Bressman SB. LRRK2 G2019S as a cause of Parkinson’s disease in Ashkenazi Jews. N Engl J Med. 2006;354:424–425. doi: 10.1056/NEJMc055509. [DOI] [PubMed] [Google Scholar]
- Paisan-Ruiz C, Jain S, Evans EW, Gilks WP, Simon J, van der BM, Lopez de MA, Aparicio S, Gil AM, Khan N, Johnson J, Martinez JR, Nicholl D, Carrera IM, Pena AS, de SR, Lees A, Marti-Masso JF, Perez-Tur J, Wood NW, Singleton AB. Cloning of the gene containing mutations that cause PARK8-linked Parkinson’s disease. Neuron. 2004;44:595–600. doi: 10.1016/j.neuron.2004.10.023. [DOI] [PubMed] [Google Scholar]
- Parkinson J. An Essay on the Shaking Palsy. Neely & Jones; London, Sherwood: 1817. [Google Scholar]
- Perez FA, Palmiter RD. Parkin-deficient mice are not a robust model of parkinsonism. Proc Natl Acad Sci USA. 2005;102:2174–2179. doi: 10.1073/pnas.0409598102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perier C, Bove J, Vila M, Przedborski S. The rotenone model of Parkinson’s disease. Trends Neurosci. 2003;26:345–346. doi: 10.1016/S0166-2236(03)00144-9. [DOI] [PubMed] [Google Scholar]
- Piccini P, Burn DJ, Ceravolo R, Maraganore D, Brooks DJ. The role of inheritance in sporadic Parkinson’s disease: evidence from a longitudinal study of dopaminergic function in twins. Ann Neurol. 1999;45:577–582. doi: 10.1002/1531-8249(199905)45:5<577::aid-ana5>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Polymeropoulos MH, Higgins JJ, Golbe LI, Johnson WG, Ide SE, Di IG, Sanges G, Stenroos ES, Pho LT, Schaffer AA, Lazzarini AM, Nussbaum RL, Duvoisin RC. Mapping of a gene for Parkinson’s disease to chromosome 4q21-q23. Science. 1996;274:1197–1199. doi: 10.1126/science.274.5290.1197. [DOI] [PubMed] [Google Scholar]
- Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di IG, Golbe LI, Nussbaum RL. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- Porter CC, Totaro JA, Burcin A. The relationship between radioactivity and norepinephrine concentrations in the brains and hearts of mice following administration of labeled methyldopa or 6-hydroxydopamine. J Pharmacol Exp Ther. 1965;150:17–22. [PubMed] [Google Scholar]
- Porter CC, Totaro JA, STONE CA. Effect of 6-hydroxydopamine and some other compounds on the concentration of norepinephrine in the hearts of mice. J Pharmacol Exp Ther. 1963;140:308–316. [PubMed] [Google Scholar]
- Przedborski S, Vila M. The 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model: a tool to explore the pathogenesis of Parkinson’s disease. Ann N Y Acad Sci. 2003;991:189–198. [PubMed] [Google Scholar]
- Richfield EK, Thiruchelvam MJ, Cory-Slechta DA, Wuertzer C, Gainetdinov RR, Caron MG, Di Monte DA, Federoff HJ. Behavioral and neuro-chemical effects of wild-type and mutated human alpha-synuclein in transgenic mice. Exp Neurol. 2002;175:35–48. doi: 10.1006/exnr.2002.7882. [DOI] [PubMed] [Google Scholar]
- Rockenstein E, Mallory M, Hashimoto M, Song D, Shults CW, Lang I, Masliah E. Differential neuropathological alterations in transgenic mice expressing alpha-synuclein from the platelet-derived growth factor and Thy-1 promoters. J Neurosci Res. 2002;68:568–578. doi: 10.1002/jnr.10231. [DOI] [PubMed] [Google Scholar]
- Ross OA, Braithwaite AT, Skipper LM, Kachergus J, Hulihan MM, Middleton FA, Nishioka K, Fuchs J, Gasser T, Maraganore DM, Adler CH, Larvor L, Chartier-Harlin MC, Nilsson C, Langston JW, Gwinn K, Hattori N, Farrer MJ. Genomic investigation of alpha-synuclein multiplication and parkinsonism. Ann Neurol. 2008;63:743–750. doi: 10.1002/ana.21380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs C, Jonsson G. Mechanisms of action of 6-hydroxydopamine. Biochem Pharmacol. 1975;24:1–8. doi: 10.1016/0006-2952(75)90304-4. [DOI] [PubMed] [Google Scholar]
- Satoh JI, Kuroda Y. A polymorphic variation of serine to tyrosine at codon 18 in the ubiquitin C-terminal hydrolase-L1 gene is associated with a reduced risk of sporadic Parkinson’s disease in a Japanese population. J Neurol Sci. 2001;189:113–117. doi: 10.1016/s0022-510x(01)00555-x. [DOI] [PubMed] [Google Scholar]
- Savettieri G, De Marco EV, Civitelli D, Salemi G, Nicoletti G, Annesi G, Ciro CI, Quattrone A. Lack of association between ubiquitin carboxy-terminal hydrolase L1 gene polymorphism and PD. Neurology. 2001;57:560–561. doi: 10.1212/wnl.57.3.560. [DOI] [PubMed] [Google Scholar]
- Schluter OM, Fornai F, Alessandri MG, Takamori S, Geppert M, Jahn R, Sudhof TC. Role of alpha-synuclein in 1-methyl-4-phenyl-1,2,3,6-tetrahydro-pyridine-induced parkinsonism in mice. Neuroscience. 2003;118:985–1002. doi: 10.1016/s0306-4522(03)00036-8. [DOI] [PubMed] [Google Scholar]
- Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, Hulihan M, Peuralinna T, Dutra A, Nussbaum R, Lincoln S, Crawley A, Hanson M, Maraganore D, Adler C, Cookson MR, Muenter M, Baptista M, Miller D, Blancato J, Hardy J, Gwinn-Hardy K. Alpha-synuclein locus triplication causes Parkinson’s disease. Science. 2003;302:841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Aalpha-synuclein in Lewy bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- Sveinbjornsdottir S, Hicks AA, Jonsson T, Petursson H, Gugmundsson G, Frigge ML, Kong A, Gulcher JR, Stefansson K. Familial aggregation of Parkinson’s disease in Iceland. N Engl J Med. 2000;343:1765–1770. doi: 10.1056/NEJM200012143432404. [DOI] [PubMed] [Google Scholar]
- Tan EK, Lee J, Lim HQ, Yuen Y, Zhao Y. Essential tremor and the common LRRK2 G2385R variant. Parkinsonism Relat Disord. 2008;14:569–571. doi: 10.1016/j.parkreldis.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Tanner CM, Ottman R, Goldman SM, Ellenberg J, Chan P, Mayeux R, Langston JW. Parkinson disease in twins: an etiologic study. JAMA. 1999;281:341–346. doi: 10.1001/jama.281.4.341. [DOI] [PubMed] [Google Scholar]
- Terzioglu M, Galter D. Parkinson’s disease: genetic versus toxin-induced rodent models. FEBS J. 2008;275:1384–1391. doi: 10.1111/j.1742-4658.2008.06302.x. [DOI] [PubMed] [Google Scholar]
- Tranzer JP, Thoenen H. Selective destruction of adrenergic nerve terminals by chemical analogues of 6-hydroxydopamine. Experientia. 1973;29:314–315. doi: 10.1007/BF01926498. [DOI] [PubMed] [Google Scholar]
- Ueda K, Fukushima H, Masliah E, Xia Y, Iwai A, Yoshimoto M, Otero DA, Kondo J, Ihara Y, Saitoh T. Molecular cloning of cDNA encoding an unrecognized component of amyloid in Alzheimer disease. Proc Natl Acad Sci USA. 1993;90:11282–11286. doi: 10.1073/pnas.90.23.11282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungerstedt U. 6-Hydroxy-dopamine induced degeneration of central mono-amine neurons. Eur J Pharmacol. 1968;5:107–110. doi: 10.1016/0014-2999(68)90164-7. [DOI] [PubMed] [Google Scholar]
- Ungerstedt U. Striatal dopamine release after amphetamine or nerve degeneration revealed by rotational behaviour. Acta Physiol Scand Suppl. 1971a;367:49–68. doi: 10.1111/j.1365-201x.1971.tb10999.x. [DOI] [PubMed] [Google Scholar]
- Ungerstedt U. Postsynaptic supersensitivity after 6-hydroxy-dopamine induced degeneration of the nigro-striatal dopamine system. Acta Physiol Scand Suppl. 1971b;367:69–93. doi: 10.1111/j.1365-201x.1971.tb11000.x. [DOI] [PubMed] [Google Scholar]
- Ungerstedt U, Arbuthnott GW. Quantitative recording of rotational behavior in rats after 6-hydroxy-dopamine lesions of the nigrostriatal dopamine system. Brain Res. 1970;24:485–493. doi: 10.1016/0006-8993(70)90187-3. [DOI] [PubMed] [Google Scholar]
- Valente EM, Abou-Sleiman PM, Caputo V, Muqit MM, Harvey K, Gispert S, Ali Z, Del Turco D, Bentivoglio AR, Healy DG, Albanese A, Nussbaum R, Gonzalez-Maldonado R, Deller T, Salvi S, Cortelli P, Gilks WP, Latchman DS, Harvey RJ, Dallapiccola B, Auburger G, Wood NW. Hereditary early-onset Parkinson’s disease caused by mutations in PINK1. Science. 2004;304:1158–1160. doi: 10.1126/science.1096284. [DOI] [PubMed] [Google Scholar]
- Valente EM, Bentivoglio AR, Dixon PH, Ferraris A, Ialongo T, Frontali M, Albanese A, Wood NW. Localization of a novel locus for autosomal recessive early-onset parkinsonism, PARK6, on human chromosome 1p35-p36. Am J Hum Genet. 2001;68:895–900. doi: 10.1086/319522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valente EM, Salvi S, Ialongo T, Marongiu R, Elia AE, Caputo V, Romito L, Albanese A, Dallapiccola B, Bentivoglio AR. PINK1 mutations are associated with sporadic early-onset parkinsonism. Ann Neurol. 2004;56:336–341. doi: 10.1002/ana.20256. [DOI] [PubMed] [Google Scholar]
- van Duijn CM, Dekker MC, Bonifati V, Galjaard RJ, Houwing-Duistermaat JJ, Snijders PJ, Testers L, Breedveld GJ, Horstink M, Sandkuijl LA, van Swieten JC, Oostra BA, Heutink P. Park7, a novel locus for autosomal recessive early-onset parkinsonism, on chromosome 1p36. Am J Hum Genet. 2001;69:629–634. doi: 10.1086/322996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Coelln R, Thomas B, Savitt JM, Lim KL, Sasaki M, Hess EJ, Dawson VL, Dawson TM. Loss of locus coeruleus neurons and reduced startle in parkin null mice. Proc Natl Acad Sci USA. 2004;101:10744–10749. doi: 10.1073/pnas.0401297101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, van der Walt JM, Mayhew G, Li YJ, Zuchner S, Scott WK, Martin ER, Vance JM. Variation in the miRNA-433 binding site of FGF20 confers risk for Parkinson disease by overexpression of alpha-synuclein. Am J Hum Genet. 2008;82:283–289. doi: 10.1016/j.ajhg.2007.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Zhao CY, Si YM, Liu ZL, Chen B, Yu L. ACT and UCH-L1 polymorphisms in Parkinson’s disease and age of onset. Mov Disord. 2002;17:767–771. doi: 10.1002/mds.10179. [DOI] [PubMed] [Google Scholar]
- West AB, Moore DJ, Biskup S, Bugayenko A, Smith WW, Ross CA, Dawson VL, Dawson TM. Parkinson’s disease-associated mutations in leucine-rich repeat kinase 2 augment kinase activity. Proc Natl Acad Sci USA. 2005;102:16842–16847. doi: 10.1073/pnas.0507360102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerlund M, Belin AC, Anvret A, Håkansson A, Nissbrandt H, Lind C, Sydow O, Olson L, Galter D. Cerebellar alpha-synuclein levels are decreased in Parkinson’s disease and do not correlate with SNCA polymorphisms associated with disease in a Swedish material. FASEB J. 2008;22:3509–3514. doi: 10.1096/fj.08-110148. [DOI] [PubMed] [Google Scholar]
- Wilkinson KD, Lee KM, Deshpande S, Duerksen-Hughes P, Boss JM, Pohl J. The neuron-specific protein PGP 9.5 is a ubiquitin carboxyl-terminal hydrolase. Science. 1989;246:670–673. doi: 10.1126/science.2530630. [DOI] [PubMed] [Google Scholar]
- Wintermeyer P, Kruger R, Kuhn W, Muller T, Woitalla D, Berg D, Becker G, Leroy E, Polymeropoulos M, Berger K, Przuntek H, Schols L, Epplen JT, Riess O. Mutation analysis and association studies of the UCHL1 gene in German Parkinson’s disease patients. Neuroreport. 2000;11:2079–2082. doi: 10.1097/00001756-200007140-00004. [DOI] [PubMed] [Google Scholar]
- Wirdefeldt K, Gatz M, Schalling M, Pedersen NL. No evidence for heritability of Parkinson disease in Swedish twins. Neurology. 2004;63:305–311. doi: 10.1212/01.wnl.0000129841.30587.9d. [DOI] [PubMed] [Google Scholar]
- Zabetian CP, Hutter CM, Yearout D, Lopez AN, Factor SA, Griffith A, Leis BC, Bird TD, Nutt JG, Higgins DS, Roberts JW, Kay DM, Edwards KL, Samii A, Payami H. LRRK2 G2019S in families with Parkinson disease who originated from Europe and the Middle East: evidence of two distinct founding events beginning two millennia ago. Am J Hum Genet. 2006;79:752–758. doi: 10.1086/508025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabetian CP, Morino H, Ujike H, Yamamoto M, Oda M, Maruyama H, Izumi Y, Kaji R, Griffith A, Leis BC, Roberts JW, Yearout D, Samii A, Kawakami H. Identification and haplotype analysis of LRRK2 G2019S in Japanese patients with Parkinson disease. Neurology. 2006;67:697–699. doi: 10.1212/01.wnl.0000227732.37801.d4. [DOI] [PubMed] [Google Scholar]
- Zarranz JJ, Alegre J, Gomez-Esteban JC, Lezcano E, Ros R, Ampuero I, Vidal L, Hoenicka J, Rodriguez O, Atares B, Llorens V, Gomez TE, del ST, Munoz DG, de Yebenes JG. The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann Neurol. 2004;55:164–173. doi: 10.1002/ana.10795. [DOI] [PubMed] [Google Scholar]
- Zhang J, Hattori N, Leroy E, Morris HR, Kubo S, Kobayashi T, Wood NW, Polymeropoulos MH, Mizuno Y. Association between a polymorphism of ubiquitin carboxy-terminal hydrolase L1 (UCH-L1) gene and sporadic Parkinson’s disease. Parkinsonism Relat Disord. 2000;6:195–197. doi: 10.1016/s1353-8020(00)00015-8. [DOI] [PubMed] [Google Scholar]
- Zhang L, Shimoji M, Thomas B, Moore DJ, Yu SW, Marupudi NI, Torp R, Torgner IA, Ottersen OP, Dawson TM, Dawson VL. Mitochondrial localization of the Parkinson’s disease related protein DJ-1: implications for pathogenesis. Hum Mol Genet. 2005;14:2063–2073. doi: 10.1093/hmg/ddi211. [DOI] [PubMed] [Google Scholar]
- Zimprich A, Biskup S, Leitner P, Lichtner P, Farrer M, Lincoln S, Kachergus J, Hulihan M, Uitti RJ, Calne DB, Stoessl AJ, Pfeiffer RF, Patenge N, Carbajal IC, Vieregge P, Asmus F, Muller-Myhsok B, Dickson DW, Meitinger T, Strom TM, Wszolek ZK, Gasser T. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron. 2004;44:601–607. doi: 10.1016/j.neuron.2004.11.005. [DOI] [PubMed] [Google Scholar]