Abstract
The in vitro susceptibility of pathogenic Candida species to the photodynamic effects of the clinically approved photosensitizing agent Photofrin was examined. Internalization of Photofrin by Candida was confirmed by confocal fluorescence microscopy, and the degree of uptake was dependent on incubation concentration. Uptake of Photofrin by Candida and subsequent sensitivity to irradiation was influenced by culture conditions. Photofrin uptake was poor in C. albicans blastoconidia grown in nutrient broth. However, conversion of blastoconidia to filamentous forms by incubation in defined tissue culture medium resulted in substantial Photofrin uptake. Under conditions where Photofrin was effectively taken up by Candida, irradiated organisms were damaged in a drug dose- and light-dependent manner. Uptake of Photofrin was not inhibited by azide, indicating that the mechanism of uptake was not dependent on energy provided via electron transport. Fungal damage induced by Photofrin-mediated photodynamic therapy (PDT) was determined by evaluation of metabolic activity after irradiation. A strain of C. glabrata took up Photofrin poorly and was resistant to killing after irradiation. In contrast, two different strains of C. albicans displayed comparable levels of sensitivity to PDT. Furthermore, a reference strain of C. krusei that is relatively resistant to fluconazole compared to C. albicans was equally sensitive to C. albicans at Photofrin concentrations of ≥3 μg/ml. The results indicate that photodynamic therapy may be a useful adjunct or alternative to current anti-Candida therapeutic modalities, particularly for superficial infections on surfaces amenable to illumination.
Photodynamic therapy (PDT) is a process in which cells are treated with an agent that makes them susceptible to killing by exposure to light. These agents, called photosensitizers, are generally macrocyclic compounds that exhibit no or minimal inherent toxicity but result in the generation of cytotoxic reactive oxygen species when excitation occurs with light of the appropriate wavelength. PDT has been applied most extensively in the treatment of neoplasms and shows promise as a novel therapy for some non-neoplastic disorders (4, 11). Photofrin is a photosensitizer that has been the subject of intensive investigation (4). Derived from acid treatment of hematoporphyrin, this compound has been approved by the U.S. Food and Drug Administration for the treatment of endobronchial and esophageal tumors (11) and is currently in clinical trials for several other indications.
Although PDT is becoming established as a treatment modality to augment conventional chemotherapy and radiation in the oncologic literature, much less is known about the effects of photosensitizers on fungi of medical importance. Candida species have become increasingly prevalent as causes of both mucocutaneous and systemic infection in immunocompromised patients (3). Moreover, resistance of Candida to traditional antifungals such as fluconazole is increasing, with some species such as Candida krusei showing inherent resistance to this agent (13). For example, fluconazole-resistant Candida species colonize ca. 81% of AIDS patients receiving therapy for oral candidiasis (7). These trends underscore the importance of developing novel strategies for treatment of fungal infections, since the microbiology and resistance patterns of clinical isolates evolve in response to selective pressures of current antifungal therapy.
In the present study, we have investigated the susceptibility of Candida to the phototoxic effects of Photofrin. Although some variation exists among different species of Candida, we were able to demonstrate rapid and exquisite sensitivity to killing by this compound. The adaptation of methods of PDT for therapy of mucosal and cutaneous Candida infections in humans is therefore possible.
MATERIALS AND METHODS
Organisms.
C. albicans laboratory strains 3153A (9, 14, 17, 20) (kindly provided by E. Rustchenko, Department of Biochemistry and Biophysics, University of Rochester School of Medicine and Dentistry, Rochester, N.Y.) and SC5314 (8) (kindly provided by P. Sundstrom, Ohio State University, Columbus) were used in the uptake and phototoxicity assays. C. krusei quality control strain ATCC 6258 (15, 16) and C. glabrata strain MR084-R were used in the phototoxicity assays. C. krusei ATCC 6258 was provided by the Department of Clinical Microbiology, Strong Memorial Hospital, Rochester, N.Y. MR084-R was collected as part of a prospective study of Candida colonization in mothers and neonates at Strong Memorial Hospital, Rochester, N.Y., and was kindly provided by W. Watson, formerly of the Department of Pediatrics, University of Rochester School of Medicine and Dentistry, Rochester, N.Y.
Uptake assay of Photofrin by Candida strains.
Candida species were grown overnight at 37°C on a shaker platform in liquid yeast extract-peptone-dextrose (YEPD) medium (Difco, Detroit, Mich.) with vigorous aeration (225 rpm) to stationary phase (∼2 × 108 cells/ml). Candida cells were washed with water and diluted to 105 cells/ml in either YEPD or Medium 199 (supplemented with Earle's balanced salt solution, HEPES, and glutamine; BioWhittaker, Walkersville, Md.). Cells were grown either in six-well dishes on the surface of glass coverslips or in suspension with constant agitation at 37°C for 3 h. Cells were washed with Dulbecco phosphate-buffered saline with calcium and magnesium (DPBS; Invitrogen, Carlsbad, Calif.) containing 0.1% glucose (DPBSG) and incubated in the dark for 30 min at 37°C with Photofrin (Axcan Pharma, Birmingham, Ala.) diluted either in growth medium, in DPBSG, or in DPBS. After incubation with Photofrin, adherent cells were washed with DPBS to remove excess photosensitizer. In selected assays, sodium azide (Sigma, St. Louis, Mo.) was used at a final concentration of 0.02%. Cells treated in suspension were spotted onto microscope slides, and cells grown on coverslips were dried and placed on microscope slides. Uptake of Photofrin was visualized by fluorescence microscopy with a custom porphyrin filter set and filter cube (Ex. 405/30; dichroic 440 LP; Em. OG 590; Chroma Technology Corp., Rockingham, Vt.).
Confocal microscopy.
C. albicans cells were prepared as described above for uptake assay and placed on 25-mm, round, no. 1 thickness coverslips. Confocal microscopy was performed on live organisms with a Nikon Diaphot inverted microscope equipped with home-built laser scanning confocal fluorescence imaging capability (2). Images were acquired by using a ×60, 1.4 NA oil immersion objective lens, providing an optical section thickness of <1 μm.
Phototoxicity assay of Candida species treated with Photofrin.
Candida strains were grown overnight, washed, and diluted in Medium 199 as described above for uptake assays. Cells were seeded in 96-well dishes and incubated in the dark for 30 min at 37°C with Photofrin serially diluted 1:3 in DPBSG for concentrations ranging from 10 to 0.01 μg/ml. Cells incubated in DPBSG alone were included as a control. The cover of the 96-well dish was removed, and plates were illuminated with broadband visible light from an Hg arc lamp (Olympus BH2-RFL-T2; Olympus Optical Co., Ltd., Tokyo, Japan) reflected to the sample with a cold mirror (Edmund Industrial Optics, Barrington, N.J.). Illumination was performed for 10 min at room temperature at a fluence rate of 15 mW/cm2 as measured by a power/energy meter (model 13PEM001; Melles Griot, Carlsbad, Calif.), resulting in a total fluence of 9 J/cm2. An identical plate that remained in the dark was included as a control. After illumination, toxicity to cells was measured by (2,3)-bis-(2-methoxy-4-nitro-5-sulfenyl)-(2H)-tetrazolium-5-carboxanilide (XTT; Sigma) assay by a method described previously (12). Briefly, XTT was freshly prepared in DPBS, heated at 60°C for 30 min, and filtered. coenzyme Q (Sigma) was added, and the solution was added to cells for final concentrations of 0.5 mg of XTT/ml and 40 μg of coenzyme Q/ml. Plates were incubated at 37°C for 1 h, and the intensity of the colorimetric reaction, reflecting cell metabolic activity, was measured by determining the optical density at 450 nm with an automated plate reader (Bio-Rad Laboratories, Hercules, Calif.).
Statistical analysis.
Experiments were performed in triplicate. Comparisons of toxicity from PDT among different Candida species at each Photofrin dose were made by using one-way analysis of variance. Between-group comparisons were made by applying the Newman-Keuls test, with P values of <0.05 considered to be significant.
RESULTS
Uptake of Photofrin by C. albicans.
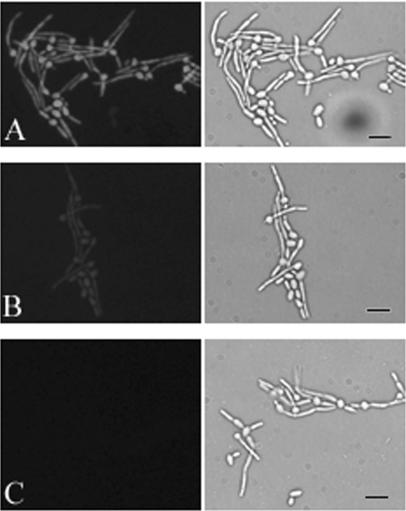
To determine whether C. albicans would take up the photosensitizing agent, cells were grown on coverslips in Medium 199 to promote germ tube formation and cell adherence. They were washed and incubated for 30 min in the presence of 10 or 1 μg of Photofrin/ml. After additional washes to remove the loosely bound agent, cells were viewed by fluorescence microscopy (Fig. 1). Bright fluorescence was observed with a Photofrin concentration of 10 μg/ml (Fig. 1A), suggesting that uptake of the photosensitizer had occurred. Fluorescence was barely discernible at 1 μg/ml (Fig. 1B). No fluorescence was seen in the absence of agent (Fig. 1C).
FIG. 1.
Uptake of Photofrin by C. albicans. Fluorescence (left) and bright-field (right) photomicrographs of the same microscopic fields of C. albicans strain 3153A are depicted after incubation with 10 μg/ml (A) or 1 μg/ml (B) Photofrin. (C) Incubation in buffer alone. Bar, 25 μm.
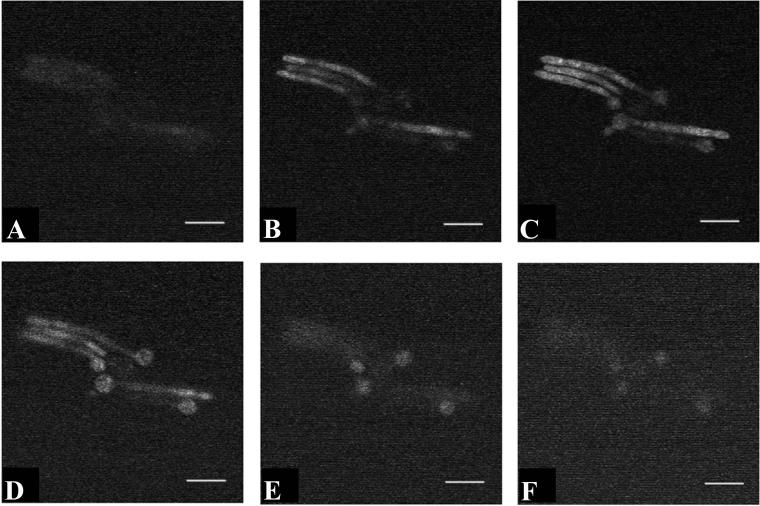
Fluorescence of C. albicans incubated in the presence of Photofrin could be indicative of stable binding of the agent to the cell surface rather than uptake into the cell itself. To distinguish between these possibilities, the uptake assay was repeated and the cells were viewed by confocal fluorescence microscopy. Optical sections at a thickness of 1 μm were obtained and are depicted in sequence in Fig. 2. Fluorescence was visible throughout these sections, suggesting that the agent had reached the interior of the cell and was not merely bound to the cell surface.
FIG. 2.
Cellular localization of Photofrin in C. albicans. Photomicrographs obtained by fluorescence confocal microscopy are depicted from a series of 1-μm-thick optical sections through viable C. albicans strain 3153A cells after uptake of Photofrin. Panels A to F were obtained in sequence through the same microscopic field. Fluorescence was visualized throughout the cell, suggesting that the compound had reached the cell interior. Bar, 15 μm.
Effect of growth conditions on uptake of Photofrin by C. albicans.
To determine whether growth conditions affected uptake of Photofrin by C. albicans, cells were grown either on coverslips in Medium 199 as germ tubes or in suspension in YEPD as blastoconidia. After 3 h, cells were washed and Photofrin was added at a concentration of 10 μg/ml in either DPBS, DPBS plus glucose, or the original medium in which the cells were grown. Cells were examined by fluorescence microscopy after 30, 60, and 120 min of uptake. The results at 30 min are shown in the table. Cells grown in Medium 199 were brightly fluorescent under all uptake conditions and at all time points. Cells grown in YEPD displayed no visible fluorescence at any time point if they were kept in YEPD. When cells grown in YEPD were put in DPBS or DPBS plus glucose, fluorescence was not detected at 30 min. Fluorescence was detectable at 60 min and was considerably brighter at 120 min. To confirm that the effect was due to growth medium and not related to adherence versus growth in suspension, C. albicans was grown in suspension in Medium 199 prior to the uptake assay. Planktonic cells took up Photofrin in an equivalent fashion to adherent cells as judged by fluorescence microscopy (data not shown).
To determine whether uptake of Photofrin is an active process, C. albicans 3153A grown in Medium 199 was incubated for 30 min with 10 μg of Photofrin/ml in either the presence or absence of sodium azide (Table 1). Cells treated with azide before incubation with Photofrin, during incubation with Photofrin, or both showed fluorescence equivalent to that of cells that were incubated in the absence of azide. These results suggest that uptake of the agent is not driven by electron transport.
TABLE 1.
Effect of culture conditions on uptake of Photofrin by C. albicans 3153A
| Growth medium | Uptake mediuma
|
||||
|---|---|---|---|---|---|
| Medium 199 | YEPD | DPBS | DPBS + glu | DPBS + glu + azide | |
| Medium 199 | + | ND | + | + | + |
| YEPD | ND | − | − | − | ND |
Supplemented with 10 μg of Photofrin/ml. Uptake was evaluated by fluorescence microscopy after 30 min of incubation. Glu, 0.1% glucose; azide, 0.02% sodium azide; ND, not determined.
Phototoxicity of C. albicans after treatment with Photofrin.
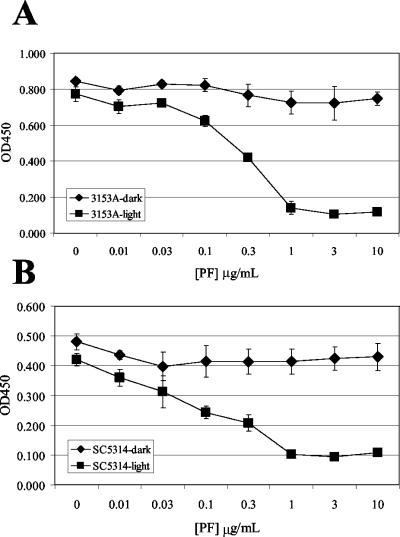
C. albicans strains 3153A and SC5314 were grown in 96-well dishes in Medium 199 under the same conditions as in the uptake experiments. Cells were washed and incubated with varied concentrations of Photofrin for 30 min in the dark. One plate from each strain was then illuminated with visible light for 10 min, while the other was kept in the dark, and the metabolic activity was measured by XTT assay (Fig. 3). Irradiation of photosensitized cells led to a dose-dependent inhibition of metabolic activity in both strains of C. albicans. Cells treated with Photofrin but not exposed to light (Fig. 3) showed XTT activity that was equivalent to control cells from the respective strain that were not treated with Photofrin (data not shown).
FIG. 3.
Phototoxicity of Photofrin to C. albicans. XTT assays were performed to quantitate toxicity to C. albicans strains 3153A (A) and SC5314 (B) after treatment with Photofrin and exposure to light. Identically treated cells that were kept in darkness are included for comparison. The intensity of color generated by XTT assay as measured by optical density at 450 nm reflects cell metabolic activity and was plotted against Photofrin dose ([PF]). Points are calculated means from triplicate samples, and error bars represent standard deviation.
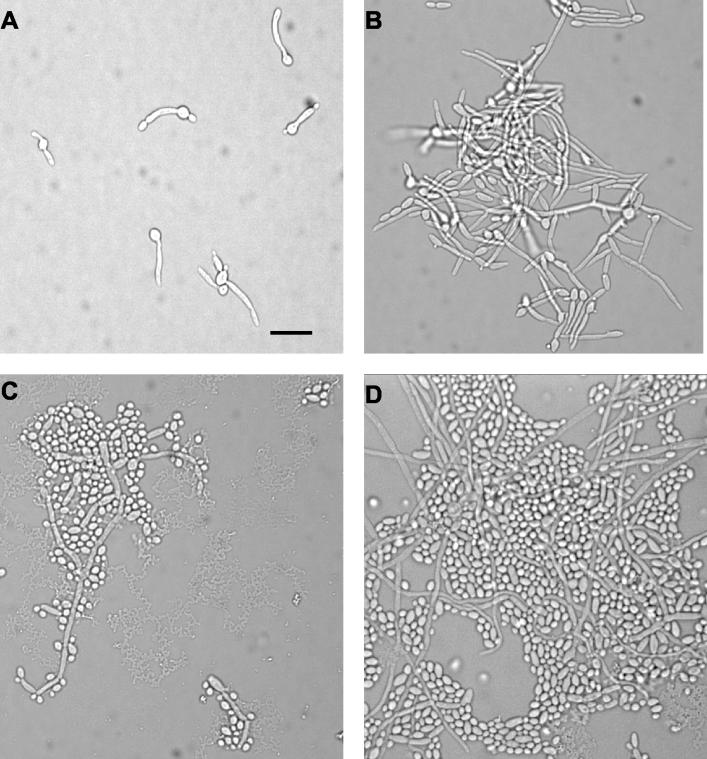
The morphology of cells treated with Photofrin-PDT was observed over time by light microscopy. Cells that were treated with Photofrin at 10, 3, or 1 μg/ml and illuminated showed little recovery of growth after several hours compared to cells that were not exposed to light. After overnight growth, a few microcolonies had formed in the 10- and 3-μg/ml wells that contained abnormal-appearing cells. At 1 μg/ml, the few colonies seen were a bit larger, while at 0.3 μg/ml ca. 50 colonies had formed overnight. Minimal hyphal growth was apparent after overnight incubation down to a concentration of 0.3 μg of Photofrin/ml, whereas the cells incubated with Photofrin and kept in darkness had numerous long hyphae after overnight incubation. Cells subjected to concentrations of Photofrin of <0.3 μg/ml looked very similar to the nonilluminated control cells. Representative photomicrographs of cells incubated with 10 μg of Photofrin/ml are shown in Fig. 4. These observations correlate well with the quantitative data provided by the XTT assay (Fig. 3).
FIG. 4.
Microscopic evaluation of phototoxicity of Photofrin to C. albicans. Organisms were exposed to 10 μg of Photofrin/ml and either irradiated (A and C) or kept in the dark (B and D). Photomicrographs were taken either 3 (A and B) or 24 (C and D) h postincubation. Bar, 20 μm.
Phototoxicity of other Candida species to Photofrin.
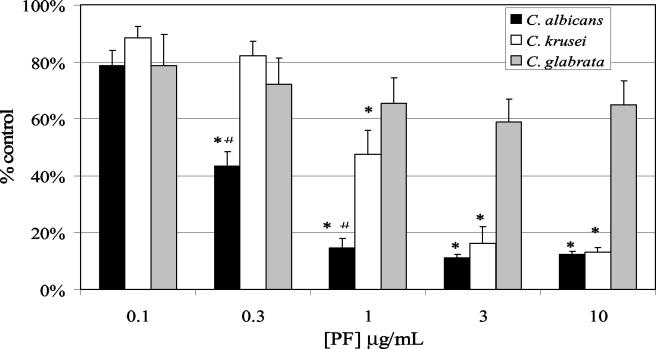
Candida species differ in their inherent susceptibility to antifungal agents (3). To determine whether Candida species also vary in their photosensitivity after treatment with Photofrin, C. krusei 6258 and C. glabrata strain MR084-R were treated with Photofrin in medium 199 in parallel with C. albicans 3153A. The metabolic activity of each species after PDT treatment was quantified by XTT assay. Figure 5 shows the XTT activity of these strains over various concentrations of Photofrin as a percent reduction in XTT activity relative to untreated control cells. Similar to earlier results, C. albicans 3153A was reduced to background XTT activity after PDT at a Photofrin concentration of 1 μg/ml. C. krusei 6528 was slightly more resistant than C. albicans in that XTT activity was reduced to ca. 50% of untreated control cells at 1 μg/ml, and Photofrin concentrations of 3 μg/ml or higher were required to reduce activity to background levels. C. glabrata MRO84-R was particularly resistant to Photofrin-PDT in these assays, with only modest reductions in XTT activity even at the highest concentrations of agent. C. glabrata cells examined by fluorescence microscopy after incubation with 10 μg of Photofrin/ml showed minimal fluorescence (data not shown).
FIG. 5.
Phototoxicity of Photofrin to other Candida species. XTT assays were performed to quantitate toxicity to C. albicans strain 3153A, C. krusei strain ATCC 6258, and C. glabrata strain MR084-R after treatment with Photofrin and exposure to light. The percent reduction in XTT activity relative to untreated cells was plotted against Photofrin concentration ([PF]). An asterisk indicates a statistically significant decrease in XTT activity relative to C. glabrata. A number symbol (#) indicates a statistically significant decrease in XTT activity relative to C. krusei. All P values were ≤0.002.
DISCUSSION
The application of PDT to the treatment of neoplasms and its effect on other mammalian cells has been an area of intensive study (4). The susceptibility of medically relevant fungi to the effects of photosensitizers has received much less attention. Friedberg et al. demonstrated an in vitro fungicidal effect of the photosensitizer Green 2W to Aspergillus fumigatus that was both light dose and inoculum dependent (5). These authors suggested that PDT may be an effective treatment option for localized cavitary infections with this organism. PDT has also been demonstrated against the fungal dermatophyte Trichophyton rubrum (18). Other in vitro studies have demonstrated the possibility of photosensitizing C. albicans to light-induced cellular damage by using a variety of compounds (1, 22, 25). We found that C. albicans was exquisitely sensitive to the phototoxic effects of Photofrin, a compound that is already approved for clinical use, in concentrations as low as 1 μg/ml in vitro. Further, the sensitivity profile suggests that therapy could be effective at concentrations that that have been obtained in vivo after systemic administration (21). For mucocutaneous candidiasis, topical application of the photosensitizer is a viable alternative, and effective concentrations should be obtainable with a minimum of side effects.
The effectiveness of PDT for fungal infections in vivo is largely untested. One study investigated the effect of topical methylene blue, followed by laser light in a murine model of oral candidiasis (19). In that study, SCID mice were infected orally with C. albicans and treated topically with increasing concentrations of methylene blue, followed by 687.5 s of illumination with laser light at 664 nm. These authors were able to demonstrate eradication of the infection in a dose-dependent manner, supporting the feasibility of such an approach for mucosal infections.
Candida species other than C. albicans have become increasingly problematic among the immunocompromised (13). Each species carries a unique antifungal resistance profile that must be considered in decisions regarding therapy of these infections. We chose to investigate the effects of PDT on C. krusei and C. glabrata, since these species are clinically important and are frequently resistant to antifungals. C. krusei is recognized as inherently resistant to fluconazole, one of the most commonly used antifungal agents for Candida infection. The fluconazole MIC for the laboratory strains of C. albicans used in the present study has been reported to be <1 μg/ml (10), whereas the MIC for the reference strain of C. krusei ATCC 6258 is 16 to 64 μg/ml (16). These two species showed similar susceptibilities to PDT in the present study, with C. krusei being only slightly more resistant. These observations support the notion that PDT may be an alternative therapy in the setting of resistance to conventional antifungals. Interestingly, C. glabrata was dramatically more resistant to Photofrin-PDT in these assays and appeared to take up little of the photosensitizer based on fluorescence microscopy. The factors influencing Photofrin uptake may provide insight into the limitations of PDT in fungi, as well as other cell types, and will be the subject of further study.
Growth conditions and cell morphology had a dramatic effect on the susceptibility of C. albicans to PDT. C. albicans grown as a filamentous form in the defined Medium 199 without serum were much more sensitive than blastoconidia grown in the nutrient medium YEPD, and the sensitivity was correlated with the uptake of Photofrin by both the parent blastoconidia and the new filament (Fig. 1). No detectable Photofrin uptake occurred in blastoconidia when the assay was performed in YEPD, raising concern that the agent was bound to a medium component and thus unavailable to the cells. However, even when C. albicans was washed after growth in YEPD and then incubated with Photofrin in DPBS for uptake, no uptake was observed at 30 min, and incubations of 60 min or longer were required to detect uptake by fluorescence microscopy. These observations suggest that the cells require particular environmental conditions to take up Photofrin. One possible explanation is that Photofrin is actively transported into the cell through an uptake mechanism that is induced by growth under more nutritionally restrictive conditions. However, since uptake was not inhibited by the presence of azide, an electron transport-dependent uptake process is unlikely. Another possible mechanism is that changes in the composition of the cell wall occur under different physiological conditions that allow for more efficient passive entry of the compound into the cell. Since Photofrin is a fairly lipid-soluble molecule, the extent of cell wall hydrophobicity may be an important determinant of uptake in these assays. The hydrophobic properties of the cell wall have been shown to be strongly influenced by growth conditions (6) and may contribute to the patterns we observed. The surface properties and composition of C. albicans are complex and dynamic, however, and relative hydrophobicity is one of many potential factors that may influence uptake. Nonetheless, the strain of C. glabrata tested demonstrated minimal Photofrin uptake under conditions favorable for uptake by C. albicans and C. krusei. Hence, genetic determinants inherent in different Candida species may also influence uptake, in addition to physiological responses to environmental changes.
The demonstration of susceptibility of Candida to PDT by using an agent that is already in use clinically is an important step in the potential application of a novel therapeutic strategy to fungal infection. Clearly, the issue of selectivity will be an important one, as healthy human cells can be susceptible to damage by these agents. The potential for topical application to affected areas in mucocutaneous candidiasis and application of light only to affected areas makes these infections particularly amenable to approach by PDT. Zeina et al. have demonstrated that PDT with methylene blue under conditions that lead to effective killing of typical skin microbes, including C. albicans cause neither cytotoxicity (24) nor DNA damage to keratinocytes in vitro (23). These observations lend support to the notion that selectivity for the microbe may be possible for mucocutaneous candidiasis, but gathering in vivo data will also be important. Deeply seated or systemic Candida infections are more problematic since potential toxicity, as well as mechanisms for light delivery, become additional obstacles. With the development of newer, more selective photosensitizers and optical fiber based means to deliver excitatory energy, treatment of fungal infections may become readily amenable to this novel therapeutic approach.
Acknowledgments
We thank David Conover for assistance in the set up of the photoillumination apparatus and James Padbury for assistance with statistical analyses.
This study was supported by NIH grant CA68409 (T.H.F.) and T32 AI07464 (J.M.B.). We thank Yeissa Chabrier Rosello for technical assistance.
REFERENCES
- 1.Bertoloni, G., E. Reddi, M. Gatta, C. Burlini, and G. Jori. 1989. Factors influencing the hematoporphyrin-sensitized photoinactivation of Candida albicans. J. Gen. Microbiol. 135:957-966. [DOI] [PubMed] [Google Scholar]
- 2.Bigelow, C. E., C. J. Harkrider, D. L. Conover, T. H. Foster, I. Georgakoudi, S. Mitra, M. G. Nichols, and M. Rajadhyaksha. 2001. Retrofitted confocal laser scanner for a commercial inverted fluorescence microscope. Rev. Sci. Instrum. 72:3407-3410. [Google Scholar]
- 3.Calderone, R. A. 2002. Candida and candidiasis. ASM Press, Washington D.C.
- 4.Dougherty, T. J., C. J. Gomer, B. W. Henderson, G. Jori, D. Kessel, M. Korbelik, J. Moan, and Q. Peng. 1998. Photodynamic therapy. J. Natl. Cancer Inst. 90:889-905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Friedberg, J. S., C. Skema, E. D. Baum, J. Burdick, S. A. Vinogradov, D. F. Wilson, A. D. Horan, and I. Nachamkin. 2001. In vitro effects of photodynamic therapy on Aspergillus fumigatus. J. Antimicrob. Chemother. 48:105-107. [DOI] [PubMed] [Google Scholar]
- 6.Hazen, K. C., J. G. Wu, and J. Masuoka. 2001. Comparison of the hydrophobic properties of Candida albicans and Candida dubliniensis. Infect. Immun. 69:779-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson, E. M., D. W. Warnock, J. Luker, S. R. Porter, and C. Scully. 1995. Emergence of azole drug resistance in Candida species from HIV-infected patients receiving prolonged fluconazole therapy for oral candidosis. J. Antimicrob. Chemother. 35:103-114. [DOI] [PubMed] [Google Scholar]
- 8.Kurtz, M. B., M. W. Cortelyou, and D. R. Kirsch. 1986. Integrative transformation of Candida albicans, using a cloned Candida ADE2 gene. Mol. Cell. Biol. 6:142-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manavathu, M., S. Gunasekaran, Q. Porte, E. Manavathu, and M. Gunasekaran. 1996. Changes in glutathione metabolic enzymes during yeast-to-mycelium conversion of Candida albicans. Can. J. Microbiol. 42:76-79. [DOI] [PubMed] [Google Scholar]
- 10.Marr, K. A., C. N. Lyons, K. Ha, T. R. Rustad, and T. C. White. 2001. Inducible azole resistance associated with a heterogeneous phenotype in Candida albicans. Antimicrob. Agents Chemother. 45:52-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCaughan, J. S., Jr. 1999. Photodynamic therapy: a review. Drugs Aging 15:49-68. [DOI] [PubMed] [Google Scholar]
- 12.Meshulam, T., S. M. Levitz, L. Christin, and R. D. Diamond. 1995. A simplified new assay for assessment of fungal cell damage with the tetrazolium dye, (2,3)-bis-(2-methoxy-4-nitro-5-sulphenyl)-(2H)-tetrazolium-5-carboxanilide (XTT). J. Infect. Dis. 172:1153-1156. [DOI] [PubMed] [Google Scholar]
- 13.Moran, G. P., D. J. Sullivan, and D. C. Coleman. 2002. Emergence of non-Candida albicans Candida species as pathogens, p. 37-53. In R. A. Calderone (ed.), Candida and candidiasis. ASM Press, Washington, D.C.
- 14.Morrow, B., H. Ramsey, and D. R. Soll. 1994. Regulation of phase-specific genes in the more general switching system of Candida albicans strain 3153A. J. Med. Vet. Mycol. 32:287-294. [DOI] [PubMed] [Google Scholar]
- 15.Pfaller, M. A., M. Bale, B. Buschelman, M. Lancaster, A. Espinel-Ingroff, J. H. Rex, and M. G. Rinaldi. 1994. Selection of candidate quality control isolates and tentative quality control ranges for in vitro susceptibility testing of yeast isolates by National Committee for Clinical Laboratory Standards proposed standard methods. J. Clin. Microbiol. 32:1650-1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pfaller, M. A., M. Bale, B. Buschelman, M. Lancaster, A. Espinel-Ingroff, J. H. Rex, M. G. Rinaldi, C. R. Cooper, and M. R. McGinnis. 1995. Quality control guidelines for National Committee for Clinical Laboratory Standards recommended broth macrodilution testing of amphotericin B, fluconazole, and flucytosine. J. Clin. Microbiol. 33:1104-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rustchenko, E. P., D. H. Howard, and F. Sherman. 1997. Variation in assimilating functions occurs in spontaneous Candida albicans mutants having chromosomal alterations. Microbiology 143:1765-1778. [DOI] [PubMed] [Google Scholar]
- 18.Smijs, T. G., and H. J. Schuitmaker. 2003. Photodynamic inactivation of the dermatophyte Trichophyton rubrum. Photochem. Photobiol. 77:556-560. [DOI] [PubMed] [Google Scholar]
- 19.Teichert, M. C., J. W. Jones, M. N. Usacheva, and M. A. Biel. 2002. Treatment of oral candidiasis with methylene blue-mediated photodynamic therapy in an immunodeficient murine model. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 93:155-160. [DOI] [PubMed] [Google Scholar]
- 20.Vargas, K., P. W. Wertz, D. Drake, B. Morrow, and D. R. Soll. 1994. Differences in adhesion of Candida albicans 3153A cells exhibiting switch phenotypes to buccal epithelium and stratum corneum. Infect. Immun. 62:1328-1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vernon, D. I., J. A. Holroyd, S. M. Stribbling, and S. B. Brown. 1995. The quantitative determination of Photofrin and Polyhematoporphyrin in plasma: pitfalls and inaccuracies. J. Photochem. Photobiol. B 27:209-217. [DOI] [PubMed] [Google Scholar]
- 22.Wilson, M., and N. Mia. 1993. Sensitisation of Candida albicans to killing by low-power laser light. J. Oral Pathol. Med. 22:354-357. [DOI] [PubMed] [Google Scholar]
- 23.Zeina, B., J. Greenman, D. Corry, and W. M. Purcell. 2003. Antimicrobial photodynamic therapy: assessment of genotoxic effects on keratinocytes in vitro. Br. J. Dermatol. 148:229-232. [DOI] [PubMed] [Google Scholar]
- 24.Zeina, B., J. Greenman, D. Corry, and W. M. Purcell. 2002. Cytotoxic effects of antimicrobial photodynamic therapy on keratinocytes in vitro. Br. J. Dermatol. 146:568-573. [DOI] [PubMed] [Google Scholar]
- 25.Zeina, B., J. Greenman, W. M. Purcell, and B. Das. 2001. Killing of cutaneous microbial species by photodynamic therapy. Br. J. Dermatol. 144:274-278. [DOI] [PubMed] [Google Scholar]