Abstract
Recent observations have shown that wide-scale vaccination with pneumococcal conjugate vaccines was associated with a reduction in invasive disease, supporting the expectation that vaccination could help reduce carriage of Streptococcus pneumoniae and control the spread of resistant strains. However, it is too early to assess whether these effects can be sustained in the long term. Here, we used mathematical modeling to investigate time changes in pneumococcal colonization and resistance induced by conjugate vaccination in an environment where antibiotic exposure is high and resistance is widespread. According to model predictions, vaccination induced a decrease in carriage of vaccine-type pneumococci to very low levels, typically in 10 to 15 years under epidemiologically realistic conditions. Almost simultaneously, non-vaccine-type pneumococci spread in the community. Consequently, while there was a short-term decrease in the overall carriage rate, it was followed after a few years by a renewed, although limited, increase. Vaccination with a heptavalent vaccine did not affect the extent to which antibiotic resistance was selected: in all cases, the distribution of resistance levels peaked at high levels (MIC > 2 μg/ml) after 20 years. With a vaccine optimally designed to include all serotypes currently exhibiting decreased susceptibility to penicillin G, the selection of resistance was slowed down, although not prevented. These results suggest that because of serotype replacement, the effects of vaccination observed today may not be sustained in the long term. As a consequence, vaccination alone may not be successful in controlling selection for resistance in S. pneumoniae.
Streptococcus pneumoniae is a leading cause of respiratory infections associated with high morbidity and mortality (4). In recent decades, penicillin-resistant and multiresistant strains of S. pneumoniae have spread widely in the community. In several countries, the rate of decreased penicillin G susceptibility is >50%. This constitutes a major public health problem as consequences of antibiotic resistance begin to be documented in terms of increased morbidity, mortality, and hospitalization costs (19, 24).
A new conjugate pneumococcal vaccine that protects against both invasive disease and pharyngeal colonization has been used in the United States since early 2000 and is now recommended for young children in other countries. Recent observations in the United States have suggested that this vaccine is highly effective in preventing invasive disease in infants and young children (29). Clinical trials of conjugate vaccines have demonstrated their ability to reduce colonization by vaccine-related serotypes among vaccinated individuals (6). Because asymptomatic carriers of S. pneumoniae, especially children, are the main reservoir for transmission, a decrease in colonization by vaccine-related serotypes in unvaccinated individuals is expected as well, through herd immunity (22). Furthermore, as the vaccine formulation covers most pneumococcal serotypes currently associated with antibiotic resistance, it has also been suggested that conjugate vaccination could play a role in controlling resistance (22), and preliminary studies have shown a reduction in the carriage of resistant strains after vaccination (5, 16).
However, the serotypes of the most frequent and the most resistant strains of S. pneumoniae circulating in some European countries only partially match those used in the vaccine formulation (27). Many are concerned that the differences between the American and European serotype distributions may impede vaccine efficacy in Europe (25, 28).
Given that pneumococcal conjugate vaccines have been used in the general populations of only a few countries and for a short period, it is too early to assess their long-term effects on carriage and on resistance selection from field data. Mathematical modeling provides a useful tool for exploring the impact of vaccination. Major consequences may be investigated in this way. For example, a model suggested that because the vaccine does not protect against all existing bacterial serotypes, an increase in carriage of nonvaccine serotypes was likely (18). This is consistent with recent data suggesting that colonization with nonvaccine serotypes of S. pneumoniae has been increasing in the United States since the introduction of the vaccine and that nonsusceptible nonvaccine serogroups, such as serogroup 35, have emerged (S. Pelton, C. Marchant, D. Christiansen, and A. Loughlin, Abstr. 43rd Intersci. Conf. Antimicrob. Agents Chemother., abstr. 890, 2003).
Here, we focus on the impact of vaccination on antibiotic resistance. We have developed a mathematical model of selection for S. pneumoniae resistance to penicillin G in an age-structured population. In this paper, we study the effect of a vaccination program carried out on children <2 years old with the currently available seven-valent conjugate vaccine and with a hypothetical “optimized” vaccine designed to incorporate most resistant serotypes. More precisely, we investigated two questions: (i) what are the epidemiological characteristics of S. pneumoniae colonization in a vaccinated population, and (ii) how does the distribution of resistance levels among children and adult carriers change after the introduction of vaccination?
(This work was presented, in part, at the 13th European Conference on Clinical Microbiology and Infectious Diseases, Glasgow, 2003, abstract O72.)
MATERIALS AND METHODS
Data.
We used initial age- and serotype-specific data on penicillin resistance of S. pneumoniae in France based on annual reports from the French Reference Center for Pneumococci (Centre National de Réference des Pneumocoques [NRC]) (10, 27). The organization of the NRC has been described in detail elsewhere (9). In short, 40 to 50 centers throughout France collect and send S. pneumoniae strains to the NRC. Each year, ∼2,000 strains are typed and evaluated for susceptibility to various antibiotics.
We used these data to compute global characteristics for vaccine-type and non-vaccine-type S. pneumoniae. More precisely, we computed global MIC distributions and colonization rates for vaccine-type (or non-vaccine-type) pneumococci in the French population according to age by averaging the corresponding serotype-specific data over all serotypes included (or not included) in the vaccine weighted by the frequency of each serotype.
Vaccines.
The currently available seven-valent conjugate vaccine includes serotypes 4, 6B, 9V, 14, 18C, 19F, and 23F. We also imagined an optimized vaccine that would include in its formulation all serotypes currently exhibiting reduced susceptibility to penicillin G (>15% of MICs over 0.1 μg/ml). This amounted to adding four serotypes (serotypes 6A, 15, 19A, and 24) to the seven-valent vaccine (10, 27).
For both these vaccines, we called S. pneumoniae strains with one of the serotypes included in the vaccine type V pneumococci and S. pneumoniae strains with a serotype not included in the vaccine type NV pneumococci.
Model.
This work builds on a previously published model of selection for penicillin-resistant S. pneumoniae in the community (26).
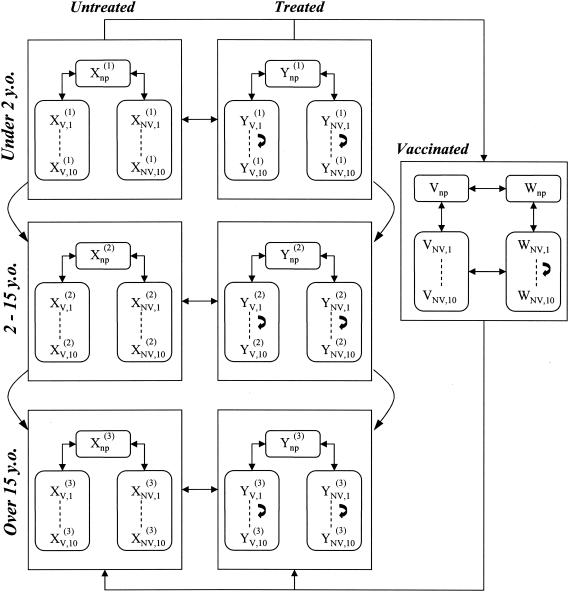
The model structure is shown in Fig. 1. Hosts enter the population at birth as noncarriers at a constant rate, μN.
FIG. 1.
Structure of the model. Xnp, number of untreated noncolonized individuals; Ynp, number of treated noncolonized individuals; XV,1, XV,2, etc. (or YV,1, YV,2, etc.), numbers of untreated (or treated) individuals colonized by vaccine-type pneumococci with one of the 10 resistance levels (see text); XNV,1, XNV,2, etc., and YNV,1, YNV,2, etc., numbers of individuals colonized by non-vaccine-type pneumococci. The model is structured into three age classes. A portion of children <2 years old are vaccinated, in which case they can be untreated (V) or treated (W); vaccinated children can be colonized only with non-vaccine-type pneumococci.
In order to take into account differences according to age, the population is structured into three age classes corresponding to young children (≤2 years old), older children (2 to 15 years old), and adults (≥15 years old). To incorporate vaccination, the model assumes that a fraction, v, of children <2 years old are vaccinated each year, in which case they are protected against carriage for an average time, dv.
Susceptible hosts may be colonized with either type V or type NV pneumococci. Vaccinated hosts can be colonized only with type NV pneumococci, and dual colonization is excluded. Colonization occurs following contact with hosts carrying type V or NV pneumococci; infectious contacts are more frequent between children than between adults but do not depend on the serotype of the strains. The rates of infectious contacts between individuals belonging to various age classes were calibrated in order to reproduce currently observed proportions of pneumococcal carriers in France in these age classes (26). In the absence of antibiotic treatment, natural decolonization occurs after a time, 1/λ, independently of the pneumococcal serotype.
The resistance levels of all bacteria colonizing one host are represented by a single MIC, which takes 1 of 10 possible values: 0.06, 0.125, 0.25, 0.5, 1, 2, 4, 8, 16, or 32.
Independently of their carrier status, hosts are exposed to antibiotics with a certain frequency: α1 for young children, α2 for older children, and α3 for adults. In vaccinated hosts, this rate is reduced to a value of α4. During antibiotic treatment, contacts with carriers are more liable to lead to colonization if the involved strains are resistant, and bacterial colonization is cleared with a probability of σ.
In hosts in whom colonization is not eliminated, bacteria with a mutation toward a higher resistance level may replace the original strains (26). We take into account the possibility of the occurrence of both point mutations, leading to a small increase in the MIC for pneumococci, and the acquisition of genetic material from other bacterial species, leading to higher levels of resistance, with the hypothesis that small increments are more frequent than large increments. To capture this feature, we randomly selected an increase in the MIC according to the following seminormal law when antibiotic exposure failed to clear pneumococcal colonization:
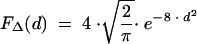 |
Considering that in this study, MICs take only 1 of 10 possible values, this law of increase can be restricted to probabilities of increase, pij, from 1 of these 10 levels, i, toward any higher level, j, in case of antibiotic exposure. These probabilities are detailed in Table 1.
TABLE 1.
Probabilities of increment of the penicillin MIC for pneumococcal strains from one level to another following a genetic event when the colonized host is exposed to antibioticsa
| Initial MIC | Probability with final MIC of:
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0.0625 | 0.125 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | |
| 0.0625 | 0.1924 | 0.3256 | 0.3214 | 0.0614 | 0.0001 | 5 · 10−15 | <10−20 | <10−20 | <10−20 | |
| 0.125 | 0.3678 | 0.4278 | 0.1048 | 0.0003 | 4 · 10−14 | <10−20 | <10−20 | <10−20 | ||
| 0.25 | 0.5886 | 0.2148 | 0.0013 | 9 · 10−13 | <10−20 | <10−20 | <10−20 | |||
| 0.5 | 0.6151 | 0.0162 | 4 · 10−10 | <10−20 | <10−20 | <10−20 | ||||
| 1 | 0.3905 | 7 · 10−6 | <10−20 | <10−20 | <10−20 | |||||
| 2 | 0.1995 | 1.10−16 | <10−20 | <10−20 | ||||||
| 4 | 0.0998 | <10−20 | <10−20 | |||||||
| 8 | 0.0499 | <10−20 | ||||||||
| 16 | 0.0249 | |||||||||
| 32 | ||||||||||
The sum of all probabilities on a given line of the table is equal to 1 minus the probability that the initial MIC remains unchanged.
Antibiotic treatment lasts for 1/γ days on average.
Finally, the mortality rate is μ1 for young children, μ2 for older children, and μ3 for adults.
Parameter values consistent with the French situation were estimated from the literature and are detailed in Table 2, together with the relevant references.
TABLE 2.
List of model parameters and their values
| Parameter (at MIC m) | Variable | Value | Reference(s) |
|---|---|---|---|
| Birth rate | μN | 0.000250 week−1 | 2 |
| Death rate in young children (≤2 years old) | μ1 | 0.000112 week−1 | 2 |
| Death rate in older children (2-15 years old) | μ2 | 0.000047 week−1 | 2 |
| Death rate in adults (≥15 years old) | μ3 | 0.000295 week−1 | 2 |
| Duration of treatment | 1/γ | 8 days | 13, 14 |
| Duration of vaccine immunity | dv | 15 years | |
| Frequency of treatment in young children (≤2 years old) | α1 | 1/30 weeks | 13, 14 |
| Frequency of treatment in older children (2-15 years old) | α2 | 1/2 year | 13, 14 |
| Frequency of treatment in adults (≥15 years old) | α3 | 1/4 year | 13, 14 |
| Frequency of treatment in vaccinated children | α4 | 1/2 year | 7 |
| Duration of carriage | 1/λ | 2.2 months | 23 |
| Infectious-contact rate between young children | β11 | 0.60 week−1 person−1 | 21, 26 |
| Infectious-contact rate between older children | β22 | 0.25 week−1 person−1 | 21, 26 |
| Infectious-contact rate between adults | β33 | 0.06 week−1 person−1 | 21, 26 |
| Infectious-contact rate between young and older children | β12 | 0.18 week−1 person−1 | 21, 26 |
| Infectious-contact rate between young children and adults | β13 | 0.30 week−1 person−1 | 21, 26 |
| Infectious-contact rate between older children and adults | β23 | 0.25 week−1 person−1 | 21, 26 |
| Probability of decolonization following treatment | σ (m) |  |
26 |
Simulations.
We studied vaccination coverage levels from 30 to 80%. We used the model to predict the changes in carriage of S. pneumoniae due to conjugate vaccination with the seven-valent vaccine, starting from the present French situation in terms of pneumococcal carriage, serotype frequencies, and penicillin susceptibility (10). We also computed the distribution of resistance levels reached after 20 years of vaccination with both the 7-valent and the optimized 11-valent vaccine, and in order to study the impacts of these vaccines on resistance selection, the distribution of resistance levels that would be obtained in 20 years if vaccination was never introduced in the population.
Sensitivity analysis.
In order to evaluate the robustness of our results, we performed an uncertainty and sensitivity analysis of the model, using the Latin hypercube sampling-partial rank correlation coefficients technique (3). We conducted this analysis for two outcomes: the overall pneumococcal colonization in the population 10 years after the introduction of vaccination and the proportion of resistant strains 20 years after the introduction of vaccination.
RESULTS
Vaccination and carriage.
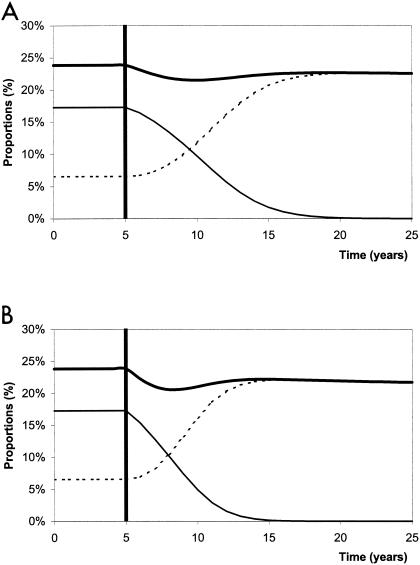
Changes in carriage over time following the introduction of vaccination with the seven-valent conjugate vaccine are presented in Fig. 2 for two different scenarios: vaccination of 30% (Fig. 2A) and 80% (Fig. 2B) of children <2 years old.
FIG. 2.
Proportion of carriers of S. pneumoniae in the general population before (5 years) and after (20 years) vaccination of 30% (A) and 80% (B) of children <2 years old. The thick solid line represents the overall carriage rate, while the thin solid line represents carriage of vaccine serotypes, and the dotted line represents carriage of nonvaccine serotypes.
After the introduction of vaccination, carriage of type V pneumococci decreased to very low levels, all the more rapidly if vaccine coverage was wide. It was <1% after ∼15 years for a 30% vaccination coverage level and after only ∼10 years for an 80% vaccination coverage level. However, almost simultaneously, carriage of type NV pneumococci increased, although this increase never compensated for the decrease in type V strains. The speed of this serotype replacement was also greater when the vaccination coverage was wide.
These two opposite trends led to a sharp decrease in overall pneumococcal carriage shortly after the introduction of vaccination, followed by a renewed increase toward a value less than the prevaccination level. Finally, when the vaccination coverage was wide enough, a slight decrease in pneumococcal colonization could be noted after the original bacterial population had been entirely replaced by type NV bacteria.
Vaccination and resistance.
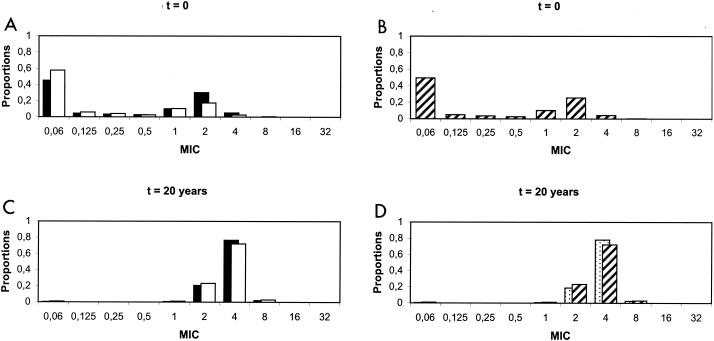
Currently observed and predicted distributions of pneumococcal resistance levels after 20 years of vaccination with the seven-valent conjugate vaccine (with a 30% coverage level) are presented in Fig. 3.
FIG. 3.
Currently observed distributions of penicillin MICs in France (A and B) and predicted distributions after 20 years of vaccination with a seven-valent conjugate vaccine (C and D). (A and C) Solid bars represent the distributions of MICs for vaccine serotypes of S. pneumoniae, and open bars represent those for nonvaccine serotypes. (B and D) Overall distributions of MICs for S. pneumoniae. In panel D, the hatched bars represent predictions with vaccination, whereas the stippled bars show predictions without vaccination.
In France, as shown in Fig. 3A, a majority of type V pneumococci are currently intermediate or resistant to penicillin G (MIC > 0.1 μg/ml), whereas most type NV pneumococci are still susceptible to penicillin G, even though some exhibit reduced susceptibility. As a majority of S. pneumoniae strains are covered by the vaccine (Fig. 2, before vaccination), the overall distribution of resistance levels peaks at high MICs (Fig. 3B).
With the present high levels of antibiotic exposure in France, resistant strains tend to be selected over time for all serotypes, irrespective of vaccination. Initially, as vaccine serotypes exhibit more decreased susceptibility than others, high levels of resistance are selected faster for type V pneumococci. However, after a while, this progression is slowed down, causing the distribution of resistance levels in type NV pneumococci to catch up. After 20 years, both distributions are very similar, as mostly highly resistant strains are left (Fig. 3C).
Serotype replacement occurs concurrently with the selection of resistance. The portion of type NV pneumococci increases along with their mean resistance level, and no faster, so that the overall distribution is not affected by vaccination.
After 20 years, the overall distribution presents only high resistance levels, irrespective of vaccination (Fig. 3D).
Vaccine “completion.”
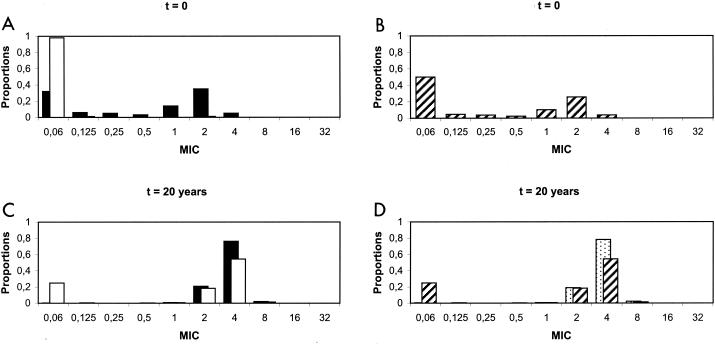
The currently observed and predicted distributions of pneumococcal resistance levels after 20 years of vaccination with the optimized 11-valent conjugate vaccine (with a 30% coverage level) are presented in Fig. 4.
FIG. 4.
Currently observed distributions of penicillin MICs in France (A and B) and predicted distributions after 20 years of vaccination with an optimized (11-valent) conjugate vaccine (C and D). (A and C) Solid bars represent the distributions of MICs for vaccine serotypes of S. pneumoniae; open bars represent those for nonvaccine serotypes. (B and D) Overall distributions of MICs for S. pneumoniae. In panel D, the hatched bars show predictions with vaccination, whereas the stippled bars show predictions without vaccination.
Contrary to the previous case, the initial distributions differ strikingly from each other, as nearly all type NV pneumococci are susceptible to penicillin. After 20 years of vaccination, this difference is reduced; however, nonvaccine serotypes are still notably more susceptible to penicillin than vaccine serotypes. Consequently, the overall distribution of MICs following 20 years of vaccination using this optimized vaccine is bimodal, with both a peak at low MICs (≤0.06 μg/ml) and a peak at high MICs, thus differing from the distribution centered on high MICs obtained without vaccination.
Sensitivity analysis.
The sensitivity analysis showed that for predicting the overall pneumococcal colonization rate in a vaccinated community, the most critical parameter was the duration of pneumococcal colonization (positively linked) (Table 3), followed by the rates of infectious contacts, especially between adults and between children and adults (positively linked).
TABLE 3.
Sensitivity analysis of the modela
| Parameter | Variable | PRCC |
|---|---|---|
| (Duration of pneumococcal carriage)−1 | λ | −0.971 |
| Contact rate between adults | β33 | 0.885 |
| Contact rate between adults and children aged 2-15 yr | β23 | 0.866 |
Key factors that increase (partial rank correlation coefficient [PRCC] > 0) or decrease (PRCC < 0) the rate of pneumococcal colonization in a vaccinated population.
For predicting the proportion of resistant S. pneumoniae strains, the most critical parameters were those defining the characteristics of antibiotic exposure, that is, the duration of antibiotic exposure (positively linked) (Table 4) and the frequencies of antibiotic exposure in various age classes (all positively linked), followed by the duration of pneumococcal colonization (negatively linked) and the rates of infectious contacts.
TABLE 4.
Sensitivity analysis of the modela
| Parameters | Variable | PRCC |
|---|---|---|
| (Duration of treatment)−1 | γ | −0.891 |
| Frequency of treatment in young children (≤2 yr) | α1 | 0.746 |
| Frequency of treatment in older children (2-15 yr) | α2 | 0.916 |
| Frequency of treatment in adults | α3 | 0.695 |
| (Duration of pneumococcal carriage)−1 | λ | 0.504 |
| Contact rate between adults | β33 | −0.802 |
| Contact rate between adults and children aged 0-2 yr | β13 | 0.498 |
| Contact rate between adults and children aged 2-15 yr | β23 | −0.404 |
Key factors that increase (partial rank correlation coefficient [PRCC] > 0) or decrease (PRCC < 0) the proportion of penicillin-resistant pneumococcal strains in a vaccinated population.
DISCUSSION
Results.
Because they prevent carriage and therefore lessen antibiotic pressure on S. pneumoniae, as well as transmission of pneumococcal strains, conjugate vaccines are expected to control the selection of resistance. The currently available vaccine, however, includes only 7 of the most frequently carried among the >90 existing serotypes of S. pneumoniae. In this study, we showed that this partial coverage prevents the vaccine from affecting more than marginally either the overall carriage rate or the selection of resistance, in the sense that in a country where antibiotic exposure is high and resistance is widespread, the distribution of resistance levels in 20 years should be concentrated on high MICs irrespective of the introduction of vaccination.
One might think that the reason this seven-valent conjugate vaccine is unable to slow resistance selection is that some of the strains that are not included in its formulation already exhibit some decreased susceptibility to penicillin G. To investigate this issue, we imagined an optimized 11-valent vaccine including all strains that currently exhibit decreased susceptibility to penicillin. With this optimized vaccine, resistance selection was slowed down, and the distribution of MICs in the midterm was affected by the use of the vaccine. However, selection for resistance was not prevented, and the effect of vaccination on resistance decreased with time (data not shown). This can be explained by an increased risk of emergence and transmission of resistant strains among nonvaccine serotypes due to their increased carriage and exposure to antibiotics.
In a Finnish study of the efficacy of a seven-valent pneumococcal vaccine against otitis media (8), episodes attributed to serotypes that were cross-reactive with those included in the vaccine were shown to decrease significantly following vaccination. Therefore, cross-immunity induced by the vaccine could reduce carriage of serotypes 6A, 9N, 18B, 19A, and 23A, as well as that of the seven serotypes included in its formulation, and put the effect of the vaccine midway between our simulations of the 7-valent vaccine and the optimized 11-valent vaccine.
Serotype replacement following vaccination is a consistent prediction from mathematical models (17) and has been observed in several trials (8, 20). However, in some studies, the decrease in carriage was noted in all serotypes of S. pneumoniae rather than in vaccine serotypes only. This finding is consistent with the simulations presented here, in which we observed a transient decrease in the overall carriage shortly after the introduction of vaccination (Fig. 2).
Finally, recent data on pneumococcal colonization and resistance in the United States since the introduction of conjugate vaccination lend support to our predictions. Indeed, these data show an increase in the proportion of nonvaccine S. pneumoniae over the period 2000 to 2003, as well as an increase in antibiotic resistance and the emergence of nonsusceptible nonvaccine serogroups (35 and NT), in total agreement with our predictions (S. Pelton, C. Marchant, D. Christiansen, and A. Loughlin, Abstr. 43rd Intersci. Conf. Antimicrob. Agents Chemother., abstr. 890, 2003).
Hypotheses.
The French Public Hygiene Committee recently recommended the use of a seven-valent pneumococcal conjugate vaccine for all children <2 years old in day care, which represents ∼30% of all children in this age group. That is why we studied vaccination coverage levels between 30 and 80%, which is consistent with Advisory Committee on Immunization Practices recommendations in the United States of vaccinating all children aged <23 months (1), as well as with the number of doses sold by the manufacturer (15.5 million in 2001) (29).
We supposed that colonized hosts carried either type V or type NV pneumococci, excluding dual colonization. However, simultaneous colonization with up to six different serotypes of S. pneumoniae has been observed in previous studies, with the consequence that serotype replacement under vaccination was favored and vaccine efficacy was decreased (11, 12, 15).
In this study, we did not consider that nonvaccine serotypes had reduced fitness compared to vaccine serotypes. Indeed, even though there may be true differences among the aptitudes for colonization of different serotypes (for instance, differing durations of carriage), there is no evidence that vaccine serotypes as a whole are more adapted for colonization than others (27). We numerically investigated the impact of a difference between carriage durations in vaccine serotypes and other serotypes; we found that this difference would not change our results on resistance selection. However, the speed of serotype replacement was slightly decreased if nonvaccine serotypes had a shorter carriage duration than vaccine serotypes, implying that our results may underestimate the short-term effect of vaccination on the overall pneumococcal colonization rate. Indeed, with a duration of carriage of nonvaccine serotypes 5% (or 10%) shorter than the duration of carriage of vaccine serotypes, serotype replacement would be fully completed 5 years (or 15 years) later, but the proportion of resistant strains after 20 years would remain the same.
Another hypothesis consistent with currently observed epidemiological differences between vaccine and nonvaccine serotypes is that nonvaccine serotypes exhibit a lower likelihood of resistance acquisition. We investigated this possibility by decreasing probabilities of MIC increments, pij (for j > i), for nonvaccine serotypes. We found that this had no impact on the predictions concerning pneumococcal colonization and little impact on those regarding resistance selection. Indeed, even if we suppose that the probabilities of genetic events for nonvaccine serotypes are half the corresponding probabilities for vaccine serotypes, the proportion of resistant strains is only 5% lower than in our predictions after 10 years of vaccination, and this difference is reduced to 1.7% after 20 years.
Sensitivity analysis.
Several of the parameters chosen were derived from direct measurments in the community or in vitro, but others required indirect evaluation. We performed a sensitivity analysis using the Latin hypercube sampling technique on the model to evaluate the impact of these uncertainties on the predictions (3).
All in all, uncertainties in estimating the values of four groups of parameters (the carriage duration, λ; contact rates, βij; and treatment characteristics, αi and γ) have the most critical effects on the predictions. The analysis also confirmed that a reasonable range of values for the duration of vaccine immunity, as well as the choice of the constants and of the exponent of m in the probability of nondecolonization following treatment, σ(m), have little effect on model outcomes.
Developments.
Considering the problem of resistance selection in serotypes not included in the vaccine formulation, a dynamic updating of the vaccine could be studied: nonvaccine serotypes would be tested for high levels of resistance at regular time intervals and, if need be, added to the vaccine formulation.
The model as we built it does not allow an evaluation of this solution, as it features only two types of S. pneumoniae and therefore does not allow the detection of newly resistant serotypes among nonvaccine serotypes. However, our study of the increased efficacy of an optimized vaccine is a first step in demonstrating the possibilities of such policies.
Acknowledgments
Laura Temime was supported by the Délégation Générale pour l'Armement and Centre National de la Recherche Scientifique. Part of this work was supported by grant 1A048G from Ministère de la Recherche/Institut National de la Santé et de la Recherche Médicale.
APPENDIX
Changes over time in the subpopulations of model compartments are driven by the following set of differential equations:
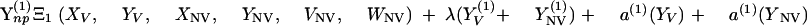 |
 |
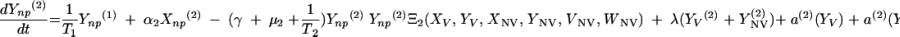 |
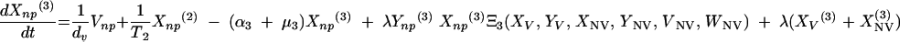 |
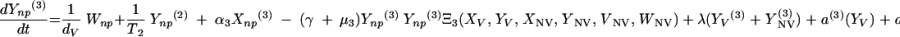 |
 |
 |
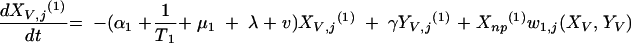 |
and for all j
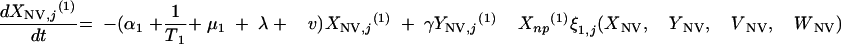 |
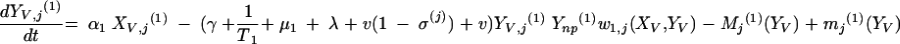 |
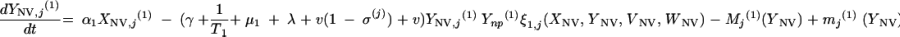 |
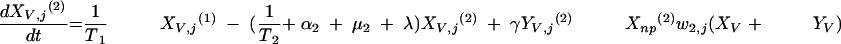 |
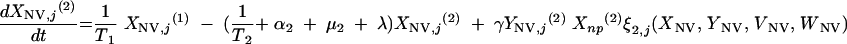 |
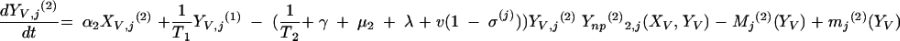 |
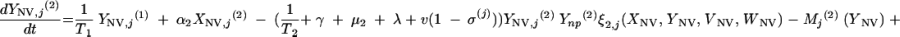 |
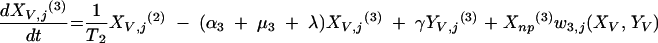 |
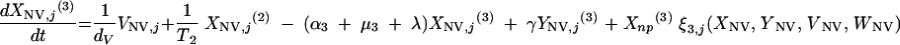 |
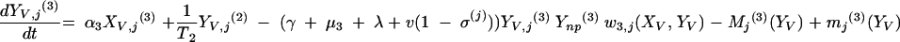 |
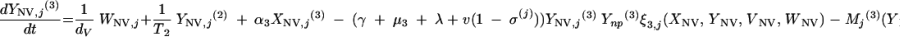 |
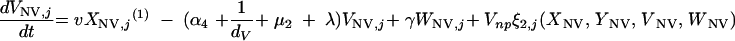 |
 |
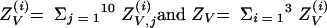 |
where for all Z, we defined
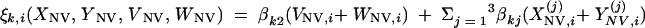 |
and the same for quantities indexed by NV.
We also introduced the following terms:
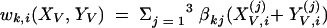 |
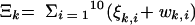 |
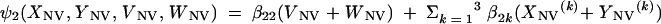 |
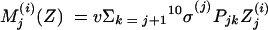 |
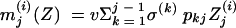 |
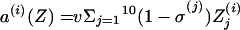 |
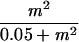 |
The three age classes are defined by the superscript i (i = 1 to 3) (the durations of the two first classes are 2 [T1] and 13 [T2] years). At time t, Xnp(i)(t) is the proportion of individuals in age class (i) in the population uncolonized and unexposed to antibiotics, Ynp(i)(t) is the proportion of individuals in age class (i) uncolonized and exposed to antibiotics, XV,j(i)(t) is the proportion of individuals in age class (i) colonized with vaccine-type pneumococci for which the MIC was 2(j−5) and unexposed to antibiotics, XNV,j(i)(t) is the proportion of individuals in age class (i) colonized with non-vaccine-type pneumococci for which the MIC was 2(j−5) and unexposed to antibiotics, YV,j(i)(t) is the proportion of individuals in age class (i) colonized with vaccine-type pneumococci for which the MIC was 2(j− 5) and exposed to antibiotics, YNV,j(i)(t) is the proportion of individuals in age class (i) colonized with non-vaccine-type pneumococci for which the MIC was 2(j−5) and exposed to antibiotics, Vnp(t) is the proportion of vaccinated individuals uncolonized and unexposed to antibiotics, Wnp(t) is the proportion of vaccinated individuals uncolonized and exposed to antibiotics, VNV,j(t) is the proportion of vaccinated individuals colonized with (non-vaccine-type) pneumococci for which the MIC was 2(j−5) and unexposed to antibiotics, and WNV,j(t) is the proportion of vaccinated individuals colonized with (non-vaccine-type) pneumococci for which the MIC was 2(j−5) and exposed to antibiotics.
Model parameters are as shown in Table 1 and Table 2, and the rate of vaccination is v.
REFERENCES
- 1.Advisory Committee on Immunization Practices. 2000. Preventing pneumococcal disease among infants and young children. Recommendations of the Advisory Committee on Immunization Practices (ACIP). Morb. Mortal. Wkly. Rep. Recomm. Rep. 49(RR-9):1-35. [PubMed] [Google Scholar]
- 2.Beaumel, C., L. Doisneau, and M. Vatan. 2003. La situation démographique en 2001. INSEE Résultats 18:11-34.
- 3.Blower, S. M., and H. Dowlatabadi. 1994. Sensitivity and uncertainty analysis of complex models of disease transmission: an HIV model, as an example. Int. Stat. Rev. 2:229-243. [Google Scholar]
- 4.Cartwright, K. 2002. Pneumococcal disease in western Europe: burden of disease, antibiotic resistance and management. Eur. J. Pediatr. 161:188-195. [DOI] [PubMed] [Google Scholar]
- 5.Dagan, R., and D. Fraser. 2000. Conjugate pneumococcal vaccine and antibiotic-resistant Streptococcus pneumoniae: herd immunity and reduction of otitis morbidity. Pediatr. Infect. Dis. J. 19:S79-S88. [DOI] [PubMed] [Google Scholar]
- 6.Dagan, R., N. Givon-Lavi, O. Zamir, M. Sikuler-Cohen, L. Guy, J. Janco, P. Yagupsky, and D. Fraser. 2002. Reduction of nasopharyngeal carriage of Streptococcus pneumoniae after administration of a 9-valent pneumococcal conjugate vaccine to toddlers attending day care centers. J. Infect. Dis. 185:927-936. [DOI] [PubMed] [Google Scholar]
- 7.Dagan, R., M. Sikuler-Cohen, O. Zamir, J. Janco, N. Givon-Lavi, and D. Fraser. 2001. Effect of a conjugate pneumococcal vaccine on the occurrence of respiratory infections and antibiotic use in day-care center attendees. Pediatr. Infect. Dis. J. 20:951-958. [DOI] [PubMed] [Google Scholar]
- 8.Eskola, J., T. Kilpi, A. Palmu, J. Jokinen, J. Haapakoski, E. Herva, A. Takala, H. Kayhty, P. Karma, R. Kohberger, G. Siber, and P. H. Makela. 2001. Efficacy of a pneumococcal conjugate vaccine against acute otitis media. N. Engl. J. Med. 344:403-409. [DOI] [PubMed] [Google Scholar]
- 9.Geslin, P., A. Fremaux, and G. Sissia. 1996. Epidemiology of Streptococcus pneumoniae antibiotic resistance. Arch. Pediatr. 3:93s-95s. [DOI] [PubMed] [Google Scholar]
- 10.Geslin, P., A. Fremaux, G. Sissia, and C. Spicq. 1998. Streptococcus pneumoniae: serotypes, invasive and antibiotic resistant strains. Current situation in France. Presse Med. 27(Suppl. 1):21-27. (In French.) [PubMed] [Google Scholar]
- 11.Gratten, M., J. Montgomery, G. Gerega, H. Gratten, H. Siwi, A. Poli, and G. Koki. 1989. Multiple colonization of the upper respiratory tract of Papua New Guinea children with Haemophilus influenzae and Streptococcus pneumoniae. Southeast Asian J. Trop. Med. Public Health 20:501-509. [PubMed] [Google Scholar]
- 12.Gray, B. M., G. M. Converse III, and H. C. Dillon, Jr. 1980. Epidemiologic studies of Streptococcus pneumoniae in infants: acquisition, carriage, and infection during the first 24 months of life. J. Infect. Dis. 142:923-933. [DOI] [PubMed] [Google Scholar]
- 13.Guillemot, D., C. Carbon, F. Vauzelle-Kervroedan, B. Balkau, P. Maison, G. Bouvenot, and E. Eschwege. 1998. Inappropriateness and variability of antibiotic prescription among French office-based physicians. J. Clin. Epidemiol. 51:61-68. [DOI] [PubMed] [Google Scholar]
- 14.Guillemot, D., P. Maison, C. Carbon, B. Balkau, F. Vauzelle-Kervroedan, C. Sermet, G. Bouvenot, and E. Eschwege. 1998. Trends in antimicrobial drug use in the community—France, 1981-1992. J. Infect. Dis. 177:492-497. [DOI] [PubMed] [Google Scholar]
- 15.Hansman, D., S. Morris, M. Gregory, and B. McDonald. 1985. Pneumococcal carriage amongst Australian aborigines in Alice Springs, Northern Territory. J. Hyg. (London) 95:677-684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klugman, K. P. 2001. Efficacy of pneumococcal conjugate vaccines and their effect on carriage and antimicrobial resistance. Lancet Infect. Dis. 1:85-91. [DOI] [PubMed] [Google Scholar]
- 17.Lipsitch, M. 1999. Bacterial vaccines and serotype replacement: lessons from Haemophilus influenzae and prospects for Streptococcus pneumoniae. Emerg. Infect. Dis. 5:336-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lipsitch, M. 1997. Vaccination against colonizing bacteria with multiple serotypes. Proc. Natl. Acad. Sci. USA 94:6571-6576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Metlay, J. P., J. Hofmann, M. S. Cetron, M. J. Fine, M. M. Farley, C. Whitney, and R. F. Breiman. 2000. Impact of penicillin susceptibility on medical outcomes for adult patients with bacteremic pneumococcal pneumonia. Clin. Infect. Dis. 30:520-528. [DOI] [PubMed] [Google Scholar]
- 20.Obaro, S. K., R. A. Adegbola, W. A. Banya, and B. M. Greenwood. 1996. Carriage of pneumococci after pneumococcal vaccination. Lancet 348:271-272. [DOI] [PubMed] [Google Scholar]
- 21.Obaro, S. K., M. A. Monteil, and D. C. Henderson. 1996. The pneumococcal problem. BMJ 312:1521-1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O'Brien, K. L., and R. Dagan. 2003. The potential indirect effect of conjugate pneumococcal vaccines. Vaccine 21:1815-1825. [DOI] [PubMed] [Google Scholar]
- 23.Raymond, J., I. Le Thomas, F. Moulin, A. Commeau, D. Gendrel, and P. Berche. 2000. Sequential colonization by Streptococcus pneumoniae of healthy children living in an orphanage. J. Infect. Dis. 181:1983-1988. [DOI] [PubMed] [Google Scholar]
- 24.Rowland, K. E., and J. D. Turnidge. 2000. The impact of penicillin resistance on the outcome of invasive Streptococcus pneumoniae infection in children. Aust. N. Z. J. Med. 30:441-449. [DOI] [PubMed] [Google Scholar]
- 25.Spratt, B. G., and B. M. Greenwood. 2000. Prevention of pneumococcal disease by vaccination: does serotype replacement matter? Lancet 356:1210-1211. [DOI] [PubMed] [Google Scholar]
- 26.Temime, L., P. Y. Boelle, P. Courvalin, and D. Guillemot. 2003. Bacterial resistance to penicillin G by decreased affinity of penicillin-binding proteins: a mathematical model. Emerg. Infect. Dis. 9:411-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Varon, E., and L. Gutmann. 2001. Rapport d'activité du CNRP. Centre National de Référence des Pneumocoques, Paris, France.
- 28.von Kries, R., A. Siedler, H. J. Schmitt, and R. R. Reinert. 2000. Proportion of invasive pneumococcal infections in German children preventable by pneumococcal conjugate vaccines. Clin. Infect. Dis. 31:482-487. [DOI] [PubMed] [Google Scholar]
- 29.Whitney, C. G., M. M. Farley, J. Hadler, L. H. Harrison, N. M. Bennett, R. Lynfield, A. Reingold, P. R. Cieslak, T. Pilishvili, D. Jackson, R. R. Facklam, J. H. Jorgensen, and A. Schuchat. 2003. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N. Engl. J. Med. 348:1737-1746. [DOI] [PubMed] [Google Scholar]