Abstract
Purpose
In this study, we introduce a methodology for preparing 18F-labeled Affibody protein, specifically 18F-Anti-HER2 dimeric Affibody (14 kDa), for in vivo imaging of HER2neu with positron emission tomography (PET).
Procedures
We have used 4-[18F]fluorobenzaldehyde as a synthon to prepare 18F-Anti-HER2 Affibody. Aminooxy-functionalized Affibody (Anti-HER2-ONH2) was incubated with 4-[18F] fluorobenzaldehyde in ammonium acetate buffer at pH 4 in the presence of methanol at 70°C for 15 min. The resulting 18F-labeled Affibody molecule was evaluated as a PET probe in xenograft models expressing HER2.
Results
We have successfully prepared 18F-Anti-HER2 dimeric Affibody (14 kDa), N-(4-[18F] fluorobenzylidine)oxime-Anti-HER2 Affibody, [18F]FBO-Anti-HER2, in 26–30% radiochemical yields (decay corrected). High-contrast small-animal PET images with relatively moderate tumor uptake (1.79±0.40% ID/g) were observed for the 18F-Anti-HER2 Affibody.
Conclusion
Site-specific 18F-labeled Affibody against HER2 has been synthesized via chemo-selective oxime formation between an aminooxy-functionalized Affibody and 18F-fluorobenzaldehyde. The results have implications for radiolabeling of other affibodies and macromolecules and should also be important for advancing Affibody imaging with PET.
Keywords: Affibody, HER2, Positron emission tomography (PET), Imaging, 18F
Introduction
Despite the high potential and interest in targeted [18F]-labeled macromolecules (e.g., proteins) for positron emission tomography (PET)-based molecular imaging of living subjects [1–2], imaging probes have been infrequent. The major reasons include (1) incompatibility of the short radioactive half-life of 18F with the pharmacokinetics of the biological molecules and (2) the difficulty of directly radiofluorinating typically fragile biomolecules and, furthermore, site-specifically to retain affinity. Affibody molecules [3–4], a novel class of proteins with molecular weight range of 6.5 to 14 kDa, have been engineered via phage display technology and have the potential to bind a variety of targets. Affibodies, specifically targeted against human epidermal growth factor receptor 2 (HER2, also known as HER2neu) labeled primarily with single-photon isotopes, including 99mTc [5], 125I [6], and 111In [7], have been reported, all of which show the affibodies to have in vivo pharmacokinetics compatible with short-lived isotopes. The molecular size in combination with the high in vivo stability of the affibodies show both blood clearance and target uptake in the minutes time frame with imaging possible in an hour or less. Furthermore, their three-helical bundle structure provides very high chemical stability at various pH and temperatures that may enable more direct [18F]-labeling of the molecules. Herein, we report a direct and high-yield synthetic approach that allows for site-specific [18F]-labeling of an Affibody molecule targeted against HER2, using oxime chemistry and preservation of the Affibody’s biological activity. The [18F]-labeled Affibody is also used to image tumors in vivo with microPET in living mice carrying tumor xenografts.
Recently, Poethko et al. [8] reported oxime formation between aminooxy-functionalized small peptides (decamers or less) and 18F-fluorobenzaldehyde as a high-yield two-step synthesis method to generate [18F]-labeled peptides that can be potentially translated to proteins. Unlike aminooxy functionalities, thiol moieties are more readily engineered into biologically generated proteins and have also been used for site-selective radiolableing via maleimide-based 18F-synthons. Examples include N-[4-(4-[18F]fluorobenzylidine) aminooxybutyl]maleimide (18F-FBABM) [9], N-[6-(4-[18F] fluorobenzylidine)aminooxyhexyl]maleimide (18F-FBAHM) [10], and N-[2-(4-18F-fluorobenzamido)ethyl]maleimide (18F-FBEM) [11]. While both aminooxy and maleimide chemistry appear relatively chemo-selective, the latter class of synthons suffer from laborious multi-step synthetic preparations leading to low yields compared to the former, where Poethko et al. consistently obtained yields of 60–80%, under tracer chemistry conditions [8]. To achieve both site selectivity and high yield of a diagnostically relevant protein, we sought to combine the two strategies [12–16]. However, compared to small peptides, a protein with distinct tertiary structural folds and multiple reactive groups is potentially more susceptible to loss of function post high exposure to organic reagents (e.g., methanol), acidic pH (or pH close to the protein’s isoelectric point), heat, or combinations thereof as used during 18F-aminooxy chemistry. Hence, an empirical demonstration that both chemoselectivity and Affibody’s target-binding ability are retained after 18F-aminooxy chemistry is warranted.
To test the feasibility of generating a functional 18F-Affibody protein, an anti-HER2 aminooxy-functionalized Affibody (Anti-HER2-ONH2, 3) was prepared from its corresponding 14 kDa, bivalent Anti-HER2 Affibody 1 construct (Scheme 1). The presence of an engineered and distal C-terminal cysteine residue of the Affibody molecule provides a thiol moiety upon which site-selectivity chemistry can be effected. In order to introduce the aminooxy group, a bifunctional linker (2) was synthesized consisting of two orthogonal groups: a thiol-reactive maleimide group for conjugation to the engineered cysteine and an 18F-aldehydereactive aminooxy group. This linker was prepared in one step by reacting N-(2-aminoethyl)malemide with 2-(tert-butoxycarbonylaminooxy)acetic acid using carbodiimide-mediated coupling conditions. On a subsequent step, the aminooxy group was exposed by cleavage of the Boc group under acidic conditions (3 M HCl). Introduction of the linker to the Affibody with a deprotected aminooxy does not additionally expose the protein to harsh conditions used for Boc-removal or produce observed adducts to the aminoxy group when using alternate acids such as trifluoroacetic acid. The linker was selectively conjugated to the Affibody by reduction of the disulfide-bridged Affibody dimer using dithiothreitol, followed by coupling at pH 7.4. Matrix-assisted laser desorption ionization mass spectroscopy (MALDI-MS) detected the presence of the expected product (calculated MW, 14431.26; found, 14431.45 Da).
Scheme 1.
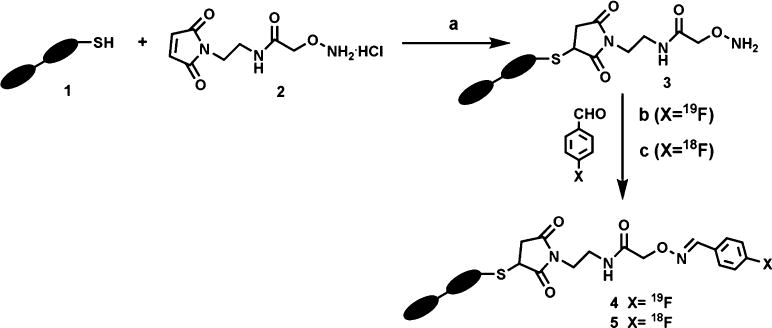
Reagents and conditions (yields): a 1% DMSO, phosphate-buffered saline, pH 7.4, 2 h (79%); b 1% DMSO, NH4OAc, pH 4, 1 h, 70°C (78%); c 33% CH3OH, NH4OAc, pH 4, 15 min, 70°C (26–32% decay corrected).
Subsequently, we wanted to understand the selectivity of the aldehyde towards the oxime over more predominant amine and other reactive groups on the Affibody. Aldehydes can selectively react with aminooxy groups to form an oxime bond, but amine groups can also potentially react with the aldehyde-forming Schiff bases. Given the difference in pKa between lysines and oximes (10.8 and 5, respectively) [17], tuning the pH reaction conditions can contribute to better selectivity. In addition, the reversible nature of Schiff bases against the non-reversible formation of oxime bonds supported the possible success in larger proteins [12–16].
To identify conditions favoring oxime formation over imines, a simple peptide model system utilizing “cold” chemistry that could be easily characterized was prepared. Two aminooxy-functionalized Arg-Gly-Asp (RGD) peptides and a control RGD peptide were designed and synthesized: NH2OCH2CO-Arg-Gly-Asp-NH2 (6) and NH2OCH2CO-Lys-Arg-Gly-Asp-NH2 (7), and Lys-Arg-Gly-Asp-NH2 (8) (Table 1), and their reactions with 4-fluorobenzaldehyde under various conditions were studied. Peptide 6 contains only a single aminooxy group, peptide 7 bears an aminooxy and an ε-amine (Lys), and 8 contains an ε-amine and two free N-terminal amines. When both 6 and 7 were reacted at the same conditions (8.5–150 equivalents, 45–60 min, 70°C, acetate buffer pH 4) with 4-fluorobenzaldehyde and studied by analytical high-performance liquid chromatography (HPLC), in each case only one product was observed. Electronspray ionization mass spectrometry (ESI-MS) detected the HPLC-corresponding expected products (calculated MH+ of P1 from 6, 523.21, found 523.30; and calculated MH+ of P2 from 7, 653.31, found 653.43; Table 1). To confirm the formation of the oxime and not a Schiff base, control peptide 8 was also reacted under the same oxime-forming conditions, and no imine product was detected under analysis conditions employed. It is also noteworthy to mention that only one product corresponding to a single addition of 4-fluorobenzaldehyde was observed when the aminooxy was in the presence of an amine even when the reaction was pushed with 150 equivalents of fluorobenzaldehyde. The fact that no imine formation was observed with control 8 even with this large excess leads us to believe that the one detectable product formed with models 6 and 7 indeed corresponds to the oxime product. More importantly, it strongly suggests that in a scenario where multiple amines are present with respect to aminooxy groups and tracer level chemistry, the formation of the oxime will still be the preferred product under these conditions. Similar results were observed by Poethko et al. [8], where they studied the specificity of amino acids argenine, histidine, serine, and lysine toward 4-[18F]fluorbenzaldehyde both in the presence and the absence of 2-aminooxyacetic acid.
Table 1.
Summary of results from control experiments performed with RGD peptides used as a model system to test the selectivity of 19F-fluorobenzaldehyde for aminooxy group vs. amine groups under specified conditions
| ||
|---|---|---|
| Peptides | Reaction time (min) | Product |
| NH2OCH2CO-Arg-Gly-Asp-NH2a,b | 60 | P1 |
| NH2OCH2CO-Arg-Gly-Asp-NH2 | 45 | P1 |
| NH2OCH2CO-Lys-Arg-Gly-Asp-NH2 | 90 | P2 |
| Lys-Arg-Gly-Asp-NH2 | 90 | No product |
Reaction was performed at pH 3.
8.5 Equivalents of 4-fluorobenzaldehyde was used.
We subsequently demonstrated the predominant selectivity of 4-fluorobenzaldehyde for aminooxy over the other groups in an Affibody under the identified conditions. Conjugations of Anti-HER2-ONH2 Affibody 3 with 4-fluorobenzaldehyde at different concentration (1, 2.5, 100, and 1,000 equivalents) at 25–70°C from 30 min to 1 h were performed in 1% dimethyl sulfoxide (DMSO) in acetate buffer pH 4 (Scheme 1b). We found that under these conditions, conjugate 4 formed only by addition of a single molecule of 4-flurobenzaldehyde to the Affibody molecule. No multiple fluorobenzaldehyde-labeled affibodies were observed as characterized by high-resolution MALDI-MS as well as ESI-MS, where in both techniques at least the qualitative detection of various Affibody adducts has been found in our lab to be similar. Given the results obtained with the previous model peptides, it is reasonable to believe that the one predominant product observed is the desired oxime product at the known position.
Surface Plasmon Resonance analysis using Biacore (GEHC) confirmed that the binding affinity of Anti-HER2-ONH2 3 is not significantly affected upon introduction of the fluorobenzaldehyde (FBO-Anti-HER2, 4). Addition of the bifunctional linker 2 is expected to have minimal and comparable results to blocking the thiol group using, for example, N-methyl-maleimide to prevent dimerization during BIAcore analysis. Figure 1 shows sensorgrams for the relative binding against HER2 at 100 nM concentration using chimeric HER2/Fc protein as a ligand. The HER2 binding affinities (Koff/Kon) of the conjugates were analyzed and determined from the kinetic analysis using BIAevaluation (GEHC) software. The fast on rate (7.9×105 and 9.3×105) and slow off rate (1.4×10−4 and 1.9×10−4) of conjugates 3 and 4, respectively, exhibit a high and comparable binding affinity of ~200 pM. These results suggest that conditions (acetate buffer pH 4 at 70°C) used for preparation of conjugate 4 did not affect its binding affinity. Stable 111In-labeled Affibody with high binding affinity has been prepared at high temperature (up to 90°C) [7].
Fig. 1.
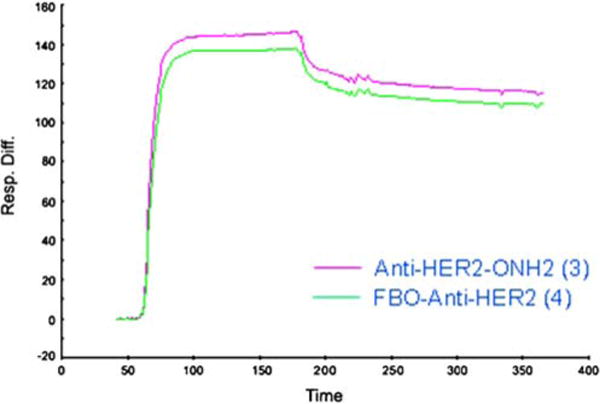
Relative binding sensorgram of Biacore surface plasmon resonance analysis of 100 nM Anti-HER2-ONH2 3 and 100 nM FBO-Anti-HER2 4 against chimeric HER2/Fc protein used as a ligand. Full kinetic analysis was performed on both conjugates at concentrations ranging from 0 nM to 100 nM to determine effect of modification of the conjugate upon modification on binding affinity.
The aminooxy-functionalized Affibody 3 was subsequently radiolabeled with 4-[18F]fluorobenzaldehyde ([18F] FBA). No carrier-added [18F] fluoride was prepared by the 18O(p,n)18F nuclear reaction on a GE PETtrace cyclotron. [18F]Fluoride processing and synthesis of 4-[18F]fluorobenzaldehyde were completed in the GE TRACERlab FX-FN synthesis module. No carrier-added [18F] fluoride trapped on a QMA cartridge (300–1,000 mCi) was washed with a solution of K2CO3 (3.5 mg) and kryptofix 2.2.2 (15 mg) in water (0.9 ml) and acetonitrile (0.1 ml; concentrations of K2CO3 and kryptofix were 25.3 mM and 39.8 mM, respectively). The solvent was removed under vacuum, and to the anhydrous residue was added a solution of 4-formyl-N,N,N-trimethylanilinium triflate precursor (4–6 mg) in DMSO (0.5 ml). The mixture was heated for 10 min at 85°C, and after cooling to room temperature, the reaction mixture was diluted with 5 ml of water. The mixture was loaded onto a preconditioned C18 cartridge. The cartridge was washed with 10 ml of 0.1 N HCl, and 4-[18F] fluorobenzaldehyde was eluted with 2 ml of methanol into a vial. The decay-corrected radiochemical yield was 50–70% (actual end of synthesis yields, 116–540 mCi), and the synthesis was completed in 40 min. To generate the radiolabeled probe, aminooxy-functionalized Anti-HER2-ONH2 Affibody 3 (14 kDa, 0.5 mg) in 100 μl of ammonium acetate at pH 4 (Scheme 1c) was incubated with 4-[18F] fluorobenzaldehyde (5–7 mCi in 50 μl of methanol) at 70°C for 15 min to afford [18F]FBO-Anti-HER2, 14 kDa (5) in 26–30% non-optimized decay-corrected radiochemical yield (actual end of synthesis yields, 1.0–1.6 mCi). Our radiochemical yield of [18F] Anti-HER2 Affibody (5) is lower than that of the [18F]-labeled peptides (60–80%) reported by Poethko et al. [8], despite our use of approximately 1.4 times the nanomole amount of Affibody. Likely explanations include (a) the concentration of aminooxy precursor (Anti-HER2-ONH2 Affibody 3, 0.23 mmol/l) that we used is less than the amount used for aminooxy-peptide precursor (0.5 mmol/l) or (b) the pH was higher (pH 4) in the case of [18F]-Anti-HER2 Affibody compared to those found to be optimal in the [18F]-peptides case (pH 2–3).
In order to gain initial insight into the in vivo imaging performance of [18F]-labeled Affibody molecules, we evaluated 5 in xenograft models expressing HER2. In vivo biological studies of [18F]-Anti-HER2 Affibody in SKOV3 xenograft-bearing nude mice (N=9) were investigated. Human ovarian cancer cells (SKOV3) have been well documented to express HER2 [18]. Table 2 shows the biodistribution of 5 in SKOV3 xenograft-bearing nude mice. The ratio of tumor to blood was 3.00±0.48 at 3 h of post-injection. High-contrast small animal PET images, with relatively moderate tumor uptake (1.79±0.40% ID/g) and low muscle uptake (0.44±0.01% ID/g) after 2 h of injection of 5, were observed (Fig. 2). However, heptabobiliary excretion was high and produced high accumulation of radioactivity in the abdomen. Although the cause of this observation is not clear, use of 18F-labeled smaller monovalent Affibody constructs (7 kDa) or reducing the lipophilicity of radiolabeled Affibody conjugates should be explored as supported by Affibody-related literature [6]. It was found that substitution of glycyl residues in the chelating sequence with more hydrophilic seryl residues is a promising method to alter the biodistribution of 99mTc-labelled Affibody molecules [19].
Table 2.
Biodistribution of [18F]Anti-HER2-Affibody 5 in mice bearing SKOV3 xenografts
| Organ (% ID/g) | 0.5 h | 1 h | 3 h |
|---|---|---|---|
| Blood | 3.81±0.5 | 1.56±0.29 | 0.36±0.03 |
| Liver | 12.02±1.57 | 4.28±0.55 | 1.35±0.19 |
| Muscle | 1.01±0.9 | 0.54±0.14 | 0.19±0.06 |
| Kidney | 20.90±3.37 | 7.41±0.92 | 2.08±0.45 |
| Intestine | 8.61±11.54 | 9.75±12.10 | 13.87±10.80 |
| Tumor | 2.03±0.31 | 2.23±0.51 | 1.08±0.15 |
Data are expressed as percentage of injected radioactivity per gram of organ or tissue (% ID/g) after intravenous injection of 5 (20–45 μCi) at different time post injection (N=3 for each group).
Fig. 2.
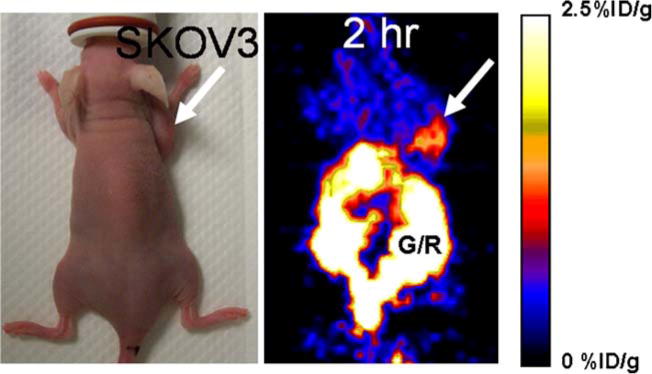
Decay-corrected coronal microPET images of a nude mouse (photograph shown on left) bearing SKOV3 xenograft at 2 h after tail injection of 50 μCi of [18F]Anti-HER2-Affibody 5. Arrow indicates location of tumor, and G/R is gastrointestinal/renal activity. Color bar represents % ID/g.
In conclusion, site-specific [18F]-labeled Affibody, [18F]-Anti-HER2 Affibody 5, 14 kDa, has been successfully generated utilizing oxime chemistry and used to image tumors in vivo. While our non-optimized yields were somewhat lower than previously demonstrated using similar chemistry for small peptides, a number of parameters exist for further exploration, e.g., smaller monovalent Affibody constructs, concentrations, etc. The combination of the oxime-based radiochemistry and highly stable, both chemically and in vivo, Affibody molecules suggests potential use of this platform to rapidly generate targeted [18F]-labeled molecular imaging probes for PET imaging.
Acknowledgments
This work was supported, in part, by Medical Diagnostics, GE Healthcare, National Cancer Institute (NCI) Small Animal Imaging Resource Program (SAIRP) grant R24 CA93862, and NCI In Vivo Cellular Molecular Imaging Center (ICMIC) grant P50 CA114747 (SSG). We also thank Dr. David Dick for [18F] production, Dr. Frederick T. Chin for modification of a GE TRACERlab FX-FN synthetic module for radiosynthesis of 4-[18F]FBA, and Dr. Alan Cuthbertson and Dr. Alex Gibson of GE Healthcare for their review of the manuscript.
References
- 1.Gambhir SS. Molecular imaging of cancer with positron emission tomography. Nat Rev Cancer. 2002;2:683–693. doi: 10.1038/nrc882. [DOI] [PubMed] [Google Scholar]
- 2.Varagnolo L, Stokkel MPM, Mazzi U, Pauwels EK. 18F-Labeled radiopharmaceuticals for PET in oncology, excluding FDG. J Nuc Med Biol. 2000;27:103–112. doi: 10.1016/s0969-8051(99)00109-2. [DOI] [PubMed] [Google Scholar]
- 3.Gunneriussoin E, Nord K, Uhlen M, Nygren P. Affinity maturation of a Taq DNA polymerase specific Affibody by helix shuffling. Protein Eng. 1999;12:873–878. doi: 10.1093/protein/12.10.873. [DOI] [PubMed] [Google Scholar]
- 4.Nord K, Gunneriusson E, Ringdahl J, Stahl S, Uhlen M, Nygren P-A. Binding protein selected from combinatorial libraries on an alpha-helical bacterial receptor domain. Nat Biotechnol. 1997;15:772–777. doi: 10.1038/nbt0897-772. [DOI] [PubMed] [Google Scholar]
- 5.Tran T, Engfeld T, Orlova A, et al. In vivo evaluation of cystine-based chelators for attachment of 99mTc to tumor-targeting Affibody molecules. Bioconjugate Chem. 2007;18:549–558. doi: 10.1021/bc060291m. [DOI] [PubMed] [Google Scholar]
- 6.Orlaova A, Magnusson M, Eriksson TL, et al. Tumor imaging using a picomolar affinity HER2 binding Affibody molecule. Cancer Res. 2006;66:4339–4348. doi: 10.1158/0008-5472.CAN-05-3521. [DOI] [PubMed] [Google Scholar]
- 7.Orlova A, Tolmachev V, Pehrson R, et al. Synthetic Affibody molecules: a novel class of affinity ligands for molecular imaging of HER2-expressing malignant tumors. Cancer Res. 2007;67:21788–2186. doi: 10.1158/0008-5472.CAN-06-2887. [DOI] [PubMed] [Google Scholar]
- 8.Poethko T, Schottelius M, Thumshirn G, et al. Two-step methodology for high yield routine radiohalogenation of peptides: 18F-labeled RGD and octreotide analogs. J Nucl Med. 2004;45:892–902. [PubMed] [Google Scholar]
- 9.Toyokuni T, Walsh JC, Dominguez A, Phelps ME, Barrio JR, Gambhir SS, Satyamurthy N. Synthesis of a new hetrobifunctional linker, N-[4-(aminooxy)butyl]maleimide, for facile access to a thiol-reactive 18F-labeling agent. Bioconjug Chem. 2003;14:1253–1259. doi: 10.1021/bc034107y. [DOI] [PubMed] [Google Scholar]
- 10.Berndt M, Pietzsch J, Wuest F. Labeling of low-density lipoproteins 18F-labeled thiol-reactive reagent N-[6-[18F]fluorobenzylidine)aminooxyhexyl]maeimide. Nucl Med Biol. 2007;34:5–25. doi: 10.1016/j.nucmedbio.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 11.Cai W, Zhang X, Wu Y, Chen X. A thiol-reactive 18F-labeling agent, N-[2-(4-18F-fluorobenzamido)ethyl]maleimide, and synthesis of RGD peptides-based tracer for PET imaging of αvβ3 integrine expression. J Nucl Med. 2006;47:172–180. [PMC free article] [PubMed] [Google Scholar]
- 12.Calzadilla M, Cordovoa T, Malpica A. Effect of structure on reactivity in oxime formation of benzaldehyde. J Phys Org Chem. 1990;12:408–712. [Google Scholar]
- 13.Rosenbeg S, Silver SM, Sayer JM, Jacks WP. Evidence for two concurrent mechanisms and a kinetically significant proton transfer in avid-catalyzed O-methyloxime formation. J Am Chem Soc. 1974;96:798. [Google Scholar]
- 14.Hang HC, Bertozzi CR. Chemoselective approaches to glycoprotein assembly. Acc Chem Res. 2001;34:727–736. doi: 10.1021/ar9901570. [DOI] [PubMed] [Google Scholar]
- 15.Cornish VW, Hahn KM, Schultz PG. Site-specific protein modification using a ketone handle. J Am Chem Soc. 1996;118:8150–8151. [Google Scholar]
- 16.Gilmore JM, Scheck RA, Esser-Kahn AP, Joshi NA, Francis MB. N-terminal protein modification through a biomimetic transamination reaction. Angew Chem Int Ed. 2006;45:5307–5311. doi: 10.1002/anie.200600368. [DOI] [PubMed] [Google Scholar]
- 17.Ruiz-Chica AJ, Medina MA, Sanchez-Jimenez F, Ramirez FJ. On the interpretation of Raman spectra of 1-aminooxy-spermine/DNA complex. Nucleic Acids Res. 2004;32:579–589. doi: 10.1093/nar/gkh232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu D, Wolf JK, Scanlon M, et al. Enhanced c-erbB-2/neu expression in human ovarian cancer cells correlates with more severe malignancy that can be suppressed by E1A. Cancer Research. 1993;53:891–898. [PubMed] [Google Scholar]
- 19.Engfeldt T, Tran T, Orlova A, et al. (99m)Tc-chelator engineering to improve tumor targeting properties of a HER2-specific Affibody molecule. Eur J Nucl Med Mol Imaging. 2007;34:1843–1853. doi: 10.1007/s00259-007-0474-6. [DOI] [PubMed] [Google Scholar]


