Abstract
This pilot study examined the effects of freeze-dried mango (Mangifera indica L.) supplementation on anthropometrics, body composition, and biochemical parameters in obese individuals. Twenty obese adults (11 males and 9 females) ages 20- to 50-years old, received 10 g/day of ground freeze-dried mango pulp for 12 weeks. Anthropometrics, biochemical parameters, and body composition were assessed at baseline and final visits of the study. After 12 weeks, mango supplementation significantly reduced blood glucose in both male (−4.45 mg/dL, P = 0.018) and female (−3.56 mg/dL, P = 0.003) participants. In addition, hip circumference was reduced in male (−3.3 cm, P = 0.048) but not in female participants. However, there were no significant changes in body weight or composition in either gender. Our findings indicate that regular consumption of freeze-dried mango by obese individuals does not negatively impact body weight but provides a positive effect on fasting blood glucose.
Keywords: mango, glucose, body composition, obesity, diabetes
Introduction
Obesity, defined as excessive adipose tissue and a body mass index (BMI) greater than 30 kg/m2, is a serious worldwide health problem with a complex multifactorial etiology.1 In 2005, the World Health Organization (WHO) estimated that 400 million adults were obese and approximately 1.6 billion adults were overweight. From 2007 to 2008, the prevalence of obesity in the USA alone was estimated to be approximately 34% among adults and 17.1% of children and adolescents were classified as overweight.2,3 The WHO projects that, by the year 2015, more than 700 million adults will be obese and another 2.3 billion adults will be overweight.1
Health concerns related to obesity extend not only from the presence of excessive adipose tissue but also include the potential complications and unprecedented parallel rise in several obesity-related chronic diseases, such as type 2 diabetes (T2DM) and metabolic syndrome.4 Currently, around 197 million individuals are glucose intolerant, which is mostly secondary to obesity and metabolic syndrome, with 90% of T2DM prevalence related to excess adiposity.5 Predictions are that, by the year 2025, 420 million individuals will have impairment in glucose metabolism. Therefore, identification of natural strategies that can reduce adiposity has the potential to effectively decrease the occurrence of obesity-related health complications, which can positively influence both the quality of life and life expectancy of obese individuals.
Dietary interventions focused on diets rich in fruits and vegetables that provide natural sources of bioactive compounds have demonstrated beneficial effects on body weight and blood glucose control.6,7 Among these fruits, mango (Mangifera indica L.) provides a number of well-known bioactive compounds that include carotenoids, tocopherols, ascorbic acid, dietary fiber, and the phenolic compounds mangiferin, gallic acid, and quercetin.8 Findings from our recent study demonstrate that freeze-dried mango pulp supplementation positively influenced body composition and improved blood glucose and lipid profile in mice fed a high-fat diet.9 In human studies, consumption of mango pulp, when compared to other fruits, favorably affected postprandial glucose and insulin responses in individuals with T2DM.10,11 In addition to the pulp, other parts of the mango plant also exhibit glucose-lowering properties. For example, an extract of the mango stem bark and foliage with a high concentration of mangiferin effectively lowered blood glucose in streptozotocin-induced diabetic rats and glucose-induced hyperglycemia in rodent models.12–14
Although the glucose-lowering properties of mango have been demonstrated in animal models and postprandially in human studies, research specifically designed to assess the effects of chronic mango supplementation on body composition and glucose parameters in obese adults has yet to be conducted. Therefore, this pilot study was designed to investigate the effects of 12-week ground freeze-dried mango pulp supplementation on anthropometric measurements, biochemical parameters, and body composition in obese individuals. In light of our previous findings in mice fed a high-fat diet and the results of others examining the effects of mango on adiposity and glucose tolerance, we hypothesized that the incorporation of mango pulp into the diet of obese adults would reduce body fat and improve glucose parameters.14–16 If dietary supplementation with mango favorably affects body weight and blood glucose, the findings of this study may provide obese individuals with another strategy to help reduce body fat and the risk of obesity-related chronic conditions, such as T2DM and metabolic syndrome.
Materials and Methods
Study design and subjects
Adults, 20- to 50-years old with a BMI of 30–45 kg/m2, were recruited from Stillwater, Oklahoma and surrounding communities. Individuals who met the BMI inclusion criteria with normal liver and kidney function, regardless of gender or ethnicity, were included in the study. Exclusion criteria included pre-existing medical conditions (e.g., diabetes, cancer, heart disease, liver, or renal disorders), pregnant or lactating individuals, and consumption of antioxidants and/or fish oil supplements greater than 1 g/day. Individuals who smoked or used any form of tobacco, consumed on average more than one ounce of alcohol per day, or had a known allergy to mango, or high sensitivity to poison ivy, poison oak, or poison sumac, were also excluded from the study.
The study protocol required four visits to the study site: pre-screening, baseline, and visits after 6 and 12 weeks of mango supplementation. Following the initial pre-screening interview, individuals underwent anthropometric measurements and completed medical history and physical activity questionnaires. Upon medical clearance and voluntary written informed consent, 22 obese adults (males, n = 13; females, n = 9) were enrolled in the study. Subjects were instructed to maintain their usual diet, exercise habits, and regimen of regularly prescribed medications. This pilot study was approved by the Oklahoma State University (OSU) Institutional Review Board.
Dietary intervention
The “Tommy Atkins” variety of mango was chosen for this study since this is the variety that demonstrated improved body composition and glucose response in our earlier animal study.9 Additionally, this mango variety is readily available in the US market, particularly from March to July and October to January. To prepare the powder supplement, ripe mangoes were purchased from a local grocery store, peeled, and the pulp was freeze-dried and ground. The ground freeze-dried mango powder was pre-weighed, placed in opaque plastic storage bags (10 g per bag), and stored in the freezer until ready for consumption. The rationale for choosing the dose of mango (10 g per day) is based on our animal findings in which 1% (by weight) freeze-dried mango added to a standard mouse diet was efficacious in reducing body fat.9
Subjects were provided a 6-week supply of pre-packaged ground freeze-dried ripe “Tommy Atkins” mango fruit powder at baseline and at midpoint of the study. Each subject was directed to consume one packet per day in whatever food form they prefer (excluding heating, cooking, or baking). At completion of the 12-week study, subjects underwent their final or post-intervention evaluation. Compliance was monitored by asking each study participant: (1) to record the day and time of mango consumption in a provided study calendar; (2) to return empty packets and any unused packets of mango powder at the midpoint and final visits; and (3) to report their status and any problems with mango powder consumption when telephoned or emailed on random dates between visits.
Dietary and physical activity analyses
The study participants were asked to maintain their normal dietary habits, except for the addition of the mango powder, during the 12-week study. To monitor dietary intake, a 3-day food record was assessed at baseline, and after 6 and 12 weeks of mango supplementation. Participants received instruction from trained study personnel on maintaining a diary of all food and beverage intake for three consecutive days (two weekdays and one weekend day) during each 6-week interval. Diet Analysis Plus 10.0 software (Cengage Learning, Inc., Independence, KY) was used for analysis of macronutrient and micronutrient intake.
To ensure that study participants did not significantly alter their exercise regimen throughout the study, a physical activity questionnaire (Physical Activity Scale 2) was administered at baseline and at 6- and 12-week visits by trained study personnel.17 The physical activity questionnaire was developed by Andersen and colleagues17 and assesses physical activity as daily time devoted to sleep, sitting, standing or walking, heavy physical work, transportation to and from work, and TV-viewing/reading. In addition, it assesses the amount of time spent weekly on performing light, moderately strenuous, and strenuous physical activity.
Anthropometric, blood pressure, and body composition measurements
While wearing minimal indoor clothing and without shoes, participants underwent measurements of height, weight, and circumferences of the waist and hip at baseline and after 6 and 12 weeks of mango supplementation. At each visit, participants were weighed on a flat surface with the same calibrated Healthometer Physician Balance Beam weight scale (Continental Scale Corp., Chicago, IL, USA) to the nearest ±0.1 kg. Height was measured to the nearest 0.1 cm with a scale-mounted Shorr Board stadiometer (Shorr Productions, Olney, MD, USA). BMI (kg/m2) was calculated from the weight and height measurements using appropriate conversions. Waist and hip circumferences were measured using a tape measure to the nearest 0.1 cm. The waist circumference was defined as the midpoint between the highest point of the iliac crest and the inferior portion of the costal margin in the mid-axillary plane. The hip circumference was determined at the level of the maximum posterior extension of the buttocks. After participants relaxed for a few minutes in a quiet room, blood pressure was measured twice using a digital blood pressure monitor (ReliOn, Oncue HealthCare Inc, Bennockburn, IL, USA).
Body composition was evaluated by dual-energy X-ray absorptiometry (DXA; Discovery A S/N 84671, Hologic Inc, Bedford, MA) at baseline and at the end of 12 weeks of mango supplementation. Participants dressed in light indoor clothing were scanned while lying in the standardized supine position with arms at their sides. Measurements of body composition from the DXA scan included percentage of fat mass and lean mass.
Laboratory measurements
For determination of biochemical parameters, a venous blood sample was drawn by a certified phlebotomist or registered nurse at baseline and after 12 weeks of mango supplementation. Blood analyses of fasting blood triglyceride, high-density lipoprotein (HDL)-cholesterol, glucose, and hemoglobin A1c were performed by an independent laboratory (Stillwater Medical Center, Still-water, OK). Plasma insulin concentration was assessed using a commercially available ELISA kit (EMD Millipore Corporation, St. Charles, MO). As an index of insulin resistance, the Homeostasis Model of Assessment-Insulin Resistance (HOMA-IR) was calculated using the formula, HOMA-IR = [fasting blood glucose level (mg/dL) × insulin (μU/mL)]/405.
Statistical analyses
Statistical analyses were conducted with SAS Version 9.2 (SAS Institute, Cary, NC). Analysis of variance methods were used (PROC GLM) to assess the effects of the main unit factor (either weight gain or gender performed in separate analysis) and time (baseline and after 12 weeks of the study). A repeated measures model was utilized, with subject as the main experimental unit. Simple effects of the main unit factor (either weight gain or gender) given time and time given the main unit factor were analyzed with planned comparisons. P value <0.05 was considered statistically significant. Means and standard deviations for each combination of main unit factor and time were reported.
Results
Anthropometric measurements, body composition, dietary intake, and physical activity
Dietary intervention for this study consisted of participants consuming 10 g of freeze-dried mango powder supplement daily while maintaining their usual eating and physical activity patterns. Mango contains a number of known bioactive compounds, which include the carotenoids, tocopherols, ascorbic acid, dietary fiber, and the phenolic compounds of mangiferin, gallic acid, and quercetin.8 The macronutrient composition of the mango powder used in the study (carbohydrate, protein, fat, and fiber contents by percent of weight are 89.6%, 4.01%, 1.62%, and 13.4%, respectively) was analyzed by NP Analytical Laboratories (St. Louis, MO). Each 10 g bag of mango powder provided 39 kcal.
Twenty-two subjects were recruited to receive the mango supplementation (13 males and 9 females). However, only 20 subjects completed the study; 55% were males (n = 11) and 45% females (n = 9). The two male participants who were excluded from the study did not show up during scheduled appointments or failed to report mango consumption data. Mean age of all participants was 36.5 ± 9.1 years with male subjects at 34.8 ± 9.6 years and female participants being 38.6 ± 8.6 years. There were no significant differences between male and female subjects for their age or baseline weight, BMI, and hip or waist circumference (Table 1). The effects of mango supplementation on anthropometric measurements, body composition, blood pressure, and serum HDL and triglyceride concentrations are presented in Table 1. After 12 weeks of mango supplementation, no changes were observed in overall body weight, hip or waist circumference, waist to hip ratio, and percentage of fat mass and lean mass of the study participants. However, there were differences by gender in hip circumference. Hip circumference was significantly lower in male subjects (−3.3 cm, P = 0.048) but not female subjects after mango supplementation (Table 1). Additionally, BMI tended to be higher in female subjects (+0.9 kg/m2, P = 0.062) but not in male subjects after mango supplementation. Overall and by gender, there were also no significant changes in blood pressure or serum HDL and triglyceride concentrations.
Table 1.
Effects of 12-week freeze-dried mango supplementation on anthropometric measurements, body composition, blood pressure, and serum HDL and triglyceride concentrations.1
| VARIABLES | OVERALL | GENDER | |||||||
|---|---|---|---|---|---|---|---|---|---|
| ALL SUBJECTS (n = 20) |
MALE (n = 11) |
FEMALE (n = 9) |
|||||||
| BASELINE | FINAL | P-VALUE | BASELINE | FINAL | P-VALUE | BASELINE | FINAL | P-VALUE | |
| Weight (kg) | 99.6 ± 15 | 99.8 ± 15 | 0.698 | 104 ± 13 | 103.8 ± 14 | 0.326 | 94 ± 15 | 95.0 ± 16 | 0.215 |
| BMI (kg/m2) | 34.6 ± 4.0 | 34.9 ± 4.3 | 0.257 | 34.2 ± 3.1 | 34.0 ± 3.4 | 0.351 | 35.1 ± 4.8 | 36.0 ± 5.1 | 0.062 |
| Waist circumference (cm) | 112.3 ± 11.2 | 112.2 ± 9.3 | 0.931 | 115.0 ± 9.3 | 113.1 ± 10.1 | 0.124 | 109.1 ± 13.0 | 111.1 ± 8.7 | 0.485 |
| Hip circumference (cm) | 118.9 ± 9.9 | 117.8 ± 10.7 | 0.366 | 116.1 ± 7.3 | 112.8 ± 6.8 | 0.048* | 122.4 ± 11.9 | 123.8 ± 11.8 | 0.438 |
| Waist:hip ratio | 0.95 ± 0.07 | 0.96 ± 0.07 | 0.336 | 0.99 ± 0.04 | 1.00 ± 0.06 | 0.401 | 0.89 ± 0.07 | 0.90 ± 0.03 | 0.643 |
| % Fat mass | 36.4 ± 6.7 | 36.6 ± 7.04 | 0.586 | 32.1 ± 5.4 | 32.0 ± 5.4 | 0.855 | 41.7 ± 3.4 | 42.3 ± 4.0 | 0.318 |
| Lean mass (kg) | 60.33 ± 10.02 | 60.39 ± 10.06 | 0.899 | 67.30 ± 4.99 | 67.30 ± 5.47 | 1.000 | 51.82 ± 7.70 | 51.95 ± 7.58 | 0.884 |
| Systolic blood pressure (mmHg) | 131 ± 13 | 129 ± 10 | 0.256 | 132 ± 13 | 129 ± 10 | 0.233 | 131 ± 15 | 128 ± 10 | 0.576 |
| Diastolic blood pressure (mmHg) | 85 ± 8 | 84 ± 7 | 0.405 | 84 ± 6 | 82 ± 5 | 0.125 | 86 ± 11 | 86 ± 10 | 0.922 |
| HDL (mg/dL) | 48.2 ± 12.8 | 48.2 ± 10.6 | 0.972 | 44.1 ± 9.8 | 44.4 ± 7.5 | 0.860 | 53.3 ± 14.6 | 52.8 ± 12.4 | 0.794 |
| Triglyceride (mg/dL) | 140.4 ± 65.6 | 121.3 ± 66.5 | 0.127 | 140.7 ± 60.6 | 117.3 ± 66.6 | 0.246 | 140.0 ± 75.1 | 126.2 ± 70.2 | 0.355 |
Notes:
Data are mean ± SD.
Indicates a statistically significant difference (P ≤0.05) from baseline to final values.
Abbreviations: BMI, body mass index; HDL, high-density lipoprotein.
From assessments of the 3-day food records and physical activity questionnaires, there were no significant changes in food intake and physical activity before and after 12 weeks of mango supplementation (Tables 2 and 3). More specifically, no significant differences were observed in either male or female dietary intake of macronutrients, saturated fat, total dietary fiber, vitamin C, or vitamin A (Table 2).
Table 2.
Dietary intake before and after 12 weeks of mango supplementation.1
| VARIABLES | OVERALL | GENDER | |||||||
|---|---|---|---|---|---|---|---|---|---|
| ALL SUBJECTS (n = 15) |
MALE (n = 8) |
FEMALE (n = 7) |
|||||||
| BASELINE | FINAL | P-VALUE | BASELINE | FINAL | P-VALUE | BASELINE | FINAL | P-VALUE | |
| Total energy (kcal) | 2200 ± 640 | 1995 ± 472 | 0.235 | 2341 ± 858 | 2102 ± 372 | 0.656 | 2096 ± 545 | 1823 ± 545 | 0.183 |
| Protein (g) | 82.3 ± 18.7 | 79.0 ± 14.4 | 0.535 | 91.8 ± 35.8 | 83.2 ± 12.7 | 0.797 | 84.4 ± 20.6 | 73.3 ± 14.6 | 0.113 |
| CHO (g) | 261 ± 92.7 | 234 ± 81.1 | 0.159 | 294 ± 101.6 | 249 ± 72.9 | 0.273 | 233 ± 72.4 | 211 ± 87.3 | 0.443 |
| Total fat (g) | 90.5 ± 34.6 | 79.1 ± 18.8 | 0.255 | 86.1 ± 43.7 | 79.1 ± 17.6 | 0.825 | 92.3 ± 36.7 | 77.2 ± 20.5 | 0.168 |
| Saturated fat (g) | 27.5 ± 10.5 | 26.0 ± 7.1 | 0.635 | 28.5 ± 16.4 | 26.0 ± 6.7 | 0.961 | 27.1 ± 9.0 | 25.5 ± 7.7 | 0.390 |
| Total dietary fiber (g) | 19.1 ± 8.77 | 17.7 ± 6.2 | 0.597 | 21.1 ± 10.2 | 19.5 ± 8.0 | 0.679 | 16.2 ± 3.1 | 15.7 ± 2.2 | 0.710 |
| Vitamin C (mg) | 76.1 ± 61.8 | 66.2 ± 35.3 | 0.577 | 71.5 ± 67.7 | 69.1 ± 41.3 | 0.727 | 73.5 ± 47.3 | 58.6 ± 30.3 | 0.607 |
| Vitamin A (IU) | 387 ± 207 | 277 ± 219 | 0.067 | 463 ± 240 | 246 ± 181 | 0.104 | 403 ± 230 | 319 ± 248 | 0.438 |
Notes:
Data are mean ± SD. Not including mango supplements.
Table 3.
Time spent on physical activity before and after 12 weeks of mango supplementation.1
| TIME (MINUTES) | OVERALL | GENDER | |||||||
|---|---|---|---|---|---|---|---|---|---|
| ALL SUBJECTS (n = 20) |
MALE (n = 11) |
FEMALE (n = 9) |
|||||||
| BASELINE | FINAL | P-VALUE | BASELINE | FINAL | P-VALUE | BASELINE | FINAL | P-VALUE | |
| Daily | |||||||||
| Sleep | 421 ± 59 | 415 ± 61 | 0.544 | 423 ± 45 | 428 ± 54 | 0.659 | 418 ± 76 | 399 ± 68 | 0.211 |
| Sedentary work | 341 ± 220 | 379 ± 229 | 0.597 | 326 ± 205 | 327 ± 190 | 0.663 | 360 ± 253 | 445 ± 268 | 0.212 |
| Standing/Walking | 188 ± 163 | 201 ± 169 | 0.36 | 205 ± 180 | 230 ± 176 | 0.316 | 165 ± 145 | 165 ± 165 | 1.000 |
| Heavy work | 26 ± 40 | 52 ± 90 | 0.257 | 21 ± 38 | 78 ± 113 | 0.415 | 33 ± 44 | 19 ± 27 | 0.258 |
| Bike/Walk to work | 13 ± 17 | 20 ± 25 | 0.221 | 14 ± 18 | 28 ± 29 | 0.059 | 13 ± 15 | 9 ± 14 | 0.663 |
| Leisure time | 211 ± 135 | 164 ± 126 | 0.168 | 217 ± 116 | 180 ± 156 | 0.478 | 203 ± 163 | 145 ± 79 | 0.190 |
| Weekly | |||||||||
| Light physical activity | 318 ± 456 | 350 ± 552 | 0.795 | 218 ± 249 | 335 ± 546 | 0.552 | 440 ± 622 | 367 ± 592 | 0.594 |
| Moderate activity | 168 ± 262 | 100 ± 90 | 0.167 | 105 ± 78 | 89 ± 84 | 0.581 | 245 ± 379 | 113 ± 100 | 0.219 |
| Heavy activity | 113 ± 117 | 96 ± 103 | 0.570 | 100 ± 126 | 69 ± 91 | 0.537 | 130 ± 110 | 130 ± 111 | 1.000 |
Note:
Data are mean ± SD.
Evaluation of self-reported physical activity revealed no overall change as well as no change in both genders on daily or weekly physical activities, whether categorized as light, moderate, or heavy. However, daily minutes spent on bike/walk to work tended (P = 0.059) to increase in male but not female participants during the 12-week study. There was also no significant difference apparent in time spent sleeping for either gender (Table 3).
Glucose parameters
A 12-week dietary supplementation of mango significantly reduced overall blood glucose (−4.1 mg/dL, P < 0.001) in obese individuals (Table 4). The glucose-lowering effect of mango was observed in both male (−4.5 mg/dL, P = 0.018) and female (−3.6 mg/dL, P = 0.003) participants (Table 4). The mean change in blood glucose concentrations by gender after 12 weeks of mango supplementation is presented in Figure 1. Along with the reduction in blood glucose levels, insulin levels in males (+2.2 μU/mL, P = 0.032) were significantly increased after the 12-week supplementation period though similar reductions were not observed among female participants. After mango supplementation, there was no change in glycated hemoglobin or in HOMA-IR (Table 4).
Table 4.
Effects of 12-week freeze-dried mango supplementation on glucose parameters.1
| VARIABLES | OVERALL | GENDER | |||||||
|---|---|---|---|---|---|---|---|---|---|
| ALL SUBJECTS (n = 20) |
MALE (n = 11) |
FEMALE (n = 9) |
|||||||
| BASELINE | FINAL | P-VALUE | BASELINE | FINAL | P-VALUE | BASELINE | FINAL | P-VALUE | |
| Glucose (mg/dL) | 94.4 ± 7.2 | 90.3 ± 5.5 | <0.001* | 95.6 ± 7.4 | 91.1 ± 5.6 | 0.018* | 92.9 ± 7.1 | 89.3 ± 5.7 | 0.003* |
| Hgb A1C (%) | 5.3 ± 0.3 | 5.3 ± 0.3 | 0.970 | 5.3 ± 0.4 | 5.3 ± 0.4 | 0.427 | 5.2 ± 0.1 | 5.3 ± 0.2 | 0.361 |
| Insulin (μU/mL) | 14.5 ± 9.6 | 14.9 ± 7.9 | 0.847 | 10.1 ± 4.5 | 12.3 ± 1.8 | 0.032* | 20.0 ± 12 | 18.0 ± 9.2 | 0.553 |
| HOMA-IR | 3.4 ± 2.2 | 3.3 ± 1.7 | 0.833 | 2.4 ± 1.0 | 2.7 ± 1.3 | 0.119 | 4.6 ± 2.6 | 4.0 ± 2.0 | 0.418 |
Notes:
Data are mean ± SD.
Statistically different (P ≤ 0.05) from baseline to final values.
Abbreviations: Hgb A1C, hemoglobin A1C; HOMA-IR, Homeostasis Model of Assessment-Insulin Resistance.
Figure 1.
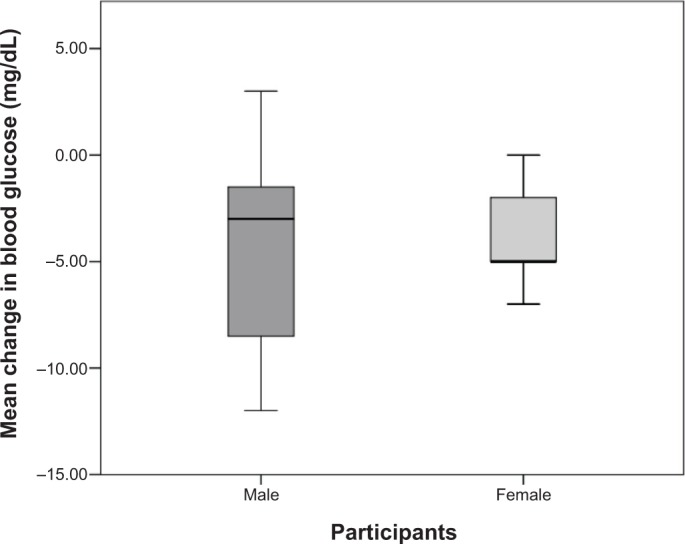
Mean change in blood glucose after 12 weeks of mango supplementation by gender.
Discussion
Current dietary recommendations promote the increased consumption of fruits and vegetables that are rich in antioxidants to reduce the risk of developing chronic conditions, such as cardiovascular disease, obesity, T2DM, and/or metabolic syndrome.18 Perhaps one protective benefit of increased fruit and vegetable consumption is a result of bioactive food components that exhibit unique biological properties to aid in the reduction of body fat and lowering of blood glucose. For example, anthocyanins found in blueberries and cyanidin 3-0-beta-D-glucoside-rich purple corn color diminish the development of obesity in mice, while the consumption of raisins may lower the risk of diabetes or heart disease in humans.6,7,19 In our mouse model of diet-induced obesity, mango supplementation (1% by weight) reduced adiposity, lowered fasting blood glucose, and improved glucose tolerance.9
Due to the increased incidence of obesity and obesity-related chronic conditions, such as T2DM and metabolic syndrome, the beneficial effects of bioactive substances and phyto-chemicals from fruits and vegetables to reduce body fat and blood glucose are of considerable interest.9 This pilot study was designed to explore the extent to which the beneficial effects of mango on body composition and blood glucose, as demonstrated in our earlier animal study, could be observed in humans. In the current study, we investigated the effects of a 12-week ground freeze-dried mango supplementation on anthropometrics, biochemical parameters, and body composition in obese individuals at a dose of 10 g/day. This dose of mango is approximately equivalent to about one half of fresh mango fruit (100 g) and is about 1% dried mango when calculated on a per total dietary intake on a per dry weight basis, a dose we found effective in our earlier animal study.9 Due to differences in micronutrient content and bioactive food components among different mango varieties, we utilized the same Tommy Atkins variety of mango used in our previous animal study.9 Furthermore, the “Tommy Atkins” mango is one of the most widely grown and readily available varieties in the USA with a long shelf life and low tolerance to deterioration.
Mango, a tropical fruit, is a rich dietary source of carotenoids, ascorbic acid, soluble fiber, flavonoids, and a variety of phenolic compounds known to exhibit antioxidant and antidiabetic properties in animal models.14,15,20 Among the bioactive compounds found in mango, mangiferin is thought to be the primary active substance responsible for its hypoglycemic properties. Mangiferin, present in the seeds (0.42 mg/kg), peel (1690.4 mg/kg), and pulp (4.4 mg/kg) of the mango, is a xanthone with high antioxidative activity that contributes to the beneficial effects of mango on blood glucose.20 For example, extract of mango stem bark and leaves, which is rich in mangiferin, was found to effectively lower blood glucose in streptozotocin-induced diabetic rats and glucose-induced hyperglycemia in rats and mice.12,13 From the study using the rat model, the hypoglycemic effect is speculated to result from mangiferin’s interference with the glucosidase enzymes of sucrase, isomaltase, and maltase that are responsible for carbohydrate digestion, which results in a decrease in glucose intestinal absorption.20 In addition to the phenolic compounds, mango is also a rich source of dietary fiber, which can also reduce digestion of carbohydrate and lower glucose absorption. The precise component and molecular mechanisms for the glucose-lowering properties of mango have yet to be elucidated.
In agreement with our previous findings in animals, dietary mango supplementation reduced blood glucose in obese individuals, which is of importance since this population is at an increased risk for T2DM. It is interesting to note that the decrease in fasting glucose occurred without a significant reduction in body weight, which may suggest that mango acts independently of changes in body weight or fat. Despite the beneficial effects of mango supplementation on fasting blood glucose, we did not observe any effects of mango supplementation on HOMA-IR, an index of insulin resistance. These inconsistencies between our current and previous studies may indicate that mango has more effect on the day-to-day blood glucose concentrations and may exhibit modest, if any, effects on long-term glucose control. In support of this notion are the findings of Roongpisuthpong and colleagues indicating that mango significantly reduces the glucose area postprandial response in T2DM subjects.10
In the present study, we also demonstrate that mango supplementation was not able to modulate body composition in obese individuals, unlike in our animal model of diet-induced obesity (i.e., mice fed a high-fat diet).9 Modulating body composition may require a longer duration of mango supplementation to show a significant change. Additionally, mango might be able to prevent an increase in body fat but is unable to reduce the fat once it has accumulated in our body. There is also evidence of other functional food, such as brindleberry (Garcinia cambogia), with anti-obesity effects in animal studies that has failed to have similar effects in human subjects.21 The findings of the present study demonstrate that mango consumption neither increase nor decrease body weight or fat in obese individuals but may still prove beneficial as a result of improving blood glucose.
To the best of our knowledge, the present study is the first to investigate the effects of mango supplementation on body fat and blood glucose levels in obese individuals. The findings related to the improvement of blood glucose levels in individuals with obesity are promising when considering the close relationship of obesity with T2DM. Mango supplementation may offer an innovative dietary intervention in modulating blood glucose without negative effects on body composition.
This pilot study is not without limitations. Study results could have been influenced by various factors, such as the small sample size, the lack of a control group, the duration of the mango supplementation, or from an inaccuracy in subjects self-reporting dietary intake, and physical activity level. A small sample size may allow the presence of any outliers to have a greater influence on the study outcomes. Being an exploratory study, there was no control group in this study, which may affect control of unforeseen variables and eliminate placebo effect. Being a 12-week intervention of mango supplementation, there may have been not enough time for some individuals to experience the metabolic or physiologic changes necessary to show an effect. In regards to diet and activity, subjects may under- or overestimate their dietary intake or physical activity levels, which could mask the effects of mango supplementation. A limitation could have also occurred if compliance with daily mango supplementation was not maintained as per study protocol, which would result in a skewed effect.
Conclusion
Although it did not induce weight loss, our findings indicate that regular consumption of mango by obese adults provides a positive effect on their blood glucose. Further clinical trials with larger sample sizes and longer duration of mango supplementation are necessary.
Acknowledgments
We would like to thank the study participants, the staff at the Stillwater Medical Center, and the Oklahoma State University Department of Nutritional Sciences for support with this research.
Footnotes
Author Contributions
Experimental design: EL, BS, SC, and PP. Preparation of the freeze-dried mango powder: PP. Statistical analyses: MP. Research procedures: EL, MM, SE, MM, HE, and SP. First draft of the manuscript: SE. Joint revisions and final approved version of paper: EL, SE, SC, BS, and PP. All authors reviewed and approved of the final manuscript.
ACADEMIC EDITOR: Joseph Zhou, Editor in Chief
FUNDING: This study was supported by the Oklahoma State University College of Human Sciences and through a grant from the National Mango Board. The National Mango Board had no involvement in the study design, data collection, data analysis, manuscript preparation, or decision in manuscript submission.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
This paper was subject to independent, expert peer review by a minimum of two blind peer reviewers. All editorial decisions were made by the independent academic editor. All authors have provided signed confirmation of their compliance with ethical and legal obligations including (but not limited to) use of any copyrighted material, compliance with ICMJE authorship and competing interests disclosure guidelines and, where applicable, compliance with legal and ethical guidelines on human and animal research participants.
REFERENCES
- 1.WHO Obesity. 2008. Available at http://www.who.int/topics/obesity/en/
- 2.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295(13):1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 3.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA. 2012;307(5):491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 4.Malnick SDH, Knobler H. The medical complications of obesity. QJM. 2006;99(9):565–579. doi: 10.1093/qjmed/hcl085. [DOI] [PubMed] [Google Scholar]
- 5.Hossain P. Obesity and diabetes in the developing world—a growing challenge (vol 356, pg 213, 2007) N Engl J Med. 2007;356(9):973–973. doi: 10.1056/NEJMp068177. [DOI] [PubMed] [Google Scholar]
- 6.Prior RL, Wilkes SE, Rogers TR, Khanal RC, Wu XL, Howard LR. Purified blueberry anthocyanins and blueberry juice alter development of obesity in mice fed an obesogenic high-fat diet. J Agric Food Chem. 2010;58(7):3970–3976. doi: 10.1021/jf902852d. [DOI] [PubMed] [Google Scholar]
- 7.Tsuda T, Horio F, Uchida K, Aoki H, Osawa T. Dietary cyanidin 3-O-beta-D-glucoside-rich purple corn color prevents obesity and ameliorates hyperglycemia in mice. J Nutr. 2003;133(7):2125–2130. doi: 10.1093/jn/133.7.2125. [DOI] [PubMed] [Google Scholar]
- 8.U.S. Department of Agriculture ARS USDA National Nutrient Database for Standard Reference, Release 18. 2005. Available at http://www.ars.usda.gov/ba/bhnrc/ndl.
- 9.Lucas EA, Li W, Peterson SK, et al. Mango modulates body fat and plasma glucose and lipids in mice fed a high-fat diet. Br J Nutr. 2011;106(10):1495–1505. doi: 10.1017/S0007114511002066. [DOI] [PubMed] [Google Scholar]
- 10.Roongpisuthipong C, Banphotkasem S, Komindr S, Tanphaichitr V. Postprandial glucose and insulin responses to various tropical fruits of equivalent carbohydrate content in non-insulin-dependent diabetes-mellitus. Diabetes Res Clin Pract. 1991;14(2):123–131. doi: 10.1016/0168-8227(91)90118-w. [DOI] [PubMed] [Google Scholar]
- 11.Contractor Z, Hussain F, Jabbar A. Postprandial glucose response to mango, banana and sapota. JPMA. 1999;49(9):215–216. [PubMed] [Google Scholar]
- 12.Aderibigbe AO, Emudianughe TS, Lawal BA. Evaluation of the antidiabetic action of Mangifera indica in mice. Phytother Res. 2001;15(5):456–458. doi: 10.1002/ptr.859. [DOI] [PubMed] [Google Scholar]
- 13.Aderibigbe AO, Emudianughe TS, Lawal BA. Antihyperglycaemic effect of Mangifera indica in rat. Phytother Res. 1999;13(6):504–507. doi: 10.1002/(sici)1099-1573(199909)13:6<504::aid-ptr533>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 14.Ojewole JAO. Antiinflammatory, analgesic and hypoglycemic effects of Mangifera indica Linn. (Anacardiaceae) stem-bark aqueous extract. Methods Find Exp Clin Pharmacol. 2005;27(8):547–554. doi: 10.1358/mf.2005.27.8.928308. [DOI] [PubMed] [Google Scholar]
- 15.Muruganandan S, Srinivasan K, Gupta S, Gupta PK, Lal J. Effect of mangiferin on hyperglycemia and atherogenicity in streptozotocin diabetic rats. J Ethnopharmacol. 2005;97(3):497–501. doi: 10.1016/j.jep.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 16.Beltran AE, Alvarez Y, Xavier FE, et al. Vascular effects of the Mangifera indica L. extract (Vimang) Eur J Pharmacol. 2004;499(3):297–305. doi: 10.1016/j.ejphar.2004.07.073. [DOI] [PubMed] [Google Scholar]
- 17.Andersen LG, Groenvold M, Jorgensen T, Aadahl M. Construct validity of a revised physical activity scale and testing by cognitive interviewing. Scand J Public Health. 2010;38(7):707–714. doi: 10.1177/1403494810380099. [DOI] [PubMed] [Google Scholar]
- 18.Castanho GKF, Marsola FC, McLellan KCP, Nicola M, Moreto F, Burini RC. Consumption of fruit and vegetables associated with the Metabolic syndrome and its components in an adult population sample. Cienc Saude Coletiva. 2013;18(2):385–392. doi: 10.1590/s1413-81232013000200010. [DOI] [PubMed] [Google Scholar]
- 19.Anderson JW, Waters AR. Raisin consumption by humans: effects on glycemia and insulinemia and cardiovascular risk factors. J Food Sci. 2013;78(suppl 1):A11–A17. doi: 10.1111/1750-3841.12071. [DOI] [PubMed] [Google Scholar]
- 20.Masibo M, Qian H. Major mango polyphenols and their potential significance to human health. Compr Rev Food Sci Food Saf. 2008;7:309–319. doi: 10.1111/j.1541-4337.2008.00047.x. [DOI] [PubMed] [Google Scholar]
- 21.Heymsfield SB, Allison DB, Vasselli JR, Pietrobelli A, Greenfield D, Nunez C. Garcinia cambogia (hydroxycitric acid) as a potential antiobesity agent: a randomized controlled trial. JAMA. 1998;280(18):1596–1600. doi: 10.1001/jama.280.18.1596. [DOI] [PubMed] [Google Scholar]


