Abstract
Objective
To evaluate the efficacy and safety of subcutaneously administered fasinumab (REGN475), a nerve growth factor-neutralizing antibody, in patients with acute sciatic pain receiving standard of care therapy.
Methods
This was a double-blind, parallel-group, proof-of-concept study. Patients with unilateral, moderate-to-severe sciatic pain of 2–16 weeks’ duration were randomized to a subcutaneous dose of placebo (n=51), fasinumab 0.1 mg/kg (n=53), or 0.3 mg/kg (n=53); follow-up was 12 weeks. Pain was assessed in a daily diary using a numerical rating scale (NRS) (0= no pain, 10= worst pain) for average and worst leg and back pain. The primary efficacy end point was the area under the curve of NRS scores for average leg pain from baseline to week 4. Key secondary end points included changes in average and worst leg and back pain from baseline to the end of week 4 and to each weekly study visit. Patient functioning (Oswestry Disability Index) and concomitant analgesic use were also assessed. Safety and tolerability were evaluated by treatment-emergent adverse events (TEAEs).
Results
Demographic and clinical characteristics were similar among the treatment groups; 141 (88.7%) patients completed the study. For the primary end point, mean ± standard deviation area under the curve values from baseline to week 4 were not significantly different between placebo (96.8±6.0) and fasinumab 0.1 mg/kg (112.7±58.3; P=0.0610) or fasinumab 0.3 mg/kg (112.4±55.8; P=0.0923). All secondary efficacy end points of changes in pain and function demonstrated responses that were similar between placebo and fasinumab groups. Incidence of TEAEs was 45.1%, 50.9%, and 64.8% in the placebo, fasinumab 0.1mg/kg, and fasinumab 0.3 mg/kg groups, respectively. The most commonly reported TEAEs included paresthesia, arthralgia, pain in extremity, and headache.
Conclusion
Administration of fasinumab provided no significant clinical benefit compared with placebo for the pain or functional limitations associated with acute sciatica. Fasinumab was generally well tolerated and incidence of TEAEs appeared to be dose related.
Keywords: fasinumab, monoclonal antibody, nerve growth factor, sciatica, lumbar radiculopathy
Introduction
Sciatica, also known as lumbar radiculopathy, is a set of symptoms usually caused by nerve root compression and irritation or inflammation of the sciatic nerve or one or more of its five nerve roots.1,2 Although accurate data on the prevalence of sciatica are lacking, studies have reported a range of 1.2%–43%,3 and estimates suggest an annual incidence of 1%–5% for acute episodes.1
Sciatica is most often characterized by acute pain, usually confined to one side of the body, which may be present in the lower back, buttocks, and various parts of the leg, including the foot.4 In addition to pain, symptoms may include numbness, muscular weakness, and difficulty moving and controlling the leg.4 Sciatica alone or combined with concurrent low back pain may be treated with nonsteroidal anti-inflammatory drugs (NSAIDs),5 systemic corticosteroids,6,7 and other pharmacologic and nonpharmacologic supportive therapies, with variable efficacy.8 The currently available drugs for sciatic pain provide only modest, short-term benefits at best and are often associated with safety concerns.6,7,9,10 Consequently, a need exists for therapies with improved efficacy and safety.
To meet this need, biologic agents that target tumor necrosis factor-α (TNF-α), a cytokine integral to the inflammatory response in musculoskeletal conditions, were evaluated in sciatica, but with mixed results. Early open-label studies of intravenous infliximab (an anti-TNF monoclonal antibody) and subcutaneous etanercept (a soluble form of the TNF receptor) suggested benefits,11,12 but subsequent randomized trials of these drugs failed to demonstrate maintenance of long-term efficacy,13–15 albeit these trials had low numbers of patients. Although one trial of the anti-TNF-α monoclonal antibody adalimumab suggested improvement among 31 patients with radicular leg pain due to lumbar disc herniation, the effect size was small.16
Neurotrophins are a family of polypeptide growth factors that help regulate pathways of development, differentiation, survival, and death of neuronal and non-neuronal cells.17 The first neurotrophin to be identified was nerve growth factor (NGF), and its role in the development and survival of peripheral and central neurons in the developing nervous system has been characterized in vivo.18,19 However, in the normal adult, NGF is not required as a survival factor but acts as a pain mediator that sensitizes neurons.20–22
NGF activity is mediated through two different membrane-bound receptors, the high-affinity tropomyosin receptor kinase A (TrkA) receptor and the low-affinity p75 common neurotrophin receptor.23,24 The NGF/TrkA system appears to play a major role in the control of inflammation and pain, and blockade of this pathway normalizes pain sensitivity.21,25
Following tissue injury or inflammation, NGF appears to modulate pain in chronic musculoskeletal pain disorders where inflammation is involved.26 A study by Purmessur et al27 on expression of the neurotrophins NGF and brain-derived neurotrophic factor in the human intervertebral disc suggested that these factors may contribute to the type of pain typically seen in sciatica. Because NGF may be associated with nerve compression and sciatic pain, it was hypothesized that anti-NGF activity could reduce the pain and associated disability.
Fasinumab (REGN475) is a recombinant, fully human, anti-NGF monoclonal antibody (immunoglobulin G4) that binds to NGF and blocks its signaling through TrkA and p75 receptors (Regeneron Pharmaceuticals, Inc., data on file). This placebo-controlled proof-of-concept study was undertaken to evaluate the efficacy of fasinumab as an adjunct to standard of care in patients with moderate-to-severe acute sciatic pain.
Methods
Study design and patients
This was a randomized, double-blind, parallel-group, single-dose study to evaluate the efficacy and safety of two dose levels of fasinumab compared with placebo in patients with acute sciatic pain. The study received approval from the appropriate institutional review boards and was performed in accordance with the revised Declaration of Helsinki; all patients provided written informed consent prior to participation.
Screening occurred from day −14 to day −3, and on day 1 eligible patients were randomized 1:1:1 to receive a single subcutaneous dose of fasinumab 0.1 mg/kg or fasinumab 0.3 mg/kg or placebo. Randomization was stratified by the duration of pain at the time of the screening visit (2–8 weeks and >8–16 weeks). Study drug was administered on day 1, and patients returned to the clinic for study visits at the end of weeks 1, 2, 3, 4, 6, 8, 10, and 12.
The use of NSAIDs (aspirin, naproxen, and ibuprofen), commonly used opioid/opioid combination analgesics (eg, morphine immediate release, hydrocodone with acetaminophen, and tramadol), or other drugs for pain, such as the anticonvulsant gabapentin, was permitted as standard of care. Any nonpharmacologic modality (eg, physical therapy or chiropractic procedures) administered from the time the informed consent was signed to the final study visit was considered concomitant therapy.
Eligible patients were adult men and women aged 18–65 years and weighing <120 kg who were experiencing unilateral, moderate-to-severe sciatic pain, defined as a score ≥4 on an eleven-point pain numerical rating scale (NRS) (0= no pain; 10= worst possible pain) at both the screening and baseline visits while receiving standard of care treatment administered by their treating physician. Patients were required to have a confirmed diagnosis of sciatica or lumbosacral radiculopathy at the screening visit based upon leg pain radiating to or below the knee in a dermatomal pattern consistent with L4, L5, or S1, and a positive straight leg raising test (<60°). They were also required to have had a radiographic examination (plain film or computed tomography) to exclude other conditions. In order to evaluate the treatment of patients with acute sciatica, the onset of pain had to have been within 2–16 weeks prior to the screening visit. For patients with recurrent sciatic pain, the prior episode must have resolved ≥3 months prior to the onset of the current episode.
Patients were excluded for back surgery within 6 months of the screening visit, radiating leg pain resulting from piriformis syndrome, neurological deficits from any cause other than sciatica, and other current neurological conditions that could confound the study results. Any other medical condition that could have interfered with the conduct of the study was also reason for exclusion.
Outcomes
Patients rated their average and worst leg and back pain using the NRS at the screening and the baseline clinic visits daily for 6 weeks following baseline (collected using an interactive voice response system) and at each study visit thereafter. Patients also completed the Oswestry Disability Index (ODI) questionnaire28 at each clinic visit and the Patient Global Impression of Change (PGIC) at each postbaseline clinic visit. The ODI is a condition-specific functional outcome measure that assesses the impact of low back pain on functional abilities and activities. The PGIC is a global assessment scale that rates the patients’ perception of their response to treatment using a seven-point Likert scale (1= very much improved to 7= very much worse).29
The primary efficacy end point was the area under the curve (AUC) of pain versus time for average leg pain from baseline to the end of week 4 as measured using the daily NRS. Key secondary end points included the AUC for average leg pain from baseline to the end of week 6, as well as changes in NRS average leg pain, NRS worst leg pain, and ODI from baseline to the end of week 4 and to each study visit. The proportion of patients with 30% and 50% reductions in leg pain from baseline to the end of week 4 was also evaluated; 30% and 50% pain reductions, which represent changes of moderate and substantial clinical importance, respectively,30 are recommended by the Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT) as outcomes in pain clinical trials.31
Safety and tolerability were evaluated based on incidence of treatment-related adverse events (TEAEs). In addition, vital signs were recorded and a neurological examination was performed at each clinic visit. A physical examination and electrocardiogram (ECG) was performed at the screening and week 12 visits, and laboratory samples for hematology, chemistry, and urinalysis were collected at specified times throughout the study.
Statistical analysis
It was estimated that enrolment of 50 patients per treatment group would provide approximately 96% power to detect a clinically relevant change of 1.4 in the NRS score from baseline between fasinumab and the placebo treatment group, assuming the common standard deviation (SD) was 1.75 points with a two-sided test at the 0.05 significance level.32
The efficacy population included all randomized patients who received at least one dose of study medication and had both baseline assessment and at least one postbaseline assessment. The safety population included all randomized patients who received study medication.
Demographic and baseline characteristics were summarized using descriptive statistics with means and SDs for continuous variables, and frequencies and percentages for categorical variables. An analysis of covariance (ANCOVA) model was used to evaluate the primary efficacy end point, AUC for average leg pain between baseline and week 4 (postbaseline day 28), as well as the secondary end point of AUC from baseline to week 6. The ANCOVA model included treatment and stratum of duration of pain as fixed factors, and baseline NRS as covariate. Fisher’s exact test was used to compare the responder rates (proportion of patients with 30% and 50% pain reductions). Other efficacy end points were evaluated using a mixed-effect model repeated measure approach. The model included factors (fixed effects) for treatment, stratum of pain duration, treatment-by-visit interaction, and baseline value as a covariate. Missing NRS values were imputed with the postbaseline value during the on-treatment period by the last observation carried forward procedure. Least squares means by treatment group were estimated with their 95% confidence intervals (95% CI). Student’s t-tests were used for comparisons of each dose with placebo.
Results
Patients
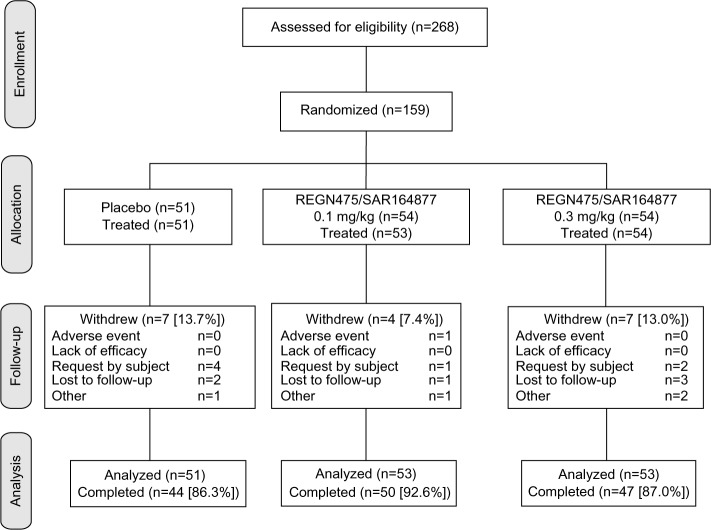
Of 268 patients screened, 159 were randomized, 158 received treatment, and 141 (88.7%) completed the study (Figure 1). The most common reasons for study withdrawal were patient request (4.4%) and lost to follow-up (3.8%), and only one patient withdrew due to adverse events. Demographic and clinical characteristics were similar among the three treatment groups (Table 1); treatment groups were balanced in respect of sex (47.1% male), were primarily white (73.9%), and had a tendency toward obesity (mean ± SD body mass index 29.7±5.2 kg/m2). The majority of patients (62.7%–71.7%) had experienced their sciatic pain for 2–8 weeks, and the mean baseline NRS scores for average leg pain ranged between 6.0 and 6.2 across the treatment groups (Table 1).
Figure 1.
Patient disposition.
Table 1.
Baseline demographic and clinical characteristics
| Variable | Value
|
||
|---|---|---|---|
| Placebo (n=51) |
Fasinumab
|
||
| 0.1 mg/kg (n=53) |
0.3 mg/kg (n=53) |
||
| Age, years, mean ± SD | 47.3±11.9 | 45.6±12.9 | 42.6±11.6 |
| Sex, n (%) | |||
| Male | 23 (45.1) | 25 (47.2) | 26 (49.1) |
| Female | 28 (54.9) | 28 (52.8) | 27 (50.9) |
| Weight, kg, mean ± SD | 90.8±14.6 | 83.3±15.8 | 84.5±18.6 |
| Body mass index, kg/m2, mean ± SD | 31.5±5.2 | 28.3±4.3 | 29.4±5.6 |
| Hispanic or Latino ethnicity, n (%) | 7 (13.7) | 12 (22.6) | 9 (17.0) |
| Race, n (%) | |||
| White | 37 (72.5) | 40 (75.5) | 39 (73.6) |
| African American | 11 (21.6) | 10 (18.9) | 12 (20.8) |
| Asian | 0 | 1 (1.9) | 1 (1.9) |
| Other | 3 (5.9) | 2 (3.7) | 2 (3.7) |
| Duration of sciatic pain, n (%) | |||
| 2–8 weeks | 32 (62.7) | 37 (69.8) | 38 (71.7) |
| 8–16 weeks | 19 (37.3) | 16 (30.2) | 15 (28.3) |
| Average leg pain, NRS, mean ± SD | 6.2±1.5 | 6.0±1.2 | 6.1±1.4 |
| Worst leg pain, NRS, mean ± SD | 7.7±1.5 | 7.7±1.2 | 7.8±1.4 |
| Average back pain, NRS, mean ± SD | 6.2±1.9 | 6.0±1.6 | 6.1±1.8 |
| Worst back pain, NRS, mean ± SD | 7.4±2.0 | 7.8±1.5 | 7.7±2.0 |
| ODI, mean ± SD | 0.4±0.1 | 0.4±0.1 | 0.4±0.2 |
Abbreviations: NRS, numeric rating scale; ODI, Oswestry Disability Index; SD, standard deviation.
Efficacy
At week 4 there was no significant difference between the fasinumab and placebo groups for the primary efficacy end point (Table 2). The least squares mean difference between placebo and fasinumab was 19.9 (95% CI −0.9, 40.7; P=0.0610) for the 0.1 mg/kg dose and 17.8 (95% CI −3.0, 38.6; P=0.0923) for the 0.3 mg/kg dose. Results were similar when stratified by pain duration (Table 2). Secondary pain end points at week 4 paralleled the primary end point, with values that showed similar reductions from baseline for placebo and fasinumab (Table 2).
Table 2.
Efficacy at week 4
| Outcome | Mean ± SD
|
||
|---|---|---|---|
| Placebo (n=51) |
Fasinumab
|
||
| 0.1 mg/kg (n=53) |
0.3 mg/kg (n=53) |
||
| AUC of average leg pain (baseline to week 4) | |||
| All patients | 96.8±56.0 | 112.7±58.3a | 112.4±55.8b |
| Patients with pain duration 2–8 weeks | 95.3±56.3 | 114.9±60.5 | 111.9±54.7 |
| Patients with pain duration >8–16 weeks | 99.3±57.0 | 107.6±54.3 | 113.6±60.6 |
| Change in average leg pain NRS | −3.0±2.5 | −2.3±2.2 | −2.9±2.4 |
| Change in worst leg pain NRS | −3.7±2.9 | −3.2±2.5 | −3.7±2.8 |
| Change in average back pain NRS | −2.6±2.3 | −2.1±2.3 | −2.8±2.2 |
| Change in worst back pain NRS | −3.1±2.8 | −3.1±2.7 | −3.6±2.5 |
| Change in ODI | −0.1±0.2 | −0.1±0.1 | −0.2±0.1 |
| PGIC score | 3.0±1.1 | 2.8±1.2 | 2.6±1.0 |
Notes:
P=0.0610 and
P=0.0923 using an analysis of covariance model with treatment and duration of pain as fixed factors and baseline pain score as a covariate.
Abbreviations: AUC, area under the curve; NRS, numerical rating scale; ODI, Oswestry Disability Index; PGIC, Patient Global Impression of Change; SD, standard deviation.
The AUC of average daily leg pain through week 6 demonstrated a similar response to that observed at week 4, although the absolute values at week 6 were higher: 138.9±85.6, 162.2±92.6, and 153.6±83.0 for placebo, fasinumab 0.1 mg/kg, and fasinumab 0.3 mg/kg, respectively.
A reduction from baseline in average daily leg pain NRS score was observed in all treatment groups at 1 week after initiating therapy, and these scores continued to decline gradually throughout the assessment period in all treatment groups (Figure 2). Although the ODI also showed a gradual decrease over time, indicating improvement in function, changes from baseline were similar in all treatment groups (data not shown). Similar patterns were observed over the study duration for average back pain, as well as worst leg and back pain (data not shown). At week 4, the proportions of patients who had experienced 30% and 50% reductions in average and worst leg pain were numerically highest in the placebo group. In contrast, for average and worst back pain, the fasinumab 0.3 mg/kg group had the numerically highest proportions of patients achieving 30% and 50% pain reductions (Table 3).
Figure 2.
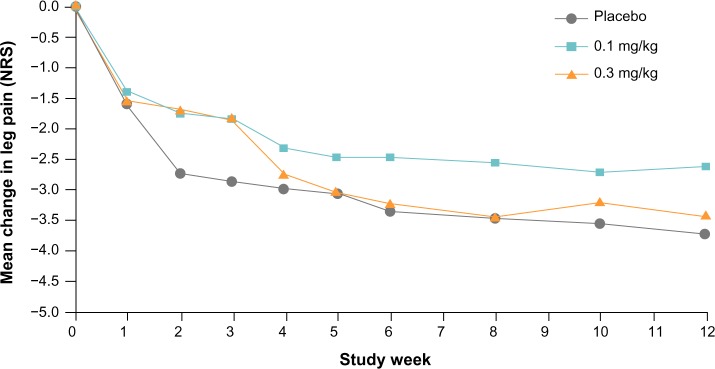
Weekly least squares mean change from baseline in average daily leg pain numerical rating scale (NRS) score. Values are for the full analysis set using last observation carried forward.
Table 3.
Proportion of patients with 30% and 50% reduction in pain at week 4
| Response category | Number of patients (%)
|
||
|---|---|---|---|
| Placebo (n=51) |
Fasinumab
|
||
| 0.1 mg/kg (n=53) |
0.3 mg/kg (n=53) |
||
| 30% reduction in pain | |||
| Average leg pain | 33 (64.7) | 32 (60.4) | 33 (62.3) |
| Worst leg pain | 34 (66.7) | 33 (62.3) | 35 (66.0) |
| Average back pain | 32 (62.7) | 28 (52.8) | 38 (71.7) |
| Worst back pain | 34 (66.7) | 30 (56.6) | 37 (69.8) |
| 50% reduction in pain | |||
| Average leg pain | 28 (54.9) | 22 (41.5) | 24 (45.3) |
| Worst leg pain | 30 (58.8) | 21 (39.6) | 26 (49.1) |
| Average back pain | 22 (43.1) | 19 (35.8) | 26 (49.1) |
| Worst back pain | 19 (37.3) | 21 (39.6) | 26 (49.1) |
The mean PGIC scores at week 1 were 2.8±1.2 in the fasinumab 0.3 mg/kg group and 2.8±1.1 in both the fasinumab 0.1 mg/kg and placebo groups, indicating that patients considered their overall status to range between “minimally improved” to “much improved”. This level of improvement was sustained throughout the 12-week assessment period, with final scores of 2.9±1.6, 3.3±1.5, and 2.9±1.5 for placebo, fasinumab 0.1 mg/kg, and fasinumab 0.3 mg/kg, respectively.
The two most common classes of medication that were used concomitantly by the patients in this study were 1) anti-inflammatory and antirheumatic products, with propionic acid derivatives such as naproxen and ibuprofen the most frequent agents, and 2) analgesics, with opioid and opioid combination therapies the most commonly used agents in this class. Both classes of medication were used with a similar frequency among the treatment groups and ranged from 51.0% (placebo) to 60.4% (fasinumab 0.1 mg/kg) for the former, and from 46.3% (fasinumab 0.3 mg/kg) to 50.9% (fasinumab 0.1 mg/kg) in the latter. Mean duration of concomitant analgesic use was 68.6 days in the 0.3 mg/kg treatment group, 80.6 days in the 0.1 mg/kg treatment group, and 76.2 days with placebo. The median duration of analgesic use was 85 days in all groups.
Safety and tolerability
The incidence of TEAEs was 45.1% in the placebo group, 50.9% in the 0.1 mg/kg group, and 64.8% in the 0.3 mg/kg group (Table 4). Most TEAEs were transient in nature and of mild-to-moderate severity; only six patients reported severe TEAEs, two in the placebo group and four with fasinumab 0.3 mg/kg. Serious AEs were reported by one patient in the placebo group (paranoia), two patients in the fasinumab 0.1 mg/kg group (hepatitis B and intervertebral disc protrusion), and two patients in the fasinumab 0.3 mg/kg group (major depression and intervertebral disc protrusion), but none was considered related to treatment. There was only one discontinuation due to a TEAE: moderate back pain in a patient in the fasinumab 0.1 mg/kg group.
Table 4.
Treatment-emergent adverse events (TEAEs)
| TEAE | Number of patients (%)*
|
|||
|---|---|---|---|---|
| Placebo (n=51) |
Fasinumab
|
|||
| 0.1 mg/kg (n=53) |
0.3 mg/kg (n=54) |
All doses (n=107) |
||
| Any TEAE | 23 (45.1) | 27 (50.9) | 35 (64.8) | 62 (57.9) |
| Serious TEAEs | 1 (2.0) | 1 (1.9) | 2 (3.7) | 3 (2.8) |
| TEAEs resulting in study discontinuation | 0 | 1 (1.9) | 0 | 1 (1.9) |
| Treatment-related TEAEs | 6 (11.8) | 7 (13.2) | 13 (24.1) | 20 (18.7) |
| Most common TEAEs, occurring in ≥2% of combined fasinumab doses | ||||
| Paresthesia | 0 | 1 (1.9) | 10 (18.5) | 11 (10.3) |
| Arthralgia | 3 (5.9) | 2 (3.8) | 8 (14.8) | 10 (9.3) |
| Pain in extremity | 1 (2.0) | 0 | 8 (14.8) | 8 (7.5) |
| Headache | 2 (3.9) | 2 (3.8) | 5 (9.3) | 7 (6.5) |
| Anxiety | 0 | 2 (3.8) | 3 (5.6) | 5 (4.7) |
| Nausea | 1 (2.0) | 3 (5.7) | 2 (3.7) | 5 (4.7) |
| Upper respiratory tract infection | 3 (5.9) | 1 (1.9) | 4 (7.4) | 5 (4.7) |
| Nasopharyngitis | 1 (2.0) | 1 (1.9) | 3 (5.6) | 4 (3.7) |
| Decreased vibratory sense | 1 (2.0) | 2 (3.8) | 1 (1.9) | 3 (2.8) |
| Dizziness | 1 (2.0) | 2 (3.8) | 1 (1.9) | 3 (2.8) |
| Hypoesthesia | 0 | 1 (1.9) | 2 (3.7) | 3 (2.8) |
| Muscle spasms | 0 | 0 | 3 (5.6) | 3 (2.8) |
| Myalgia | 0 | 2 (3.8) | 1 (1.9) | 3 (2.8) |
| Urinary tract infection | 1 (2.0) | 2 (3.8) | 1 (1.9) | 3 (2.8) |
Note:
Safety analysis set.
The most common AEs were paresthesia, arthralgia, pain in an extremity, and headache (Table 4), and their incidence was highest with fasinumab 0.3 mg/kg. Adverse events related to abnormal peripheral or musculoskeletal sensation (hypoesthesia, paresthesia, myalgia, and arthralgia) were also reported as common TEAEs, and most appeared to be dose related (Table 4). No clinically significant changes in laboratory assessments, vital signs, ECG, and physical examinations were observed.
Discussion
The results of this study demonstrated that treatment with fasinumab for sciatic pain, at doses that were effective for reducing pain in osteoarthritis of the knee,33 provided no significant improvement in measures of leg and back pain or daily function compared with placebo. Fasinumab was generally well tolerated, although a possible dose-related increase in the incidence of TEAEs was observed. In particular, abnormal peripheral and musculoskeletal sensations were reported following administration of fasinumab. A similar pattern of neurosensory and neuromuscular adverse events has been reported with fasinumab,33 as well as with other anti-NGF antibodies.34–36 The mechanisms underlying these transient events are not yet understood, but they may represent a class effect.
The results from this trial are somewhat disappointing in light of the suggestion that anti-NGF therapy may be appropriate for sciatica based on expression of neurotrophins in relevant anatomical regions of the human intervertebral disc.27 However, these results are consistent with a meta-analysis of the few studies of anti-NGF therapy for chronic low back pain, which reported only low evidence for anti-NGF therapy for this condition.37 It should also be noted that the studies of anti-TNF-α biologic agents have, for the most part, failed to demonstrate efficacy for pain relief in sciatica.13–15 In contrast to sciatica and chronic low back pain, efficacy for the reduction of pain in osteoarthritis has been demonstrated with the anti-NGF agents fasinumab,33 tanezumab,34,38,39 and fulranumab.36
Several reasons can be proposed for the lack of efficacy with fasinumab and most other biologic drugs in sciatic pain. This condition may represent mixed pain states consisting of pathways different from those involved in other painful musculoskeletal conditions. Nevertheless, there was no prior reason to suggest that fasinumab would not work on either nociceptive or neuropathic pain, as anti-NGF agents have demonstrated efficacy for osteoarthritis,33,34,38,39 primarily a nociceptive pain condition, and limited efficacy for at least some neuropathic pain conditions: eg, painful diabetic peripheral neuropathy.40,41 Additionally, it is possible that these drugs do not effectively reach the site of action mediating the sciatic pain. As another explanation that may have accounted for the lack of an effect in the earlier studies was the timing of the assessments, efficacy in the current study was assessed at week 4 using both time-integrated (AUC) and landmark analyses. This time point was chosen both because of the self-limiting nature of many cases of sciatica and because it was hypothesized that evaluation at an early time point might enhance the ability to demonstrate treatment benefit. A previous clinical trial of infliximab that showed no difference between active and placebo treatment used a 12-week primary end point.13 However, the results of the current study do not support the earlier assessment hypothesis.
The diagnostic criteria for this study relied on the clinical signs and symptoms associated with the syndrome of sciatic pain that are commonly used by general practitioners to diagnose this condition. The diagnosis did not require confirmation of disc herniation based upon imaging (magnetic resonance or computerized tomography), as radiological confirmation is rarely conducted in the general practice setting unless infection or neoplasm is suspected. Thus, based upon this diagnostic approach, it is possible that at least some of these patients did not have radiculopathy due to disc herniation but had low back pain with leg radiation.
Taken together, the available data suggest that sciatica is a complex and variable disorder, and that the pathogenesis of acute sciatic pain is not yet sufficiently understood for effective targeted treatment.
Acknowledgments
The authors would like to acknowledge the editorial assistance of E Jay Bienen, who was funded by Regeneron Pharmaceuticals, Inc., in the preparation of this manuscript.
Footnotes
Disclosure
Paul J Tiseo, Haobo Ren, and Scott Mellis are employees and stockholders of Regeneron Pharmaceuticals, Inc. The authors have no further conflicts of interest in this work.
References
- 1.Stafford MA, Peng P, Hill DA. Sciatica: a review of history, epidemiology, pathogenesis, and the role of epidural steroid injection in management. Br J Anaesth. 2007;99(4):461–473. doi: 10.1093/bja/aem238. [DOI] [PubMed] [Google Scholar]
- 2.Freemont AJ. The cellular pathobiology of the degenerate intervertebral disc and discogenic back pain. Rheumatology (Oxford) 2009;48(1):5–10. doi: 10.1093/rheumatology/ken396. [DOI] [PubMed] [Google Scholar]
- 3.Konstantinou K, Dunn KM. Sciatica: review of epidemiological studies and prevalence estimates. Spine (Phila Pa 1976) 2008;33(22):2464–2472. doi: 10.1097/BRS.0b013e318183a4a2. [DOI] [PubMed] [Google Scholar]
- 4.Valat JP, Genevay S, Marty M, Rozenberg S, Koes B. Sciatica. Best Pract Res Clin Rheumatol. 2010;24(2):241–252. doi: 10.1016/j.berh.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 5.Roelofs PD, Deyo RA, Koes BW, Scholten RJ, van Tulder MW. Non-steroidal anti-inflammatory drugs for low back pain. Cochrane Database Syst Rev. 2008;(1):CD000396. doi: 10.1002/14651858.CD000396.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holve RL, Barkan H. Oral steroids in initial treatment of acute sciatica. J Am Board Fam Med. 2008;21(5):469–474. doi: 10.3122/jabfm.2008.05.070220. [DOI] [PubMed] [Google Scholar]
- 7.Roncoroni C, Baillet A, Durand M, Gaudin P, Juvin R. Efficacy and tolerance of systemic steroids in sciatica: a systematic review and meta-analysis. Rheumatology (Oxford) 2011;50(9):1603–1611. doi: 10.1093/rheumatology/ker151. [DOI] [PubMed] [Google Scholar]
- 8.Lewis RA, Williams NH, Sutton AJ, et al. Comparative clinical effectiveness of management strategies for sciatica: systematic review and network meta-analyses. Spine J. 2013 Oct 4; doi: 10.1016/j.spinee.2013.08.049. Epub. [DOI] [PubMed] [Google Scholar]
- 9.Pinto RZ, Maher CG, Ferreira ML, et al. Drugs for relief of pain in patients with sciatica: systematic review and meta-analysis. BMJ. 2012;344:e497. doi: 10.1136/bmj.e497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chou R, Huffman LH. Medications for acute and chronic low back pain: a review of the evidence for an American Pain Society/American College of Physicians clinical practice guideline. Ann Intern Med. 2007;147(7):505–514. doi: 10.7326/0003-4819-147-7-200710020-00008. [DOI] [PubMed] [Google Scholar]
- 11.Karppinen J, Korhonen T, Malmivaara A, et al. Tumor necrosis factor-alpha monoclonal antibody, infliximab, used to manage severe sciatica. Spine (Phila Pa 1976) 2003;28(8):750–753. [PubMed] [Google Scholar]
- 12.Genevay S, Stingelin S, Gabay C. Efficacy of etanercept in the treatment of acute, severe sciatica: a pilot study. Ann Rheum Dis. 2004;63(9):1120–1123. doi: 10.1136/ard.2003.016451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Korhonen T, Karppinen J, Paimela L, et al. The treatment of disc herniation-induced sciatica with infliximab: results of a randomized, controlled, 3-month follow-up study. Spine (Phila Pa 1976) 2005;30(24):2724–2728. doi: 10.1097/01.brs.0000190815.13764.64. [DOI] [PubMed] [Google Scholar]
- 14.Korhonen T, Karppinen J, Paimela L, et al. The treatment of disc-herniation-induced sciatica with infliximab: one-year follow-up results of FIRST II, a randomized controlled trial. Spine (Phila Pa 1976) 2006;31(24):2759–2766. doi: 10.1097/01.brs.0000245873.23876.1e. [DOI] [PubMed] [Google Scholar]
- 15.Okoro T, Tafazal SI, Longworth S, Sell PJ. Tumor necrosis alpha-blocking agent (etanercept): a triple blind randomized controlled trial of its use in treatment of sciatica. J Spinal Disord Tech. 2010;23(1):74–77. doi: 10.1097/BSD.0b013e31819afdc4. [DOI] [PubMed] [Google Scholar]
- 16.Genevay S, Viatte S, Finckh A, Zufferey P, Balague F, Gabay C. Adalimumab in severe and acute sciatica: a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2010;62(8):2339–2346. doi: 10.1002/art.27499. [DOI] [PubMed] [Google Scholar]
- 17.Chao MV, Rajagopal R, Lee FS. Neurotrophin signalling in health and disease. Clin Sci (Lond) 2006;110(2):167–173. doi: 10.1042/CS20050163. [DOI] [PubMed] [Google Scholar]
- 18.Crowley C, Spencer SD, Nishimura MC, et al. Mice lacking nerve growth factor display perinatal loss of sensory and sympathetic neurons yet develop basal forebrain cholinergic neurons. Cell. 1994;76(6):1001–1011. doi: 10.1016/0092-8674(94)90378-6. [DOI] [PubMed] [Google Scholar]
- 19.Smeyne RJ, Klein R, Schnapp A, et al. Severe sensory and sympathetic neuropathies in mice carrying a disrupted Trk/NGF receptor gene. Nature. 1994;368(6468):246–249. doi: 10.1038/368246a0. [DOI] [PubMed] [Google Scholar]
- 20.McGonagle D, Tan AL, Shankaranarayana S, Madden J, Emery P, McDermott MF. Management of treatment resistant inflammation of acute on chronic tophaceous gout with anakinra. Ann Rheum Dis. 2007;66(12):1683–1684. doi: 10.1136/ard.2007.073759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McMahon SB, Bennett DL, Priestley JV, Shelton DL. The biological effects of endogenous nerve growth factor on adult sensory neurons revealed by a trkA-IgG fusion molecule. Nat Med. 1995;1(8):774–780. doi: 10.1038/nm0895-774. [DOI] [PubMed] [Google Scholar]
- 22.Pezet S, McMahon SB. Neurotrophins: mediators and modulators of pain. Annu Rev Neurosci. 2006;29:507–538. doi: 10.1146/annurev.neuro.29.051605.112929. [DOI] [PubMed] [Google Scholar]
- 23.Kaplan DR, Miller FD. Neurotrophin signal transduction in the nervous system. Curr Opin Neurobiol. 2000;10(3):381–391. doi: 10.1016/s0959-4388(00)00092-1. [DOI] [PubMed] [Google Scholar]
- 24.Allen SJ, Dawbarn D. Clinical relevance of the neurotrophins and their receptors. Clin Sci. 2006;110:175–191. doi: 10.1042/CS20050161. [DOI] [PubMed] [Google Scholar]
- 25.Woolf CJ, Safieh-Garabedian B, Ma QP, Crilly P, Winter J. Nerve growth factor contributes to the generation of inflammatory sensory hypersensitivity. Neuroscience. 1994;62(2):327–331. doi: 10.1016/0306-4522(94)90366-2. [DOI] [PubMed] [Google Scholar]
- 26.Seidel MF, Herguijuela M, Forkert R, Otten U. Nerve growth factor in rheumatic diseases. Semin Arthritis Rheum. 2010;40(2):109–126. doi: 10.1016/j.semarthrit.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 27.Purmessur D, Freemont AJ, Hoyland JA. Expression and regulation of neurotrophins in the nondegenerate and degenerate human intervertebral disc. Arthritis Res Ther. 2008;10(4):R99. doi: 10.1186/ar2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fairbank JC, Couper J, Davies JB, O’Brien JP. The Oswestry low back pain disability questionnaire. Physiotherapy. 1980;66(8):271–273. [PubMed] [Google Scholar]
- 29.Guy W, US Department of Health, Education, and Welfare publication (ADM) ECDEU Assessment Manual for Psychopharmacology, Revised. Rockville, MD: National Institute of Mental Health; 1976. [Google Scholar]
- 30.Dworkin RH, Turk DC, Wyrwich KW, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. 2008;9(2):105–121. doi: 10.1016/j.jpain.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 31.Dworkin RH, Turk DC, Farrar JT, et al. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. 2005;113:9–19. doi: 10.1016/j.pain.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 32.Katz N, Borenstein D, Birbara C, et al. Tanezumab, an anti-nerve growth factor (NGF) antibody, for the treatment of chronic low back pain (CLBP): a randomized, controlled, double-blind, phase 2 trial [abstract] J Pain. 2009;10(Suppl):S42. [Google Scholar]
- 33.Tiseo PJ, Kivitz AJ, Ervin JE, Ren H, Mellis SJ. Fasinumab (REGN475), an antibody against nerve growth factor for the treatment of pain: results from a double-blind, randomized, placebo-controlled exploratory study in osteoarthritis of the knee. Pain. 2014;155(7):1245–1252. doi: 10.1016/j.pain.2014.03.018. [DOI] [PubMed] [Google Scholar]
- 34.Lane NE, Schnitzer TJ, Birbara CA, et al. Tanezumab for the treatment of pain from osteoarthritis of the knee. N Engl J Med. 2010;363(16):1521–1531. doi: 10.1056/NEJMoa0901510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sanga P, Karcher K, Wang S, Kelly K, Oh C, Thipphawong J. Efficacy, safety, and tolerability of fulranumab in treatment of patients with moderate-to-severe, chronic low back pain [abstract] J Pain. 2011;12(Suppl):P53. [Google Scholar]
- 36.Sanga P, Katz N, Polverejan E, et al. Efficacy, safety, and tolerability of fulranumab, an anti-nerve growth factor antibody, in the treatment of patients with moderate to severe osteoarthritis pain. Pain. 2013;154(10):1910–1919. doi: 10.1016/j.pain.2013.05.051. [DOI] [PubMed] [Google Scholar]
- 37.Leite VF, Buehler AM, El Abd O, et al. Anti-nerve growth factor in the treatment of low back pain and radiculopathy: a systematic review and a meta-analysis. Pain Physician. 2014;17(1):E45–E60. [PubMed] [Google Scholar]
- 38.Brown MT, Murphy FT, Radin DM, Davignon I, Smith MD, West CR. Tanezumab reduces osteoarthritic knee pain: results of a randomized, double-blind, placebo-controlled phase III trial. J Pain. 2013;13(8):790–798. doi: 10.1016/j.jpain.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 39.Schnitzer TJ, Ekman EF, Spierings EL, et al. Efficacy and safety of tanezumab monotherapy or combined with non-steroidal anti-inflammatory drugs in the treatment of knee or hip osteoarthritis pain. Ann Rheum Dis. 2014 Mar 13; doi: 10.1136/annrheumdis-2013-204905. Epub. [DOI] [PubMed] [Google Scholar]
- 40.Bramson C, Herrmann D, Biton V, et al. Efficacy and safety of subcutaneous tanezumab in patients with pain related to diabetic peripheral neuropathy ( NCT01087203) [abstract] J Pain. 2013;14(Suppl 4):S68. [Google Scholar]
- 41.Wang H, Romano G, Frustaci ME, et al. Analgesic efficacy of fulranumab in patients with painful diabetic peripheral neuropathy in a randomized, placebo-controlled, double-blind study [abstract] J Neurol Sci. 2013:e522–e523. [Google Scholar]