Abstract
To investigate their potential mechanisms of action, the nucleoside analogue ribavirin and a TLR9 agonist were compared. The CpG oligodeoxynucleotides (ODN) demonstrated strong TLR9-related Th1-type effects, and ribavirin appeared only to mediate signaling in TLR-transfected cells. CpG ODN represent a promising new type of therapeutic drug for hepatitis C or other infectious diseases.
Ribavirin is used for the treatment of chronic hepatitis C virus (HCV) infection in combination with recombinant alpha interferon (IFN-α) (2). Chronic HCV infection appears to result from Th2-biased immune responses against HCV antigens. The resultant lack of an efficient immune response is probably due to direct infection of immune cells and especially dendritic cells (DC) by HCV that might interfere with the development of Th1 responses (4, 10). The mechanism by which ribavirin contributes to the effects of IFN-α in HCV therapy is not well understood, but several possibilities of direct or indirect effects induced by ribavirin have been proposed (8). These include the possibilities that ribavirin acts directly as a viral inhibitor or as an RNA mutagen, indirectly enhances host T-cell-mediated immunity against viral infections, or inhibits the host enzyme IMP dehydrogenase (11). Few reports have investigated in more detail the possibility of ribavirin acting by itself as a direct immune modulator and its potential mechanism of action.
Short oligodeoxynucleotides (ODN) containing unmethylated CpG dinucleotides are potent Th1 immune stimulators and act via Toll-like receptor 9 (TLR9) (6, 13). In this study, we compared and contrasted the direct and indirect immune effects of both ribavirin and a TLR9 ligand, CpG ODN 2006.
Human peripheral blood mononuclear cells (PBMC; 5 × 106cells/ml) purified from buffy coats of healthy volunteers (University of Düsseldorf, Düsseldorf, Germany) were cultured with phosphorothioate CpG ODN 2006 (TCGTCGTTTTGTCGTTTTGTCGTT), control ODN 1982 (TCCAGGACTTCTCTCAGGTT), or 5177 (TCCGCCTGTGACATGCATT) (BioSpring, Frankfurt, Germany), ribavirin (Rebetol; Schering Plough), or lipopolysaccharide (LPS; Sigma, Deisenhofen, Germany) for 24 h (interleukin-6 [IL-6]), 48 h (IFN-inducible protein 10 [IP-10], IL-10, IFN-γ, and IFN-α) or 5 days (for B-cell proliferation, measured by decreasing 5- [and 6-] carboxyfluorescein diacetate succinimidyl ester label of CD19+ B cells [monoclonal antibody from Becton Dickinson, Heidelberg, Germany]) by flow cytometry (Becton Dickinson). Protein levels were measured using commercially available enzyme-linked immunosorbent assay (ELISA) kits (Diaclone, Besançon, France) or an in-house ELISA for IFN-α (PBL, New Brunswick, N.J.). Ribavirin, in contrast to the CpG ODN, had no effect on B cells or the secretion of any tested cytokine or chemokine, suggesting that it does not have stimulatory effects alone, at least not for these read-outs and at the concentrations used (Fig. 1 and 2).
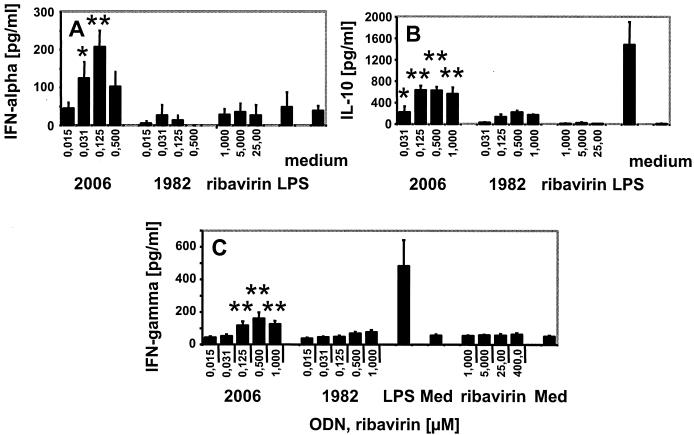
FIG. 1.
Ribavirin does not induce secretion of IFN-α, IL-10, or IFN-γ from human PBMC. Mean cytokine secretion ± the standard error of the mean of three (ribavirin in panel C), four (A and B), or six (C) donors with CpG ODN (2006), control ODN (1982), or LPS (100 ng/ml). Med, medium control. *, P ≤ 0.1; **, P < 0.05 for ODN 2006 compared to medium control (Student's t test; Sigma Plot, SPSS Inc., Chicago, Ill.).
FIG. 2.
IL-6 and IP-10 secretion or B-cell proliferation are induced by CpG ODN. Human PBMC were incubated with CpG ODN (2006), control ODN (5177), ribavirin, or 100 ng of LPS/ml. Data are the mean protein secretion ± the standard error of the mean (SEM) of four donors and the mean percentage ± SEM of proliferating cells of four donors. *, P < 0.1; **, P < 0.05 (see Fig. 1 legend for further information).
The CpG ODN stimulated significant secretion of Th1-like cytokines and chemokines (Fig. 1 and 2). The bell-shaped nature of some of these effects may be explained by negative feedback loops (14). CpG-stimulated IFN-α is derived from TLR9-expressing plasmacytoid DC (pDC) (13). It is tempting to speculate that a potent and direct TLR-dependent stimulation of pDC and Th1-like cytokine production might at least partially overcome weak cellular immune responses observed in chronic virus infections (4, 10).
We also compared the effects of CpG ODN with those of another microbial stimulus, the TLR4 ligand LPS (15). LPS induced a distinct pattern of cytokine secretion when compared to the TLR9 ligand (Fig. 1 and 2). In contrast to LPS, CpG ODN 2006 produced lower amounts of IL-6 or IL-10 but stimulated strong IFN-α and IP-10 secretion and B-cell proliferation. This indicates that TLR9 and TLR4 ligands act, at least in part, through distinct intracellular signaling pathways and that CpG ODN promote strong Th1 and humoral immune responses.
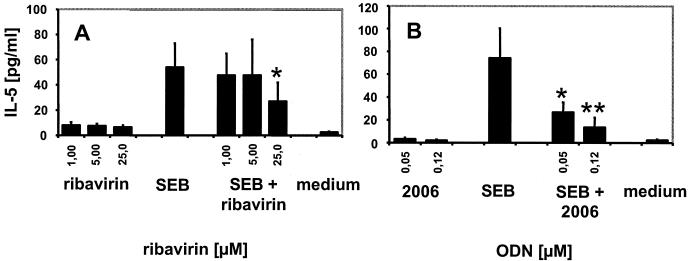
It has been noted previously that in vitro ribavirin has T-cell-polarizing effects and does dampen Th2 cytokine secretion while enhancing Th1 cytokine production (9, 12). Upon culture of human PBMC for 48 h in the presence of the superantigen staphylococcal enterotoxin B (SEB; 50 ng/ml; Sigma), addition of ribavirin or of CpG ODN appeared to result in decreased IL-5 secretion (Fig. 3). The observation that this effect was obtained only at 25 μM ribavirin might be explained by the usage of PBMC instead of the purified T cells in previous reports that required lower concentrations (8). Another possibility is that ribavirin becomes toxic at higher concentrations, although up to 400 μM ribavirin was previously reported to be noncytolytic on human PBMC (9) and serum drug concentrations of up to 50 μM are found in patients receiving the drug (3).
FIG. 3.
Ribavirin affects SEB-mediated IL-5 production. Mean IL-5 secretion ± standard error of the mean of five (A) or three (B) donors. *, P ≤ 0.2; **, P < 0.1 compared to SEB. The lower detection limit of the IL-5 ELISA was 4 pg/ml.
Nucleic acid derivatives like R-848 or substituted guanosine nucleosides can stimulate immune effects via TLR7 or TLR8 (5, 7). Ribavirin could possibly, as a purine analogue, induce TLR7- or TLR8-mediated signaling. We, therefore, incubated nonresponsive HEK293 cells stably expressing human TLR7 or TLR8 (1, 7) and a 6× NF-κB-luciferase reporter with increasing concentrations of the TLR7 and TLR8 agonist R-848 (commercially synthesized by GLSynthesis, Worchester, Mass.) or ribavirin for 16 h (Fig. 4). Each data point was done in triplicate, and cells were lysed and assayed for luciferase gene activity (BriteLite kit; Perkin-Elmer, Zaventem, Belgium). Stimulation indices were calculated in reference to medium alone. R-848 stimulated strong TLR7- and TLR8-mediated signaling. Ribavirin appeared to stimulate NF-κB activation in these transfectants in a dose-dependent manner, although the stimulation index did not exceed twofold and NF-κB stimulation could be observed only at high concentrations. Detectable NF-κB activation was not observed in TLR9-transfected HEK293 cells that were efficiently stimulated by ODN 2006 (data not shown) (14). Nevertheless, ribavirin did not stimulate considerable NF-κB activation in the TRL7 and TLR8 transfectants at the biologically active in vitro concentrations (1 to 5 μM) or at concentrations present in patients receiving the drug (about 50 μM) (3, 9, 12). Further studies have to evaluate if the observed effects at high concentrations indeed reflect stimulation mediated by TLR7 and TLR8.
FIG. 4.
Ribavirin stimulates NF-κB activation in TLR7 and TLR8 transfectants. Stimulation indices ± standard deviations of NF-κB activation of one representative experiment out of two experiments are shown.
In summary, CpG ODN were demonstrated to be potent immune activators which preferentially promote antiviral and Th1-like cytokine patterns in vitro. In contrast, ribavirin appeared not to induce detectable immune effects in the absence of a strong stimulus. Nevertheless, our data suggest another potential mechanism of action of ribavirin mediated via TLR7 and TLR8. The detailed mechanism by which ribavirin contributes to IFN-α therapy in HCV remains to be further elucidated. CpG ODN are promising candidates for the treatment of HCV or other viral diseases because they directly stimulate TLR9-mediated pDC activation, promote potent Th1-type responses, and reduce Th2 immune activity.
Acknowledgments
We thank Heather Davis for carefully reading the manuscript and helpful discussions.
REFERENCES
- 1.Bauer, S., C. J. Kirschning, H. Hacker, V. Redecke, S. Hausmann, S. Akira, H. Wagner, and G. B. Lipford. 2001. Human TLR9 confers responsiveness to bacterial DNA via species-specific CpG motif recognition. Proc. Natl. Acad. Sci. USA 98:9237-9242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Colgan, R., R. Michocki, L. Greisman, and T. A. Moore. 2003. Antiviral drugs in the immunocompetent host. I. Treatment of hepatitis, cytomegalovirus, and herpes infections. Am. Fam. Physician 67:757-762. [PubMed] [Google Scholar]
- 3.Fernandez, H., G. Banks, and R. Smith. 1986. Ribavirin: a clinical overview. Eur. J. Epidemiol. 2:1-14. [DOI] [PubMed] [Google Scholar]
- 4.Goutagny, N., A. Fatmi, V. De Ledinghen, F. Penin, P. Couzigou, G. Inchauspe, and C. Bain. 2003. Evidence of viral replication in circulating dendritic cells during hepatitis C virus infection. J. Infect. Dis. 187:1951-1958. [DOI] [PubMed] [Google Scholar]
- 5.Heil, F., P. Ahmad-Nejad, H. Hemmi, H. Hochrein, F. Ampenberger, T. Gellert, H. Dietrich, G. Lipford, K. Takeda, S. Akira, H. Wagner, and S. Bauer. 2003. The Toll-like receptor 7 (TLR7)-specific stimulus loxoribine uncovers a strong relationship within the TLR7, 8 and 9 subfamily. Eur. J. Immunol. 33:2987-2997. [DOI] [PubMed] [Google Scholar]
- 6.Hemmi, H., O. Takeuchi, T. Kawai, T. Kaisho, S. Sato, H. Sanjo, M. Matsumoto, K. Hoshino, H. Wagner, K. Takeda, and S. Akira. 2000. A Toll-like receptor recognizes bacterial DNA. Nature 408:740-745. [DOI] [PubMed] [Google Scholar]
- 7.Jurk, M., F. Heil, J. Vollmer, C. Schetter, A. M. Krieg, H. Wagner, G. Lipford, and S. Bauer. 2002. Human TLR7 or TLR8 independently confer responsiveness to the antiviral compound R-848. Nat. Immunol. 3:499. [DOI] [PubMed] [Google Scholar]
- 8.Lau, J. Y. N., R. C. Tam, J. Liang, and Z. Hong. 2002. Mechanism of action of ribavirin in the combination treatment of chronic HCV infection. Hepatology 35:1002-1009. [DOI] [PubMed] [Google Scholar]
- 9.Martin, J., S. Navas, J. A. Quiroga, M. Pardo, and V. Carreno. 1998. Effects of the ribavirin-interferon alpha combination on cultured peripheral blood mononuclear cells from chronic hepatitis C patients. Cytokine 10:635-644. [DOI] [PubMed] [Google Scholar]
- 10.Pavio, N., and M. M. Lai. 2003. The hepatitis C virus persistence: how to evade the immune system? J. Biosci. 28:287-304. [DOI] [PubMed] [Google Scholar]
- 11.Prosise, G. L., J. Z. Wu, and H. Luecke. 2002. Crystal structure of Tritrichomonas foetus inosine monophosphate dehydrogenase in complex with the inhibitor ribavirin monophosphate reveals a catalysis-dependent ion-binding site. J. Biol. Chem. 277:50654-50659. [DOI] [PubMed] [Google Scholar]
- 12.Tam, R. C., B. Pai, J. Bard, C. Lim, D. R. Averett, U. T. Phan, and T. Milovanovic. 1999. Ribavirin polarizes human T cell responses towards a type 1 cytokine profile. J. Hepatol. 30:376-382. [DOI] [PubMed] [Google Scholar]
- 13.Uhlmann, E., and J. Vollmer. 2003. Recent advances in the development of immunostimulatory oligonucleotides. Curr. Opin. Drug Discov. Dev. 6:204-217. [PubMed] [Google Scholar]
- 14.Vollmer, J., R. Weeratna, P. Payette, M. Jurk, C. Schetter, M. Laucht, T. Wader, S. Tluk, M. Liu, H. L. Davis, and A. M. Krieg. 2004. Characterization of three CpG oligodeoxynucleotide classes with distinct immunostimulatory activities. Eur. J. Immunol. 34:251-262. [DOI] [PubMed] [Google Scholar]
- 15.Zuany-Amorim, C., J. Hastewell, and C. Walker. 2002. Toll-like receptors as potential therapeutic targets for multiple diseases. Nat. Rev. Drug Discov. 1:797-807. [DOI] [PubMed] [Google Scholar]