Abstract
Epigallocatechin-gallate (EGCg), the major catechin present in green tea extracts, has been shown to have several antibacterial activities, limiting bacterial growth and invasion and acting in synergy with β-lactam antibiotics. In this article, we report that EGCg at doses half and below its calculated MIC of 100 μg/ml, is able to reverse tetracycline resistance in staphylococcal isolates expressing the specific efflux pump Tet(K) and appears to improve the MICs of tetracycline for susceptible staphylococcal isolates as well. The visible effect of EGCg is an increased accumulation of tetracycline inside bacterial cells. This effect is likely due to the inhibition of pump activity, and it is evident not only for Tet(K) pumps but also for efflux pumps of a different class [Tet(B)]. In summary, our data indicate that the observed dramatic enhancement by EGCg of tetracycline activity for resistant staphylococcal isolates is caused by impairment of tetracycline efflux pump activity and increased intracellular retention of the drug, suggesting a possible use of EGCg as an adjuvant in antibacterial therapy.
Tetracyclines are antibiotics which inhibit bacterial growth through the inhibition of protein synthesis. They bind to the 30S ribosome subunit and thus prevent the association of aminoacyl-tRNA with the bacterial ribosome (2, 14). However, since their binding is transient, these agents are bacteriostatic.
Tetracyclines have been widely used for the past 40 years as therapeutic agents in human and veterinary medicine and also as growth promoters in animal husbandry. However, the emergence of bacterial resistance to these antibiotics has limited their present-day use. Tetracycline resistance genes were identified in producing Streptomyces spp. (and designated tet for tetracycline resistance) and in nonproducing Mycobacterium spp. (and designated otr for oxytetracycline resistance). At least 29 different tet genes and 3 otr genes have been described to date, and there is no intrinsic difference between the two gene categories (3).
Three different specific mechanisms of tetracycline resistance have been identified so far: (i) ribosome protection (7 tet genes and 1 otr gene) (12, 20), (ii) enzymatic modification (1 tet gene) (16), and (iii) increased efflux (18 tet genes and 1 otr gene). Efflux proteins have been divided into six groups based on amino acid sequence similarities (9); the majority of the most common resistance determinants are classified in groups 1 and 2. Group 1 includes 11 different Tet proteins, characterized by 12 transmembrane segments. Ten of these proteins are expressed only by gram-negative bacteria, while Tet(Z) is also found in gram-positive species (19). Tet(K) and Tet(L) are the components of group 2; they display 14 transmembrane segments and are found primarily in gram-positive species. The tet(K) gene is most commonly found in Staphylococcus aureus but is also present in other staphylococcal species (15). The export protein has been shown to function as an electroneutral antiport system which catalyzes the exchange of a tetracycline-divalent metal cation complex for a proton (23).
A different efflux pump was characterized in Bacillus subtilis and named Bmr (bacterial multidrug resistance). The protein encoded by the bmr gene displays a remarkable sequence similarity to tetracycline efflux pumps (Tet proteins) and an intriguing functional similarity to mammalian multidrug transporters, since both are inhibited by reserpine and verapamil (10).
Green tea polyphenols possess many beneficial properties, such as chemoprevention, anticarcinogenicity, and antiatherogenicity, and have antioxidant actions (5). Blanco et al. (1) recently showed that the main polyphenol component of green tea, epigallocatechin-gallate (EGCg), beside having direct antibacterial properties, can decrease bacterial invasion due to the inhibition of bacterial gelatinase activities. EGCg has also been shown to be able to inhibit mammalian multidrug resistance P-glycoprotein (6). Furthermore, EGCg can act in synergy with penicillins and modulate the resistance of methicillin-resistant staphylococci (18).
Based on these observations, we set out to investigate whether EGCg (at various concentrations below the MIC) would be able to decrease the tetracycline MICs for two susceptible and two resistant staphylococcal isolates (one Staphylococcus epidermidis and one S. aureus) expressing the tet(K) resistance gene. The effect of tetracycline and EGCg in combination was evaluated by MIC determinations and time-kill assays with tetracycline-resistant and -susceptible isolates. It was found that EGCg at concentrations below the MIC (between 50 and 15 μg/ml) could effectively enhance the effect of tetracycline on susceptible bacteria and dramatically sensitize resistant microorganisms. Spectrofluorometric analyses suggested that this effect could be due to the inhibition of Tet(K) efflux pump activity.
MATERIALS AND METHODS
Bacterial isolates and growth conditions.
Four different staphylococcal isolates, two S. epidermidis and two S. aureus, belonging to our private collection were used throughout this study. Both a tetracycline-susceptible isolate and a tetracycline-resistant isolate, in which resistance was due to the expression of the tetracycline efflux pump Tet(K), were chosen for each species.
All isolates were cultured in Mueller-Hinton broth (MHB), which was supplemented with tetracycline at 10 μg/ml for the resistant bacteria.
Antimicrobial assays.
The MICs of tetracycline alone, tetracycline-EGCg combinations, and EGCg alone were determined by the broth dilution method. Bacterial cells (4 × 106 CFU/ml) from overnight cultures were used to inoculate 4 ml of MHB containing twofold serial dilutions of EGCg alone, tetracycline alone, or tetracycline plus various concentrations of EGCg (0, 15, 30, and 50 μg/ml), and the mixtures were incubated at 37°C for 18 to 20 h. The MIC was determined as the lowest concentration of twofold serially diluted tetracycline and/or EGCg at which no visible growth occurred.
Time-kill assays.
Two different S. epidermidis isolates (4 × 106/ml), one tetracycline susceptible and the other expressing the tet(K) gene, were used to inoculate tubes containing 8 ml of MHB with tetracycline (at one-half and one-fourth the MICs) and/or EGCg (50 μg/ml). A tube with MHB alone was inoculated as a control. The bacterial suspensions were incubated at 37°C with gentle shaking for defined times (0, 4, 8, 12, and 24 h), and 1 ml of bacterial suspension was withdrawn and serially diluted in MHB. Twenty-five microliters of each dilution was spotted on Mueller-Hinton agar plates, and the CFU were counted after overnight incubation of the plates at 37°C.
Spectrofluorometric assays.
After overnight culturing, about 2 × 107 bacterial cells (as estimated by measuring the optical density at 590 nm) were resuspended in 2-ml aliquots of Mg2+ buffer (50% methanol, 10 mM Tris-HCl [pH 7.0], 0.1 mM MgCl2, 0.2% glucose) (11). Each vial was preincubated for 15 min at 28°C with either EGCg at 50 μg/ml or buffer alone. Tetracycline was added at 100 μg/ml, and immediately the fluorescence (excitation at 400 nm and emission at 520 nm) was recorded for at least 15 min with a spectrofluorophotometer (Shimadzu). A blank baseline curve was obtained with buffer and tetracycline alone. When required, the experiment was performed with protoplasts prepared by lysozyme treatment (1 mg/ml, 1 h, 37°C) (10).
In other experiments, bacteria were preincubated with or without EGCg and loaded with tetracycline for 15 min as described above. Bacterial suspensions were centrifuged, the pellets were resuspended in 2.0 ml of Mg2+ buffer, and the released fluorescence was immediately quantitated with the spectrofluorophotometer.
RESULTS
MICs for EGCg, tetracycline, and their combinations.
Table 1 shows the MICs of tetracycline and EGCg, alone or in combination, for the four bacterial strains. The MIC of EGCg was 100 μg/ml for the four strains. The MICs of tetracycline were 1 μg/ml for the susceptible strains and 128 and 64 μg/ml for the resistant strains of S. epidermidis and S. aureus, respectively. The concomitant presence of EGCg at concentrations below the MIC (15, 30, and 50 μg/ml) effectively decreased the tetracycline MICs; the effect was most dramatic at 50 μg/ml, with which the MICs for the resistant strains of S. epidermidis and S. aureus were 256- and 128-fold lower, respectively. A decrease (16-fold) in the MIC in the presence of 50 μg of EGCg/ml also occurred for the susceptible strain of S. epidermidis, while the susceptible strain of S. aureus appeared to be less sensitive to the effect of EGCg (8-fold decrease in the MIC).
TABLE 1.
MICs of tetracycline and EGCg alone and in combination
| Isolates | MIC (μg/ml)
|
||||
|---|---|---|---|---|---|
| Tetracycline | EGCg | Tetracycline-EGCg combination with EGCg at the following concn (μg/ml):
|
|||
| 15 | 30 | 50 | |||
| Tet(K) resistant | |||||
| S. epidermidis | 128 | 100 | 64 | 32 | 0.5 |
| S. aureus | 64 | 100 | 32 | 8 | 0.5 |
| Susceptible | |||||
| S. epidermidis | 1 | 100 | 0.25 | 0.125 | 0.0625 |
| S. aureus | 1 | 100 | 0.25 | 0.125 | 0.125 |
Time kill assays.
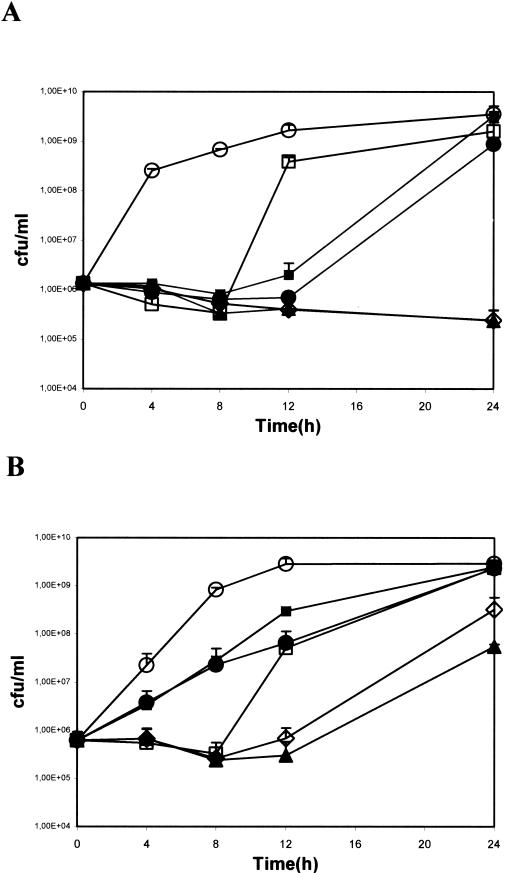
Given the similar responses to EGCg and tetracycline of S. epidermidis and S. aureus, we report next, for the sake of brevity, only the results obtained with S. epidermidis. Figure 1 shows the growth kinetics of susceptible and resistant S. epidermidis strains in the presence of tetracycline and EGCg, alone or in combination. Tetracycline was used at two doses, corresponding to one-fourth and one-half the MICs (16 and 32 μg/ml for the resistant strain [Fig. 1A] and 0.25 and 0.5 μg/ml for the susceptible strain [Fig. 1B], respectively), and EGCg was used at 50 μg/ml, corresponding to one-half the MIC.
FIG. 1.
Time kill-curves for tet(K)-resistant (A) and tet(K)-susceptible (B) S. epidermidis. Bacteria were grown in the presence of one-half and one-fourth the MICs of tetracycline (32 and 16 μg/ml for resistant strains and 0.5 and 0.25 μg/ml for susceptible strains, respectively), with and without EGCg at 50 μg/ml (corresponding to one-half the MIC). Symbols: ○, control; •, tetracycline (one-half the MIC); ▪, tetracycline (one-fourth the MIC); □, EGCg at 50 μg/ml (one-half the MIC); ▴, tetracycline (one-half the MIC) plus EGCg at 50 μg/ml; ⋄, tetracycline (one-fourth the MIC) plus EGCg at 50 μg/ml. Error bars indicate standard deviations.
In the resistant strain (Fig. 1A), tetracycline or EGCg alone had a bacteriostatic effect. This effect, however, was limited to 8 h for EGCg and to 12 h for tetracycline, after which time the bacteria started to grow exponentially, reaching plateau levels at 24 h. However, the association of EGCg with tetracycline had a clear potentiating effect. In fact, the bacteria remained at the inoculum density after 24 h of incubation as well, indicating a growth inhibition rate of more than 2 log units with respect to the growth of bacteria treated with either drug alone.
In the susceptible strain (Fig. 1B), EGCg alone had a bacteriostatic effect, although it was still limited to the first 8 h of incubation, whereas tetracycline alone at concentrations below the MICs resulted only in a lower growth rate. The association of EGCg with either dose of tetracycline still enhanced the antibiotic effect, although the final growth inhibition rate was less than 2 log units.
Spectrofluorometric assay.
We next investigated whether inhibition of the Tet(K) efflux pump was the reason why EGCg enhanced tetracycline activity in these bacterial strains. Taking advantage of the fact that only internalized tetracycline becomes fluorescent (11), we analyzed the kinetics of tetracycline uptake and release by resistant and susceptible staphylococcal strains.
Figure 2A shows the effects of EGCg on the kinetics of fluorescent tetracycline release from resistant and susceptible S. epidermidis strains. Under these experimental conditions, bacterial cells were preincubated with EGCg (50 μg/ml) and then treated with tetracycline (100 μg/ml). Immediately after the tetracycline addition, the released fluorescence was detected over a continuous period of 15 min and reported as a curve. In the absence of EGCg pretreatment, the release curve for resistant bacteria was steeper than that for susceptible bacteria, indicating, as expected, more rapid efflux of tetracycline from the former. EGCg pretreatment resulted in a fall in both curves, implying that more tetracycline was now retained within the cells. Under these conditions, values for resistant bacteria became closer to those for nonpretreated susceptible bacteria, and the values for susceptible bacteria became closer to the blank values. Almost identical results were obtained with S. aureus strains (data not shown).
FIG. 2.
Dynamics of tetracycline uptake and release. Intact tetracycline-resistant or tetracycline-susceptible S. epidermidis bacterial cells (A) or resistant S. epidermidis cells stripped of the cell wall (B) were incubated with tetracycline (100 μg/ml), with or without EGCg preincubation (50 μg/ml), in Mg2+ buffer. Under these conditions, tetracycline that enters the cells becomes fluorescent, and once extruded from the cell, it can be detected and quantitated with a spectrofluorophotometer. The curves shown thus represent the relative amounts of tetracycline pumped out of the cells under the various incubation conditions.
In order to confirm that the effects of EGCg were directed against Tet(K) proteins and were independent of EGCg binding to cell wall peptidoglycan (25), we repeated the same experiment with protoplasts prepared from resistant S. epidermidis cells (Fig. 2B). The effects of EGCg pretreatment on tetracycline efflux were unaffected by cell wall removal, indicating that in this scenario, interactions with the peptidoglycan had no influence on the accumulation of and release curve for tetracycline.
Finally, in the experiment shown in Fig. 3, S. epidermidis cells were preloaded with tetracycline (100 μg/ml) during a 15-min incubation, pelleted, and resuspended in tetracycline-free buffer. In this experiment, intracellular fluorescent tetracycline was immediately released into the buffer and could be detected by a spectrofluorophotometer (11), providing a quantitative estimate of the amount of tetracycline accumulated during the 15-min loading phase. Under these conditions, tetracycline-resistant bacteria accumulated the smallest amount of tetracycline, as expected, because of their expression of Tet(K) efflux pump proteins. However, when tetracycline was added after EGCg preincubation, the amount of tetracycline retained by the resistant bacteria was doubled and was close to that detected in the susceptible bacteria. EGCg pretreatment also caused a moderate increase in tetracycline accumulation in susceptible cells.
FIG. 3.
Tetracycline accumulation and release by Tet(K)-resistant and Tet(K)-susceptible S. epidermidis strains. Bacterial suspensions were incubated in triplicate for 15 min with tetracycline (100 μg/ml), with or without EGCg preincubation (50 μg/ml). Under these conditions, tetracycline is dynamically accumulated within the cells, depending on the activity of their efflux pumps. After a rapid rinsing, the cells are resuspended in Mg2+ buffer, in which released fluorescence (a direct measurement of accumulated tetracycline) is immediately detected with a spectrofluorophotometer. From top to bottom, the bars represent tetracycline-resistant cells, tetracycline-resistant cells in the presence of EGCg, tetracycline-susceptible cells, and tetracycline-susceptible cells in the presence of EGCg. Error bars indicate standard deviations.
DISCUSSION
Antibiotic resistance is increasing worldwide at an accelerating pace, forcing health agencies to recognize it as a pressing priority for intervention (4, 22). Staphylococci are responsible for many nosocomial infections, and often they express resistance to methicillin and/or tetracyclines (Santos Sanches, 2000, 32). Green tea polyphenols, on the other hand, have long been known to have antibacterial properties (7, 17). More recently, some of these properties were ascribed to the action of EGCg which, besides having bacteriostatic and bactericidal activities (8), can also act in synergy with β-lactams (25) and decrease the invasive ability of gelatinase-producing strains (1).
This article describes yet another antibacterial effect of EGCg, the inhibition of a tetracycline efflux pump, Tet(K), resulting in the sensitization of Tet(K)-resistant bacteria to the effects of tetracycline. The potentiating effect of EGCg on tetracycline activity is evident in the experiments reported in Table 1 and Fig. 1, where it is shown that 50 μg of EGCg/ml (one-half the MIC) can dramatically decrease both the MICs of tetracycline for resistant strains of S. aureus and S. epidermidis (Table 1) and the rate of growth of S. epidermidis (Fig. 1). It is interesting that EGCg alone, even at this sub-MIC, is still bacteriostatic, although this effect vanishes quite early; after 8 h, normal growth is resumed (Fig. 1). However, when fresh EGCg is added just before 8 h, growth remains partially inhibited until 24 h (data not shown); this result suggests that the loss of the inhibitory effect is likely due to the inactivation of EGCg which, being a powerful antioxidant, is rapidly oxidized.
We cannot completely rule out the possibility that this initial bacteriostatic effect partially contributes to the observed enhancing effect of EGCg on tetracycline; however, that effect is better explained by the inhibition of the tetracycline efflux pump, clearly illustrated by the experiments reported in Fig. 2 and 3. In fact, since the binding of tetracycline to its targets is transient, the resulting toxic effect strictly depends on the amount of accumulated drug, which is the result of a dynamic equilibrium between influx and efflux. The influx of tetracycline into bacterial cells appears to occur by passive diffusion, since gram-positive bacteria are easily permeated by small molecules, such as tetracycline (13, 14). Tetracycline efflux, on the other hand, is responsible for high-level resistance, is energy dependent, and occurs through two different types of efflux pumps: multidrug resistance pumps and tetracycline-specific transporters belonging to the tet gene family (14). Therefore, it is expected that the inhibition of efflux mechanisms should enhance the antibacterial effects of tetracycline.
A similar synergy has already been described for EGCg and β-lactams, apparently depending on both the binding of EGCg to the cell wall peptidoglycan (25) and the inhibition of penicillinase activity (24). Therefore, this ability of EGCg to bind to peptidoglycan, which can induce some damage to the cell wall and thus decrease the tolerance of staphylococci for low osmotic pressure (25), also may increase the transit of tetracycline. However, the experiments in Fig. 2 show that the effect of EGCg in decreasing the efflux of tetracycline is similarly achieved both in intact bacteria and in their protoplasts (Fig. 2); this result indicates that an interaction with efflux pumps is the major event responsible for the observed enhanced accumulation of tetracycline. Moreover, other experiments (data not shown) indicated that EGCg has a similar blocking effect on the Tet(B) efflux pump; these data lend further support to the functional similarity described for tetracycline efflux pumps and mammalian multidrug resistance proteins (10). On the contrary, EGCg had no effect on the Tet(K)-resistant staphylococcal response to minocycline treatment (MICs remained at 0.5 to 1.0 μg/ml). Since minocycline is a semisynthetic tetracycline not extruded by efflux pumps (21), this result indicates that the sensitizing effect of EGCg mainly involves interactions with efflux pumps.
In conclusion, the data reported in this article show that EGCg can dramatically sensitize staphylococci to the action of tetracycline, efficiently reversing resistance in strains in which the resistance is due to the expression of Tet(K) [and likely also Tet(B)] efflux pump proteins. Moreover, preliminary data show that Tet proteins acting at the level of ribosome protection [e.g., Tet(M)] can be inhibited by EGCg and thus broaden further the possibility of using EGCg in combination with tetracycline to overcome resistance due to the expression of Tet determinants.
Acknowledgments
We thank Ken Mugridge for critical reading of the manuscript.
REFERENCES
- 1.Blanco, A. R., S. La Terra Mule, G. Babini, S. Garbisa, V. Enea, and D. Rusciano. 2003. (−)Epigallocatechin-3-gallate inhibits gelatinase activity of some bacterial isolates from ocular infection, and limits their invasion through gelatine. Biochim. Biophys. Acta 1620:273-281. [DOI] [PubMed]
- 2.Chopra, I., P. M. Hawkey, and M. Hinton. 1992. Tetracyclines: molecular and clinical aspects. J. Antimicrob. Chemother. 29:245-277. [DOI] [PubMed] [Google Scholar]
- 3.Chopra, I., and M. Roberts. 2001. Tetracycline antibiotics: mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol. Mol. Biol. Rev. 65:232-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen, M. L. 1992. Epidemiology of drug resistance: implications for a post-antimicrobial era. Science 257:1050-1055. [DOI] [PubMed] [Google Scholar]
- 5.Higdon, J. V., and B. Frei. 2003. Tea catechins and polyphenols: health effects, metabolism, and antioxidant functions. Crit. Rev. Food Sci. Nutr. 43:89-143. [DOI] [PubMed] [Google Scholar]
- 6.Jodoin, J., M. Demeule, and R. Beliveau. 2002. Inhibition of the multidrug resistance P-glycoprotein activity by green tea polyphenols. Biochim. Biophys. Acta 1542:149-159. [DOI] [PubMed] [Google Scholar]
- 7.Klaunberg, H. J. (ed.). 1955. Tea: a symposium on the pharmacology and physiologic and psychologic effects of tea. The Biological Science Foundation, Washington, D.C.
- 8.Kono, K., I. Tatara, S. Takeda, K. Arakawa, and Y. Hara. 1994. Antibacterial activity of epigallocatechin gallate against methicillin-resistant Staphylococcus aureus. Kansenshogaku Zasshi 68:1518-1522. [DOI] [PubMed] [Google Scholar]
- 9.McMurry, L. M., and S. B. Levy. 2000. Tetracycline resistance in gram-positive bacteria, p. 660-672. In V. A. Fischetti, R. P. Novick, J. J. Ferretti, D. A. Portnoy, and J. I. Rood (ed.), Gram-positive pathogens. American Society for Microbiology, Washington D.C.
- 10.Neyfakh, A. A., V. E. Bidnenko, and L. B. Chen. 1991. Efflux-mediated multidrug resistance in Bacillus subtilis: similarities and dissimilarities with the mammalian system. Proc. Natl. Acad. Sci. USA 88:4781-4785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Samra, Z., J. Krausz-Steinmetz, and D. Sompolinsky. 1978. Transport of tetracyclines through the bacterial cell membrane assayed by fluorescence: a study with susceptible and resistant strains of Staphylococcus aureus and Escherichia coli. Microbios 21:7-21. [PubMed] [Google Scholar]
- 12.Sanchez-Pescador, R., J. T. Brown, M. Roberts, and M. S. Urdea. 1988. Homology of TetM with translational elongation factors: implications for potential modes of tetM-conferred tetracycline resistance. Nucleic Acids Res. 16:1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scherrer, R., and P. Gerhardt. 1971. Molecular sieving by the Bacillus megaterium cell wall and protoplast. J. Bacteriol. 107:718-735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schnappinger, D., and W. Hillen. 1996. Tetracyclines: antibiotic action, uptake, and resistance mechanisms. Arch. Microbiol. 165:359-369. [DOI] [PubMed] [Google Scholar]
- 15.Schwarz, S., M. C. Roberts, C. Werckenthin, Y. Pang, and C. Lange. 1998. Tetracycline resistance in Staphylococcus spp. from domestic animals. Vet. Microbiol. 63:217-227. [DOI] [PubMed] [Google Scholar]
- 16.Speer, B. S., L. Bedzyk, and A. A. Salyers. 1991. Evidence that a novel tetracycline resistance gene found on two Bacteroides transposons encodes an NADP-requiring oxidoreductase. J. Bacteriol. 173:176-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stagg, G. V., and D. J. Millin. 1975. The nutritional and therapeutic value of tea: a review. J. Sci. Food Agric. 26:1439-1459. [Google Scholar]
- 18.Stapleton, P. D., and P. W. Taylor. 2002. Methicillin resistance in Staphylococcus aureus: mechanisms and modulation. Sci. Prog. 85:57-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tauch, A., A. Puhler, J. Kalinowski, and G. Thierbach. 2000. TetZ, a new tetracycline resistance determinant discovered in gram-positive bacteria, shows high homology to gram-negative regulated efflux systems. Plasmid 44:285-291. [DOI] [PubMed] [Google Scholar]
- 20.Taylor, D. E., and A. Chau. 1996. Tetracycline resistance mediated by ribosomal protection. Antimicrob. Agents Chemother. 40:1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trzcinski, K., B. S. Cooper, W. Hryniewicz, and C. G. Dowson. 2000. Expression of resistance to tetracyclines in strains of methicillin-resistant Staphylococcus aureus. J. Antimicrob. Chemother. 45:763-770. [DOI] [PubMed] [Google Scholar]
- 22.World Health Organization. 2001. Global strategy for containment of antimicrobial resistance. [Online.] World Health Organization, Geneva, Switzerland. http://who.int/emc/amr.html.
- 23.Yamaguchi, A., N. Ono, T. Akasaka, T. Noumi, and T. Sawai. 1990. Metal-tetracycline/H+ antiporter of Escherichia coli encoded by a transposon, Tn10. The role of the conserved dipeptide, Ser65-Asp66, in tetracycline transport. J. Biol. Chem. 265:15525-15530. [PubMed] [Google Scholar]
- 24.Zhao, W. H., Z. Q. Hu, Y. Hara, and T. Shimamura. 2002. Inhibition of penicillinase by epigallocatechin gallate resulting in restoration of antibacterial activity of penicillin against penicillinase-producing Staphylococcus aureus. Antimicrob. Agents Chemother. 46:2266-2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao, W. H., Z. Q. Hu, S. Okubo, Y. Hara, and T. Shimamura. 2001. Mechanism of synergy between epigallocatechin gallate and beta-lactams against methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 45:1737-1742. [DOI] [PMC free article] [PubMed] [Google Scholar]