Abstract
Purpose: Recent studies suggest a potential application for digoxin in the prevention and/or treatment of prostate cancer. However, the effect of digoxin on androgen receptor (AR)‐positive prostate tumor in vivo is not clear. This study is designed to determine if digoxin can inhibit AR‐positive xenograft prostate tumors.
Materials and Methods: Athymic male nude mice were utilized to establish subcutaneous C4‐2 castration‐resistant prostate tumors. The animals were castrated and then treated with daily intraperitoneal (i.p.) injection of digoxin at 2mg/kg along with vehicle controls for 7 consecutive days. Tumor growth was determined by measuring tumor volume changes, blood vessel density by immunostaining of CD31, and cell proliferation by BrdU labeling. The expression of HIF‐1a in C4‐2 tumors was measured by Western blot and real‐time RT‐PCR.
Results: Digoxin inhibited blood vessel density about fourfold and down‐regulated HIF‐1a expression at both mRNA and protein levels. However, digoxin did not inhibit C4‐2 tumor growth.
Conclusions: Digoxin is a potent inhibitor of HIF‐1a signaling pathway and blood vessel formation in C4‐2 castration‐resistant prostate tumors. Clin Trans Sci 2012; Volume #: 1–4
Keywords: castration resistant prostate cancer, digoxin, angiogenesis, hypoxia‐inducible factor 1a (HIF‐1a)
Introduction
Nearly 217,730 men were diagnosed with prostate cancer in the US in 2010. 1 Prostate cancer claimed 32,050 lives in 2010, and is the second leading cause of cancer death in US men. 1 Hormone refractory prostate cancer (HRPC) is the deadliest form of the disease. Management of HRPC still represents a therapeutic challenge despite the development of new and effective treatment options. 2 , 3 New modalities for treatment and prevention of prostate cancer are currently being investigated.
Cardiac glycosides have been used in medicine to treat heart failure and cardiac arrhythmias. The use of cardiac glycosides in the field of cancer therapy has been in place since the 1980s when Stenkvist et al. reported that breast cancer cells obtained from women on digitalis therapy were characterized by a series of more benign features compared with cancer cells from control patients. Long‐term follow‐up (22.3 years) of 175 patients with breast carcinoma, of which 32 were on digitalis treatment, demonstrated a lower death rate (6%) from breast carcinoma among the patients on digitalis, when compared with patients not on digitalis (34%). 4 , 5 Digoxin, another drug in the class of cardiac glycosides, could also represent a new potential agent for the prevention and treatment of prostate cancer. It was reported that men on digoxin displayed a 25% reduction in prostate cancer incidence. 6 A pilot phase II clinical trial is in place to evaluate the effect of digoxin in patients with recurring prostate cancer. 7
Svensson et al. tested digoxin on blood vessel growth in neuroblastoma cells using a CAM assay. 8 When combining digoxin and fibroblast growth factor, blood vessel growth was significantly reduced. Yeh et al. evaluated the inhibitory effects of digitalis on the proliferation of androgen‐sensitive LNCaP and androgen‐independent DU145 and PC3 prostate cancer cells. 9 All three prostate cancer cells were significantly inhibited by different types of cardiac glycosides. Recently, digoxin was reported to inhibit the tumor growth and the expression of Hypoxia‐inducible factor‐1alpha (HIF‐1α), a key regulator of angiogenesis, in androgen receptor (AR)‐negative PC3 xenograft prostate tumors. 10 , 11 , 12 , 13 However, it is not clear if digoxin can inhibit angiogenesis in AR‐positive prostate cancer, which is the predominant form of prostate cancer in patients. We present results on the effect of digoxin on blood vessel density in AR‐positive castrate resistant C4‐2 xenograft prostate tumor model. 14 , 15
Material and Methods
Animal studies
Four‐to six‐week‐old nu/nu athymic male mice were purchased from Harlan Labs (Indianapolis, IN, USA). Experiments were carried out under approval through an Institutional Animal Care and Use Committee approved protocol. Guidelines for the proper and humane use of animals in research were followed. The mice were allowed to sit 1 week after arrival and subcutaneous injection of 1×106 C4‐2 cells at a 1:1 ratio in matrigel (BD Bioscience, San Jose, CA, USA). Tumors were allowed to establish and grow to a diameter of 7 mm. Mice were castrated and then kept for 10 days before digoxin injection. Tumors were measured three times weekly with vernier calipers to calculate the tumor volume (largest diameter × smallest diameter). 16 After 10 days the mice received daily i.p. injections of digoxin (2mg/kg; Sigma Aldrich, St. Louis, MO, USA) for 7 consecutive days. The mice were allowed to rest for 2 days at which time they were sacrificed by CO2 euthanasia. Tumor tissue was harvested in three parts, two of which were snap frozen in liquid nitrogen for RNA and western blot analysis. One‐third of the tumor tissue was fixed in 10% formalin for histology.
Western blot analysis
Tumor tissue was lysed in radioimmunoprecipitation assay (RIPA) buffer containing 10mM NaPO4, 1% Triton‐X‐100, 1 mM ethylenediaminetetraacetic acid (EDTA), 150mM NaCl, 2mM PMSF, 1mM Na3VO4, and protease inhibitor cocktail (Sigma Aldrich). Lysates were boiled and concentrations were determined using Protein Assay Dye (Bio‐Rad, Hercules, CA, USA). Samples were run on a 7% polyacrylamide gel and subjected to immunoblot detection using antibodies against HIF‐1a (NB100–449 Novus Biologicals, Littleton, CO, USA) and glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) (FL‐335, Santa Cruz Biotechnology, Santa Cruz, CA). As a positive control for HIF‐1a, LNCaP cells were cultured in normoxia or hypoxia conditions and lysed in RIPA buffer. Images were captured on a Versadoc Molecular Imager (Bio‐Rad) using enhanced chemiluminescence (ECL) Plus Detection Reagents (Amersham, Piscataway, NJ, USA). Dosimetric analysis was determined using the Quantity One 1‐D Analysis Software (Bio‐Rad).
RNA isolation and real‐time PCR
Tumor tissue was harvested and snap frozen in LN2 to prevent degradation of RNA. Tissue was homogenized on ice and isolated using Trizol Reagent (Invitrogen, Carlsbad, CA, USA). Quality of RNA was confirmed on an agarose gel. Reverse Transcription was performed using Cells Direct Superscript III cDNA Synthesis Kit (Invitrogen) and subsequnt quantitative PCR was performed from pooled samples using SYBR Green PCR Master Mix (Invitrogen). Primers to HIF‐1a, GAPDH, and vascular endothelial growth factor (VEGF) were designed using Primer3 software (Primer3, Totowa, NJ, USA) and were as follows: HIF‐1a forward 59 CCAGTTACGTTCCTTCGATCAGT 39, HIF‐1a reverse 59 TTTGAGGACTTGCGCTTTCA 39, GAPDH forward 59ATGTTCGTCATGGGTGTGA 39, GAPDH reverse 59 GGTGCTAAGCAGTTGGTGGT 39, VEGF forward 59 CCTTGCTGCTCTACCTCCAC 39, VEGF reverse 59 ATGATTCTGCCCTCCTCCTT 39. The primers were optimized within suitable ranges for efficiency and correlation coefficient using a standard curve on the ABI Step‐One Plus thermocycler (Applied Biosystems, Foster City, CA, USA) as well as tested to make sure genomic DNA was not amplified. Data are shown as relative expression to GAPDH normalized to vehicle‐treated mice using the delta–delta Ct method.
Immunohistochemistry
Tissue was fixed immediately in 10% formalin at 4°C overnight followed by embedding in paraffin. Serial 10μm sections were incubated at 1:500 with rat monoclonal anti‐CD31 (BD Pharmingen, San Diego, CA, USA). Anti‐rat IgG conjugated to HRP (Santa Cruz Biotechnology) was used as the secondary antibody. Diaminobenzidine tetrahydrochloride (DAB) (DAKO, Carpinteria, CA, USA) was used as the chromagen to view the localization of the antigen. Sections were lightly counterstained with hematoxylin. The CD31 index was determined according to presence or absence of staining compared to specimen controls. CD31‐positive vessel density was determined based on microvessel count as described from four different xenograft tumors. 17 Six random fields were chosen from each specimen and vessels were counted at 10× magnification to determine the average number of vessels per field.
Blood vessel and branches were counted by a blind observer who was unaware of the treatment groups.
5‐Bromo‐2‐deoxyuridine (BrdU) was performed by injecting mice i.p. with 1mL BrdU labeling reagent (Zymed, San Francisco, CA, USA) per 100g of body weight. Animals were sacrificed 2 hours after injection and tissues were immediately fixed in 10% formalin overnight at 4°C. 10 μm serial sections were incubated with BrdU monoclonal antibody (Sigma Aldrich) at 1:500 for 60 minutes at room temperature. The slides were placed in Rodent Decloaker (Biocare Medical, Concord, CA, USA) antigen retrieval buffer in a decloaking chamber. Endogenous peroxidase was quenched using 3% hydrogen peroxide. Biocare Medical Mouse on Mouse HRP secondary antibody was used and DAB (DAKO) applied for 10 minutes to visualize antigen.
Results
Digoxin did not inhibit growth of C4‐2 xenograft tumors in nude mice
In our study, mice were castrated once the subcutaneous tumor reached a diameter of 7 mm. There was no significant difference in animal body weight ( Figure 1a ) as well in the tumor volume increase between the digoxin‐treated group and the controlled groups ( Figure 1b ). BrdU labeling experiment showed active proliferation in both control and digoxin‐treated xenograft tumors ( Figure 2 ).
Figure 1.
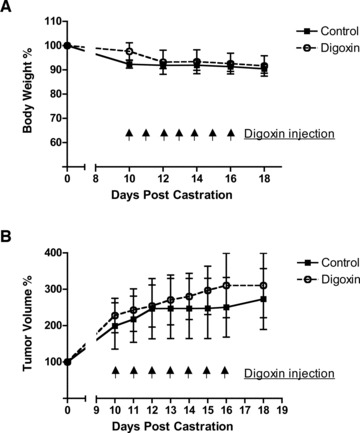
(A) Digoxin injection versus mouse body weight relation. Mice were castrated once the tumors reached a diameter of 7mm. Beginning at 10 days postcastration, digoxin was given i.p. daily at 2mg/kg for 7 days. Body weight was set to 100% at the time of castration. (B) Association of digoxin Injection and size of C4‐2 xenograft tumor. Tumor volume was set to 100% at the time of castration. Data represents six mice in each group.
Figure 2.
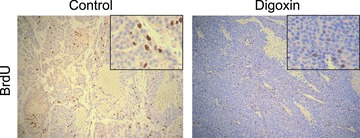
C4‐2 tumor cell proliferation and effects of digoxin. C4‐2 cell xenograft tumors were excised after 7 days of treatment with digoxin or control. Two hours prior to sacrifice, mice were injected with BrdU labeling reagent and stained for BrdU. Photomicrographs are representative of both control‐ and digoxin‐treated tissue with five mice each.
Digoxin‐inhibited blood vessel density and C4‐2 xenograft tumors
Although digoxin did not inhibit C4‐2 tumor growth, it significantly inhibited blood vessel formation in the tumors. Based on the gross appearance of the freshly dissected tissues, the dimethyl sulfoxide (DMSO)‐treated tumors were bloody and well vascularized, whereas the digoxin‐treated tumors were pale and poorly vascularized (data not shown). This difference was confirmed by immunostaining of CD31, an endothelial blood vessel marker, in the tumor sections from digoxin‐treated and the DMSO‐treated control groups ( Figure 3A ). CD31 staining depicted the vast amount of blood vessels in the tumors of the control group, and the digoxin treated group had a significant (p= 0.0004) fourfold reduction in the number of blood vessels ( Figure 3A and B ).
Figure 3.
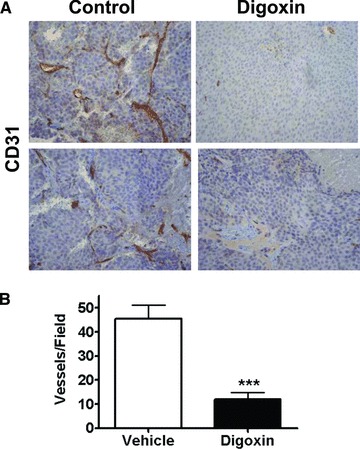
Digoxin inhibition of blood vessel density in C4‐2 xenogaft tumor. Nude mice were injected subcutaneously with 1× 106 C4‐2 cells in a 1: 1 ratio with matrigel. Tumors were established and upon reaching 7mm in diameter were castrated. Mice were allowed to recover for 10 days before starting intraperitoneal treatment with either digoxin (2mg/kg) or vehicle alone for 7 days. Tumors were harvested 2 days after treatment stopped. IHC was performed on the tumor tissue for CD31 to view microvascular density. (A) Displays a representative view at 20×. (B) Shows the scoring of the tumor tissue counting CD31 positive vessels in each field. The graph displays the average of six random fields per mouse with four mice in each group. The digoxin treated group had a significant fourfold reduction in the number of blood vessels (***p= 0.0004).
Digoxin inhibited HIF‐1α expression in C4‐2 tumors
To determine if digoxin modulates HIF‐1α in C4‐2 xenograft tumors, we analyzed HIF‐1α protein levels in digoxin‐treated C4‐2 tumors and the DMSO vehicle treated control groups. Two out of three control tumors exhibited high levels of HIF‐1α, whereas all of the digoxin‐treated tumors exhibited low or no detectable HIF‐1α protein level ( Figure 4a ). We also tested the expression level of HIF‐1α mRNA in digoxin‐treated and the control C4‐2 tumors using real‐time reverse transcription polymerase chain reaction (RT‐PCR). Figure 4b showed reduced HIF‐1α mRNA expression in digoxin‐treated tumors.
Figure 4.
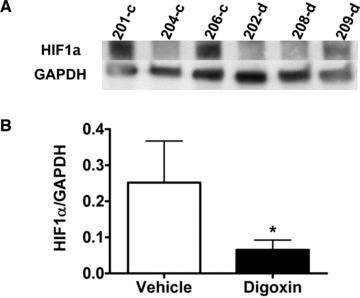
Expression of HIF‐1a in C4‐2 xenograft tumor downregulated by digoxin. Tumor tissue was frozen at the time of sacrifice to test levels of HIF‐1a by (A) western blot and (B) real‐time PCR. Western blot shows three samples of either control‐ (‐c) or digoxin (‐d)‐treated mice. HIF‐1a protein is downregulated in three digoxin‐treated tumors. Real‐time PCR represents data from three mice in the control‐treated group and six mice in the digoxin‐treated group. Gene expression is relative to GAPDH. Difference seen in HIF‐1a is statistically significant (*p<0.05).
Discussion
Recent findings in the literature suggest that digoxin may have efficacy in prostate cancer prevention and treatment. 6 By analyzing digoxin's influence on prostate tumors, particularly in AR‐positive prostate tumor in vivo, we were able to better evaluate the potential efficacy of digoxin in prostate cancer patients. Our current study indicates digoxin as a potent inhibitor of angiogenesis and HIF‐1α in prostate cancer.
Increased blood vessel density and HIF‐1α levels have been associated with prostate cancer progression. 12 Digoxin significantly reduced the tumor blood vessel density in C4‐2 castrate resistant prostate tumors in the treated mice, by a fourfold reduction when compared to controls. Not only was blood vessel formation significantly inhibited, but also was associated with inhibition of HIF‐1α synthesis.
Zhang et al. reported digoxin inhibited HIF‐1α protein synthesis. 13 However, it was unclear whether digoxin can also affect the level of HIF‐1α mRNA. Results in Figure 4 suggested that digoxin also inhibited the expression of HIF‐1α mRNA. Taken together, digoxin could inhibit HIF‐1α expression at both protein and mRNA levels.
Although digoxin treatment caused a fourfold reduction in blood vessel density in C4‐2 xenograft tumors, it did not reduce the growth of the tumor. In fact, C4‐2 tumor cells in vivo were still undergoing active proliferation as demonstrated by BrdU staining ( Figure 2 ). Previous in vitro studies showed that digoxin can significantly inhibit the proliferation of LNCaP, DU145, and PC3 prostate cancer cell lines. 9 The lack of growth inhibition by digoxin in C4‐2 xenograft tumors may reflect differences of in vivo and in vitro conditions. Furthermore, digoxin treatment had no effect on the body weight of the animals, which is in agreement with the published observations. 13 In the C4‐2 xenograft tumors, the dosage for digoxin to inhibit angiogenesis and HIF‐1α pathway may not be sufficient to inhibit cell proliferation.
It was unexpected that digoxin inhibited angiogenesis but not tumor growth in C4‐2 tumors in this study. One possibility is that the mice were only treated with digoxin for 7 days, which may not be long enough to allow for detecting the effect of digoxin on tumor growth. Another possibility is that C4‐2 tumor growth rate was slower than PC3 tumors which exhibited growth inhibition in response to digoxin. It may be more difficult to detect growth inhibition in less aggressive tumors. Furthermore, digoxin, in the human setting, requires a loading dose to achieve therapeutic levels in cardiac patients. We did not employ a loading dose in our mice. Thus, the digoxin level may not have been at a high enough level to induce tumor reduction although it inhibited angiogenesis in the C4‐2 model.
Finally, Digoxin, as well as other cardiac glycosides, carries with it a very slim therapeutic window. Side effects can be detrimental if digoxin levels are not within this therapeutic window. We do acknowledge pharmacological testing needs to be done to develop or alter the chemical composition that would limit or abolish this side effect profile.
Digoxin's role in inhibiting HIF‐1α and angiogenesis in prostate cancer may provide a new approach to prevent and/or treat prostate cancer. Our findings substantiated the efficacy of digoxin as a potent inhibitor of HIF‐1α signaling and angiogenesis in prostate cancers. The findings of this study also suggest that HIF‐1α pathway and blood vessel density can be used as readout for evaluating the efficacy of digoxin in prostate cancer.
Conclusions
Results of this study showed digoxin as a potent inhibitor of HIF‐1α expression and angiogenesis in castration resistant C4‐2 xenograft tumors. Digoxin could have efficacy in prevention and/or treatment of prostate cancer by inhibiting blood vessel formation. Further research and trials are warranted to evaluate the efficacy of digoxin in the treatment of prostate cancer.
Acknowledgments
This work was supported in part by National Institutes of Health Grants 5 R37 DK51193, R01 CA 108675, and 1 P50 CA90386. This project used the UPCI Animal Facility and Tissue and Research Pathology Services (TARPS), and was supported in part by award P30CA047904. We thank Marie Acquafondata for technical assistance.
References
- 1. Jemal A, Siegel R, Xu J, Ward E. Cancer Statistics, 2010. Ca Cancer J Clin. 2010; 60: 277–300. [DOI] [PubMed] [Google Scholar]
- 2. Fitzpatrick JM, Anderson J, Sternberg CN, Fleshner N, Fizazi K, Rébillard X, Dogliotti L, Conti G, Turesson I, James N, et al. Optimizing treatment for men with advanced prostate cancer: expert recommendations and the multidisciplinary approach. Crit Rev Oncol Hematol. 2008; 68(Suppl 1): S9–S22. [DOI] [PubMed] [Google Scholar]
- 3. Heidenreich A, Aus G, Bolla M, Joniau S, Matveev VB, Schmid HP, Zattoni F. EAU guidelines on prostate cancer. Eur Urol. 2008; 53(1): 68–80. [DOI] [PubMed] [Google Scholar]
- 4. Stenkvist B, Bengtsson E, Eklund G, Eriksson O, Holmquist J, Nordin B, Westman‐Naeser S. Evidence of a modifying influence of heart glucosides on the development of breast cancer. Anal Quant Cytol. 1980; 2(1): 49–54. [PubMed] [Google Scholar]
- 5. Stenkvist B. Is digitalis a therapy for breast carcinoma? Oncol Rep. 1999; 6: 493–496. [PubMed] [Google Scholar]
- 6. Platz E. Digoxin: a heart drug may help prevent or treat prostate cancer. Prostate Cancer Discovery. 2010; 6: 13–14 [Google Scholar]
- 7. Available at: http://clinicaltrials.gov/ct2/show/NCT01162135. Accessed January 1, 2012.
- 8. Svensson A, Azarbayjani F, Bäckman U, Matsumoto T, Christofferson R. Digoxin inhibits neuroblastoma tumor growth in mice. Anticancer Res. 2005; 25(1A): 207–212. [PubMed] [Google Scholar]
- 9. Yeh JY, Huang WJ, Kan SF, Wang PS. Inhibitory effects of digitalis on the proliferation of androgen dependent and independent prostate cancer cells. J Urol. 2001; 166(5): 1937–1942. [PubMed] [Google Scholar]
- 10. Lekas A, Lazaris AC, Deliveliotis C, Chrisofos M, Zoubouli C, Lapas D, Papathomas T, Fokitis I, Nakopoulou L. The expression of hypoxia‐inducible factor‐1alpha (HIF‐1alpha) and angiogenesis markers in hyperplastic and malignant prostate tissue. Anticancer Res. 2006; 26(4B): 2989–2993. [PubMed] [Google Scholar]
- 11. Pipinikas CP, Carter ND, Corbishley CM, Fenske CD. HIF‐1alpha mRNA gene expression levels in improved diagnosis of early stages of prostate cancer. Biomarkers. 2008; 13(7): 680–691. [DOI] [PubMed] [Google Scholar]
- 12. Zhong H, Semenza GL, Simons JW, De Marzo AM. Up‐regulation of hypoxia‐inducible factor 1alpha is an early event in prostate carcinogenesis. Cancer Detect Prev. 2004; 28(2): 88–93. [DOI] [PubMed] [Google Scholar]
- 13. Zhang H, Qian DZ, Tan YS, Lee K, Gao P, Ren YR, Rey S, Hammers H, Chang D, Pili R, et al. Digoxin and other cardiac glycosides inhibit HIF‐1alpha synthesis and block tumor growth. Proc Natl Acad Sci USA 2008; 105(50): 19,579–19,586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wu HC, Hsieh JT, Gleave ME, Brown NM, Pathak S, Chung LW. Derivation of androgen‐independent human LNCaP prostatic cancer cell sublines: role of bone stromal cells. Int J Cancer. 1994; 57(3): 406–412. [DOI] [PubMed] [Google Scholar]
- 15. Thalmann GN, Anezinis PE, Chang SM, Zhau HE, Kim EE, Hopwood VL, Pathak S, von Eschenbach AC, Chung LW. Androgen‐independent cancer progression and bone metastasis in the LNCaP model of human prostate cancer. Cancer Res. 1994; 54(10): 2577–2581. [PubMed] [Google Scholar]
- 16. Euhus DM, Hudd C, LaRegina MC, Johnson FE. Tumor measurement in the nude mouse. J Surg Oncol. 1986; 31: 229–234 [DOI] [PubMed] [Google Scholar]
- 17. Maeda K, Chung YS, Takatsuka S, Ogawa Y, Onoda N, Sawada T, Kato Y, Nitta A, Arimoto Y, Kondo Y, et al. Tumour angiogenesis and tumour cell proliferation as prognostic indicators in gastric carcinoma. Br J Cancer. 1995; 72: 319–323. [DOI] [PMC free article] [PubMed] [Google Scholar]


