Background: Kinases, involved in all processes of life, are enzymes capable of self-activation. MAP kinases, a kinase subfamily, do not self-activate.
Results: We found that p38β is a unique MAP kinase that can self-activate.
Significance: Through this we disclosed the element responsible for self-activation.
Conclusion: Minor differences between kinases impose dramatic differences on their self-activation property.
Keywords: Mitogen-activated Protein Kinase (MAPK), p38, p38 MAPK, Phosphorylation, Signal Transduction, Autophosphorylation
Abstract
Protein kinases are regulated by a large number of mechanisms that vary from one kinase to another. However, a fundamental activation mechanism shared by all protein kinases is phosphorylation of a conserved activation loop threonine residue. This is achieved in many cases via autophosphorylation. The mechanism and structural basis for autophosphorylation are not clear and are in fact enigmatic because this phosphorylation occurs when the kinase is in its inactive conformation. Unlike most protein kinases, MAP kinases are not commonly activated by autophosphorylation but rather by MEK-dependent phosphorylation. Here we show that p38β, a p38 isoform that is almost identical to p38α, is exceptional and spontaneously autoactivates by autophosphorylation. We identified a 13-residue-long region composed of part of the αG-helix and the MAPK insert that triggers the intrinsic autophosphorylation activity of p38β. When inserted into p38α, this fragment renders it spontaneously active in vitro and in mammalian cells. We further found that an interaction between the N terminus and a particular region of the C-terminal extension suppresses the intrinsic autophosphorylation of p38β in mammalian cells. Thus, this study identified the structural motif responsible for the unique autophosphorylation capability of p38β and the motif inhibiting this activity in living cells. It shows that the MAPK insert and C-terminal extension, structural motifs that are unique to MAPKs, play a critical role in controlling autophosphorylation.
Introduction
Protein phosphorylation is a central biochemical mechanism for controlling all biological processes. 518 genes, ∼1.7% of all genes in the human genome, encode protein kinases that catalyze these phospho-group transfers (1). All eukaryotic protein kinases (EPKs)2 adopt a similar topology that enables ATP binding and subsequent group transfer (2). This shared and highly conserved fold serves as a scaffold for additional loops and subdomains that appeared during the course of evolution in different kinases (2–5) and play various roles, mainly in regulation and substrate recognition. Some of these elements, including the activation loop and the GHI helices subdomain (5, 6), are common to all EPKs (2–5). Other elements are family-specific. Kinases of the MAPK family, for example, contain two motifs known as the MAPK insert and the C-terminal extension (1, 5, 7). These motifs impose dramatic effects on the proteins structure, but their roles in catalysis and regulation are not clear (see “Discussion”).
EPK activity is regulated through a multitude of mechanisms, unique to each subfamily, or even to a single kinase. However, a regulatory mechanism common to almost all EPKs is activation via phosphorylation of a conserved activation loop threonine (5). In many protein kinases, this phosphorylation/activation event occurs by autophosphorylation, making activation loop autophosphorylation a fundamental reaction of living cells. The mechanism of the autophosphorylation reaction is poorly understood, but because it occurs when the kinase is in its inactive form, it must be different from that of substrate phosphorylation by active kinases (8, 9).
The mammalian p38 family of MAPKs is composed of four isoforms; p38α, p38β, p38γ, and p38δ. This MAPK family is critical in mediating the cellular response to a wide variety of stresses, including DNA damage, oncogenic activity, and pro-inflammatory cytokines. Like all MAPKs, p38s have, in addition to the conserved activation loop threonine, a proximal tyrosine phospho-acceptor, composing a unique TGY phosphorylation motif. Phosphorylation of both sites is required for full catalytic ability and is mediated mainly by the MAPK kinases (MAPKK) MKK3 and MKK6 (10, 11). Unlike many other EPKs, MAPKs do not manifest spontaneous autophosphorylation. However, it is now clear that p38s are capable of TGY autophosphorylation, but this reaction is tightly regulated and can occur only under particular situations (12–18). For example, p38α autophosphorylation is induced when it is in complex with TAB1 (17, 19) or when phosphorylated on Tyr323 by Zap70 (15). Furthermore, specific point mutations, D176A and F327(S\L), also render p38α capable of autophosphorylation and autoactivation (13, 14, 20). p38α autophosphorylation has been shown to be physiologically relevant in the immune system (21, 22) and in ischemic preconditioning (17, 19) and to be sufficient to induce cellular senescence (23). The finding that MAPKs can autophosphorylate suggests that they are similar to most EPKs in this respect, but evolution provided them with inherent inhibition that prevents spontaneous autophosphorylation. Little is known about the structural basis that prevents MAPK spontaneous autophosphorylation and activates it when necessary.
We show here that when purified as a recombinant protein, p38β manifests spontaneous catalytic activity, which is a consequence of autophosphorylation. This observation is particularly surprising because of the high sequence identity between p38β and p38α, which does not autophosphorylate spontaneously. We took advantage of the high sequence similarity on one hand and the dramatic difference in activity between p38β and p38α on the other to map the motif responsible for autophosphorylation and autoactivation. We have identified a C-terminal region, Arg233–Val246, which composes part of the G-helix and the MAPK insert (MKI), to be responsible for triggering the intrinsic autophosphorylation activity of p38β. Inserting this region into p38α rendered it intrinsically active both in vitro and in HEK293 cells. We further show that the autophosphorylation activity of p38β is regulated in mammalian cells. Its regulation is mediated by part of the MKI and via an interaction between the N-lobe and C-tail. Thus, the autophosphorylation activity of p38β is regulated and executed by a number of C-terminal elements that were so far unnoticed.
EXPERIMENTAL PROCEDURES
Mammalian Cell Culture
HEK293 were grown in DMEM supplemented with 10% fetal bovine serum, sodium pyruvate, and antibiotics. Cells were grown at 37 °C and 5% CO2. The cells were transfected with the calcium phosphate method.
Protein Expression and Plasmid Construction
For bacterial expression, the pET15b and pET28b vectors were used, with the ORFs hexahistidine-tagged N-terminally, as described previously (14). For expression, the Rosetta strain (Novagene) was used. For mammalian expression, the pcDNA3 (Stratagene) and pCEFL (Addgene) vectors were used with the ORFs tagged N-terminally with 3× HA or Flag tag. Site-directed mutagenesis, using the Stratagene QuikChange® kit, was performed according to the manufacturer's instructions. Truncation mutants were obtained by inserting stop codons by PCR with specific primers to p38β cDNAs. p38α\p38β chimeras were obtained without changing the native sequence by using five restriction sites: (i) the Kpn1 site in p38β; (ii) the Bsu36i site, p38β; (iii) the Sal1 site, p38α; (iv) the Xho1 site, which was generated by to p38β by introducing a silent point mutation (CTGGAG → CTCGAG, CTC and CTG both encode Ile); and (v) the Mfe1 site, which was generated in p38α by introducing a silent mutation (CAGTTG → CAATTG). The corresponding restriction sites were generated in the other isoform by site-directed mutagenesis. Chimeras that could not be obtained by restriction\ligation were generated by PCR with the Phusion site-directed mutagenesis kit (thermo-scientific) according to the manufacturer's instructions.
Protein Purification
Protein purification from Escherichia coli cells was performed using nickel-nitrilotriacetic acid beads (Hadar Biotech, Rehovot, Israel) as previously described (14). Protein concentrations were determined by the Bradford method.
In Vitro Kinase Assay
All reactions with GST-ATF2 as a substrate were conducted in 96-well plates in triplicate. To initialize the reaction, 45 μl of the reaction mixture were added to 5 μl of p38 enzyme (0.2 μg, 100 nm, of purified hexahistidine tag p38). Final reaction conditions were 25 mm Hepes, pH 7.5, 20 mm MgCl2, 20 mm 2-glycerolphosphate, 0.1 mm Na3VO4, 1 mm dithiothreitol, 40 μg of GST-ATF2, 50 μm ATP (kinase buffer), and 0.5 μCi of [γ-32P]ATP. The reactions proceeded for 10 min at 30 °C with agitation and were terminated with 50 μl of 0.5 m EDTA, pH 8 (final concentration, 250 mm). Following reaction termination, 15 μl from each set of samples were subjected to SDS-PAGE, stained with Coomassie, dried, and exposed to film, and aliquots of 85 μl from each well were spotted onto 3X3-cm Whatman 3MM paper squares and air-dried. Each square was rinsed three times with 10% trichloroacetic acid and 3% sodium pyrophosphate (10 ml/square) for 1.5 h (each time) with gentle agitation, and a fourth wash for overnight was given without shaking. The following day, the squares were rinsed twice with 100% ethanol (4 ml/square) for 20 min each time and air-dried. The radioactivity of each square was counted using a scintillation counter running a 32P Cherenkov program. Autophosphorylation reactions of proteins purified from E. coli were conducted under similar conditions, with 1 μg of the tested p38 protein and without GST-ATF2. These reactions were analyzed by SDS-PAGE. For activation of p38 with MKK6, an active mutant of MKK6 in which Ser207 and Thr211 were both mutated to Glu (termed MKK6EE) was used under similar conditions. Immune complex kinase assay was preformed as previously described (24).
In-Solution Tryptic Protein Digest
5 μg of p38αWT, p38αD176A+F327S, p38βWT, and p38βD176A proteins were diluted to 8 m urea and 100 mm ammonium bicarbonate. Before tryptic digestion, cysteine residues were reduced with 2.8 mm DTT (Sigma; D9163-5G) for 30 min at 60 °C and carboxymethylated with 8.8 mm iodoacetamide (Sigma; I6125-100G) for 30 min at room temperature in the dark. Trypsin (sequencing grade modified; Promega V511A) was added, after the samples were diluted in 2 m urea, in a ratio of 1:50 and incubated overnight at 37 °C. A second dose of trypsin was added the following morning for 4 h at 37 °C. The samples were then acidified with 0.1% TFA and desalted with micro TipColumn C-18 (Harvard Apparatus 74–5201).
Analysis by Liquid Chromatography Tandem MS and Interpretation of MS Data
Peptides were resolved by reversed phase chromatography on 0.075 ID 200-mm fused silica capillaries (Agilent 160-2644-10) packed with Reprosil-Aqua C18 reversed phase material (Dr. Maisch, GmbH, Germany). The peptides were eluted with linear gradient, 7–45% acetonitrile in 0.1% formic acid, at a flow rate of 0.25 μl/min, using an Eksigent Nano capillary HPLC. Mass spectrometry was performed with an OrbitrapXP mass spectrometer (Thermo-Fisher) using the positive mode and repetitively full MS scan, followed by collision induced dissociation with multistage activation of the 10 most dominant ion selected from the full MS scan. The mass spectrometry data were analyzed using MaxQuant version 1.3.0.5 (25) with Andromeda (26) search engines, searching against the E. coli section of the UniProt database from September 2012 including human p38 proteins and their mutated variants with mass tolerance of 6 ppm for the precursor masses and 0.5 Da for the fragments. Identifications of the proteins and phosphorylation sites were filtered to 1% false discovery rate. The resulting identified phospho-sites table was filtered to include only phospho-sites on p38 proteins and their mutated variants.
Western Blot Analysis
For recombinant proteins, 100 ng were mixed with X4 Laemmli buffer and boiled at 100 °C for 4 min. Mammalian cell protein lysates were prepared by decanting the growth medium, washing cells with PBS, and adding Laemmli buffer directly to the plate. Lysates were then collected and boiled for 10 min. The different samples were separated via a 10% SDS-PAGE and transferred to a nitrocellulose paper. Antibodies used in this study were: anti-phospho-p38 (Cell Signaling; #9211), anti-HA antibodies (obtained from the 12CA5 hybridoma cell line), anti-MAPKAPK2 (Cell Signaling; #3042) anti-phospho MAPKAPK2 (Cell Signaling; #3007), anti-Flag (Sigma; A2220), and anti-polyhistidine (Sigma; H1029).
RESULTS
p38β, but Not p38α, Possesses an Intrinsic Catalytic Activity Caused by Autophosphorylation on Thr180
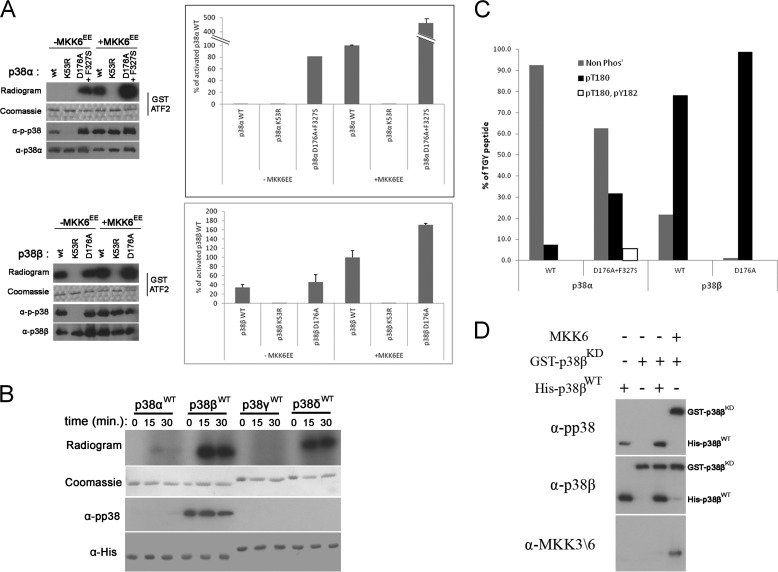
MAPKs do not manifest basal intrinsic activity but require MEK-mediated phosphorylation for activation. Surprisingly, we found that purified p38βWT is spontaneously active and able to phosphorylate GST-ATF2 in an in vitro kinase assay (Fig. 1A). To analyze and quantify this activity, we compared the ability of purified WT, kinase-dead (KD), and intrinsically active variants of p38α and p38β to phosphorylate GST-ATF2 in an in vitro kinase assay. p38βWT, but not p38αWT, was purified in an active state, demonstrated by its ability to phosphorylate ATF2 (Fig. 1A). The intrinsic activity of p38βWT is very significant, reaching a level of 35% of that of MKK6-phosphorylated p38βWT (Fig. 1A). The activity of the active mutant p38βD176A (12) is just slightly higher (Fig. 1A). Following incubation with MKK6EE, the active variants of the p38 MAPKK, both p38αWT and p38βWT, were able to efficiently phosphorylate ATF2, indicating that both proteins can acquire a catalytically active conformation. The high intrinsic activity of p38βWT is unexpected because this protein is very similar to p38α (Fig. 2A), which shows very low intrinsic activity, ∼0.5% of the activity shown by MKK6-activated p38αWT (Fig. 1A).
FIGURE 1.
p38β, but not p38α, is intrinsically active. A, in vitro kinase assay was preformed with the indicated p38 proteins with GST-ATF2 as a substrate. Activity is shown in graphs, expressed as percentages of the activity of MKK6-activated wild type p38α or p38β (100%), and by autoradiograms. 100 ng of each protein was also analyzed by Western blot with the indicated antibodies. B, in vitro autophosphorylation assay was preformed with the indicated p38 isoforms. Activity is shown by autoradiograms. 100 ng of each protein was also analyzed by Western blot with the indicated antibody. C, 5 μg of p38αWT, p38αD761A+F327S, p38βWT, and p38βD176A were incubated with ATP for 1 h in a kinase reaction mixture with no other substrate (autophosphorylation). Proteins were then cleaved into tryptic peptides and analyzed for phosphorylation sites by tandem MS. The relative intensities of the TGY motif-containing peptides in the different phosphorylation states: nonphosphorylated (Non Phos), Thr180-phosphorylated, and Thr180 + Tyr182-phosphorylated (0P, 1P, and 2P) are displayed as the percentages of all TGY peptides intensities (0P + 1P + 2P). D, 250 nm of purified His-tagged p38βWT or of MKK6EE were incubated with 250 nm GST-tagged p38βKD in a kinase reaction mixture for 1 h. Samples of each reaction were analyzed by Western blot with the indicated antibodies.
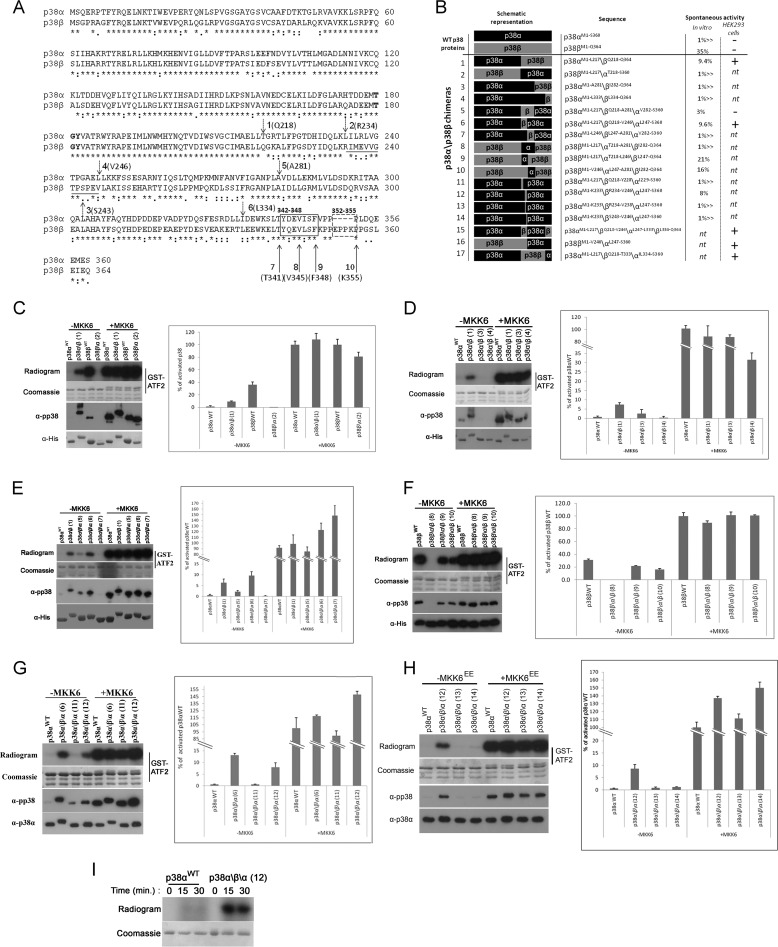
FIGURE 2.
A 13-amino acid region, Arg234–Val246, which composes part of the G-helix and the MKI of p38β, is responsible for its autophosphorylation activity. A, p38α and p38β linear sequences were aligned using the ClustalW server. Bold type, TGY motif; dashed arrows 1–6, chimera swap points; black line under alignment, Arg234–Val246 region; solid arrows 7–10, truncation sites; solid and dashed boxes, Tyr342–Phe348 and Glu352–Lys355 regions. B, summary of the chimeras used in this study, showing their measured activity in in vitro kinase assays as percentages of the activity of MKK6-activated p38 and their activity in HEK293 cells. C–H, results of kinase assays and Western blots preformed with p38α\p38β chimeras. The results are shown in gels and graphs as described in legend of Fig. 1. p38 chimeras are numbered according to B. C, activities of chimeras 1 and 2 compared with those of MKK6-activated p38αWT and p38βWT. D, activities of chimeras 1, 3, and 4 compared with MKK6-activated p38αWT. E, activities of chimeras 1, 5, and 6 compared with MKK6-activated p38αWT. F, activities of chimeras 8, 9, and 10 compared with MKK6-activated p38βWT. G, activities of chimeras 6, 11, and 13 compared with MKK6-activated p38βWT. H, activities of chimeras 12, 13, and 14 compared with MKK6-activated p38αWT. I, p38αWT and p38α\β\α chimera 12 were tested in an autophosphorylation assay. Samples were collected at the indicated times, separated by SDS-PAGE, and exposed to film.
The catalytic activity of the p38 proteins is expected to be correlated with phosphorylation of their TGY motif. Indeed, Western blot analysis showed that p38βWT reacted more efficiently with anti-phospho-p38 (α-pp38) antibody than did p38αWT (Fig. 1A). On the other hand, the kinase dead (p38βKD) variant, p38βK53R, reacted with the α-pp38 antibody only after MKK6EE phosphorylation. It implies that the efficient phosphorylation of purified p38βWT is a result of an autophosphorylation activity of the TGY motif that is more efficient than that of p38αWT.
To test this notion and to see whether this autophosphorylation activity is unique to p38β, we conducted an in vitro autophosphorylation assay with all p38 isoforms. Indeed, of the four isoforms tested, only p38βWT was phosphorylated on the TGY motif (Fig. 1B). Curiously, p38δWT manifested some autophosphorylation activity, as indicated by the radiogram (Fig. 1B). However, this phosphorylation does not occur on the TGY motif, as shown by Western blot (Fig. 1B). Thus, p38β is the only p38 isoform capable of spontaneous autophosphorylation of the TGY motif, explaining the observation that it is the only p38 isofom that manifests spontaneous activity.
The α-pp38 antibody, raised against dual phosphorylation of the TGY motif, also detects threonine 180-monophosphorylated p38 (24). Therefore, to unequivocally determine which residues are phosphorylated by autophosphorylation, we preformed an MS/MS analysis. p38βWT was found to be monophosphorylated on Thr180 (Thr of the TGY motif) at very high levels compared with p38αWT. ∼80% of the peptides derived from p38βWT that contain the TGY motif had a phosphate group on Thr180, compared with only ∼7% of the corresponding peptides derived from p38αWT (Fig. 1C). Thr180 was found to be phosphorylated at higher levels in p38βD176A and p38αD176A+F327S than in their WT counterparts (>98% and ∼40%, respectively). Interestingly, although no phosphorylation was detected on Tyr182 of p38βWT or p38βD176A, ∼5% phosphorylation on this residue was found in p38αD176A+F327S, indicating that this protein possesses some tyrosine kinase activity. Although Thr + Tyr dual phosphorylation is considered an absolute requirement for MAPK activity, we and others have shown that p38 and ERK molecules are active to various degrees even when monophosphorylated on the threonine phospho-acceptor (16, 24, 27). The intrinsic activity of monophosphorylated p38β (35% of maximum) seems exceptional because the activity of monophosphorylated p38α, or ERK2 is much lower (16, 24).
p38 autophosphorylation of the regulatory threonine, Thr180 in p38β and p38α, has been proposed to occur both by cis (19) and by trans (28) mechanisms. To determine by which mechanism the autophosphorylation of p38βWT occurs, we co-incubated purified His-tagged p38βWT with GST-tagged kinase dead p38β variant (p38βKD) and monitored the phosphorylation status of these proteins via Western blot with the α-pp38 antibody. We found that although GST-p38βKD was phosphorylated by MKK6EE, showing that it is a legitimate substrate, it was not phosphorylated by p38βWT, which was efficiently phosphorylated in this assay (Fig. 1D). Thus, the autophosphorylation of Thr180 of p38β occurs in cis.
A Region Containing Part of the α-G Helix and the MKI Is Responsible for the Intrinsic Activity of p38β
p38α and p38β are 75% identical and 88% similar (Fig. 2A). Therefore, to identify the region responsible for the intrinsic activity of p38β, we constructed a series of p38α\β chimeras (chimera swapping points are presented in Fig. 2A as dashed arrows numbered 1–6. A schematic representation and chimera numeration is presented in Fig. 2B). We first constructed two chimeras: p38α\β, composed of the N terminus of p38α fused to the 164 C-terminal amino acids of p38β and a reciprocal p38β\α chimera (Fig. 2B, chimeras 1 and 2). When tested for their ability to phosphorylate ATF2 in vitro, the p38α\β chimera manifested a high basal activity that was 9.4% of MKK6-activated p38αWT. No basal activity was observed for the reciprocal chimera, p38β\α (Fig. 2C). Correspondingly, Western blot analysis showed that p38α\β was phosphorylated to higher levels than p38αWT (Fig. 2C). Importantly, both chimeras were fully activated by MKK6, indicating that they preserved regulatory and catalytic properties of their parental proteins. These chimeras disclosed that the intrinsic activity of p38β resides somewhere in its C terminus. Two additional p38α\β chimeras, composed of the N-terminal part of p38α fused to the 83 and 31 C-terminal amino acids of p38β (Fig. 2B, chimeras 3 and 4) were both inactive unless pre-incubated with MKK6 (Fig. 2D), strongly suggesting that the region responsible for the autoactivity of p38β resides between Gln218–Ala281. Indeed, a p38α\β\α chimera containing the Gln218–Ala281 region of p38β (Fig. 2B, chimera 5) was active prior to MKK6 activation (Fig. 2E). Supporting this notion, a p38β\α\β chimera protein containing the corresponding Thr218–Ala281 region of p38α (Fig. 2B, chimera 8) was active only in a MKK6-dependent manner (Fig. 2F). To determine whether this entire region (Gln218–Ala281) is required for activity, we constructed two additional p38α\β\α chimeras: the first containing Gln218–Val246 of p38β and the second containing Leu247–Ala281 (Fig. 2B, chimeras 6 and 7). The p38α\β\α protein, containing only Gln218–Val246 of p38β, but not the chimera containing Leu247–Ala281, manifested an intrinsic activity of ∼13% compared with that of MKK6-activated p38αWT and was purified as a phosphorylated protein (Fig. 2E). However, in the reciprocal experiment conducted with p38β\α\β chimeras, only the chimera with the 64-amino acid region of p38α, Thr218–Ala281, did not possess MKK6-independent activity, whereas the chimeras that contained part of this region maintained some MKK6-independent activity (Fig. 2, B, chimeras 9 and 10, and F). Therefore, the Thr218–Leu246 region of p38α can replace that of p38β in the context of the p38β protein. We next tested whether the p38β fragment Gln218–Val246 is the minimal region sufficient to render an autophosphorylating p38α protein. For this, we generated two additional chimeras, each containing approximately half of the Gln218–Val246 region (Fig. 2B, chimeras 11 and 12). Interestingly, only chimera 12, containing the Arg234–Val246 region, possessed intrinsic activity, ∼8% of MKK6-activated p38αWT (Fig. 2G), whereas chimera 11, containing Gln218–Tyr228 of p38β, was active only in an MKK6-dependent manner (Fig. 2G). Finally, two p38α\β\α chimeras, each containing part of the Arg234–Val246 region (Fig. 2B, chimeras 13 and 14), were not intrinsically active (Fig. 2H). To show that the activity rendered on p38α by the small fragment of p38β is correlated with an increase in autophosphorylation activity, we conducted an in vitro autophosphorylation assay and found that chimera 12, a p38α protein containing the Arg234–Val246 fragment of p38β indeed manifested a very high autophosphorylation activity (Fig. 2I). Thus, the Arg234–Val246 region termed hereafter G-helix-MKI motif, is the minimal region of p38β required for autophosphorylation.
p38β-specific Structural Elements Are Responsible for Regulating Its Intrinsic Activity in Mammalian Cells
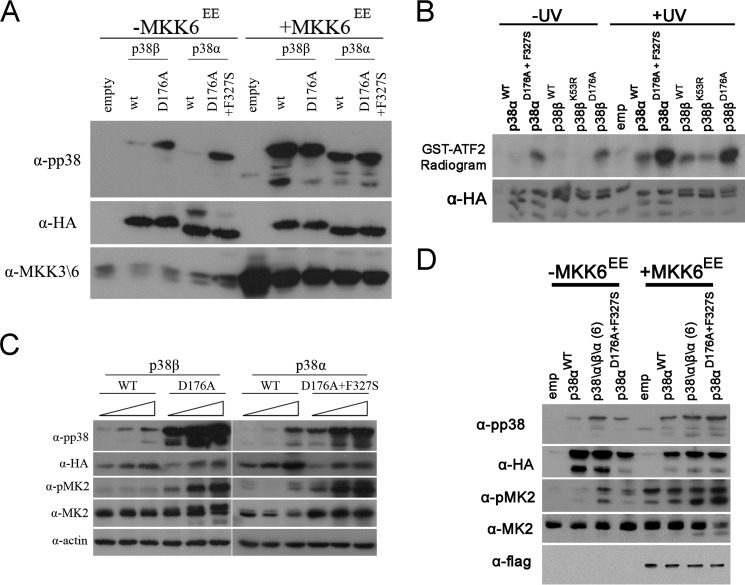
To test whether p38βWT might be spontaneously active not only as recombinant purified protein, but also in mammalian cells, we overexpressed it in HEK293 cells. Like other p38 isoforms (20), p38βWT was not phosphorylated in unprovoked cells (Fig. 3A). Accordingly, p38αWT and p38βWT showed a very low ability to phosphorylate ATF2 when immunoprecipitated from these cells, unless the cells were exposed to UV (Fig. 3B). Elevating expression levels of p38αWT and p38βWT resulted in only a slight increase in their apparent phosphorylation (Fig. 3C) that was not sufficient to induce phosphorylation of the p38 substrate MAPKAPK2 (MK2). Thus, the intrinsic activity of p38βWT is regulated in mammalian cells. On the other hand, intrinsically active variants p38αD176A+F327S and p38βD176A were found to be spontaneously phosphorylated and active toward MK2 under the same conditions (Fig. 3, A and C) and manifested elevated catalytic activity when immunoprecipitated from cells not exposed to any activating signal (Fig. 3B).
FIGURE 3.
A short segment of p38β is sufficient to render p38α spontaneously active in HEK293 cells. Cell lysates were prepared and analyzed by Western blot with the indicated antibodies 48 h post-transfection. A, transfections with 1.5 μg of each p38 expression plasmid with or without 0.5 μg of a plasmid containing MKK6EE. 30 μg of protein from each sample were prepared 48 h post-transfection, separated by SDS-PAGE, blotted, and probed with the indicated antibodies. B, cells were transfected as in A. 48 h post-transfection cells were irradiated by UV (120 J\m2) or not and lysed 1 h later. p38 proteins were immunoprecipitated with α-HA antibodies and subjected to an immune complex kinase assay with ATF2. Samples were separated by SDS-PAGE, exposed to film, and probed with an α-HA antibody. C, increasing amounts (1, 2, and 4 μg of DNA) of each p38 were transfected into HEK293 cells. Proteins were analyzed as in A. D, transfections were preformed as in A.
Several possible explanations can account for the incapability of p38β to express its intrinsic activity in vivo. A general model would be that the suppression involves cellular components (because in vitro the activity is not suppressed) that may bind to particular domains in p38β and lock the G-helix-MKI motif. If so, the p38α\β\α chimera that contains only the “activating fragment” (G helix-MKI) of p38β should not be suppressed in mammalian cells. Indeed, when expressed in HEK293 cells, the chimera containing Gln218–Val246, which possessed the highest intrinsic activity as a recombinant purified protein, was found to be spontaneously phosphorylated at high levels in HEK293 cells (Fig. 3D). Importantly, expression of this p38α\β\α chimera led to a spontaneous, signal-independent phosphorylation of MK2. Thus, the G helix-MKI region of p38β is sufficient to activate p38α not only as a recombinant purified protein, but also in mammalian cell culture. These results support the notion that in addition to the region responsible for autoactivation found in p38β, an additional region exists that is responsible for suppressing this activity in mammalian cells.
A p38β Molecule Lacking the C-terminal Tail Has Increased Spontaneous Activity Both in Mammalian Cells and in Vitro
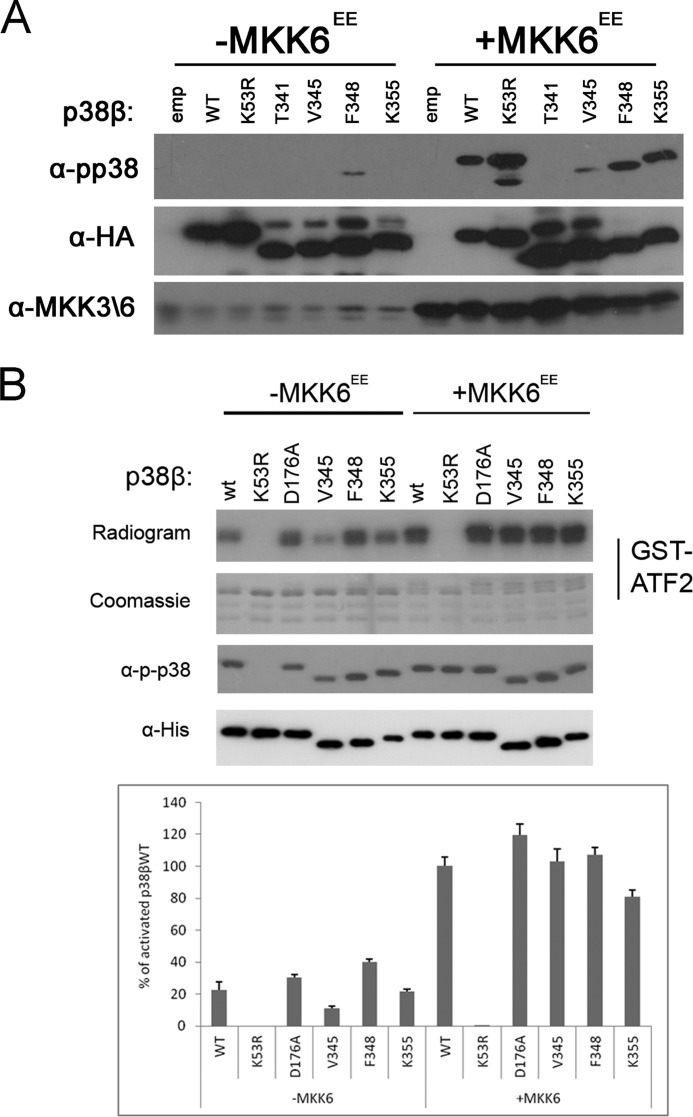
We next tried to identify the region responsible for suppressing the activity of p38β in mammalian cell culture. In previous studies, we found that the C termini of the yeast MAPKs Hog1 and Mpk1 suppress autophosphorylation activity (29, 30). The MAPK extension, a MAPK-unique C-terminal region, is therefore an attractive region for a potential regulatory domain. The function of this region is still unknown. To test whether an autophosphorylation inhibitory domain may reside in the C terminus of p38β, we generated and expressed in HEK293 cells a series of four C-terminal truncated p38β proteins (truncation points are marked by solid arrows 7–10 in Fig. 2A). The region selected for this analysis was Thr341–Gln364, composing the C-tail of p38β. Of this region, the L16 helix (Tyr342–Phe348, marked by a solid box in Fig. 2A) was shown to be necessary for autophosphorylation in p38α and Hog1 (30). Also, this region contains the only p38β unique insertion Glu352–Lys355 (Fig. 2A, dashed box). Of the series of C-terminally truncated p38β proteins, p38βK355 and p38βV345 were not found to be spontaneously phosphorylated in HEK293 cells but were phosphorylated when co-expressed with MKK6EE (Fig. 4A). p38βF348, however, showed a mild but clear elevation in its spontaneous phosphorylation levels (Fig. 4A). Thus, Lys349–Gln364, which composes an unstructured region C-terminal to the L16 helix, might be involved in suppressing autophosphorylation.
FIGURE 4.
The C tail of p38β inhibits autophosphorylation. A and B, premature stop codons were inserted into p38β generating p38β C-terminal truncation mutants p38βT341, p38βV345, p38βF348, and p38βK355. A, these proteins and p38βWT or an empty vector (emp) were transiently expressed in HEK293 cells with or without plasmids expressing MKK6EE. Cell lysates were prepared, and 30 μg of protein from each sample were separated by SDS-PAGE, blotted, and probed with the indicated antibodies. A summary of all chimeras tested is provided in Fig. 2B. B, 200 ng of recombinant p38 proteins were subjected to a kinase assay as described in the legend to Fig. 1.
The proteins lacking the putative inhibitory C terminus were also tested as recombinant purified proteins. The activity of p38βK355 (Fig. 4B) was similar to that of p38βWT. However, p38βF348 displayed an elevated spontaneous (MKK6-independent) activity, ∼160% over the spontaneous, MKK6-idependent, activity of p38βWT (Fig. 4B); this result is reminiscent of the increased spontaneous activity shown by p38βF348 in mammalian cells (Fig. 4A). This supports the notion that the Phe348–Lys355 region of the C terminus bears inhibitory properties on the autophosphorylation of p38β.
The C-terminal Part of p38β MAPK Insert Suppresses the Autophosphorylation Activity in Mammalian Cells
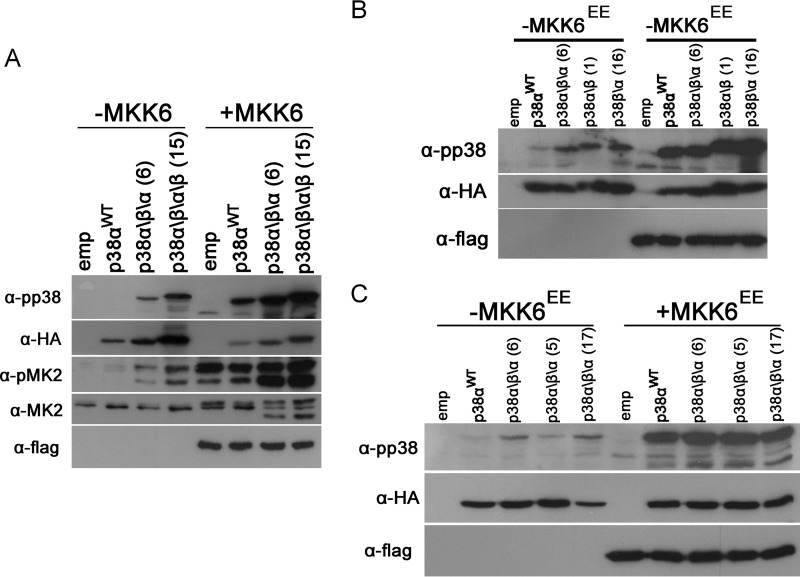
The data so far shows that: (i) replacing the G-helix and part of the MKI of p38α with that of p38β rendered this protein intrinsically active in vitro and in HEK293 cells (Figs. 2G and 3D) and (ii) truncation of the C-terminal extension of p38β up to the L16 helix (generating p38βF348) partially relieved p38β autoactivation from inhibition in HEK293 cells (Fig. 4A). We postulated that if the C-terminal extension has an inhibitory role, perhaps replacing the C-terminal extension in the active p38α\β\α chimera, which originates from p38α, with that of p38β would eliminate the spontaneous activity of this protein in HEK293 cells. Therefore, we generated a p38α\β\α\β chimera that has, in addition to the G-helix-MKI (Gln218–Val246) region from p38β, also the C terminus of p38β; Leu334–Gln364 (Fig. 2B, chimera 15). However, this p38α\β\α\β chimera not only did not lose spontaneous activity, it seemed to be more active than the “parental” chimera. p38α\β\α\β was phosphorylated at higher levels and led to higher phosphorylation of MK2 in HEK293 cells than p38α\β\α (Fig. 5A). The data combined suggest that the C terminus of p38β is an inhibitor of the autophosphorylation activity but cannot function alone. To determine which elements cooperate with the C-terminal inhibitory region, we tested the phosphorylation of a p38α\β chimera, composed of the N terminus of p38α and the C terminus of p38β and a p38β\α reciprocal chimera in HEK293 cells (Fig. 2B, chimeras 1 and 16). Of note, both chimeras contained the Gln218–Val246 activating region. Interestingly, both the p38α\β chimera and the p38β\α chimera were found to be phosphorylated at similar levels like the p38α\β\α chimera (Fig. 5B). Thus, when the N terminus and the C terminus are of different isoforms, inhibition of autophosphorylation is not achieved. In MAPKs, the C-terminal extension protrudes into the N-lobe in a unique manner (5). The function of this unique interaction is not known, but now we propose that disruption of this interaction, either by truncation of the C-terminal extension or by generating a chimera that contains an N terminus of one isoform and a C terminus of another, leads to an increase in spontaneous activity (Figs. 4A and 5A). To further test this notion, we expressed two additional p38α\β\α chimeras that contain the activating fragment in HEK293 cells. The first chimera contains a p38β fragment (Gln218–Ala281) that, in addition to the activating G-helix-MKI region, based on the crystal structure, is not in the interface of the C- to N-terminal interaction, thus maintaining the N- to C-terminal interaction of p38α. The second chimera contains a larger p38β region (Gln218–Thr333), enforcing C-tail to N-lobe interaction from the two different isoforms (Fig. 2B, chimeras 5 and 17). Indeed, although chimera 17 was found to be active, chimera 5 was not (Fig. 5C). Based on this, we suggest the following model: p38β spontaneous activity depends on a fragment composed of the G-helix and part of the MKI (Arg234–Val246). This activity is suppressed in cells via a mechanism involving the remaining part of the MKI (Leu247–Ala281), as well as the interaction between the C-terminal extension and the N-lobe (Fig. 6).
FIGURE 5.
p38β autophosphorylation suppression depends on the MAPK insert. A–C, HEK293 cells were transfected transiently with plasmids expressing the indicated p38 protein or an empty vector (emp), with or without plasmids expressing MKK6EE. Cell lysates were prepared, and 30 μg of protein from each sample were separated by SDS-PAGE, blotted, and probed with the indicated antibodies. As control, phosphorylation levels are compared with transfected p38αWT. Chimeras are numbered according to Fig. 2B. A, chimeras 6 and 15. B, chimeras 1, 6, and 16. C, chimeras 5, 6, and 17.
FIGURE 6.
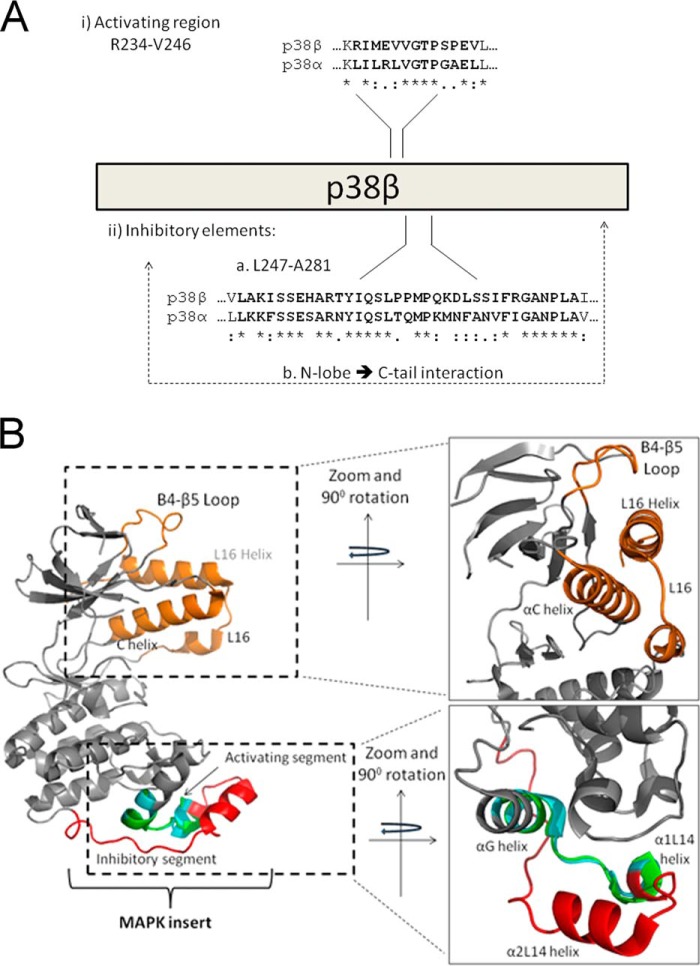
A schematic (A) and structural (B) representation of regions that regulate p38β autophosphorylation. The crystal structures of p38α (Protein Data Bank code 4E5B; gray backbone) and p38β (Protein Data Bank code 3GC8; backbone not shown) were aligned according to the Arg234–Val246 region, p38β numeration, and are presented from two angles rotated 90°. (i) A and B, the unique intrinsic autophosphorylation activity of p38β is triggered by the Arg234–Val246 region composing part of the α-G helix and MKI. B, the G-MKI region of p38α is shown in cyan, and that of p38β is shown in green. (ii) A and B, p38β intrinsic autophosphorylation is suppressed in mammalian cells via part of the MKI (shown in red in B) and depends on an interaction between the N-lobe and C-tail. The C-tail, composed of L16 and the L16 helix, the N-lobe elements that interact with the C-tail, the C helix, and the β4-β5 loop, is shown in orange.
DISCUSSION
During the course of evolution, MAPKs acquired some properties that distinguish them from other EPKs. A prominent property is the requirement of an unusual, MEK-dependent, dual phosphorylation for activation. Evolution also equipped MAPKs with two unique structural motifs: the MAPK insert (also found in CDKs) and a C-terminal extension. In this study, we show that these MAPK-specific motifs are critical players in controlling MAPK autophosphorylation. Unlike the spontaneous autophosphorylation manifested by most EPKs, such activity is rarely monitored in MAPKs. However, there are strong indications that MAPKs are capable of efficient autophosphorylation that is manifested only under very specific conditions (12, 13, 15–17, 31). Consequently, structural elements involved in executing autophosphorylation, or rather, elements blocking spontaneous autophosphorylation and thereby differing MAPKs from most EPKs, were not revealed. We were able to propose such elements through the unusual spontaneous autophosphorylation property of p38β.
Although this activity is very specific to p38β, the regions found to be responsible for it turned out to be very similar not only in p38α (Fig. 6A), but also in other p38 isoforms and even in other MAPKs. The most critical region identified, Arg234–Val246, which contains the capability to endow p38α with intrinsic autophosphorylation, is composed of the C-terminal part of the G-helix (Fig. 6B), which is a conserved structural motif in all EPKs, and the N-terminal part of the MKI, including the α1L14 helix, which is conserved in all MAPKs (1, 2, 7). This region is highly conserved within the p38 family so that bioinformatic and structural analyses could not point at it as p38β-specific. Notably, of the 13 residues that compose the G-helix-MKI fragment responsible for p38β intrinsic activity, six are identical and two are conserved in p38α (Fig. 6A), explaining why the domain was not disclosed in simple sequence analysis.
The identification of the G-helix-MKI fragment as the trigger of autophosphorylation was also unexpected because the G-helix and MKI were so far assigned roles in nuclear localization (32) and not in catalysis or in its regulation. This region also does not seem to be related to the R or C spines that regulate activity of protein kinases (5, 6, 33, 34). However, in addition to its role in nuclear translocation, the MKI was reported to serve as an allosteric site for binding of regulators, activators, substrates, and lipids (35, 36). The GHI helices subdomain, including the MKI in MAPKs, has been shown to be more flexible than other regions in a number of different kinases (5) and may also play a role in allosteric regulation of kinase activity (36). However, structure-function studies of MAPKs have mainly addressed the mechanisms and structural requirements of catalysis toward substrates and not of autophosphorylation, which must have specific requirements because it occurs when kinases are in their inactive conformation (8, 9).
There are many crystal structures available of p38α, including nonphosphorylated (37), dually phosphorylated (38), and induced to autophosphorylate, either by point mutation (28) or via interaction with TAB1 (19). Only two crystal structures of p38β (39) were reported, both in complex with a small inhibitor molecule. Therefore, structural comparison is limited. Nevertheless, comparing the structures of p38α and p38β shows an almost complete overlap of the folds of the regions identified here (Fig. 6B). Furthermore, the G-helix-MKI region does not seem to adopt an alternative conformation in the structures of p38α in complex with the TAB1 peptide or harboring activating point mutations that render it an autophosphorylating protein. Therefore, just like the sequence alignment, structural analysis could not disclose the role of this region in autophosphorylation.
Acquisition of an active conformation in p38α is accompanied with reduced mobility of various regions throughout the kinase domain (40). The Arg234–Val246 region may project on other regions in the molecule and perhaps affect their mobility, allowing ATP binding and conferring correct alignment of the catalytic spines. The activation loop is a candidate to be affected by this region because it is proximal to the G helix in the three-dimensional structure (28). Interestingly, the region responsible for the spontaneous activity of JNK2α2, the only other mammalian MAPK with reported spontaneous activity (41), does not overlap with the region we have identified in this study.
Finally, the notion that an N-terminal–C-terminal tail interaction inhibits autophosphorylation in p38β may explain results obtained with Hog1 and Mpk1 in which C-terminal truncations evoke intrinsic activity (29, 30). Perhaps in those and other MAPKs, N-terminal–C-terminal interactions suppress autophosphorylation. The C terminus proximity to the N-lobe is a unique feature of MAPKs. In most EPKs that undergo spontaneous autophosphorylation, the termini reside away from each other, supporting the notion that the role of the C-terminal protrusion into the N-lobe in MAPKs is to inhibit the autophosphorylation of MAPKs (5). This notion at least partially explains why autophosphorylation is so rarely manifested by this group of kinases.
Because p38 activity, including p38β (42), has dramatic effects on cell fate, their activation, either by MEKs or by autophosphorylation, must be tightly controlled. Indeed, we show that the autophosphorylation-dependent activation of p38β is regulated in vivo by cellular components. These components could either be an interacting protein physically inhibiting the conformation required for autophosphorylation or constitutive down-regulation by phosphatases. A candidate phosphatase could be MKP1, which is likely to bind the CD domain (43).
p38 autophosphorylation is of high physiological and clinical importance (19, 44). Because mammalian MAPK families are composed of a number of isoforms that are regulated in vivo by the same MAPKKs, autophosphorylation provides an elegant mechanism for isoform-specific activation. Given the efficient intrinsic autophosphorylation of p38β, specific activation of this isoform would require just desuppression of its intrinsic activity.
This study was supported by the Israel Science Foundation (Center of Excellence Grants 180/09 and 1772/13), the Bi-national US-Israel Science Foundation (Grant 2009116), the Israel Cancer Research Fund, and Singapore National Research Foundation under its HUJ-NUS partnership program in the Campus for Research Excellence And Technology Enterprise (CREATE).
- EPK
- eukaryotic protein kinase
- MKI
- MAPK insert.
REFERENCES
- 1. Manning G., Whyte D. B., Martinez R., Hunter T., Sudarsanam S. (2002) The protein kinase complement of the human genome. Science 298, 1912–1934 [DOI] [PubMed] [Google Scholar]
- 2. Oruganty K., Kannan N. (2012) Design principles underpinning the regulatory diversity of protein kinases. Philos. Trans. R. Soc. Lond. B Biol. Sci. 367, 2529–2539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hanks S. K., Quinn A. M., Hunter T. (1988) The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science 241, 42–52 [DOI] [PubMed] [Google Scholar]
- 4. Johnson L. N., Noble M. E., Owen D. J. (1996) Active and inactive protein kinases: structural basis for regulation. Cell 85, 149–158 [DOI] [PubMed] [Google Scholar]
- 5. Taylor S. S., Keshwani M. M., Steichen J. M., Kornev A. P. (2012) Evolution of the eukaryotic protein kinases as dynamic molecular switches. Philos. Trans. R. Soc. Lond. B Biol. Sci. 367, 2517–2528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Taylor S. S., Kornev A. P. (2011) Protein kinases: evolution of dynamic regulatory proteins. Trends Biochem. Sci. 36, 65–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kannan N., Neuwald A. F. (2004) Evolutionary constraints associated with functional specificity of the CMGC protein kinases MAPK, CDK, GSK, SRPK, DYRK, and CK2α. Protein Sci. 13, 2059–2077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lochhead P. A. (2009) Protein kinase activation loop autophosphorylation in cis: overcoming a Catch-22 situation. Sci. Signal. 2, pe4. [DOI] [PubMed] [Google Scholar]
- 9. Pike A. C., Rellos P., Niesen F. H., Turnbull A., Oliver A. W., Parker S. A., Turk B. E., Pearl L. H., Knapp S. (2008) Activation segment dimerization: a mechanism for kinase autophosphorylation of non-consensus sites. EMBO J. 27, 704–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Marshall C. J. (1994) MAP kinase kinase kinase, MAP kinase kinase and MAP kinase. Curr. Opin. Genet. Dev. 4, 82–89 [DOI] [PubMed] [Google Scholar]
- 11. Widmann C., Gibson S., Jarpe M. B., Johnson G. L. (1999) Mitogen-activated protein kinase: conservation of a three-kinase module from yeast to human. Physiol. Rev. 79, 143–180 [DOI] [PubMed] [Google Scholar]
- 12. Avitzour M., Diskin R., Raboy B., Askari N., Engelberg D., Livnah O. (2007) Intrinsically active variants of all human p38 isoforms. FEBS J. 274, 963–975 [DOI] [PubMed] [Google Scholar]
- 13. Bell M., Capone R., Pashtan I., Levitzki A., Engelberg D. (2001) Isolation of hyperactive mutants of the MAPK p38/Hog1 that are independent of MAPK kinase activation. J. Biol. Chem. 276, 25351–25358 [DOI] [PubMed] [Google Scholar]
- 14. Diskin R., Askari N., Capone R., Engelberg D., Livnah O. (2004) Active mutants of the human p38α mitogen-activated protein kinase. J. Biol. Chem. 279, 47040–47049 [DOI] [PubMed] [Google Scholar]
- 15. Salvador J. M., Mittelstadt P. R., Guszczynski T., Copeland T. D., Yamaguchi H., Appella E., Fornace A. J., Jr., Ashwell J. D. (2005) Alternative p38 activation pathway mediated by T cell receptor-proximal tyrosine kinases. Nat. Immunol. 6, 390–395 [DOI] [PubMed] [Google Scholar]
- 16. Levin-Salomon V., Kogan K., Ahn N. G., Livnah O., Engelberg D. (2008) Isolation of intrinsically active (MEK-independent) variants of the ERK family of mitogen-activated protein (MAP) kinases. J. Biol. Chem. 283, 34500–34510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ge B., Gram H., Di Padova F., Huang B., New L., Ulevitch R. J., Luo Y., Han J. (2002) MAPKK-independent activation of p38α mediated by TAB1-dependent autophosphorylation of p38α. Science 295, 1291–1294 [DOI] [PubMed] [Google Scholar]
- 18. Kang Y. J., Seit-Nebi A., Davis R. J., Han J. (2006) Multiple activation mechanisms of p38α mitogen-activated protein kinase. J. Biol. Chem. 281, 26225–26234 [DOI] [PubMed] [Google Scholar]
- 19. De Nicola G. F., Martin E. D., Chaikuad A., Bassi R., Clark J., Martino L., Verma S., Sicard P., Tata R., Atkinson R. A., Knapp S., Conte M. R., Marber M. S. (2013) Mechanism and consequence of the autoactivation of p38α mitogen-activated protein kinase promoted by TAB1. Nat. Struct. Mol. Biol. 20, 1182–1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Askari N., Diskin R., Avitzour M., Capone R., Livnah O., Engelberg D. (2007) Hyperactive variants of p38α induce, whereas hyperactive variants of p38γ suppress, activating protein 1-mediated transcription. J. Biol. Chem. 282, 91–99 [DOI] [PubMed] [Google Scholar]
- 21. Jirmanova L., Giardino Torchia M. L., Sarma N. D., Mittelstadt P. R., Ashwell J. D. (2011) Lack of the T cell-specific alternative p38 activation pathway reduces autoimmunity and inflammation. Blood 118, 3280–3289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jirmanova L., Sarma D. N., Jankovic D., Mittelstadt P. R., Ashwell J. D. (2009) Genetic disruption of p38α Tyr323 phosphorylation prevents T-cell receptor-mediated p38α activation and impairs interferon-γ production. Blood 113, 2229–2237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kwong J., Hong L., Liao R., Deng Q., Han J., Sun P. (2009) p38α and p38γ mediate oncogenic ras-induced senescence through differential mechanisms. J. Biol. Chem. 284, 11237–11246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Askari N., Beenstock J., Livnah O., Engelberg D. (2009) p38α is active in vitro and in vivo when monophosphorylated at threonine 180. Biochemistry 48, 2497–2504 [DOI] [PubMed] [Google Scholar]
- 25. Cox J., Mann M. (2008) MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 26, 1367–1372 [DOI] [PubMed] [Google Scholar]
- 26. Cox J., Neuhauser N., Michalski A., Scheltema R. A., Olsen J. V., Mann M. (2011) Andromeda: a peptide search engine integrated into the MaxQuant environment. J. Proteome Res. 10, 1794–1805 [DOI] [PubMed] [Google Scholar]
- 27. Zhang Y. Y., Mei Z. Q., Wu J. W., Wang Z. X. (2008) Enzymatic activity and substrate specificity of mitogen-activated protein kinase p38α in different phosphorylation states. J. Biol. Chem. 283, 26591–26601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Diskin R., Lebendiker M., Engelberg D., Livnah O. (2007) Structures of p38α active mutants reveal conformational changes in L16 loop that induce autophosphorylation and activation. J. Mol. Biol. 365, 66–76 [DOI] [PubMed] [Google Scholar]
- 29. Levin-Salomon V., Maayan I., Avrahami-Moyal L., Marbach I., Livnah O., Engelberg D. (2009) When expressed in yeast, mammalian mitogen-activated protein kinases lose proper regulation and become spontaneously phosphorylated. Biochem. J. 417, 331–340 [DOI] [PubMed] [Google Scholar]
- 30. Maayan I., Beenstock J., Marbach I., Tabachnick S., Livnah O., Engelberg D. (2012) Osmostress induces autophosphorylation of Hog1 via a C-terminal regulatory region that is conserved in p38α. PLoS One 7, e44749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Good M., Tang G., Singleton J., Reményi A., Lim W. A. (2009) The Ste5 scaffold directs mating signaling by catalytically unlocking the Fus3 MAP kinase for activation. Cell 136, 1085–1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chuderland D., Konson A., Seger R. (2008) Identification and characterization of a general nuclear translocation signal in signaling proteins. Mol. Cell 31, 850–861 [DOI] [PubMed] [Google Scholar]
- 33. Kornev A. P., Taylor S. S., Ten Eyck L. F. (2008) A helix scaffold for the assembly of active protein kinases. Proc. Natl. Acad. Sci. U.S.A. 105, 14377–14382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kornev A. P., Haste N. M., Taylor S. S., Eyck L. F. (2006) Surface comparison of active and inactive protein kinases identifies a conserved activation mechanism. Proc. Natl. Acad. Sci. U.S.A. 103, 17783–17788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gills J. J., Castillo S. S., Zhang C., Petukhov P. A., Memmott R. M., Hollingshead M., Warfel N., Han J., Kozikowski A. P., Dennis P. A. (2007) Phosphatidylinositol ether lipid analogues that inhibit AKT also independently activate the stress kinase, p38α, through MKK3/6-independent and -dependent mechanisms. J. Biol. Chem. 282, 27020–27029 [DOI] [PubMed] [Google Scholar]
- 36. Tzarum N., Eisenberg-Domovich Y., Gills J. J., Dennis P. A., Livnah O. (2012) Lipid molecules induce p38α activation via a novel molecular switch. J. Mol. Biol. 424, 339–353 [DOI] [PubMed] [Google Scholar]
- 37. Wang Z., Harkins P. C., Ulevitch R. J., Han J., Cobb M. H., Goldsmith E. J. (1997) The structure of mitogen-activated protein kinase p38 at 2.1-A resolution. Proc. Natl. Acad. Sci. U.S.A. 94, 2327–2332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhang Y. Y., Wu J. W., Wang Z. X. (2011) Mitogen-activated protein kinase (MAPK) phosphatase 3-mediated cross-talk between MAPKs ERK2 and p38α. J. Biol. Chem. 286, 16150–16162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Patel S. B., Cameron P. M., O'Keefe S. J., Frantz-Wattley B., Thompson J., O'Neill E. A., Tennis T., Liu L., Becker J. W., Scapin G. (2009) The three-dimensional structure of MAP kinase p38β: different features of the ATP-binding site in p38β compared with p38α. Acta Crystallogr. D Biol. Crystallogr. 65, 777–785 [DOI] [PubMed] [Google Scholar]
- 40. Sours K. M., Kwok S. C., Rachidi T., Lee T., Ring A., Hoofnagle A. N., Resing K. A., Ahn N. G. (2008) Hydrogen-exchange mass spectrometry reveals activation-induced changes in the conformational mobility of p38α MAP kinase. J. Mol. Biol. 379, 1075–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cui J., Holgado-Madruga M., Su W., Tsuiki H., Wedegaertner P., Wong A. J. (2005) Identification of a specific domain responsible for JNK2α2 autophosphorylation. J. Biol. Chem. 280, 9913–9920 [DOI] [PubMed] [Google Scholar]
- 42. Zheng M., Wang Y. H., Wu X. N., Wu S. Q., Lu B. J., Dong M. Q., Zhang H., Sun P., Lin S. C., Guan K. L., Han J. (2011) Inactivation of Rheb by PRAK-mediated phosphorylation is essential for energy-depletion-induced suppression of mTORC1. Nat. Cell Biol. 13, 263–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Huang C. Y., Tan T. H. (2012) DUSPs, to MAP kinases and beyond. Cell Biosci. 2, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ashwell J. D. (2006) The many paths to p38 mitogen-activated protein kinase activation in the immune system. Nat. Rev. Immunol. 6, 532–540 [DOI] [PubMed] [Google Scholar]