Abstract
We previously showed that moxifloxacin (MXF) exerts protective anti-inflammatory effects in immunosuppressed mice infected with Candida albicans by inhibiting interleukin-8 (IL-8) and tumor necrosis factor alpha (TNF-α) production in the lung. Immunohistochemistry demonstrated inhibition of nuclear factor (NF)-κB translocation in lung epithelium and macrophages in MXF-treated mice. In the present study we investigated the effects of MXF on the production of proinflammatory cytokines (i.e., IL-8, TNF-α, and IL-1β) by activated human peripheral blood monocytes and THP-1 cells and analyzed the effects of the drug on the major signal transduction pathways associated with inflammation: NF-κB and the mitogen-activated protein kinases ERK and c-Jun N-terminal kinase (JNK). The levels of IL-8, TNF-α, and IL-1β secretion rose 20- and 6.7-fold in lipopolysaccharide (LPS)-activated monocytes and THP-1 cells, respectively. MXF (5 to 20 μg/ml) significantly inhibited cytokine production by 14 to 80% and 15 to 73% in monocytes and THP-1 cells, respectively. In THP-1 cells, the level of NF-κB nuclear translocation increased fourfold following stimulation with LPS-phorbol myristate acetate (PMA), and this was inhibited (38%) by 10 μg of MXF per ml. We then assayed the degradation of inhibitor (I)-κB by Western blotting. LPS-PMA induced degradation of I-κB by 73%, while addition of MXF (5 μg/ml) inhibited I-κB degradation by 49%. Activation of ERK1/2 and the 46-kDa p-JNK protein was enhanced by LPS and LPS-PMA and was significantly inhibited by MXF (54 and 42%, respectively, with MXF at 10 μg/ml). We conclude that MXF suppresses the secretion of proinflammatory cytokines in human monocytes and THP-1 cells and that it exerts its anti-inflammatory effects in THP-1 cells by inhibiting NF-κB, ERK, and JNK activation. Its anti-inflammatory properties should be further assessed in clinical settings.
Fluoroquinolones are synthetic, broad-spectrum antimicrobial agents in common use for a variety of infections including systemic infections in immune-compromised hosts (11, 17). In addition to their antimicrobial properties, certain quinolones were shown to have immune-modulating activities in animal models and humans (for a review, see reference 10). Moxifloxacin (MXF) is a fluoroquinolone with activities against both gram-positive and gram-negative bacteria. It has been suggested that MXF has inhibitory and stimulatory effects on the immune system, primarily by studies that have shown that the production of several cytokines by human and murine leukocytes can be affected by this drug. It has previously been shown (34) that MXF enhances the production of granulocyte-macrophage colony-stimulating factor and interleukin-6 (IL-6) by various organs of cyclophosphamide-injected mice. Other investigators have shown that MXF significantly inhibits IL-8 production by human neutrophils (45) and tumor necrosis factor alpha (TNF-α) and IL-1α production by human monocytes stimulated in vitro with lipopolysaccharide (LPS) (3).
Recently, we performed in vivo studies and investigated the protective effect of MXF in cyclophosphamide-injected mice inoculated intratracheally with Candida albicans. In that study, control and ceftazidime-treated mice developed bronchopneumonia with significant neutrophilic infiltration in association with increased levels of TNF-α and IL-8 production in the lungs. By contrast, mice pretreated with MXF showed no bronchopneumonia and the levels of TNF-α and IL-8 secretion were significantly inhibited compared to those in the controls (35; H. Blau, I. Fabian, Y. Kletter, L. Horev, N. Kariv, I. Shechtman, H. Alteraz, Y. Ben-Neriah, and I. Shalit. Abstract, Am. J. Respir. Crit. Care Med. 165:A782, 2002). Immunohistochemistry of the lung tissue showed that nuclear translocation of nuclear factor (NF)-κB occurred in more than 50% of airway epithelial cells as well as in inflammatory cells within the lungs of both saline- and ceftazidime-treated mice. In contrast, there was a marked decrease in the levels of NF-κB activation in the lungs of MXF-treated mice (Blau et al., Abstract, Am. J. Respir. Crit. Care Med. 165:A782, 2002). The chemokine IL-8 is known to have a critical role in the recruitment of neutrophils, T lymphocytes, and monocytes to regions of tissue injury (4, 13). It has been reported that IL-8 is the chemokine responsible for the maintenance of various chronic inflammatory diseases, such as gastritis caused by Helicobacter pylori (37) and other inflammatory diseases (7, 38). In the lung, IL-8 has been demonstrated to play a crucial role in the inflammatory processes of diffuse panbronchiolitis (27) and fibrosis and adult respiratory distress syndrome (12). Neutrophil recruitment to the lungs might be a critical step in the pathogenesis of ventilator-induced lung injury (18, 33). The likely cellular sources of IL-8 in the lung are the respiratory epithelium and alveolar macrophages (16, 42).
In response to proinflammatory stimuli, IL-8 production is dependent on mitogen-activated protein kinases (MAPKs) and NF-κB pathways (16). NF-κB comprises specific heterodimeric complexes present in an inactive form in the cytoplasms of resting cells, where each is bound to one of the inhibitor (I)-κB proteins. Stimulus-induced activation of the NF-κB-inducing kinase leads to phosphorylation of the I-κB kinase complexes (for a review, see reference 19), followed by ubiquitination and proteasome-mediated degradation of I-κB, which frees NF-κB to translocate to the nucleus. In the nucleus, NF-κB interacts with its target motifs and regulates the secretion of various chemokines, including IL-8 (19, 41). I-κB kinases are phosphorylated by NF-κB-inducing kinases, which activate various MAPKs, including the stress-activated protein kinase/c-Jun N-terminal kinase (JNK) (24). Recently, the IL-6 and IL-8 genes were identified as new targets regulated by JNK (23), while other studies have shown that ERK1/2 and JNK play an important role in protein I/II-mediated synthesis of IL-6 and IL-8 in fibroblast-like synoviocytes (29).
This study aims to further elucidate the cellular mechanisms underlying the anti-inflammatory effects of MXF observed previously. We investigated the effects of MXF on IL-8, TNF-α, and IL-1β secretion by activated monocytes isolated from the peripheral blood of healthy volunteers and THP-1 cells stimulated with proinflammatory substances. Subsequently, we analyzed the effects of MXF on activation of the two major signal transduction pathways, those for NF-κB and the MAPKs ERK and JNK, known to be involved in the regulation of the proinflammatory response.
(This study was presented in part at the 43rd Interscience Conference on Antimicrobial Agents and Chemotherapy, Chicago, Ill., September 2003.)
MATERIALS AND METHODS
Human monocytes and THP-1 cells.
Peripheral blood was drawn from healthy volunteers. Peripheral blood mononuclear cells were isolated by centrifugation on Ficoll-Paque (Pharmacia Biotech, Uppsala, Sweden) and were suspended in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum (FBS; HyClone Laboratories, Logan, Utah), 2 mM l-glutamine, 100 U of penicillin per ml, and 100 μg of streptomycin per ml. Peripheral blood mononuclear cells were then plated and incubated for 1 h at 37°C in a humidified 95% air-5% CO2 atmosphere. Nonadherent cells were removed by washing with phosphate-buffered saline (PBS). More than 90% of the adherent cells were morphologically identified as monocytes. The human monocytic THP-1 cells (ATCC TIB 202) were maintained in RPMI 1640 medium supplemented with 10% heat-inactivated FBS, 2 mM l-glutamine, 100 U of penicillin per ml, and 100 μg of streptomycin per ml at 37°C in a humidified incubator with 5% CO2. The assays were performed with cells at a density of 106/cells ml.
ELISAs for IL-8, TNF-α, and IL-1β production.
Monocytes were suspended in RPMI 1640 medium supplemented with 1% FBS and placed in 24-well culture plates. MXF (Bayer AG, Wuppertal, Germany) was added at various concentrations (5 to 20 μg/ml), and the cells were incubated for 2 h and then exposed to 10 ng of LPS (Escherichia coli; Sigma Chemical Co., St. Louis, Mo.) per ml for 4 h. Cell-free supernatants were recovered by centrifugation and stored at −20°C until they were assayed. THP-1 cells suspended in RPMI 1640 medium supplemented with 1% FBS were placed in 24-well culture plates and incubated for 24 h. MXF (5 to 20 μg/ml) was added, and the cells were incubated for 4 h and then exposed to 100 ng of LPS (E. coli; Sigma Chemical Co.) per ml for 20 h. In some experiments MXF and LPS were concomitantly added to the cells. Cell-free supernatants were recovered as described above. The concentrations of IL-8, TNF-α, and IL-1β were determined by an enzyme-linked immunosorbent assay (ELISA; R&D Systems Inc., Minneapolis, Minn.). The sensitivities of the assay are >10 pg/ml for IL-8, >15 pg/ml for TNF-α, and >4 pg/ml for IL-1β.
WB analysis of ERK, I-κBα, and JNK.
THP-1 cells were preincubated in serum-free medium at 37°C for 24 h in the absence or presence of 5 to 20 μg of MXF per ml prior to the addition of 1 μg of LPS per ml alone or in combination with 100 nM phorbol ester (phorbol myristate acetate [PMA]; Consolidated Midland, Brewster, N.Y.), as described previously (28). For Western blotting (WB) analysis of ERK1/2 and I-κBα, after incubation for 0 to 60 min, the cells were collected on ice, washed twice with ice-cold PBS, and suspended in 40 μl of the lysis buffer: 50 mM Tris (pH 7.6), 150 mM NaCl, 5 mM EDTA (pH 8.0), 0.6% Nonidet P-40, 1 mM Na3VO4, 20 mM β-glycerophosphate, 1 mM phenylmethylsulfonyl fluoride, 2 mM p-nitrophenyl phosphate, and 1:25 Complete Mini Protease Inhibitor cocktail (Boehringer Mannheim, Mannheim, Germany) (32). After the lysates were kept on ice for 15 min, they were subjected to centrifugation (20,000 × g) at 4°C for 30 min to obtain a cytosolic fraction. The protein concentration was determined by a Bradford assay (Bio-Rad, Munich, Germany) before storage at −70°C. For the analysis of JNK, after incubation for 0 to 120 min, the cells were collected on ice, washed twice with ice-cold PBS, and suspended in 80 μl of the homogenization buffer: 40 mM HEPES (pH 7.5), 150 mM NaCl, 5 mM EDTA (pH 8.0), 0.1 mM Na3VO4, 50 mM β-glycerophosphate, 1 mM benzamidine, 1 mM dithiothreitol (DTT), and 1:100 Complete Mini Protease Inhibitor cocktail (Boehringer Mannheim). After incubation overnight at −70°C, the lysates were sonicated (3 s on and 1 s off; total time, 6 min) and then subjected to centrifugation (20,000 × g) at 4°C for 15 min to obtain a cytosolic fraction. The protein concentration was determined by a Bradford assay (Bio-Rad) before storage at −70°C. Aliquots of the cytosol fraction containing 50 μg of protein for ERK1/2 and JNK1/2 and 20 μg of protein for I-κBα were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis on 10, 10, and 12% polyacrylamide gels, respectively. After electrophoresis and electrophoretic transfer of the proteins to a nitrocellulose membrane (Schleicher & Schuell, Dassel, Germany), the membranes were blocked with 3% nonfat milk in Tris-buffered saline (pH 7.4) containing 0.1% Tween (TBST) for 1 h. The membranes were then rinsed three times in TBST and incubated at room temperature (RT) with mouse monoclonal anti-MAPK antibody and with activated, diphosphorylated, ERK-1/2, phospho-p44/42 MAPK antibody (Sigma Chemical Co.), and anti-phospho-JNK1/2, phospho-p46/54 JNK antibody (New England Biolabs, Beverly, Mass.) (1:20,000 and 1:1,000 dilutions, respectively) and anti-ERK-1/2 antibody and anti-JNK1/2 antibody (1:1,000 dilution) or rabbit polyclonal antibody against I-κB (1:1,000 dilution) (Santa Cruz Biotechnology, Inc., Santa Cruz, Calif.) for 1 h. Actin levels were also assessed as a loading control by using an antibody (Santa Cruz Biotechnology) that reacts with a broad range of actin isoforms. The blots were then incubated with a secondary antibody, horseradish peroxidase-linked anti-mouse immunoglobulin G (Santa Cruz Biotechnology) or rabbit polyclonal antibody against I-κBα (1:1,000 dilution) (Santa Cruz Biotechnology) for 1 h. After 1 h at RT and three washes in TBST, the blots were incubated in enhanced chemiluminescence reagent (Amersham Pharmacia Biotech). The relative densities of ERK1/2, JNK1/2, and I-κBα were determined by densitometric analysis, followed by photography of the specific bands (Kodak XLS-1 film).
Electrophoretic mobility shift assay (EMSA).
THP-1 cells were preincubated in serum-free medium at 37°C for 24 h in the absence or presence of 5 or 10 μg of MXF per ml prior to the addition of LPS (1 μg/ml) or LPS-PMA (1 μg/ml and 100 nM, respectively) for 60 or 120 min. The cells were collected on ice before isolation of nuclear extracts by the protocol reported by Aikawa et al. (1). Briefly, the cells (106/ml) were washed with ice-cold PBS, suspended in 200 μl of lysis buffer (10 mM HEPES [pH 7.9], 10 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA, 1 mM DTT) and allowed to swell on ice for 15 min, after which 12.5 μl of 10% Nonidet P-40 was added. The tube was then mixed thoroughly with a Vortex mixer for 10 s prior to centrifugation (20,000 × g) at 4°C for 8 min. The nuclear pellets thus obtained were resuspended in 25 μl of ice-cold nuclear extraction buffer (20 mM HEPES [pH 7.9], 400 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM DTT) and kept on ice for 15 min with intermittent agitation. The samples were subjected to centrifugation for 5 min at 4°C, and the supernatant was stored at −70°C after measurement of its protein content with the Bio-Rad protein assay kit, as described above.
The oligonucleotide described below was radiolabeled with T4 polynucleotide kinase (Epicentre Technologies) and [γ-32P]ATP (Sigma Chemical Co.) before being annealed to oligonucleotides whose sequences were complementary. An NF-κB consensus sequence is GGGAGGGGACTTTCCGAGAG (Santa Cruz Biotechnology). A binding reaction buffer (10 mM Tris HCl [pH 7.9], 60 mM KCl, 0.4 mM DTT, 10% glycerol, 2 μg of bovine serum albumin) (44) was used to optimize NF-κB binding. Volumes of 20 μl of the binding reaction mixtures were prepared and contained 8 μg of nuclear extract, 0.5 ng of 32P-labeled double-stranded oligonucleotide, and 1 μg of poly(dI-dC). The reaction mixtures were incubated at room temperature before electrophoresis through 5% polyacrylamide gels with 0.5× TGA (1× TGA is 25 mM Tris, 190 mM glycine, and 10 mM EDTA). For competition assays, preincubation at RT for 30 min with a 100-fold molar excess of competitor was carried out before the addition of the labeled oligonucleotides. The gels were dried under vacuum and exposed to intensifying screens at −70°C. The relative densities of NF-κB were determined by densitometric analysis followed by photography of the specific bands (Kodak XLS-1 film).
WB analysis of NF-κB.
The nuclear extracts of THP-1 cells used in the WB analyses were prepared as described above. An aliquot of the nuclear fraction containing 50 μg of protein for NF-κB was resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis on 10% polyacrylamide gels as described above. NF-κB was detected by incubating the blots with anti-NF-κB p65 rabbit polyclonal antibody (1:500 dilution; Santa Cruz Biotechnology). The blots were then incubated with a secondary antibody, horseradish peroxidase-linked anti-rabbit immunoglobulin G (Santa Cruz Biotechnology). After 1 h at 37°C and three washes in TBST, the blots were incubated in enhanced chemiluminescence reagent (Amersham Pharmacia Biotech).
Statistical significance was determined by Student's t test.
RESULTS
Effects of MXF on IL-8, TNF-α, and IL-1β secretion induced by exposure of monocytes and THP-1 cells to LPS.
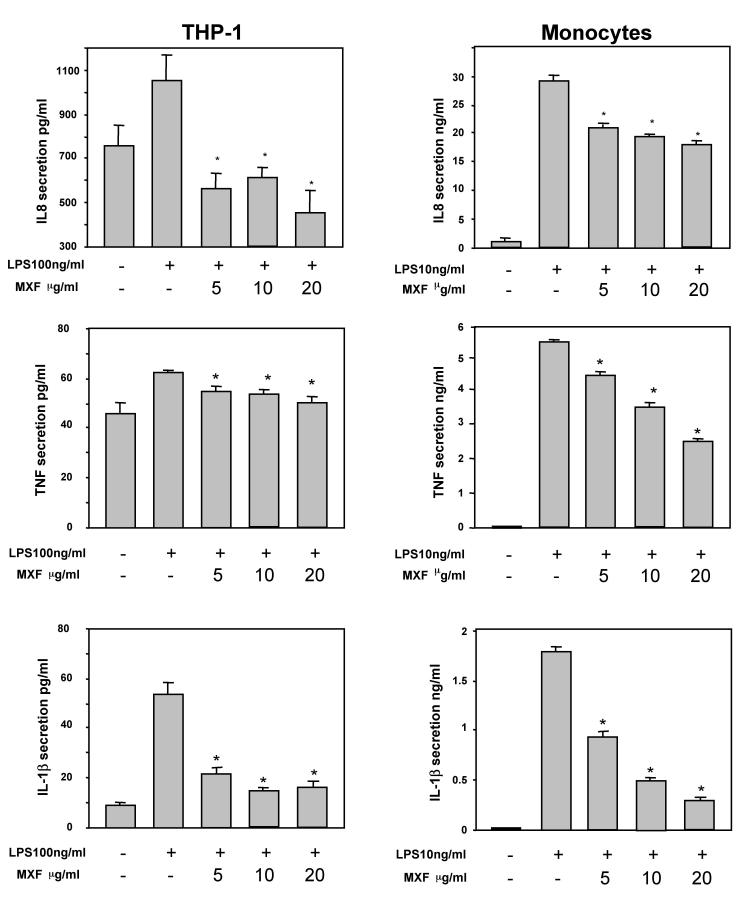
To determine the effects of MXF on the production of proinflammatory cytokines, LPS was added to the cells with or without MXF. Exposure of monocytes to 10 ng of LPS per ml for a short period (4 h) induced a marked increase in the levels of IL-8 production by the cells. MXF at concentrations of 5, 10, and 20 μg/ml inhibited the induction of IL-8 secretion by 26, 35, and 40%, respectively (P < 0.05) (Fig. 1). Similarly, preincubation of THP-1 cells with MXF suppressed the enhanced IL-8 secretion induced by exposure to 100 ng of LPS per ml (47 to 57% inhibition at 5 to 20 μg/ml) (P < 0.05) (Fig. 1). Similar results were obtained when MXF was given concomitantly with LPS (7 to 42% inhibition at 5 to 20 μg/ml).
FIG. 1.
Effects of MXF on the production of IL-8, TNF-α, and IL-1β by LPS-stimulated monocytes and THP-1 cells. Monocytes and THP-1 cells were preincubated in the presence or absence of MXF (5 to 20 μg/ml). LPS was added to the cells at 10 and 100 ng/ml, respectively, and the cells were further incubated for 4 and 24 h, respectively. The concentrations of IL-8, TNF-α, and IL-1β in the culture supernatants were measured by ELISA; and the values are the means + standard errors of three independent experiments. Asterisks denote a statistically significant difference compared with the results for the LPS-stimulated cells without MXF. (P < 0.05).
From our previous in vivo research, which indicated that MXF exerts pronounced anti-inflammatory effects when it is administered as a pretreatment to immune-suppressed animals, we conducted further in vitro studies using preincubation with MXF to elucidate the cellular mechanisms underlying the in vivo anti-inflammatory effects of the drug.
Marked increases in the levels of secretion of TNF-α and IL-1β by monocytes and THP-1 cells were observed following exposure of the cells to LPS. MXF inhibited the induction of TNF-α secretion in monocytes by 14 to 51% and that of IL-1β by 50 to 83%. Similarly, the drug inhibited the induction of TNF-α and IL-1β secretion in THP-1 cells by 15 to 73% (P < 0.05) (Fig. 1).
These data indicate that MXF suppresses the signaling that transmits LPS stimuli into the cell and suppresses proinflammatory cytokine production by monocytes and THP-1 cells. To further explore the mechanism of action of MXF, additional experiments were performed with THP-1 cells.
Effect of MXF on ERK1/2 activation.
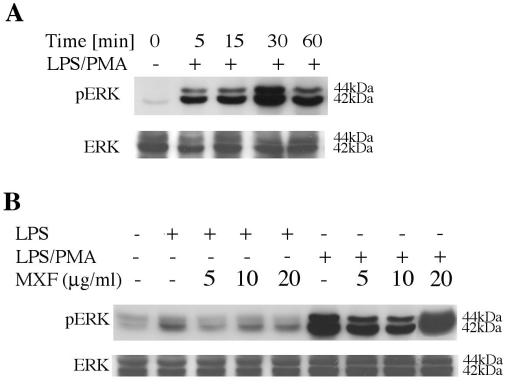
MAPKs are required for IL-8 induction mediated by LPS-PMA in THP-1 cells (25, 26, 36). On the basis of our observation that MXF inhibits the release of IL-8 induced by LPS alone, we investigated the effect of MXF on the activation of ERK1/2 in THP-1 cells exposed to LPS alone or LPS in combination with PMA (Fig. 2). Activation of ERK1/2 was examined by detecting the appearance of the 42-kDa p-ERK protein and the 44-kDa p-ERK protein.
FIG. 2.
Activation of ERK1/2 in LPS- and LPS-PMA-stimulated THP-1 cells and effects of MXF. (A) Time-dependent studies. THP-1 cells were incubated in serum-free medium and exposed to 1 μg of 100 nM LPS-PMA per ml for the indicated times. (B) Effects of MXF on LPS- and LPS-PMA-induced activation of ERK1/2. THP-1 cells were preincubated in serum-free medium in the absence or the presence of the indicated concentrations of MXF and then treated with LPS or LPS-PMA for 30 min. The cytoplasmic extracts were prepared and subjected to Western immunoblotting with an antibody specific for phosphorylated form of ERK1/2 (upper blots in panels A and B) and total ERK1/2 (lower blots in panels A and B). The experiment was repeated three times, and each experiment produced similar results.
Initially, we performed time-dependent studies. LPS-PMA ligation resulted in the rapid and time-dependent activation of ERK1/2, which was detected 5 min after LPS-PMA ligation and which was maximal at 30 min (Fig. 2A). ERK1/2 activation decreased after longer periods of stimulation. On the basis of the results of the time-dependent studies, all further experiments were performed following exposure of the cells to LPS or LPS-PMA for 30 min. Exposure to LPS resulted in the activation of the 42-kDa p-ERK protein (2.2-fold increase relative to the level of activation for unstimulated cells) (Fig. 2B, second lane). When cells were pretreated with 5 μg of MXF per ml before incubation with LPS, activation of the 42-kDa p-ERK protein was completely attenuated (Fig. 2B, third lane), while preincubation with 10 and 20 μg of MXF per ml resulted in 22 and 25% inhibition, respectively (Fig. 2B, fourth and fifth lanes, respectively). Treatment of the cells with LPS-PMA markedly induced the activation of the 42-kDa p-ERK protein and the 44-kDa p-ERK protein (threefold increase in phosphorylation compared to that with LPS alone, as determined by densitometric analysis) (Fig. 2B, sixth and second lanes, respectively). Preincubation with 5 and 10 μg of MXF per ml before exposure to LPS-PMA induced 33 and 54% reductions in the levels of activation of ERK1/2 (Fig. 2B, seventh and eighth lanes, respectively; compare the results with those in the sixth lane). No reduction in LPS-PMA-induced ERK1/2 was observed in the presence of 20 μg of MXF per ml (Fig. 2B, ninth lane). Immunoblot analysis with an antibody that detected total ERK showed equivalent levels of ERK1/2 expression in all samples (Fig. 2B, lower blot).
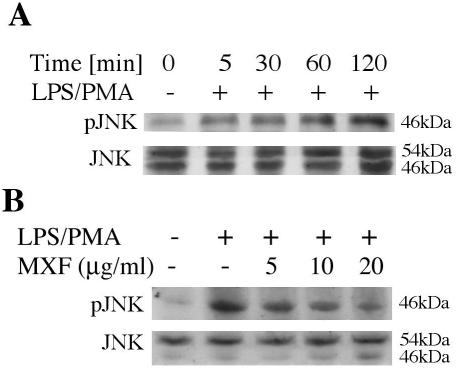
Effects of MXF on JNK activation.
Activation of p-JNK1/2 was assessed after treatment of THP-1 cells with LPS-PMA. At various times after exposure to LPS-PMA, cell lysates were subjected to immunoblot analysis with an antibody that detected dually phosphorylated JNK. Figure 3A indicates that phosphorylation of JNK1 was observed at 5 min and was maximal at 60 min. High levels of activated JNK were observed up to 120 min after exposure to LPS-PMA. Subsequent experiments were performed following exposure of the cells to LPS-PMA for 60 min. We examined the effect of MXF on LPS-PMA-induced activation of the 54-kDa p-JNK protein and the 46-kDa p-JNK protein. Figure 3B indicates that exposure of the cells to LPS-PMA induced a 3.6-fold increase in the level of activation of the activities of the 46-kDa p-JNK protein (Fig. 3B, second lane); and preincubation with MXF at concentrations of 5, 10, and 20 μg/ml for 24 h inhibited the activation of the 46-kDa p-JNK protein by 37, 42, and 50%, respectively (Fig. 3B, third to fifth lanes, respectively). Immunoblot analysis with an antibody that detected total JNK confirmed that equivalent amounts of the 54- and 46-kDa JNK proteins were present.
FIG. 3.
Activation of JNK in LPS-PMA-stimulated THP-1 cells and effects of MXF. (A) Time-dependent studies. THP-1 cells were preincubated in serum-free medium and then exposed to LPS-PMA for the indicated times. (B) Effects of MXF on LPS-PMA-induced activation of JNK. THP-1 cells were preincubated in serum-free medium in the absence or presence of the indicated concentrations of MXF and were then treated with LPS-PMA for 60 min. The cytoplasmic extracts were prepared and subjected to Western immunoblotting with an antibody specific for the phosphorylated form of JNK (upper blots in panels A and B) or total JNK (lower blots in panels A and B). The experiment was repeated three times, and each experiment produced similar results.
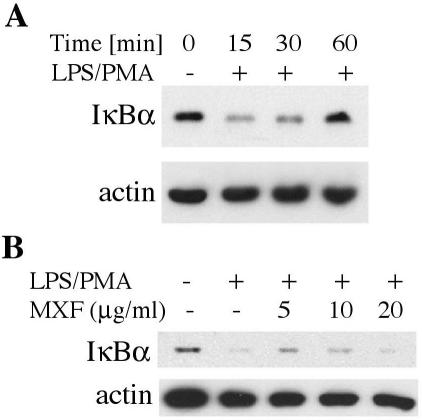
MXF inhibits LPS-PMA-induced degradation of I-κBα.
We studied the effect of MXF on LPS-PMA-induced I-κBα degradation (Fig. 4). As shown in Fig. 4A, LPS-PMA stimulation induced degradation of I-κBα. The band markedly decreased within 15 min of incubation (Fig. 4A, second lane), while by 30 and 60 min it gradually reappeared (Fig. 4A, third and fourth lanes, respectively). Figure 4B (third lane) indicates that preincubation with MXF at a concentration of 5 μg/ml for 24 h before the addition of LPS-PMA for 15 min markedly inhibited I-κBα degradation. Slight inhibition was observed with 10 μg/ml, while no inhibition was observed in the presence of 20 μg of MXF per ml (Fig. 4B, fourth and fifth lanes, respectively). Actin was also immunoblotted as an internal loading control (Fig. 4A and B). It is known that NF-κB is present in an inactive form in the cytoplasms of resting cells, where it is bound to the inhibitor I-κB. It is also well established that phosphorylation and degradation of I-κB are crucial steps in NF-κB translocation to the nucleus. Thus, our observation that MXF inhibits the degradation of I-κB indicates that the drug inhibits the release of NF-κB from the inhibitor and prevents NF-κB activation and translocation to the nucleus.
FIG. 4.
Effects of MXF on the kinetics of LPS-PMA-induced degradation of I-κBα in THP-1 cells. (A) Time-dependent studies. THP-1 cells were preincubated in serum-free medium and then treated with LPS-PMA, as indicated. (B) The cells were preincubated in serum-free medium in the absence or presence of 5, 10, or 20 μg of MXF per ml and then treated with LPS-PMA for 15 min. The cytoplasmic extracts were prepared and assayed by WB analysis for I-κBα (upper blots in panels A and B) and actin (lower blots in panels A and B).
Effects of MXF on LPS- and LPS-PMA-induced NF-κB activation (EMSA and WB studies).
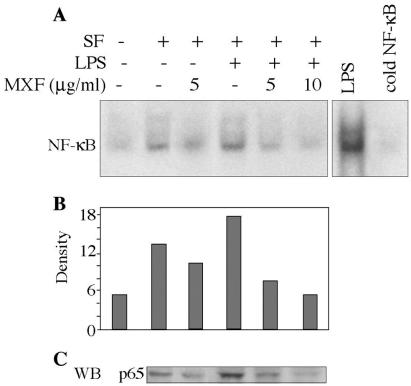
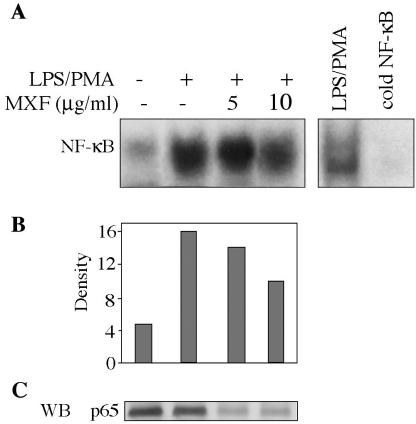
THP1 cells were preincubated for 24 h in serum-free medium in the absence or presence of MXF (5 or 10 μg/ml) and then treated with LPS (1 μg/ml) for 1 h. Cells alone showed no NF-κB activity. Growth in serum-free medium induced a slight increase in the level of NF-κB activation (Fig. 5A; compare the second and first lanes). Addition of LPS significantly induced NF-κB nuclear translocation (Fig. 5A; compare the fourth and second lanes). MXF at 5 μg/ml inhibited serum-free-induced NF-κB activity (Fig. 5A, third lane), while both concentrations of the drug (5 and 10 μg/ml) inhibited LPS-induced NF-κB activation (Fig. 5A, fifth and sixth lanes, respectively). Densitometric scans of the films revealed that the decreases induced by MXF were 60% at 5 μg/ml and 72% at 10 μg/ml compared with the levels for the LPS-stimulated samples (Fig. 5B, fifth and sixth lanes, respectively). WB analysis was performed to detect whether MXF also affects the expression of the p65 NF-κB protein within the nucleus. Nuclear extracts isolated from cells pretreated with MXF or medium and exposed to LPS were analyzed for the p65 NF-κB protein. Figure 5C shows that LPS enhances protein expression, while pretreatment with MXF decreases protein expression in a dose-dependent manner. The effect of MXF on LPS-PMA-induced activation of NF-κB was also evaluated (Fig. 6A). Cells incubated in serum-free medium showed basal levels of NF-κB activity, as shown in the first lane of Fig. 6A. LPS-PMA induced enhanced NF-κB binding activity (approximately fourfold; Fig. 6A, second lane). Densitometric scans of the films revealed that MXF at a concentration of 10 μg/ml inhibited LPS-PMA-induced NF-κB activation by about 35% compared with the level of activation in LPS-PMA-stimulated samples (Fig. 6B). Figure 6C shows that MXF also inhibited the expression of the p65 NF-κB protein under these conditions.
FIG. 5.
Effects of MXF on LPS-induced activation of NF-κB. (A) THP-1 cells were preincubated in serum-free medium in the absence or presence of MXF and then treated with LPS for 1 h. Nuclear extracts were prepared and assayed for NF-κB by EMSA on a 5% polyacrylamide gel with a double-stranded oligonucleotide containing the NF-κB consensus sequence. Binding competition assays were performed with a 100-fold excess of unlabeled NF-κB oligonucleotide as the competitor (cold NF-κB). (B) Bands were quantified by optical densitometry. (C) Expression of p65 NF-κB protein in LPS-stimulated cells. The Western blot (WB) illustrates the expression of the p65 NF-κB protein in nuclear extracts from cells exposed to LPS and preincubated in the absence or presence of MXF. Representatives of the blots from two experiments are shown.
FIG. 6.
Effects of MXF on LPS-PMA-induced activation of NF-κB. (A) THP-1 cells were preincubated in serum-free medium in the absence or presence of MXF and then treated with LPS-PMA for 2 h. Nuclear extracts were prepared and assayed for NF-κB as described in the legend to Fig. 5. (B) Bands were quantified by optical densitometry. (C) Expression of p65 NF-κB protein in LPS-PMA-stimulated cells. The Western blot (WB) illustrates the expression of the p65 NF-κB protein in nuclear extracts from cells exposed to LPS-PMA and preincubated in the absence or presence of MXF. Representatives of the blots from two experiments are shown.
DISCUSSION
The present study demonstrates that MXF inhibits the production of IL-8, TNF-α, and IL-1β in LPS-stimulated human peripheral blood monocytes and in the THP-1 monocytic cell line in a dose-dependent manner. This finding confirms those presented in previous reports showing that MXF inhibits the accumulation of cytokines from activated human leukocytes (3, 9, 45).
The inhibitory effects described above confirm the results of previous in vivo studies showing that pretreatment with MXF of mice injected with cyclophosphamide and inoculated with C. albicans significantly inhibited neutrophilic infiltration and TNF-α and IL-8 production in the lungs (35).
A wide range of antimicrobial agents, including quinolones, have been reported to modify immune and inflammatory responses both in vivo and in vitro (for a review, see reference 10). To further characterize the nature of the inhibitory effect of MXF on cytokine production, we examined the effects of MXF on the activation of the MAPKs ERK and JNK, which are known to be involved in the regulation of these cytokines, and on the activation of the transcription factor NF-κB, which regulates the expression of many immune and inflammatory genes. Activation of the ERK pathway is part of the early biochemical events that follow LPS treatment of various leukocytes. In the present study we show that LPS induces the activation of the 42-kDa p-ERK protein in THP-1 cells, while stimulation with LPS-PMA induces the activation of both the 42-kDa p-ERK protein and the 44-kDa p-ERK protein. Our findings are consistent with those in the studies reported by Procyk et al. (32), who have shown that Salmonella enterica serovar Typhimurium and LPS stimulate ERK activation in macrophages, and Bonner et al. (6), who showed that LPS activates ERK in the neutrophils of both adults and newborns. Previous studies have shown that activation of ERK results in the induction of IL-8 genes. Zhao et al. (48) reported that exposure of human colonocytes to neurotensin leads to IL-8 secretion that is mediated by both NF-κB- and ERK-dependent pathways. The study has indicated that ERK is required for neurotensin-induced IL-8 production. Other results also support a major role of MAPKs in IL-8 secretion. For example, the inhibitor of MAPK/ERK kinase, PD98059, significantly inhibited IL-8 release induced by Clostridium difficile toxin A in human monocytes (43) as well as that induced by H. pylori in gastric epithelial cells (20). We report here for the first time that MXF inhibits activation of the ERK induced by LPS and LPS-PMA in THP-1 cells. The inhibition of ERK activation by MXF could explain at least in part the inhibitory effects of MXF on IL-8 secretion by LPS-stimulated cells.
Other members of the MAPK group of kinases, such as JNK, have also been implicated in the control of cytokine production in different cell types (2). In the present study we show that stimulation with LPS-PMA induces activation of the activities of the 46-kDa p-JNK protein and that preincubation with MXF inhibits this activation in a dose-dependent manner. The basis for the selective activation and selective inhibition of the activity of the 46-kDa p-JNK protein is not known. However, activation of JNK1 (without activation of JNK2) following CD40 ligation to THP-1 cells or human monocytes was reported by other investigators (31).
In resting cells, the NF-κB protein is retained in the cytoplasm through an association with inhibitory proteins termed I-κB, which mask the nuclear localization signal. Upon stimulation of the cells with stimuli such as LPS, LPS-PMA, TNF-α, and IL-1, the complex releases I-κB by its phosphorylation and subsequent degradation to generate transcriptionally active NF-κB, which undergoes rapid translocation to the nucleus for DNA binding. MXF suppressed NF-κB binding to the DNA, as demonstrated by EMSA with the nuclear extracts of stimulated THP-1 cells and the 32P-labeled double-stranded oligonucleotide (Fig. 5). The suppression was observed over the concentration range required for the inhibition of IL-8 production.
To ascertain whether MXF inhibited the nuclear translocation of NF-κB due to an effect on I-κB degradation, we assayed I-κB degradation after stimulation with LPS-PMA in the presence of increasing concentrations of MXF. WB analysis of the I-κBα protein in the cytoplasmic extracts revealed that stimulation of THP-1 cells with LPS-PMA caused rapid depletion of I-κBα, followed by its gradual reappearance, presumably owing to a feedback mechanism which regulated its resynthesis. Treatment with 5 or 10 μg of MXF per ml inhibited I-κB degradation. It is of note that additional anti-inflammatory agents, such as sodium salicylate, acetylsalicylic acid, and sanguinarine, were also found to inhibit I-κB degradation (8, 22).
The findings from previous in vivo studies (35) and the present in vitro data, which demonstrate and expand our understanding of the anti-inflammatory effects of MXF, provide further support for other observations relating to modulation of the inflammatory response by other quinolone compounds. In vitro studies with LPS-stimulated human monocytes have shown that ciprofloxacin, trovafloxacin, moxifloxacin, grepafloxacin, and levofloxacin have inhibitory effects on various proinflammatory cytokines, mainly IL-1α, IL-1β, ΤΝF-α, IL-6, and IL-8 (3, 5, 9, 21, 30, 45, 47). Reduction of IL-8 levels was also found in stimulated human airway cells after treatment with grepafloxacin (15). In vivo studies have demonstrated that trovafloxacin, rufloxacin, and ciprofloxacin have significant anti-inflammatory activities in animal models of intra-abdominal infections caused by anaerobic organisms (with the drugs being devoid of activity against anaerobic organisms) or abscesses induced by heat-killed bacteria. It was demonstrated in those studies that the drugs have significant anti-TNF-α effects (14, 39, 40). In clinical settings it is worthwhile to mention the effect of ciprofloxacin on Crohn's disease, which is partly attributed to TNF-α inhibition by the drug (36). Sparfloxacin was also found to reduce TNF-α, IL-1, and IL-6 levels in patients with nonbacterial prostatitis. The cytokine reduction was also associated with the disappearance of symptoms (46). In some of the studies cited above it was shown that the synthesis of the mRNAs of the relevant cytokines was indeed inhibited following exposure to the quinolones.
Nonetheless, none of these studies explored the signal transduction pathways preceding the synthesis of the mRNAs of the proinflammatory cytokines. The present study offers a more comprehensive explanation and basic mechanisms for the observed anti-inflammatory effects of quinolones, based on the inhibition of the two major signal transduction pathways associated with many of the inflammatory processes studied, as described above.
Future studies should focus on basic mechanisms on the one hand and on the clinical relevance of our studies on the other. It is not clear at present how MXF inhibits the NF-κB and MAPKs in stimulated cells, and upstream events up to the Toll-like receptor level should be investigated. The known effects of quinolones on nuclear topoisomerases should also be explored in relationship to the present findings due to their possible effects on the delicate nuclear-cytosolic balance of NF-κB and various kinases.
From a clinical standpoint, the anti-inflammatory effects of MXF could be of significant importance when these effects are linked to its antimicrobial properties. The effects of MXF on lung infection, inflammation, and tissue injury should be further studied both in animal models and in the clinical setting.
REFERENCES
- 1.Aikawa, Y., M. Yamamoto, T. Yamamoto, and K. Tanaka. 2002. An anti-rheumatic agent T-614 inhibits NFkB activation in LPS-and TNF-α-stimulated THP-1 cells without interfering with IkB α degradation. Inflamm. Res. 51:188-194. [DOI] [PubMed] [Google Scholar]
- 2.Annabel, F. V., C. Monica, X. Jordi, and C. Antonio. 2000. The differential time-course of ERK activity correlates with macrophage response toward proliferation or activation. J. Biol. Chem. 275:7403-7409. [DOI] [PubMed] [Google Scholar]
- 3.Araujo, F. G., T. L. Slifer, and J. S. Remington. 2002. Effect of moxifloxacin on secretion of cytokines by human monocytes stimulated with lipopolysaccharide. Clin. Microbiol. Infect. 8:26-30. [DOI] [PubMed] [Google Scholar]
- 4.Baggiolini, M., B. Dewald, and B. Moser. 1997. Human chemokines: an update. Annu. Rev. Immunol. 15:675-705. [DOI] [PubMed] [Google Scholar]
- 5.Bailly, S., Y. Mahe, B. Ferru, M. Fay, T. Tursz, H. Wakasugi, and M. A. Gougerot-Pocidalo. 1990. Quinolone-induced differential modification of IL-1α and IL-1β production by LPS-stimulated human monocytes. Cell. Immunol. 128:277-288. [DOI] [PubMed] [Google Scholar]
- 6.Bonner, S., S. R. Yan, D. M. Byers, and R. Bortolussi. 2001. Activation of extracellular signal-related protein kinases 1 and 2 of the mitogen-activated protein kinase family by lipopolysaccharide requires plasma in neutrophils from adults and newborns. Infect. Immun. 69:3143-3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cervin, A. 2001. The anti-inflammatory effect of erythromycin and its derivatives, with special reference to nasal polyposis and chronic sinusitis. Acta Otolaryngol. 121:83-92. [DOI] [PubMed] [Google Scholar]
- 8.Chatuverdi, M. M., A. Kumar, B. G. Darnay, C. B. N. Chainy, S. Agarwal, and B. B. Aggarwal. 1997. Sanguinarine (pseudochelerythrine) is a potent inhibitor of NFkB activation, IkB phosphorylation, and degradation. J. Biol. Chem. 272:30129-30134. [DOI] [PubMed] [Google Scholar]
- 9.Choi, J. H., M. J. Song, S. H. Kim, S. M. Choi, D. G. Lee, J. H. Yoo, and W. S. Shin. 2003. Effect of moxifloxacin on production of proinflammatory cytokines from human peripheral blood mononuclear cells. Antimicrob. Agents Chemother. 47:3704-3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dalhoff, A., and I. Shalit. 2003. Immunomodulatory effects of quinolones. Lancet Infect. Dis. 3:359-371. [DOI] [PubMed] [Google Scholar]
- 11.Freifeld, A., D. Marchigiani, T. Walsh, S. Chanock, L. Lewis, J. Hiemenz, S. Hiemenz, J. E. Hicks, V. Gil, S. M. Steinberg, and P. A. Pizzo. 1999. A double blind comparison of empirical oral and intravenous antibiotic therapy for low-risk febrile patients with neutropenia during cancer chemotherapy. N. Engl. J. Med. 341:305-311. [DOI] [PubMed] [Google Scholar]
- 12.Fujimori, Y., M. Kataoka, S. Tada, H. Takehara, K. Matsuo, T. Miyake, M. Okahara, I. Yamadori, and M. Tanimoto. 2002. The role of interleukin-8 in interstitial pneumonia. Respirology 8:33-40. [DOI] [PubMed] [Google Scholar]
- 13.Gerszten, R. E., E. A. Garcia-Zepeda, Y. C. Lim, M. Yoshida, H. A. Ding, M. A. Gimbrone, Jr., A. D. Luster, F. W. Luscinskas, and A. Rosenzweig. 1999. MCP-1 and IL-8 trigger firm adhesion of monocytes to vascular endothelium under flow conditions. Nature 398:718-723. [DOI] [PubMed] [Google Scholar]
- 14.Gollapudi, S., S. K. Chuah, T. Harvey, H. D. Thadepalli, and H. Tadepalli. 1993. In vivo effects of rufloxacin and ciprofloxacin on T-cell subsets and tumor necrosis factor production in mice infected with Bacteroides fragilis. Antimicrob. Agents Chemother. 37:1711-1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hashimoto, S., K. Matsumoto, Y. Gon, S. Maruoka, S. Hayashi, Y. Asai, T. Machino, and T. Horie. 2000. Grepafloxacin inhibits tumor necrosis factor-α-induced interleukin-8 expression in human airway epithelial cells. Pharmacol. Lett. 66:77-82. [DOI] [PubMed] [Google Scholar]
- 16.Holtmann, H., R. Winzen, P. Holland, S. Eickemeier, E. Hoffmann, D. Wallach, N. L. Malinin, J. A. Cooper, K. Resch, and M. Kracht. 1999. Induction of interleukin-8 synthesis integrates effects of transcription and mRNA degradation from at least three different cytokine- or stress-activated signal transduction pathways. Mol. Cell. Biol. 19:6742-6753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hooper, D. C., and J. S. Wolfson. 1991. Fluoroquinolone antimicrobial agents. N. Engl. J. Med. 324:384-394. [DOI] [PubMed] [Google Scholar]
- 18.Imai, Y., T. Kawano, K. Miyasaka, M. Takata, T. Imai, and K. Okuyama. 1994. Inflammatory chemical mediators during conventional ventilation and during high frequency oscillatory ventilation. Am. J. Respir. Crit. Care Med. 150:1550-1554. [DOI] [PubMed] [Google Scholar]
- 19.Karin, M., and Y. Ben-Nariah. 2000. Phosphorylation meets ubiquitination: the control of NF-kappa-B activity. Annu. Rev. Immunol. 18:621-663. [DOI] [PubMed] [Google Scholar]
- 20.Keates, S., A. C. Keates, M. Warny, R. M. Peek, P. G. Murray, and C. P. Kelly. 1999. Differential activation of mitogen activated protein kinases in AGS gastric epithelial cells by cag+ and cag− Helicobacter pylori. J. Immunol. 163:5552-5559. [PubMed] [Google Scholar]
- 21.Khan, A. A., T. R. Slifer, and J. S. Remington. 1998. Effect of trovafloxacin on production of cytokines by human monocytes. Antimicrob. Agents Chemother. 42:1713-1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kopp, E., and S. Ghosh. 1994. Inhibition of NFkB by sodium salicylate and aspirin. Science 265:956-959. [DOI] [PubMed] [Google Scholar]
- 23.Krause, A., H. Holtmann, S. Eickemeier, R. Winzen, M. Szamel, K. Resch, J. Saklatvala, and M. Kracht. 1998. Stress-activated protein kinase/Jun-N-terminal kinase is required for interleukin-1-induced IL-6 and IL-8 gene expression in the human epidermal carcinoma cell line KB. J Biol. Chem. 273:23681-23689. [DOI] [PubMed] [Google Scholar]
- 24.Lin, A., A. Minden, H. Martinetto, F. X. Claret, C. Lange-Carter, F. Mercurio, G. I. Johnson, and M. Karin. 1995. Identification of a dual specificity kinase that activates the jun kinases and p38-mpk2. Science 268:286-290. [DOI] [PubMed] [Google Scholar]
- 25.Liu, R., M. O'Connell, K. Johnson, K. Pritzker, N. Mackman, and R. Terkeltaub. 2000. Extracellular signal-regulated kinase 1/extracellular signal-regulated kinase 2 mitogen-activated protein kinase signaling and activation of activator protein 1 and nuclear factor kB transcription factors play central roles in interleukin-8 expression stimulated by monosodium urate monohydrate and calcium pyrophosphate crystals in monocytic cells. Arthritis Rheum. 43:1145-1155. [DOI] [PubMed] [Google Scholar]
- 26.Marie, C., M. R. Losser, C. Fitting, N. Kermarrec, D. Payen, and J. M. Cavaillon. 1997. Cytokines and soluble cytokine receptors in pleural effusions from septic and nonseptic patients. Am. J. Respir. Crit. Care Med. 156:1515-1522. [DOI] [PubMed] [Google Scholar]
- 27.Matsunaga, Y., I. Katayama, and J. Katoda. 2001. Therapeutic effects of macrolides of patients with palmoplantar pustulosis. Jpn. J. Antibiot. 54:106-108. [PubMed] [Google Scholar]
- 28.Na, Y. J., Y. J. Jeon, J. H. Suh, J. S. Kang, K. H. Yang, and H. M. Kim. 2001. Suppression of IL-8 gene expression by radicicol is mediated through the inhibition of ERK1/2 and p38 signaling and negative regulation of NFkB and AP-1. Int. Immunopharmacol. 1:1877-1887. [DOI] [PubMed] [Google Scholar]
- 29.Neff, L., M. Zeisel, J. Sibilia, M. Scholler-Guinard, J. P. Klein, and D. Wachsmann. 2001. NFkB and the MAP kinases/AP-1 pathways are both involved in interleukin-6 and interleukin-8 expression in fibroblast-like synoviocytes stimulated by protein I/II, a modulin from oral streptococci. Cell. Microbiol. 3:703-712. [DOI] [PubMed] [Google Scholar]
- 30.Ouo, Y., Y. Ohmoto, K. Ouo, Y. Sakata, and K. Murata. 2000. Effect of grepafloxacin on cytokine production in vitro. J. Antimicrob. Chemother. 46:91-94. [DOI] [PubMed] [Google Scholar]
- 31.Pearson, L. L., B. E. Castle, and M. R. Kehry. 2001. CD40-mediated signaling in monocytic cells: up-regulation of tumor necrosis factor receptor-associated factor mRNA and activation of mitogen-activated protein kinase signaling pathways. Int. Immunol. 13:273-283. [DOI] [PubMed] [Google Scholar]
- 32.Procyk, K. J., P. Kovarik, A. Gabain, and M. Baccarini. 1999. Salmonella typhimurium and lipopolysaccharide stimulate extracellularly regulated kinase activation in macrophages by a mechanism involving phosphatidylinositol 3-kinase and phospholipase D as novel intermediates. Infect. Immun. 67:1011-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pugin, J., I. Dunn, P. Jolliet, D. Tassaux, J. L. Magnenat, L. P. Nicod, and J. C. Chevrolet. 1998. Activation of human macrophages by mechanical ventilation in vitro. Am. J. Physiol. 275:L1040-L1050. [DOI] [PubMed] [Google Scholar]
- 34.Shalit, I., Y. Kletter, D. Halperin, D. Waldman, E. Vasserman, A. Nagler, and I. Fabian. 2000. Immunology effects of moxifloxacin in comparison to ciprofloxacin and G-CSF in a murine model of cyclophosphamide-induced leukopenia. Eur. J. Haematol. 66:287-296. [DOI] [PubMed] [Google Scholar]
- 35.Shalit, I., L. Horev, I. Fabian, H. Blau, N. Kariv, I. Shechtman, H. Alteraz, and Y. Kletter. 2002. Immunomodulatory and protective effects of moxifloxacin against Candida albicans-induced bronchopneumonia in cyclophosphamide-injected mice. Antimicrob. Agents Chemother. 46:2442-2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shapiro, L., and C. A. Dinarello. 1995. Osmotic regulation of cytokine synthesis in vitro. Proc. Natl. Acad. Sci. USA 92:12230-12234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shimada, T., and A. Terano. 1998. Chemokine expression in Helicobacter pylori-infected gastric mucosa. J. Gastroenterol. 33:613-617. [DOI] [PubMed] [Google Scholar]
- 38.Takizawa, H., M. Desaki, T. Ohtoshi, S. Kawasaki, T. Kohyama, and M. Sato. 1997. Erythromycin modulates IL-8 expression in normal and inflamed human bronchial epithelial cells. Am. J. Respir. Crit. Care Med. 156:266-271. [DOI] [PubMed] [Google Scholar]
- 39.Thadepalli, H., U. Reddy, S. K. Chuoh, F. Thadepalli, C. Malilay, R. J. Polzer, N Hanna, A. Esfandiari, P. Brown, and S. Gollapudi. 1997. In vivo efficacy of trovafloxacin (CP-99,217), a new quinolone, in experimental intra-abdominal abscesses caused by Bacteroides fragilis and Escherichia coli. Antimicrob. Agents Chemother. 41:583-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thadepalli, H., S. K. Chuoh, U. Reddy, N. Hanna, R. Clark, R. J. Polzer, and S. Gollapudi. 1997. Efficacy of trovafloxacin for treatment of experimental Bacteroides infection in young and senescent mice. Antimicrob. Agents Chemother. 41:1933-1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thomas, L. H., J. S. Friedland, M. Sharland, and S. Becker. 1998. Respiratory syncytial virus-induced RANTES production from human bronchial epithelial cells is dependent on NF-κB nuclear binding and inhibited by adenovirus-mediated expression of IκBα. J. Immunol. 161:1007-1016. [PubMed] [Google Scholar]
- 42.Vlahakis, N. E., M. A. Schroeder, H. A. Limper, and R. D. Hubmayr. 1999. Stretch induces cytokine release by alveolar epithelial cells in vitro. Am. J. Physiol. 277:L167-L173. [DOI] [PubMed] [Google Scholar]
- 43.Warny, M., A. C. Keates, S. Keates, I. Castagliuolo, J. K. Zacks, S. Aboudola, A. Qamar, C. Pothoulakis, J. T. LaMont, and C. P. Kelly. 2000. p38 MAP kinase activation by Clostridium difficile toxin A mediates monocyte necrosis, IL-8 production, and enteritis. J. Clin. Investig. 105:1147-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weizman, N., Y. Shiloh, and A. Barzilai. 2003. Contribution of the Atm protein to maintaining cellular homeostasis evidenced by continuous activation of the ap-1 pathway in Atm-deficient brains. J. Biol. Chem. 278:6741-6747. [DOI] [PubMed] [Google Scholar]
- 45.Williams, A. C., H. F. Galley, and N. R. Webster. 2001. The effect of moxifloxacin on release of interleukin-8 from human neutrophils. Br. J. Anaesthesiol. 87:671-672. [Google Scholar]
- 46.Yasumoto, R., M. Kawano, T. Tsujino, Y. Iwai, S. Hayashi, N. Nishisaka, A. Horii, and T. Kishimoto. 1995. Seminal plasma cytokines in non-bacterial prostatitis: changes following sparfloxacin treatment. Acta Ruol. Jpn. 41:771-774. [PubMed] [Google Scholar]
- 47.Yoshimura, T., C. Kurita, E. Usami, T. Nakao, S. Watanabe, J. Kobayashi, F. Yamazaki, and H. Nagai. 1996. Immunomodulatory action of levofloxacin on cytokine production by human peripheral blood mononuclear cells. Chemotherapy 42:459-464. [DOI] [PubMed] [Google Scholar]
- 48.Zhao, D., A. C. Keates, S. Kuhnt-Moore, M. P. Moyers, C. P. Kelly, and C. Pothoulakis. 2001. Signal transduction pathways mediating neurotensin-stimulated interleukin-8 expression in human colonocytes. J. Biol. Chem. 276:44464-44471. [DOI] [PubMed] [Google Scholar]