Background: Increased SREBP-1c level contributes to excessive triglyceride accumulation in liver of NAFLD patients.
Results: Dec1 represses Srebp-1c gene expression, thereby reducing hepatic lipogenesis and ameliorating fatty liver phenotype.
Conclusion: Dec1 negatively regulates hepatic lipid synthesis.
Significance: Dec1 is a negative regulator of Srebp-1c expression and integrates circadian clock and hepatic lipid metabolism.
Keywords: Lipid Synthesis, Liver Metabolism, Metabolic Disease, Metabolism, Transcription Repressor, DEC1, NAFLD, SREBP-1c
Abstract
Hepatic steatosis, characterized by ectopic hepatic triglyceride accumulation, is considered as the early manifestation of non-alcoholic fatty liver diseases (NAFLD). Increased SREBP-1c level and activity contribute to excessive hepatic triglyceride accumulation in NAFLD patients; however, negative regulators of Srebp-1c are not well defined. In this study, we show that Dec1, a critical regulator of circadian rhythm, negatively regulates hepatic Srebp-1c expression. Hepatic Dec1 expression levels are markedly decreased in NAFLD mouse models. Restored Dec1 gene expression levels in NAFLD mouse livers decreased the expression of Srebp-1c and lipogenic genes, subsequently ameliorating the fatty liver phenotype. Conversely, knockdown of Dec1 expression by an adenovirus expressing Dec1-specific shRNA led to an increase in hepatic TG content in normal mouse livers. Correspondingly, expression levels of lipogenic genes, including Srebp-1c, Fas, and Acc, were increased in livers of mice with Dec1 knockdown. Moreover, a functional lipogenesis assay suggested that Dec1 overexpression repressed lipid synthesis in primary hepatocytes. Finally, a luciferase reporter gene assay indicates that DEC1 inhibits Srebp-1c gene transcription via the E-box mapped to the promoter region. Chromatin immunoprecipitation confirmed that DEC1 proteins bound to the identified E-box element. Our studies indicate that DEC1 is an important regulator of Srebp-1c expression and links circadian rhythm to hepatic lipogenesis. Activation of Dec1 can alleviate the nonalcoholic fatty liver phenotype.
Introduction
Non-alcoholic fatty liver disease (NAFLD),2 which refers to a disease spectrum ranging from simple triglyceride accumulation (hepatic steatosis) to hepatic steatohepatitis (steatosis with inflammation), fibrosis, and cirrhosis, has become the most frequent etiological factor of chronic liver diseases (1).
De novo lipogenesis is markedly increased in NAFLD patients compared with healthy subjects, contributing to the excessive TG deposition in the liver (2). Moreover, hepatic lipogenesis, which is normally inhibited under fasting conditions, is relatively high in the fasted state and fails to further increase in the postprandial periods in NAFLD patients (3). Thus, the ease of hepatic lipogenesis may alleviate the NALFD phenotype. Consistent with abnormal de novo lipogenesis, hepatic expression levels of Srebp-1c, a master transcription factor controlling fatty acid synthesis, are significantly increased in the livers of obese mice and NAFLD patients (4–6).
Srebp-1c is the predominant member of the SREBP family responsible for fatty acid synthesis in mouse liver (7). It is transcriptionally controlled by multiple nutritional and hormonal factors, including insulin, and synthesized as a precursor protein bound to endoplasmic reticulum membrane. NAFLD symptoms spontaneously appeared in Srebp-1 transgenic mice (8–10). LXRs are potent activators of Srebp-1c expression through the LXR response element identified in its promoter region (11). Meanwhile, Srebp-1c is demonstrated to activate its own promoter, forming a positive feedback loop (12). However, its repressive factors are not fully defined (13).
Dec1 (differentiated embryo chondrocyte 1; also known as BHLHB2, STRA13, or SHARP2), a member of the basic helix-loop-helix (bHLH) transcription factor family, is known as a critical regulator of the circadian rhythm (14). It exhibits a circadian expression in the suprachiasmatic nucleus and various peripheral tissues and regulates circadian rhythm via suppressing the CLOCK-BMAL1-mediated activation of key components of the circadian clock system, including Per1 (14). It has been identified to bind with high affinity to class B E-box element (CANGTG) and in most cases serves as a transcriptional repressor (15).
Circadian rhythm has been demonstrated to control various physiological processes, including energy and metabolic homeostasis. Dec1 has been shown to be involved in adipogenesis. Hypoxia-induced Dec1 inhibits adipogenesis by repressing Pparγ2 expression (16). In addition, nuclear receptor LXRs and RORα also activate Dec1 expression through direct binding to its promoter region (17, 18).
In the present study, we show that Dec1 acts as a repressor of hepatic lipogenesis. Activation of Dec1 in obese mouse livers decreased the expression levels of lipogenic genes, including Srebp-1c and its target genes, subsequently alleviating the nonalcoholic fatty liver phenotype.
EXPERIMENTAL PROCEDURES
Animal Treatments
Eight-week-old male C57BL/6J, Leprdb/+ (db/m), and Leprdb/db (db/db) mice and 10-week-old Lepob/ob (ob/ob) mice were purchased from the Model Animal Research Center of Nanjing University (Nanjing, China) and housed and maintained in 12-h light and dark photoperiods.
The mouse protocol was approved by the Animal Research Committee in the Institute of Laboratory Animals, Chinese Academy of Medical Sciences and Peking Union Medical College, and conformed to criteria outlined in the National Institutes of Health (Bethesda, MD) Guide for the Care and Use of Laboratory Animals. For adenovirus treatment, mice were injected with purified adenovirus with 1.0 × 109 active viral particles in 150 μl of 0.4% NaCl solution via tail vein. Then, 5–7 days later, mice fasted for 6 h were sacrificed, and their livers and serum were collected for analysis.
Construction of Adenoviruses Expressing Dec1 and Dec1 shRNA
The full-length mouse Dec1 gene was amplified by polymerase chain reaction (PCR) from C57BL/6J mouse liver cDNA using the following primer pair: 5′-ATGGAACGGATCCCCAGCG-3′ as a forward primer and 5′-TTAGTCTTTGGTTTCTAAGTTTAAA-3′ as a reverse primer. A FLAG tag was added at the C terminus of Dec1 cDNA with restriction enzyme sites by using the forward primer 5′-AGATCTCACCATGGACTACAAAGACGATGACGACAAGATGGAACGGATCCCCAGCG-3′ and the reverse primer 5′-AAGCTTTTAGTCTTTGGTTTCTAAGTTTAAA-3′. Then it was constructed into pcDNA3.1 and pAd-Track-CMV vector with the same restriction sites. For RNA interference application, the DNA sequences corresponding with the short hairpin RNA (shRNA) sequences of 5′-GATCTCCGCACGTGAAAGCATTGACATTCAAGAGATGTCAATGCTTTCACGTGCTTTTT-3′ (top) and 5′-AGCTTAAAAAGCACGTGAAAGCATTGACATCTCTTGAATGTCAATGCTTTCACGTGCGG-3′(bottom), which are against Dec1, were constructed into pAd-Track-U6 vector. Recombinant adenoviruses were generated according to the manufacturer's instructions (Invitrogen) and purified by the cesium chloride method. As a negative control, a recombinant adenovirus vector expressing a scrambled shRNA (shScram) was generated as well. Adenovirus was purified by the cesium chloride method and dialyzed against phosphate-buffered saline containing 10% glycerol.
Isolation and Culture of Mouse Primary Hepatocytes
Primary mouse hepatocytes were obtained from the livers of male C57BL/6J mice. Briefly, the mice were anesthetized, and their livers were perfused with 0.5 mg/ml type II collagenase (Sigma-Aldrich) via the inferior vena cava to isolate hepatocytes. Mouse hepatocytes were cultured in RPMI 1640 containing 10% FBS, 100 units/ml penicillin, and 0.1 mg/ml streptomycin for further study.
Chromatin Immunoprecipitation (ChIP) Assay
ChIP assays were performed as described previously (19). Briefly, the tissue or cells were cross-linked in 1% formaldehyde at 37 °C for 10 min and resuspended in 200 ml of lysis buffer (1% SDS, 10 mm EDTA, 50 mm Tris-HCl (pH 8.1)). Lysates were sonicated and then diluted with ChIP dilution buffer (0.01% SDS, 1.1% Triton X-100, 1.2 mm EDTA, 16.7 mm Tris-HCl (pH 8.1), and 167 mm NaCl). The diluted lysates were immunoprecipitated with anti-DEC1 (Sigma) or anti-FLAG antibody (Sigma) or normal mouse IgG. The immunoprecipitates were washed and then eluted with 300 ml of elution buffer (1% SDS, 0.1 m NaHCO3) and reversed. The promoter region of Srebp-1c was amplified by PCR using the following primer pair: 5′-GATTGGCCATGTGCGCTCA-3′ as a forward primer and 5′-CTGGCAAAGTAATAGAGTG-3′ as a reverse primer.
Quantitative Real-time Reverse Transcription Polymerase Chain Reaction
Total RNA was isolated from cells or pulverized liver using TRIzol (Invitrogen). For real-time PCR analysis, cDNA was synthesized using random primers and the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems). Gene expression analysis was performed using a Bio-Rad IQ5. The mRNA abundance of various transcripts was measured using the Q-PCR SYBR Green kits (Promega). All gene expression data were normalized to β-actin expression levels. All gene expression experiments were performed in at least three independent runs, each performed in triplicate. Primer sequences are shown in Table 1.
TABLE 1.
Real-time PCR primers used in this work
All of the primers are listed in the 5′–3′ direction.
| Mouse gene | Forward primer | Reverse primer |
|---|---|---|
| Dec1 | TTGTCGGGAAGAAATCTCGAGGCA | AGTGTTCTCATGCTTCGCCAGGTA |
| Dec2 | AAAGCGCGCGAGGTATTGCAAGAC | ATTGCTTTACAGAATGGGGAGCG |
| Srebp-1c | GGAGCCATGGATTGCACATT | GGCCCGGGAAGTCACTGT |
| Fas | GTAAGTTCTGTGGCTCCAGAG | GCCCTCCCGTACACTCACTC |
| Acc | AGGAAGATGGCGTCCGCTCTG | GGTGAGATGTGCTGGGTCAT |
| Cpt1a | GAACCCCAACATCCCCAAAC | TCCTGGCATTCTCCTGGAAT |
| Pparα | ACAAGGCCTCAGGGTACCA | GCCGAAAGAAGCCCTTACAG |
| Mcad | AACACTTACTATGCCTCGATTGCA | CCATAGCCTCCGAAAATCTGAA |
| Mttp | GGGACTGGATGTGGCAGA | CACGCTGTCTTGCGGTTT |
| β-actin | AAATCGTGCGTGACATCAAA | AAGGAAGGCTGGAAAAGAGC |
Histological Analysis
For Oil red O staining, liver tissue was frozen in liquid nitrogen and cut into 10-μm sections. Sections were stained and analyzed at ×20 magnification using a microscope.
Hepatic Very Low Density Lipoprotein Secretion Assay
Hepatic VLDL secretion assays were performed as described previously (20). Briefly, overnight-fasted mice were injected with 500 mg/kg body weight of tyloxapol via tail vein. Blood samples were collected by tail bleeding at several time points for serum triglyceride measurement.
Lipogenesis Assays
The rate of lipogenesis in hepatocytes was measured as described previously (21). Briefly, primary hepatocytes were isolated and treated with the indicated adenovirus and subsequently exposed to 5 μm TO901317 for 48 h. The cells were then incubated for additional 4 h in Williams Medium E (Sigma) supplemented with 4 μCi/ml [3H]acetate (PerkinElmer), 0.5 mm unlabeled acetate, and 0.5% BSA. Lipids were extracted from cell lysate with chloroform/methanol (2:1). The organic phase was first dried by evaporation at 50 °C and then dissolved with 50 μl of hexane and 200 μl of 1.8% H2SO4 in methanol and heated at 100 °C for 30 min. 125 μl of water was added to the mixtures, and they were extracted with 250 μl of petroleum. The petroleum phase was collected for measurement of 3H radioactivity. Hepatocyte lipogenesis rates were normalized to cell protein levels.
Fatty Acid Oxidation Assays
The assays were performed as described previously (21). Primary hepatocytes infected with the indicated adenovirus were incubated with 0.4 μCi/ml [9,10-3H]oleic acid (PerkinElmer Life Sciences) and 100 μm unlabeled oleic acid (conjugated with BSA) in Krebs-Ringer buffer (119 mm NaCl, 5 mm KCl, 2 mm CaCl2, 2.6 mm MgSO4, 24.6 mm NaHCO3, 2.6 mm KH2PO4, 10 mm HEPES, pH 7.4) for 1 h at 37 °C. Supernatants were collected and incubated with 1.3 m perchloric acid. All the solutions were then centrifuged at 16,000 × g for 10 min. Supernatants were neutralized with 2 m KOH, 0.6 m MOPS. 3 ml of scintillation liquid was then added, and [3H]radioactivity was measured.
Microarray Analysis
Chow-fed ob/ob and C57BL/6J mice were housed in a 12-h light and 12-h dark cycle and fed ad libitum a regular chow diet. Mice were sacrificed at 16:00 (Zeitgeber time 8 during light phase). Total RNA was prepared from each liver using TRIzol (Invitrogen). Equal aliquots of total RNA from each of four mouse livers in each group were pooled and used for biotin labeling as described in the Affymetrix technical bulletin. Then the transcriptional profiles of samples were probed using the Gene-Chip Mouse Genome 430 2.0 arrays (Affymetrix). Significance analysis of microarrays was used to identify differences between different samples (22). Gene Ontology (GO) classification was performed with the DAVID tool (23). Data were processed for the heat maps with cluster analysis (24).
Statistical Analysis
Data are presented as means ± S.D. and were compared between or among groups by a two-tailed unpaired Student's t test or by a one-way analysis of variance followed by a Fisher least significant difference test. p < 0.05 was considered statistically significant.
RESULTS
Dec1 Is a Candidate Gene in Non-alcohol Fatty Liver Disease
To identify novel transcriptional factors involved in dysfunctional hepatic lipid homeostasis in obesity, we performed mRNA microarray analysis of livers of ob/ob mice, a widely used obese model, and C57BL/6J control mice (the data sets have been submitted to the ArrayExpress database under accession number E-MTAB-2678).
Preliminary microarray data revealed that many genes known to be involved in metabolism and circadian rhythm display a change of at least 2-fold, of which 42 genes, 20 genes, and 18 genes are involved in lipid metabolism (including transcriptional factor SREBF1 (also known as SREBP-1) and its downstream target genes SCD-1 and Fasn (also known as Fas)), glucose metabolism (including G6pc and Pck1 (also known as Pepck1)), and rhythmic processes (including Per1 and Cry1) respectively (Fig. 1, A–C). A real-time PCR assay verified the decreased mRNA levels of Dec1 in livers of ob/ob mice compared with C57BL/6J control mice (Fig. 1D). We also observed a similar decrease in hepatic Dec1 expression in other NAFLD mouse models, including db/db mice (Fig. 1E) and high-fat diet-induced obese mice (Fig. 1F). Correspondingly, DEC1 protein levels were also decreased in the livers of these mice, as revealed by Western blot analysis (Fig. 1G). All of the NAFLD mice exhibited a high level of hepatic triglyceride content. These data suggest that Dec1 may be involved in hepatic dysfunctional lipid metabolism.
FIGURE 1.
Dec1 expression levels are down-regulated in NAFLD mouse livers. A–C, Affymetrix microarray analysis of liver RNA from ob/ob obese mice and C57BL/6J control mice was performed as described under “Experimental Procedures.” The heat maps show differentially expressed genes involved in lipid metabolism (A), glucose metabolism (B), and the circadian process (C). D–G, mice of various genotypes fasted for 6 h were sacrificed at 16:00. D, relative Dec1 mRNA levels in livers of ob/ob mice versus C57BL/6J mice (n = 5/group); E, Dec1 mRNA levels in livers of db/db mice versus db/m mice (n = 5/group); F, Dec1 mRNA levels in livers of C57BL/6J mice versus diet-induced obesity (DIO) mice (n = 5/group). G, Western blot analysis showing the DEC1 protein levels in livers of ob/ob mice versus C57BL/6J mice (top), db/db mice versus db/m mice (middle), and C57BL/6J mice versus diet-induced obesity mice (bottom). H, 8-week-old db/db diabetic and db/m control mice were housed in a 12-h light/12-h dark cycle with the lights on (defined as ZT0) at 8:00 a.m. and fed ad libitum. After 2 weeks, these mice were sacrificed at 6-h intervals over a 24-h period. mRNA levels of Dec1 at each time point were measured by real-time PCR analysis as described under “Experimental Procedures.” The data were expressed relative to the peak value of Dec1 mRNA levels of db/m mice (taken as 1). The data shown are the means ± S.D. (error bars) and were compared between groups by a two-tailed unpaired Student's t test. *, p < 0.05.
Dec1 is a clock gene with rhythmic expression in liver and other peripheral tissues of wild-type mice. We next measured its expression levels in db/db diabetic and db/m control mouse livers at different Zeitgeber time (ZT) points (ZT0, light on; ZT12, light off). Our data indicate that Dec1 also exhibits a rhythmic expression pattern in these mouse livers. In db/m mice, Dec1 levels rose in the light cycle and peaked at ZT8 (16:00). However, in db/db mice, its expression mildly fluctuated (Fig. 1H).
Restoring Dec1 Expression Ameliorates Hepatic Steatosis in db/db and ob/ob Mice
Hepatic steatosis is characterized with excessive TG accumulation (4). We hypothesized that hepatic Dec1 deficiency contributed to fatty liver disease progress in these obese mice. To test this hypothesis, we generated recombinant adenovirus expressing DEC1-FLAG fusion protein (Ad-DEC1) and performed genetic constitution experiments in NAFLD mice, including db/db mice and ob/ob mice. Ad-DEC1 and the control adenovirus (Ad-GFP), which expresses GFP only, were infused into mice via tail vein. Ad-DEC1 efficiently expressed the exogenous DEC1-FLAG fusion protein in mouse livers. Consequently, histological analysis, Oil red O staining (Fig. 2A), and H&E staining (Fig. 2B) suggested that Ad-DEC1 treatment markedly ameliorated the fatty liver phenotype in db/db mice. In Ad-DEC1-infected db/db mice, oil drop size in hepatocytes became smaller, and hepatic tissues showed increased density compared with that in Ad-GFP-infected db/db mice. Gross morphological changes showed that the liver of Ad-DEC1-infected db/db mice appeared fresh red and smaller in size compared with the mice injected with Ad-GFP (Fig. 2C). Biochemical analysis indicated that adenovirus-mediated overexpression of Dec1 significantly reduced hepatic (Fig. 2D, top) and serum triglyceride levels (Fig. 2D, bottom). However, the hepatic and serum cholesterol levels remained unchanged (Table 2), indicating that Dec1 specifically regulated TG metabolism. Additionally, hepatic overexpression of Dec1 did not significantly affect mouse body weight (Table 2).
FIGURE 2.
Restoring Dec1 expression ameliorates hepatic steatosis in db/db and ob/ob mice. A–D, adenoviruses expressing exogenous DEC1-FLAG fusion protein (Ad-DEC1) or GFP (Ad-GFP), as a control, were injected into db/db mice to restore expression levels of Dec1 in liver as described under “Experimental Procedures.” Mice fasted for 6 h were sacrificed at 6 days after adenovirus injection. All samples were collected at 16:00 for further analysis. A and B, the representative Oil Red O staining (A) and H&E staining (B) of liver sections from db/db mice treated with the indicated adenovirus. C, representative morphology of livers from db/db mice treated as above (photographs were taken immediately when livers were picked on ice). D, biochemical analysis showing hepatic TG content (top) and serum TG content (bottom) in db/db mice treated as above. E–H, adenovirus Ad-DEC1 or Ad-GFP was injected into ob/ob mice as described under “Experimental Procedures.” E and F, the representative Oil Red O staining (E) and H&E staining (F) of liver sections from ob/ob mice treated with the indicated adenovirus. G, representative morphology of livers from ob/ob mice treated as above. H, biochemical analysis showing hepatic TG content (top) and serum TG content (bottom) in ob/ob mice treated as above. The data shown are means ± S.D. (error bars) (individual points refer to each sample) and were compared between groups by a two-tailed unpaired Student's t test. p values are shown in each graph.
TABLE 2.
Metabolic characteristics of NAFLD mice (db/db and ob/ob mice) treated with adenovirus containing exogenous Dec1 (Ad-DEC1) and Ad-GFP for 6 days or normal mice (C57/BL6J) treated with Dec1-specific shRNA (Ad-shDEC1) and Ad-shScram for 7 days. All samples were collected at 16:00
Data are means ± S.D. (n = 6/group) and were compared between groups by a two-tailed unpaired Student's t test.
| db/db mice |
ob/ob mice |
C57/BL6J mice |
||||
|---|---|---|---|---|---|---|
| Ad-GFP | AD-DEC1 | Ad-GFP | Ad-DEC1 | Ad-shScram | Ad-shDEC1 | |
| Body weight (g) | 48.9 ± 1.4 | 43.2 ± 3.4 | 51.9 ± 2.1 | 46.6 ± 4.4 | 28.4 ± 3.4 | 27.0 ± 2.5 |
| Body weight gain (g) | 4.95 ± 3.03 | −1.08 ± 3.51 | 5.09 ± 0.39 | 0.86 ± 0.44 | 0.88 ± 0.97 | 0.22 ± 0.49 |
| Liver/body weight (× 100) | 7.59 ± 1.02 | 5.46 ± 1.34a | 5.33 ± 1.65 | 4.97 ± 1.26 | 5.43 ± 0.92 | 5.77 ± 0.52 |
| Liver cholesterol (mg/g of liver weight) | 16.8 ± 4.2 | 15.4 ± 3.3 | 20.0 ± 6.3 | 19.0 ± 5.3 | 11.8 ± 2.0 | 12.3 ± 2.1 |
| Serum alanine aminotransferase (units/liter) | 952 ± 231 | 860 ± 362 | 886 ± 173 | 900 ± 89 | 437 ± 91 | 400 ± 82 |
| Serum aspartate aminotransferase (units/liter) | 1087 ± 269 | 771 ± 275 | 855 ± 174 | 832 ± 159 | 402 ± 85 | 363 ± 78 |
| Serum cholesterol (mmol/liter) | 4.83 ± 0.24 | 4.51 ± 0.43 | 5.54 ± 1.10 | 5.93 ± 0.66 | 3.12 ± 0.26 | 2.56 ± 1.43 |
| Serum free fatty acid (mmol/liter) | 1.80 ± 0.05 | 1.58 ± 0.16a | 2.51 ± 0.51 | 2.20 ± 0.501a | 0.97 ± 0.07 | 0.91 ± 0.05 |
a p < 0.05.
We also injected Ad-DEC1 into ob/ob mice. Similar results were obtained as that in db/db mice. Overexpression of Dec1 alleviated the fatty liver phenotype in ob/ob mice as well (Fig. 2, E–G). Biochemical analysis also confirmed that adenovirus-mediated overexpression of Dec1 significantly reduced hepatic and serum triglyceride levels (Fig. 2H). Again, adenovirus Ad-DEC1 treatment did not markedly change hepatic or serum cholesterol contents (Table 2).
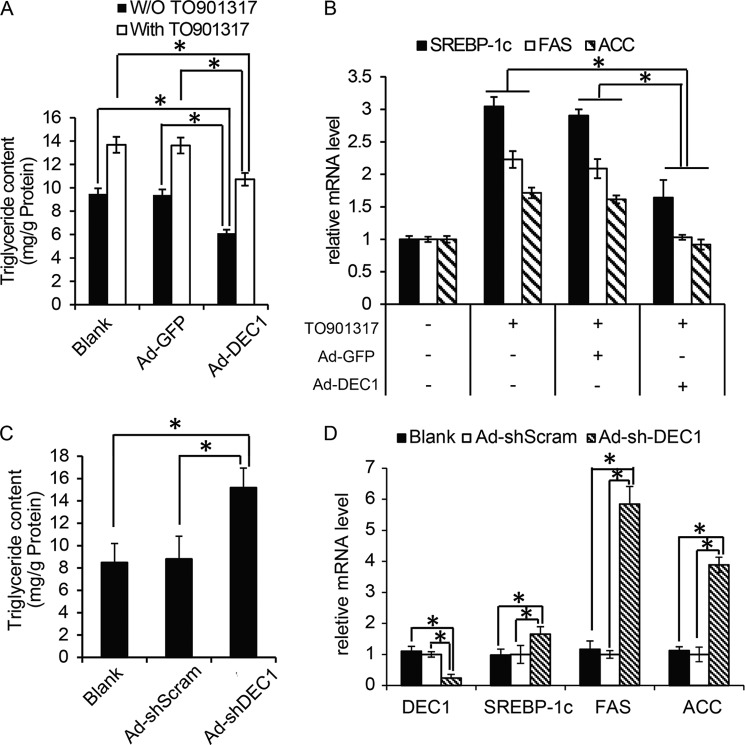
Knockdown of Dec1 Expression Raised Hepatic Triglyceride Contents in Normal Mice
To further confirm the inhibitory effects of Dec1 on hepatic TG contents and test whether Dec1 deficiency in normal mice liver could affect hepatic TG accumulation, we generated an adenovirus expressing Dec1-specific shRNA (Ad-shDEC1). Preliminary data indicated that this adenovirus could effectively knock down endogenous Dec1 gene expression in primary hepatocytes (Fig. 3A). Next, we injected Ad-shDEC1 into C57BL/6J normal mice via tail vein. As a result, Ad-shDEC1-infected C57 mice showed increased hepatic (Fig. 3B) and serum (Fig. 3C) triglyceride content compared with Ad-shScram-infected control mice. However, Ad-shDEC1 treatment of C57BL/6J mice did not significantly influence body weight or hepatic cholesterol contents (Table 2).
FIGURE 3.
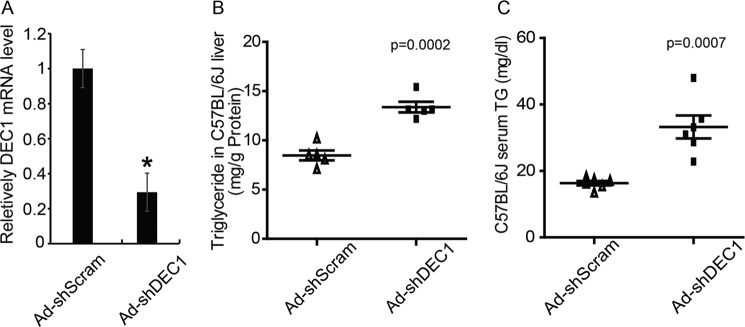
Knockdown of hepatic Dec1 in C57BL/6J mice increases hepatic and serum TG levels. Adenoviruses expressing Dec1-specific shRNA (Ad-shDEC1) or a scrambled shRNA (AdshScram), as a control, were injected into normal C57BL/6J mice to interfere endogenous Dec1 expression. Mice fasted for 6 h were sacrificed at 7 days after adenovirus injection. All samples were collected at 16:00 for further analysis. A, real-time PCR analysis showing the hepatic mRNA level of Dec1 in mice injected with the indicated adenovirus. B and C, biochemical analysis showing hepatic TG (B) content and serum TG (C) content in C57BL/6J mice treated with the indicated adenovirus. Hepatic or serum TG contents were measured by an enzymatic assay. The data shown are means ± S.D. (error bars) (individual points refer to each sample) and were compared between groups by a two-tailed unpaired Student's t test. *, p < 0.05.
Dec1 Negatively Regulates the Expression of Hepatic Srebp-1c and Other Lipogenic Genes
Theoretically, a decrease in the hepatic TG could be caused by increased VLDL secretion, fatty acid oxidation, or decreased TG synthesis or lipid uptake. We measured some key genes involved in these physiological processes, including Pparα, Mcad, and Cpt1 (fatty acid oxidation), Cd36 (fatty acid uptake), and Mttp and ApoB (VLDL secretion). We did not observe significant changes in expression levels of these genes (data not shown). A hepatic VLDL secretion assay also confirmed that Dec1 overexpression did not influence this physiological process in db/db or ob/ob mice (Fig. 4A). In addition, adenovirus-mediated activation of Dec1 did not affect fatty acid β-oxidation in primary hepatocytes (Fig. 4B).
FIGURE 4.
Dec1 negatively regulates expression of hepatic Srebp-1c and lipogenic genes. A, hepatic VLDL secretion in db/db or ob/ob mice injected with the indicated adenovirus was measured at 5 days after adenovirus treatments as described under “Experimental Procedures.” B, β-oxidation rates in primary hepatocytes were measured as described under “Experimental Procedures.” The NAFLD mice were infected with adenoviruses expressing exogenous DEC1-FLAG fusion protein (Ad-DEC1) or GFP (Ad-GFP) (C–F) as described in the legend to Fig. 2. In addition, normal mice were infected with adenoviruses expressing DEC1-specific shRNA (Ad-shDEC1) or a scrambled shRNA (Ad-shScram) (H and I) as described in the legend to Fig. 3. C, D, and H, Western blot analysis showing the endogenous DEC1 and exogenous DEC1-FLAG protein levels using antibody against DEC1 or FLAG and SREBP-1 (precursor form) protein level in livers of db/db mice (C), ob/ob mice (D), and the normal C57BL/6J mice (H) treated with indicated adenovirus. E, F, and I, real-time PCR analysis showing mRNA levels of Dec1, Srebp-1c, Fas, and Acc in livers of db/db mice (E), ob/ob mice (F), and the normal C57BL/6J mice (I) treated with the indicated adenovirus. G, lipogenesis rates in primary hepatocytes were measured as described under “Experimental Procedures.” The data shown are the means ± S.D. (error bars) and were compared between groups by a two-tailed unpaired Student's t test. *, p < 0.05.
However, our data suggested that overexpression of Dec1 effectively repressed Srebp-1c expression in livers of db/db (Fig. 4C) and ob/ob mice (Fig. 4D). In addition, expression levels of Fas and Acc were also decreased in Ad-DEC1-infected db/db (Fig. 4E) and ob/ob mouse livers (Fig. 4F). Consistent with these effects, Ad-DEC1 treatment of primary hepatocytes decreased the rate of lipogenesis compared with Ad-GFP treatment (Fig. 4G).
In contrast, Ad-shDEC1 adenovirus efficiently silenced endogenous Dec1 mRNA expression levels in C57 mouse livers compared with the control Ad-shScram adenovirus (Fig. 4H). As expected, knockdown of Dec1 expression in C57 mouse livers resulted in increases in expression levels of lipogenic genes, including Srebp-1c and its downstream target genes (Fig. 4I).
Dec1 Regulates SREBP-1c Expression and TG Synthesis in a Cell-autonomous Manner
To determine whether Dec1 could repress Srebp-1c expression in primary hepatocytes, adenovirus Ad-DEC1 was used to treat hepatocytes in the presence of TO901317 compound, a well known agonist of LXRs. As expected, TO901317 treatment increased TG contents in primary hepatocytes (Fig. 5A), which is consistent with increased expression of Srebp-1c and its target genes (Fig. 5B). However, Ad-DEC1 treatment reduced the TG content in primary hepatocytes in the presence or absence of TO901317 (Fig. 5A). Consistent with its effect on lipogenesis rate in hepatocytes (Fig. 4G), Dec1 overexpression could repress the expression of Srebp-1c as well as its target genes under TO901317 treatment conditions (Fig. 5B).
FIGURE 5.
Dec1 regulates Srebp-1c gene expression and TG synthesis in mouse primary hepatocytes. Primary hepatocytes were isolated and treated with the indicated adenovirus and subsequently exposed to 5 μm TO901317 for 48 h. Then cells were used for further analysis. A, TG contents in the primary hepatocytes treated as described above. B, real-time PCR analysis showing mRNA levels of genes involved in lipid synthesis in primary hepatocytes treated as in A. C, TG contents in primary hepatocytes infected with the indicated adenovirus and then cultured in completed medium for 48 h before cells were harvested. D, real-time PCR analysis showing mRNA levels of Dec1 and genes involved in lipid synthesis in primary hepatocytes treated as in C. The data shown are the means ± S.D. (error bars) and were compared among groups by a one-way analysis of variance followed by a Fisher least significant difference test. *, p < 0.05.
In contrast, knockdown of endogenous Dec1 by Ad-shDEC1 in primary hepatocytes led to an increase in intracellular triglyceride content (Fig. 5C), in accordance with the increased mRNA of Srebp-1c, Fas, and Acc (Fig. 5D). These observations indicate that Dec1 regulates expression of lipogenic genes and triglyceride synthesis in a cell-autonomous manner.
Dec1 Represses Srebp-1c Expression via Binding to Its Promoter
Our above data suggested that DEC1 is an important negative regulator of Srebp-1c gene expression. Next we wondered whether DEC1 protein binds to the Srebp-1c gene promoter and regulates its expression as a transcription repressor. ChIP assays were carried out to investigate whether endogenous DEC1 protein could be recruited to the Srebp-1c promoter in vivo. A putative DEC1 binding site (CCATGTGC), which contains an E-box element, exists in the Srebp-1c promoter region at −119 bp up from the transcription start site. The Srebp-1c promoter fragment containing the putative DEC1 binding element (E-box) could be amplified from the precipitates obtained when using anti-DEC1 antibody in mouse liver tissue lysate but not when using normal mouse IgG (negative control) (Fig. 6A).
FIGURE 6.
DEC1 proteins bind to the Srebp-1c gene promoter. A, ChIP assays using anti-DEC1 or nonspecific mouse IgG (mIgG) antibodies showing the binding of endogenous DEC1 protein to the Srebp-1c promoter region in livers of C57BL/6J mice were performed as described under “Experimental Procedures.” The resultant DNA was analyzed by PCR with primers to amplify the promoter region of Srebp-1c flanking the E-box site located at about −119 bp relative to the transcription start site. B, ChIP assays using anti-FLAG antibody showing the binding of exogenous DEC1-FLAG protein to the Srebp-1c promoter region in mouse primary hepatocytes (left) and in livers of C57BL/6J (right) infected with adenovirus expressing DEC1-FLAG fusion protein (Ad-DEC1) or GFP (Ad-GFP). C, 5′-deletion series of the mouse Srebp-1c promoter (pSREBP-1c-224, pSREBP-1c-119, and pSREBP-1c-71) or the E-box mutant of the mouse Srebp-1c promoter (pSREBP-1c-224-mut and pSREBP-1c-119-mut) fused to the luciferase reporter gene and were co-transfected into HepG2 cells, together with pcDNA3.1 (control) or Dec1 expression plasmids. After 48 h, cells were harvested, and relative luciferase activity was corrected for Renilla luciferase activity and normalized to the control activity (with the basal activity of each construct (white bar) set to 1.0). D, the basal activities of the 5′-deletion series of the mouse Srebp-1c promoter were measured as in C and normalized to the activity of pSREBP-1c-224 (taken as 1.0). The data shown are the means ± S.D. (error bars) and were compared between groups by a two-tailed unpaired Student's t test. *, p < 0.05.
We also performed ChIP assay to study whether exogenous DEC1 protein could bind to the Srebp-1c promoter. As a result, the Srebp-1c promoter fragment containing the E-box could be amplified while precipitating the DNA complex with anti-FLAG antibody either in primary hepatocytes (Fig. 6B, left) or in liver tissue lysates (Fig. 6B, right) but not when using normal mouse IgG. These data suggest that both endogenous and exogenous DEC1 proteins could bind to the Srebp-1c gene promoter.
Finally, we cloned the mouse Srebp-1c gene promoter and generated a series of luciferase reporter constructs with or without deletion of the DEC1 binding site or site mutation (from CCATGTGC to CAAAATGC) fragments of the Srebp-1c promoter (pSREBP-1c-224, pSREBP-1c-224-mut, pSREBP-1c-119, pSREBP-1c-119-mut, and pSREBP-1c-71). A luciferase reporter gene assay showed that overexpression of DEC1 inhibited pSREBP-1c-224 and pSREBP-1c-119 reporter gene transcription. However, deletion or mutation of a potential DEC1 binding element (pSREBP-1c-71, pSREBP-1c-119-mut, and pSREBP-1c-224-mut) almost abolished the DEC1-inhibitory effects (Fig. 6C). Of note, deletion of the Srebp-1c promoter to −119 bp reduced its basal luciferase activity, but further deletions do not have an advanced effect (Fig. 6D). These data suggested that the effects of DEC1 on Srebp-1c gene expression are mediated by the E-box element.
DISCUSSION
Hepatic steatosis, characterized by overstorage of TG in hepatocytes, is the first step of NAFLD (25, 26). Abnormal hepatic lipogenesis is supposed to contribute to the development of hepatic steatosis. Previous study has indicated that hepatic Dec1 expression is regulated by nutritional status; fasting decreased its expression, and refeeding after fasting restored the decreased Dec1 mRNA (27). Consistent with these data, insulin rapidly induces the expression of the Dec1 gene in primary hepatocytes (28). In the present study, we show that hepatic Dec1 expression levels are decreased in several NAFLD mouse models. The data imply that Dec1 expression levels are closely associated with the metabolic functions of the liver. Thus, we performed Dec1 genetic constitution experiments in several NAFLD mouse models. We find that restored expression of Dec1 in NAFLD mouse models reduced the TG content in liver and ameliorated fatty liver symptoms, suggesting a protective role of Dec1 in the development of NAFLD.
The Srebp-1c promoter contains the sterol regulatory element complex that includes the sterol regulatory element and E-box (12). Here we demonstrate that Dec1 proteins are able to inhibit the expression of Srebp-1c through binding to the E-box element mapped in the Srebp-1c gene promoter, serving as an important negative regulator of hepatic lipid synthesis. Our data suggest that decreased Dec1 expression might underlie the increased Srebp-1c expression and hepatic lipogenesis in NAFLD mice.
Several physiological processes, including fatty acid uptake, fatty acid oxidation, and de novo lipid synthesis, affect hepatic triglyceride contents. In this study, we found that adenovirus-mediated overexpression of Dec1 in mouse livers and primary hepatocytes inhibited the lipogensis rates and repressed expression of lipogenic genes. However, manipulation of Dec1 expression in tissues or cells did not affect the VLDL secretion or fatty acid oxidation. These data suggest that the repressing effects of Dec1 on hepatic lipogenesis accounts for decreased hepatic TG levels.
Both insulin and LXRα agonist induce Srebp-1c expression in hepatocytes (29). Meanwhile, Dec1 expression also can be induced by insulin and LXRα agonist (17, 28). Of note, we also found that overexpression of Srebp-1c also decreased Dec1 expression in primary hepatocyte, although the exact molecular mechanism remains unclear. Thus, Dec1 and Srebp-1c may interact reciprocally in vivo. These data suggest the existence of a feedback loop associated with Srebp-1c transcription in vivo, and these genes constitute a network of hepatic lipogenesis.
Metabolism and circadian rhythms are tightly linked in vivo and interact reciprocally; disruption of circadian rhythms leads to metabolic disorders (30). Accumulating evidence also suggests that key clock genes influence lipid metabolism. For example, Bmal1-deficient embryonic fibroblasts fail to differentiate into adipocytes due to a decrease in gene expression of several adipogenic/lipogenic factors, including Pparγ2, C/EBPα, and Srebp-1. In contrast, overexpression of Bmal1 in adipocytes promotes lipid synthesis (31). Liver-specific deletion of Bmal1 in mice leads to fasting hypoglycemia, insulin resistance, and loss of rhythmic expression of hepatic glucose regulatory genes (32). Similarly, Clock mutant mice having a greatly attenuated diurnal feeding rhythm are hyperphagic and obese and develop a metabolic syndrome with hyperleptinemia, hyperlipidemia, hepatic steatosis, and hyperglycemia (33).
DEC1 was previously identified as a circadian component in the central clock system (14). Five clock gene families, including Dec genes (Dec1 and Dec2), Per genes (Per1, Per2, and Per3), Cry genes (Cry1 and Cry2), Clock, and Bmal, constitute a complex transcription-translation loop that generates the circadian rhythm in mammals. The CLOCK-BMAL1 complex activates expression of Per and Cry genes by direct binding to the E-box elements of these genes. The CLOCK-BMAL1 complex also activates Dec1 gene expression; in turn, induced Dec1 blocks the stimulatory effects of CLOCK-BMAL1 on Dec1 expression through competing for binding to E-box in its promoter, thus forming a negative feedback loop of Dec1 expression. In contrast to CLOCK-BMAL1, PERs and CRYs negatively regulate Dec1 gene transcription (34). Interestingly, light induces Dec1 expression, and induced DEC1 proteins inhibit CLOCK-BMAL1-induced Per1 through interacting with BMAL1 (14). In this study, we provide evidence that Dec1 also plays an important role in lipid metabolism through regulating Srebp-1c expression, suggesting that Dec1 may link circadian rhythm to lipid metabolism.
Interestingly, in this study, we show that Dec1 gene expression in the livers of NAFLD mice also remains rhythmic, peaking in the light cycle. However, the expression levels of Srebp-1c and its target genes, including Fas and Acc, increase during the dark phase and fall during the light phase (35). These data further confirm that the expressions of Srebp-1c and Dec1 show opposite trends and that these two proteins may interact reciprocally in vivo. Of note, another clock key component, REV-ERBα, also regulates SREBP signaling (36). Like DEC1, REV-ERBα also acts as a transcriptional repressor, and CLOCK-BMAL1-induced REV-ERBα proteins feedback to inhibit Bmal1 expression through binding to ROR elements. However, REV-ERBα and DEC1 regulate expression of Srebp-1c in different manners. Our studies show that DEC1 inhibits Srebp-1c expression through direct binding to the E-box in its promoter, whereas REV-ERBα indirectly controls SREBP-1 activity through repressing Insig2 gene transcription (36). Thus, hepatic SREBP-1 signaling and lipogenesis are fine-tuned by the circadian clock system.
In addition to regulation of lipid metabolism (16, 18), Dec1 also appears to be involved in glucose metabolism. Adenovirus-mediated overexpression of Dec1 decreases mRNA levels of phosphoenolpyruvate carboxykinase (Pepck), a rate-limiting enzyme involved in gluconeogenesis, in primary hepatocytes through repressing gene transcription (37). We also found that overexpression of Dec1 inhibited expression of Pgc-1α, a master regulator of hepatic gluconeogenesis. However, the precise molecular mechanism of Dec1 regulation of these gluconeogenic genes remains unclear. Further studies are also required to clarify the roles of Dec1 in regulating hepatic glucose metabolism. We should point out that in this study, we employed the adenovirus system to effectively drive exogenous Dec1 gene expression in mouse livers, which only allowed us to observe the short term effects of hepatic Dec1 activation on lipid metabolism. Other mouse models, such as Dec1 transgenic or knock-out mice, are still required to study the roles of the Dec1 gene in regulating hepatic metabolism.
In summary, we identify Dec1 as an important negative regulator of Srebp-1c expression and hepatic lipogenesis. Thus, Dec1 integrates the circadian clock system and hepatic lipid metabolism. Our study might provide a potential target for treatment of NAFLD and other metabolic diseases.
This work was supported by Major State Basic Research Development Program of China 973 Program Grant 2012CB517504 and National Natural Science Foundation of China Grant 81170763.
- NAFLD
- nonalcoholic fatty liver disease
- LXR
- liver X receptor
- SREBP
- sterol regulatory element-binding protein
- TG
- triglyceride
- ZT
- Zeitgeber time
- Ad
- adenovirus.
REFERENCES
- 1. Brunt E. M. (2010) Pathology of nonalcoholic fatty liver disease. Nat. Rev. Gastroenterol. Hepatol. 7, 195–203 [DOI] [PubMed] [Google Scholar]
- 2. Diraison F., Moulin P., Beylot M. (2003) Contribution of hepatic de novo lipogenesis and reesterification of plasma non-esterified fatty acids to plasma triglyceride synthesis during non-alcoholic fatty liver disease. Diabetes Metab. 29, 478–485 [DOI] [PubMed] [Google Scholar]
- 3. Donnelly K. L., Smith C. I., Schwarzenberg S. J., Jessurun J., Boldt M. D., Parks E. J. (2005) Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J. Clin. Invest. 115, 1343–1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ferré P., Foufelle F. (2010) Hepatic steatosis: a role for de novo lipogenesis and the transcription factor SREBP-1c. Diabetes Obes. Metab. 12, 83–92 [DOI] [PubMed] [Google Scholar]
- 5. Kammoun H. L., Chabanon H., Hainault I., Luquet S., Magnan C., Koike T., Ferré P., Foufelle F. (2009) GRP78 expression inhibits insulin and ER stress-induced SREBP-1c activation and reduces hepatic steatosis in mice. J. Clin. Invest. 119, 1201–1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pettinelli P., Del Pozo T., Araya J., Rodrigo R., Araya A. V., Smok G., Csendes A., Gutierrez L., Rojas J., Korn O., Maluenda F., Diaz J. C., Rencoret G., Braghetto I., Castillo J., Poniachik J., Videla L. A. (2009) Enhancement in liver SREBP-1c/PPAR-α ratio and steatosis in obese patients: correlations with insulin resistance and n-3 long-chain polyunsaturated fatty acid depletion. Biochim. Biophys. Acta 1792, 1080–1086 [DOI] [PubMed] [Google Scholar]
- 7. Shimomura I., Shimano H., Horton J. D., Goldstein J. L., Brown M. S. (1997) Differential expression of exons 1a and 1c in mRNAs for sterol regulatory element binding protein-1 in human and mouse organs and cultured cells. J. Clin. Invest. 99, 838–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nakayama H., Otabe S., Ueno T., Hirota N., Yuan X., Fukutani T., Hashinaga T., Wada N., Yamada K. (2007) Transgenic mice expressing nuclear sterol regulatory element-binding protein 1c in adipose tissue exhibit liver histology similar to nonalcoholic steatohepatitis. Metabolism 56, 470–475 [DOI] [PubMed] [Google Scholar]
- 9. Shimano H., Horton J. D., Hammer R. E., Shimomura I., Brown M. S., Goldstein J. L. (1996) Overproduction of cholesterol and fatty acids causes massive liver enlargement in transgenic mice expressing truncated SREBP-1a. J. Clin. Invest. 98, 1575–1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shimano H., Yahagi N., Amemiya-Kudo M., Hasty A. H., Osuga J., Tamura Y., Shionoiri F., Iizuka Y., Ohashi K., Harada K., Gotoda T., Ishibashi S., Yamada N. (1999) Sterol regulatory element-binding protein-1 as a key transcription factor for nutritional induction of lipogenic enzyme genes. J. Biol. Chem. 274, 35832–35839 [DOI] [PubMed] [Google Scholar]
- 11. Repa J. J., Liang G., Ou J., Bashmakov Y., Lobaccaro J.-M. A., Shimomura I., Shan B., Brown M. S., Goldstein J. L., Mangelsdorf D. J. (2000) Regulation of mouse sterol regulatory element-binding protein-1c gene (SREBP-1c) by oxysterol receptors, LXRα and LXRβ. Genes Dev. 14, 2819–2830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Amemiya-Kudo M., Shimano H., Yoshikawa T., Yahagi N., Hasty A. H., Okazaki H., Tamura Y., Shionoiri F., Iizuka Y., Ohashi K., Osuga J., Harada K., Gotoda T., Sato R., Kimura S., Ishibashi S., Yamada N. (2000) Promoter analysis of the mouse sterol regulatory element-binding protein-1c gene. J. Biol. Chem. 275, 31078–31085 [DOI] [PubMed] [Google Scholar]
- 13. Jeon T.-I., Osborne T. F. (2012) SREBPs: metabolic integrators in physiology and metabolism. Trends Endocrinol. Metab. 23, 65–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Honma S., Kawamoto T., Takagi Y., Fujimoto K., Sato F., Noshiro M., Kato Y., Honma K. (2002) Dec1 and Dec2 are regulators of the mammalian molecular clock. Nature 419, 841–844 [DOI] [PubMed] [Google Scholar]
- 15. St-Pierre B., Flock G., Zacksenhaus E., Egan S. E. (2002) Stra13 homodimers repress transcription through class B E-box elements. J. Biol. Chem. 277, 46544–46551 [DOI] [PubMed] [Google Scholar]
- 16. Yun Z., Maecker H. L., Johnson R. S., Giaccia A. J. (2002) Inhibition of PPARγ2 gene expression by the HIF-1-regulated gene DEC1/Stra13: a mechanism for regulation of adipogenesis by hypoxia. Dev. Cell 2, 331–341 [DOI] [PubMed] [Google Scholar]
- 17. Noshiro M., Usui E., Kawamoto T., Sato F., Nakashima A., Ueshima T., Honda K., Fujimoto K., Honma S., Honma K., Makishima M., Kato Y. (2009) Liver X receptors (LXRα and LXRβ) are potent regulators for hepatic Dec1 expression. Genes Cells 14, 29–40 [DOI] [PubMed] [Google Scholar]
- 18. Ozaki N., Noshiro M., Kawamoto T., Nakashima A., Honda K., Fukuzaki-Dohi U., Honma S., Fujimoto K., Tanimoto K., Tanne K., Kato Y. (2012) Regulation of basic helix-loop-helix transcription factors Dec1 and Dec2 by RORα and their roles in adipogenesis. Genes Cells 17, 109–121 [DOI] [PubMed] [Google Scholar]
- 19. Choi S. M., Cho H.-J., Cho H., Kim K. H., Kim J. B., Park H. (2008) Stra13/DEC1 and DEC2 inhibit sterol regulatory element binding protein-1c in a hypoxia-inducible factor-dependent mechanism. Nucleic Acids Res. 36, 6372–6385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Qiao A., Liang J., Ke Y., Li C., Cui Y., Shen L., Zhang H., Cui A., Liu X., Liu C., Chen Y., Zhu Y., Guan Y., Fang F., Chang Y. (2011) Mouse patatin-like phospholipase domain-containing 3 influences systemic lipid and glucose homeostasis. Hepatology 54, 509–521 [DOI] [PubMed] [Google Scholar]
- 21. Sheng L., Cho K. W., Zhou Y., Shen H., Rui L. (2011) Lipocalin 13 protein protects against hepatic steatosis by both inhibiting lipogenesis and stimulating fatty acid β-oxidation. J. Biol. Chem. 286, 38128–38135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhang S. (2007) A comprehensive evaluation of SAM, the SAM R-package and a simple modification to improve its performance. BMC Bioinformatics 8, 230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Huang da W., Sherman B. T., Lempicki R. A. (2009) Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 4, 44–57 [DOI] [PubMed] [Google Scholar]
- 24. Eisen M. B., Spellman P. T., Brown P. O., Botstein D. (1998) Cluster analysis and display of genome-wide expression patterns. Proc. Natl. Acad. Sci. U.S.A. 95, 14863–14868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Browning J. D., Horton J. D. (2004) Molecular mediators of hepatic steatosis and liver injury. J. Clin. Invest. 114, 147–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tiniakos D. G., Vos M. B., Brunt E. M. (2010) Nonalcoholic fatty liver disease: pathology and pathogenesis. Annu. Rev. Pathol. 5, 145–171 [DOI] [PubMed] [Google Scholar]
- 27. Kawamoto T., Noshiro M., Furukawa M., Honda K. K., Nakashima A., Ueshima T., Usui E., Katsura Y., Fujimoto K., Honma S., Honma K., Hamada T., Kato Y. (2006) Effects of fasting and re-feeding on the expression of Dec1, Per1, and other clock-related genes. J. Biochem. 140, 401–408 [DOI] [PubMed] [Google Scholar]
- 28. Yamada K., Kawata H., Shou Z., Mizutani T., Noguchi T., Miyamoto K. (2003) Insulin induces the expression of the SHARP-2/Stra13/DEC1 gene via a phosphoinositide 3-kinase pathway. J. Biol. Chem. 278, 30719–30724 [DOI] [PubMed] [Google Scholar]
- 29. Foretz M., Guichard C., Ferré P., Foufelle F. (1999) Sterol regulatory element binding protein-1c is a major mediator of insulin action on the hepatic expression of glucokinase and lipogenesis-related genes. Proc. Natl. Acad. Sci. U.S.A. 96, 12737–12742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shimba S., Ishii N., Ohta Y., Ohno T., Watabe Y., Hayashi M., Wada T., Aoyagi T., Tezuka M. (2005) Brain and muscle Arnt-like protein-1 (BMAL1), a component of the molecular clock, regulates adipogenesis. Proc. Natl. Acad. Sci. U.S.A. 102, 12071–12076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Eckel-Mahan K., Sassone-Corsi P. (2013) Metabolism and the circadian clock converge. Physiol. Rev. 93, 107–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lamia K. A., Storch K.-F., Weitz C. J. (2008) Physiological significance of a peripheral tissue circadian clock. Proc. Natl. Acad. Sci. U.S.A. 105, 15172–15177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Turek F. W., Joshu C., Kohsaka A., Lin E., Ivanova G., McDearmon E., Laposky A., Losee-Olson S., Easton A., Jensen D. R., Eckel R. H., Takahashi J. S., Bass J. (2005) Obesity and metabolic syndrome in circadian clock mutant mice. Science 308, 1043–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kawamoto T., Noshiro M., Sato F., Maemura K., Takeda N., Nagai R., Iwata T., Fujimoto K., Furukawa M., Miyazaki K., Honma S., Honma K., Kato Y. (2004) A novel autofeedback loop of Dec1 transcription involved in circadian rhythm regulation. Biochem. Biophys. Res. Commun. 313, 117–124 [DOI] [PubMed] [Google Scholar]
- 35. Patel D. D., Knight B. L., Wiggins D., Humphreys S. M., Gibbons G. F. (2001) Disturbances in the normal regulation of SREBP-sensitive genes in PPAR α-deficient mice. J. Lipid Res. 42, 328–337 [PubMed] [Google Scholar]
- 36. Le Martelot G., Claudel T., Gatfield D., Schaad O., Kornmann B., Lo Sasso G., Moschetta A., Schibler U. (2009) REV-ERBα participates in circadian SREBP signaling and bile acid homeostasis. PLoS Biol. 7, e1000181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yamada K., Ogata-Kawata H., Matsuura K., Miyamoto K. (2005) SHARP-2/Stra13/DEC1 as a potential repressor of phosphoenolpyruvate carboxykinase gene expression. FEBS Lett. 579, 1509–1514 [DOI] [PubMed] [Google Scholar]