Background: ZapA and FtsZ interact prior to bacterial cell division to stabilize the Z-ring.
Results: The structure of E. coli ZapA reveals a charged α-helix important for FtsZ interactions.
Conclusion: Key residues in the charged α-helix of ZapA are important for FtsZ filament bundling.
Significance: ZapA facilitates FtsZ filament bundling and Z-ring stability in dividing bacterial cells.
Keywords: Bacteria, Cell Division, Crystal Structure, Electron Microscopy (EM), Escherichia coli (E. coli)
Abstract
FtsZ is an essential cell division protein in Escherichia coli, and its localization, filamentation, and bundling at the mid-cell are required for Z-ring stability. Once assembled, the Z-ring recruits a series of proteins that comprise the bacterial divisome. Zaps (FtsZ-associated proteins) stabilize the Z-ring by increasing lateral interactions between individual filaments, bundling FtsZ to provide a scaffold for divisome assembly. The x-ray crystallographic structure of E. coli ZapA was determined, identifying key structural differences from the existing ZapA structure from Pseudomonas aeruginosa, including a charged α-helix on the globular domains of the ZapA tetramer. Key helix residues in E. coli ZapA were modified using site-directed mutagenesis. These ZapA variants significantly decreased FtsZ bundling in protein sedimentation assays when compared with WT ZapA proteins. Electron micrographs of ZapA-bundled FtsZ filaments showed the modified ZapA variants altered the number of FtsZ filaments per bundle. These in vitro results were corroborated in vivo by expressing the ZapA variants in an E. coli ΔzapA strain. In vivo, ZapA variants that altered FtsZ bundling showed an elongated phenotype, indicating improper cell division. Our findings highlight the importance of key ZapA residues that influence the extent of FtsZ bundling and that ultimately affect Z-ring formation in dividing cells.
Introduction
Bacterial cell division requires the presence, accumulation and mid-cell localization of FtsZ (filamentous temperature-sensitive protein Z). FtsZ is a 40-kDa monomeric protein with GTPase activity that shows structural and functional homology to the eukaryotic protein tubulin (1–5). FtsZ binds and hydrolyzes GTP in vivo (6) and also polymerizes to form the Z-ring at mid-cell. The Z-ring forms prior to cell division and acts as a scaffold for the recruitment of all downstream cell division proteins; together these comprise the “divisome” (7–10). In Escherichia coli, the divisome includes ∼20 essential and nonessential proteins (11–13). Divisome proteins are recruited in a hierarchal manner and depend on the Z-ring for stability. Included in the divisome are the Zaps (FtsZ-associated proteins): ZapA, ZapB, ZapC, and ZapD (14–19). These Zaps stabilize the Z-ring by increasing lateral interactions between individual filaments, thus bundling FtsZ to provide a scaffold for divisome assembly (9, 20).
Our knowledge of the functional, structural, and biochemical significance of the Zaps in E. coli is limited. It has been demonstrated that they are not individually essential in vivo (14, 15, 17). However, because of their potentially overlapping function and temporal recruitment to the Z-ring, it may be the cooperative interaction of all four proteins with FtsZ that has a significant impact on cell division (14, 15). Advances in microscopy have aided in the visualization of FtsZ filaments in vitro (5, 21–25) and Z-ring formation in vivo (2, 26–30). Depending on the techniques and bacterial species used, recent superresolution fluorescence studies suggest that, in vivo, FtsZ filaments may adopt either a dynamic “bead-like” organization (31); loose, overlapping bundles comprised of multiple FtsZ filaments (30, 32); loosely packed continuous bands of bundled FtsZ filaments (33); or a combination of the arrangements described above (34, 35). Although ZapA and ZapB have been implicated in positioning FtsZ protofilaments at mid-cell (30), the precise function of the Zap proteins in vivo remains unclear.
ZapA has been shown to bundle FtsZ in vitro, and ZapA-deficient strains exhibit significant elongation, indicating a cell division defect (14, 30, 36, 37). To date, all biochemical studies on the E. coli ZapA protein (EcZapA)2 have been interpreted in the context of the crystal structure of the tetrameric Pseudomonas aeruginosa ZapA (PaZapA) (37–40). Because these two proteins share only 25% sequence identity (as determined by ClustalW(41)), a structure of E. coli ZapA may aid in advancing understanding about the lateral interactions that bundle FtsZ filaments. Recently, a critical region on E. coli ZapA was identified by a bacterial two-hybrid assay (36). This 26-amino acid region is weakly conserved across bacterial species but contains several charged residues that could potentially interact with FtsZ. Here, we report the crystal structure of the EcZapA tetramer showing several key differences from its P. aeruginosa counterpart. Additionally, by targeting charged amino acids on the α-helix of the N-terminal globular domain of EcZapA, we have identified key residues involved in the ZapA-FtsZ interaction that promote filament bundling.
EXPERIMENTAL PROCEDURES
Cloning, Site-directed Mutagenesis, and Construction of Zap Deletion Strain
Plasmids containing zapA and ftsZ were constructed by amplifying the zapA and ftsZ genes using primers 308F (TATATGAATTCATGCACCACCACCACCACCACAGCATCGAAGGTCGAAGTGGTATGTCTGCACAACCCGTCGATATC) and 308R (TATCGAAGCTTTCATTCAAAGTTTTGGTTAGTTTTTTCGGTGATGCGACCTTGTTCAAGTAACGCTTGTTCTATGG), and 400F (TTTAATACCATGGTGTTTGAACCAATGGAACTTACCAATG) and 400R (TATTATAAGCTTTTATTAATCAGCTTGCTTACGCAGGAATG), respectively. The zapA gene from E. coli W3110 was amplified and cloned into pBAD24 as an EcoRI-HindIII His tag-encoding fragment (sites underlined). The ftsZ gene was cloned into pET28a using the NcoI and HindIII cut sites (sites underlined) so as to exclude the plasmid-encoded His tag. The ΔzapA strain was made using the λ red deletion system as described previously (42, 43). The entire zapA gene was replaced with the cat region from pKD3 mediated by the λ red operon products from pSIM6 in E. coli DH5α. Site-directed mutagenesis was performed using the QuikChange® Lightning site-directed mutagenesis kit (Stratagene), and zapA variant sequences were amplified using the primers listed in Table 1.
TABLE 1.
Strains, plasmids, and primers used in this study
| Description/genome/sequence | Source | |
|---|---|---|
| E. coli strains | ||
| DH5α | F− endA1 glnV44 thi-1 recA1 relA1 gyrA96 deoR nupG Φ80dlacZΔM15Δ (lacZYA-argF)U169, hsdR17 (rK− mK+), λ− | Invitrogen |
| BL21(DE3) | F− ompT gal dcm lon hsdSB(rB− mB−) λ(DE3) pLysS(cmR)a | New England Biolabs |
| W3110 | rph-1IN (rrnD-rrnE) | Coli Genetic Stock Centre |
| ΔzapA | W3110, ΔzapA | This work |
| Plasmids | ||
| pEJR005b | Modified pET28A (no histidine tag) with WT ftsZ from E. coli | This work |
| pEJR029b | pBAD24 with WT His6-zapA from E. coli | This work |
| pEJR029b_D22A | pBAD24 with D22A His6-zapA from E. coli | This work |
| pEJR029b_R24A | pBAD24 with R24A His6-zapA from E. coli | This work |
| pEJR029b_N28A | pBAD24 with N28A His6-zapA from E. coli | This work |
| pEJR029b_D32A | pBAD24 with D32A His6-zapA from E. coli | This work |
| pEJR029b_D33A | pBAD24 with D33A His6-zapA from E. coli | This work |
| pEJR029b_D33K | pBAD24 with D33K His6-zapA from E. coli | This work |
| pEJR029b_R46A | pBAD24 with R46A His6-zapA from E. coli | This work |
| Primers for mutagenesis | ||
| D22A_F | 5′-AACTGCCCGCCTGCCCAAAGGGATGCG-3′ | This work |
| D22A_R | 5′-CGCATCCCTTTGGGCAGGCGGGCAGTT-3′ | This work |
| R24A_F | 5′-GAACTGCCCGCCTGACCAAGCGGATGCGTTGAATC-3′ | This work |
| R24A_R | 5′-GATTCAACGCATCCGCTTGGTCAGGCGGGCAGTTC-3′ | This work |
| N28A_F | 5′-CCAAAGGGATGCGTTGGCTCAGGCAGCGGACGAT-3′ | This work |
| N28A_R | 5′-ATCGTCCGCTGCCTGAGCCAACGCATCCCTTTGG-3′ | This work |
| D32A_F | 5′-TGAATCAGGCAGCGGCCGATCTGAACCAACG-3′ | This work |
| D32A_R | 5′-CGTTGGTTCAGATCGGCCGCTGCCTGATTCA-3′ | This work |
| D33A_F | 5′-CAGGCAGCGGACGCTCTGAACCAACGG-3′ | This work |
| D33A_R | 5′-CCGTTGGTTCAGAGCGTCCGCTGCCTG-3′ | This work |
| D33K_F | 5′-GAATCAGGCAGCGGACAAGCTGAACCAACGGTTGC-3′ | This work |
| D33K_R | 5′-GCAACCGTTGGTTCAGCTTGTCCGCTGCCTGATTC | This work |
| R46A_F | 5′-GGTTGCAAGATCTGAAAGAACGCACTGCAGTCACAAATACTGAA-3′ | This work |
| R46A_R | 5′-TTCAGTATTTGTGACTGCAGTGCGTTCTTTCAGATCTTGCAACC-3′ | This work |
a cmR, chloramphenicol resistance.
Protein Expression and Purification
FtsZ expression was induced by the addition of 1 mm isopropyl 1-thio-β-d-galactopyranoside (Roche) in an E. coli BL21(DE3) pLysS strain at 37 °C for 3 h in LB medium supplemented with kanamycin and chloramphenicol. Cell pellets were resuspended in PEM-KOH buffer (50 mm piperazine-N,N′-bis(ethanesulfonic acid)-KOH, 1 mm EDTA, 5 mm MgCl2, pH 6.5) and lysed by French press. Cell debris was removed by centrifugation at 8,000 × g for 15 min, and membranes were removed by centrifugation at 100,000 × g for 1 h. FtsZ was purified by two rounds of calcium cycling as described previously (23) with an additional low speed centrifugation step (8,000 × g for 5 min) before desalting using Amicon® Ultra-15 10,000 NMWL centrifugal filters (Millipore). FtsZ was then stored at 4 °C for no longer than 4 days in PEM-KOH buffer. For His6-ZapA expression, an overnight culture of E. coli BL21(DE3) pLysS cells carrying the pEJR029b plasmid was diluted 1/100 into LB medium supplemented with ampicillin. Cells were grown at 37 °C to an A600 of 1.0 (∼2 h) and then induced by the addition of 0.2% (v/v) l-arabinose (Sigma). Expression proceeded for 1 h before cell pellets were collected by centrifugation. After pelleting, cells were resuspended in A1 buffer (20 mm Tris, 50 mm NaCl, 20 mm imidazole, pH 7.4) and lysed by French press. Cell debris and membranes were removed as described for FtsZ (above). His6-ZapA was purified by immobilized metal affinity chromatography, using the BiologicTM Duoflow Chromatography System (Bio-Rad). Soluble protein fractions were loaded onto an MT2 column (Bio-Rad) packed with nickel-nitrilotriacetic acid Superflow resin (Qiagen). After washing with six column volumes of A1 buffer containing 75 mm imidazole, His6-ZapA was eluted with a linear gradient of imidazole from 75–500 mm. His6-ZapA fractions were pooled, concentrated, and desalted using Amicon® Ultra-15 3,000 NMWL centrifugal filters (Millipore). Purified His6-ZapA was stored at 4 °C in A1 buffer for no longer than 4 weeks with no loss of activity during this period.
Crystallization, Data Collection, Structure Determination, and Analysis of ZapA
We crystallized EcZapA from 0.5 m ammonium sulfate, 1.0 m lithium sulfate, and 0.1 m sodium citrate, pH 5.6. Crystals grew as hexagonal bipyramids up to 500 μm in length. After the removal of surface water by immersion in paratone N-oil, crystals were frozen in liquid nitrogen. The data were collected at the Canadian Synchrotron Light Source (the Canadian Macromolecular Crystallography Facility, Beamline 08ID-1) and processed in XDS (44). Initial attempts at molecular replacement failed in Phaser (45) with a variety of search models derived from the PaZapA dimer or protomer. Molecular replacement eventually succeeded with a single protomer search model, comprising residues 50–94. This model comprises less than 25% of the asymmetric unit, with a pairwise sequence identity of only ∼30% to the target. The top translation function scores for the second copy just exceeded the Z-score of 8.0, which generally indicates a reliable molecular replacement solution. The solution is actually out of sequence register with respect to the final model and with different offsets in the two protomers. The C-terminal helix appears to act as a generic α-helical model of approximately the correct curvature, capturing the essential structural elements independent of the underlying sequence. This initial model was subjected to autobuild in Phenix (46), which was able to correctly build most of chain A. The rest of the structure was completed manually by rebuilding in Coot (47) and refined in Phenix (46), using a translation-liberation-screw model of the atomic displacement parameters.
FtsZ Filamentation and Sedimentation Assays
FtsZ (4.8 μm) filamentation was performed in PEM-KOH buffer at 30 °C upon the addition of GTP to 1 mm; in all cases samples were preincubated for 5 min at 30 °C before filamentation. Sedimentation assays were performed by titrating His6-ZapA (0.5, 1.0, 2.1, 4.8, 8.1, and 11.4 μm) against a constant concentration of FtsZ (4.8 μm). His6-ZapA and FtsZ were diluted and mixed in PEM-KOH buffer, GTP was added to 1 mm (with the exception of the negative control), and reactions were incubated at 30 °C for 5 min. Following incubation, an aliquot of total protein was taken from each reaction and mixed with 5× SDS sample buffer for analysis. Samples were sedimented at 10,000 rpm for 15 min. Previous studies (36, 40) used 80,000 rpm for 10 min to sediment FtsZ-ZapA bundles, and we observed no differences in FtsZ-ZapA sedimentation between these conditions (data not shown). Soluble protein was obtained from the supernatant, whereas pellets were resuspended in an equal volume of PEM-KOH buffer; both were mixed with 5× SDS sample buffer for analysis. All protein samples were boiled in sample buffer and analyzed by SDS-PAGE followed by Coomassie Blue staining using the SimplyBlueTM SafeStain (Invitrogen). We performed densitometry on gels to determine the amounts of soluble and pelleted FtsZ relative to the total protein fractions from each reaction using ImageLabTM Software (Bio-Rad). Pellet fractions were compared with the initial fraction, which was set to 100%. To account for FtsZ pelleting out of solution when not bundled, control reactions were performed containing FtsZ and the ZapA variant alone, without GTP. This amount of pelleting was then subtracted from all other pellet values. Additionally, FtsZ filamented by GTP was pelleted as a control to be sure that unbundled filaments did not pellet out of solution when centrifuged at this speed.
In Vivo Complementation and Immunofluorescence
Chemically competent E. coli ΔzapA cells were made by standard methods. Each pBAD24 vector containing mutated zapA (Table 1) was transformed into these cells, after which a single colony was chosen and grown for 16 h. Cultures were then diluted 1/100 in LB medium and allowed to grow to an A600 of 1.0. Expression was then induced by the addition of l-arabinose to 0.1% (w/v). Cells were adhered to a copper 200 mesh electron microscopy grid (Canemco) and imaged by transmission electron microscopy. For each variant, ∼20 random fields of view were imaged, and >100 cells from two experimental replicates were measured using ImageJ (48). Immunofluorescence was performed as described by Hiraga et al. (49) with minor modifications. Cells were prepared and fixed as described previously. Cells were then blocked with 10% normal goat serum (Invitrogen) for 30 min at room temperature. The blocking solution was replaced with primary antibody (1:100 rabbit anti-FtsZ (Cedarlane) in 1% (w/v) BSA-PBST; PBS with 0.05% (v/v) Tween 20). Slides were incubated for 1 h at room temperature before removing the primary antibody and washing with PBST. Secondary antibody (1:200 goat anti-rabbit conjugated to FITC (Sigma-Adrich) in 1% (w/v) BSA-PBST) was added and incubated for 1 h at room temperature. Secondary antibody was removed, and samples were washed with PBST; samples were counterstained with 4′,6-diamidino-2-phenylindole (10 μg/ml) for 1 min in the dark and washed with PBST. Coverslips were mounted on the slides with SlowFade® Gold antifade reagent (Invitrogen). The slides were imaged using a Leica DM5000B fluorescent microscope equipped with a Hamamatsu ImagEM EM-CCD digital camera (Quorum Technologies). Images were analyzed using Volocity software (version 6.3; PerkinElmer Life Sciences).
Transmission Electron Microscopy (TEM)
For protein filaments and bundles, grids were subjected to 7 s of plasma cleaning in the SolarusTM advanced plasma cleaning system (Gatan). Protein samples were adhered to the grid for 45 s, washed once with PEM-KOH buffer and once with double distilled H2O, then stained with 2% uranyl acetate for 30 s, and allowed to air dry. For whole cell samples, cells were adhered to a copper grid for 10 s, stained with 1% uranyl acetate for 7 s, then washed once with double distilled H2O, and allowed to air dry. Grids were imaged using the FEI Tecnai G2 F20 operated at 120 kV.
RESULTS
X-ray Crystallographic Structure of E. coli ZapA (EcZapA)
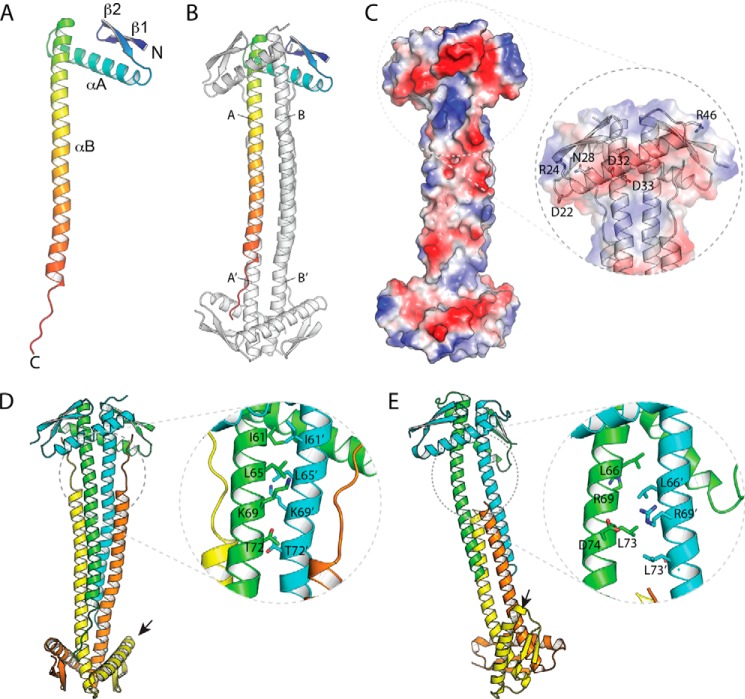
EcZapA was determined to a resolution of 1.95 Å (Fig. 1 and Table 2), with two molecules per asymmetric unit. N-terminal residues 1–3 in chain A are disordered, whereas residues 1 and 46 in chain B are too poorly defined to reliably model. Similar to PaZapA (38), the structure is organized into two distinct domains, with the first 49 residues forming a globular domain comprised of a two stranded antiparallel β-sheet plus an α-helix, whereas the C-terminal half of the protein (residues 50–102) forms a single, 14-turn α-helix. The ZapA protomer is organized into a pseudosymmetric tetramer, where chains A and B interact with their doppelgangers A′ and B′ through a crystallographic 2-fold axis (Fig. 1, B and C). The strongest interaction forms a tight dimer that is stabilized predominantly by interactions mediated by the long C-terminal helix. In EcZapA, the first seven turns of the two helices interact through a coiled-coil type packing arrangement, where the two helices wrap around one another to form a left-handed superhelix; the two helices therefore cross at a 20° angle, and equivalent residues pack against one another laterally. Interactions are also mediated by the N-terminal domains, which pack against both helices so that both the α-helix and β-strands pack onto the second protomer. Note that the two chains of EcZapA differ significantly, with an r.m.s.d. of 2.0 Å; this difference is mostly attributable to significant differences in the twist of the C-terminal helices of the respective chains required to optimize these interactions. Overall, the tight dimer interface (A to B) buries 1670 Å2 per protomer (Fig. 1B). The distal ends of the C-terminal helices splay apart from their dimeric mates, packing instead against the other dimer in a four-helix bundle-like arrangement. Residues 103–109, C-terminal to this helix, are also all ordered. They pack in an extended conformation against the C-terminal helix from a related protomer, with the last two residues of the protein interacting with the N-terminal domain of the opposite dimer. The tetramer is stabilized by burying ∼1500 Å2 per protomer between A and A′ (and also B and B′) and a further 920 Å2 between symmetry-related copies of chains A and B′ (as well as B and A′). PISA predicts that the dissociation energy of the tetramer (−54.0 kcal/mol) is larger than the combined dissociation energy of the respective dimers (−28.6 kcal/mol).
FIGURE 1.
X-ray crystallographic structure of E. coli ZapA. A, the EcZapA protomer is organized as a compact N-terminal domain, with an extended C-terminal tail that is built as a single, elongated α-helix. B, the EcZapA tetramer is comprised of two protomers that form a tight dimer, mediated mostly by coiled-coil-like interactions between the long αB helix. This dimer interacts through the C-terminal end of this α-helix to form a four-helix bundle. Chain identities referred to in the text are marked. C, the electrostatic surface of the EcZapA tetramer highlights the N-terminal α-helix, αA, which forms a prominent ridge of electronegative residues. The inset shows the cartoon representation, as well as residues chosen for more detailed studies. D and E, details in the packing of the EcZapA tetramer (D) versus packing in the PaZapA tetramer (E). Note that the two structures were oriented by superimposing the upper dimer of the structure. Although the structures do bear an overall resemblance, they differ in numerous details. In particular, the interactions between protomers of the tight dimer are very different, with EcZapA using a coiled-coil interaction, whereas PaZapA has a ladder of hydrophobic residues that interdigitate. In the latter case, this results in the two protomers of the dimer having a noticeable vertical offset from one another. The N-terminal domains also differ markedly in their orientation relative to the C-terminal helices. In EcZapA, the N-terminal domains both sit at a similar angle across the two C-terminal helices. In PaZapA, one N-terminal domain in each dimer shows a marked shift and interacts with αB at an acute angle. Differences in packing of the helical domains also result in the N-terminal domains at opposite ends of the dimer aligning to the same face in EcZapA but rotated almost 90° around the long axis in PaZapA (small arrows).
TABLE 2.
Data collection, model refinement, and final structure statistics for EcZapA
| Space group | P6122 |
| Cell dimensions | |
| a = b | 54.10 Å |
| c | 328.07 Å |
| Wavelength (Å) | 0.97949 |
| Resolution (Å) | 50-1.95 |
| Total observations | 200,730 |
| Unique observations | 22,080 |
| Completeness (last shell)a | 0.999 (0.999) |
| <I/σ(I)> (last shell)a | 19.4 (2.7) |
| Rsym (last shell)a | 0.067 (0.84) |
| Asymmetric unit contents | |
| Protein chains | 2 |
| Water molecules | 118 |
| Other molecules | 1 Cl− |
| Average B-factor (Å2) | |
| Protein | 51.8 |
| Water | 45.8 |
| Rcryst | 0.224 |
| Rfreeb | 0.27 |
| r.m.s.d. bond lengths (Å) | 0.006 |
| r.m.s.d. bond angles (°) | 0.996 |
| Ramachandran plotc | |
| Favored (%) | 98.6 |
| Disallowed (%) | 0.5 |
a The last shell includes all reflections between 2.00 and 1.95 Å.
b Rfree calculated using 5% of the data, which were chosen randomly.
c Calculated using Molprobity.
Despite an overall resemblance to the PaZapA structure, the EcZapA structure differs in many important aspects (Fig. 1, D versus E). The C-terminal helix of PaZapA exhibits much less extensive interaction between tetramers than EcZapA (five turns of helix versus nine). This may have functional implications, because the PaZapA tetramer seems capable of dissociating into a dimer. In addition, the C-terminal residues in PaZapA are disordered, and so do not form the extended interactions seen in EcZapA. The interactions between protomers within the dimer are also fundamentally different in PaZapA. In the PaZapA structure, the C-terminal helices pack in parallel, but with the helices offset by one turn, so that each residue packs above the equivalent one (Fig. 1E). The offset of the two C-terminal helices presents two different interaction surfaces to the N-terminal domain, which then packs into two clearly distinct conformations differing by a large (∼40°) rotation. Together, these differences in protomer orientation result in dimers that are structurally different from those in the EcZapA structure (Fig. 1D) and whose protomers superpose with an r.m.s.d. of 5.7 Å (a difference more typically seen between two proteins that are almost at the limits of detectable relatedness). Note that this asymmetry is seen in two independent structures (Protein Data Bank codes 1T3U and 1W2E) in different crystal forms (38) and are highly unlikely to represent an artifact of crystal packing.
ZapA Variants Show Altered FtsZ Bundling Capability in Vitro
Galli and Gerdes (36) reported that the N-terminal domain of E. coli ZapA alone was capable of interacting with FtsZ, albeit weakly (36). Based on their bacterial two-hybrid assay results, we mutated six residues of ZapA (Fig. 1C, inset, and Table 1) to assess their roles in ZapA interactions with FtsZ filaments.
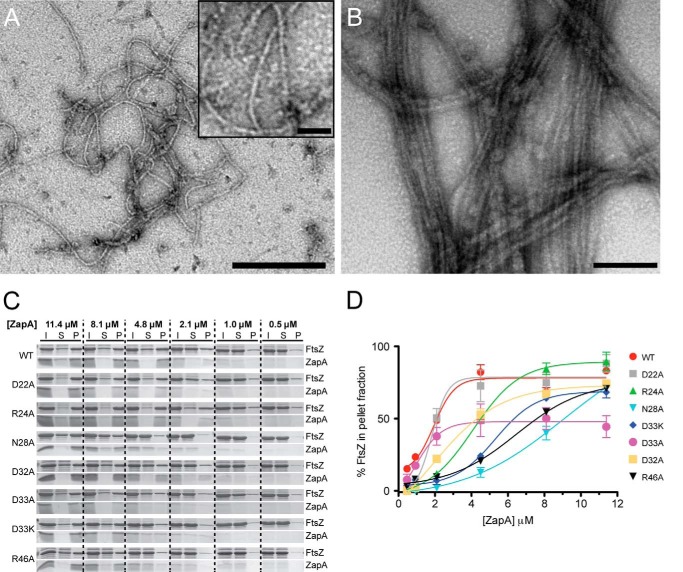
ZapA is proposed to bundle FtsZ by increasing the lateral interactions between filaments (14, 36, 38, 50, 51). This bundling interaction can be studied in vitro using a sedimentation assay. In the presence of ZapA, FtsZ filaments come out of solution, whereas in the absence of ZapA, they remain soluble (36, 40). This approach was used to titrate the sedimentation of FtsZ filaments using WT ZapA and ZapA variants generated by site-directed mutagenesis (Table 1) along the charged N-terminal α-helix (Fig. 1C, inset). First, FtsZ was allowed to form filaments in the absence of ZapA, as a control, to ensure proper filament formation (Fig. 2A). These FtsZ filaments resemble those described previously (3, 51) and are single filaments ∼5 nm in width (n = 100). Next, ZapA variants were mixed at subequimolar (0.5, 1.0, and 2.1 μm), equimolar (4.8 μm; Fig. 2B) (46), and saturated concentrations (8.1 μm and 11.4 μm) with a constant concentration of FtsZ, and filament sedimentation was monitored by SDS-PAGE (Fig. 2C). The sedimentation assays presented in this study were conducted at pH 6.5 as previously described (14, 36, 37, 40, 50, 51). However, FtsZ-ZapA bundles have also been reported at the more physiologically relevant pH of 7.5 (50). For each reaction, the initial ZapA-FtsZ mixture was incubated for 2 min and centrifuged to separate the soluble and pellet fractions (Fig. 2C). These experiments were performed in triplicate, and the pelleted fractions were analyzed by densitometry (Fig. 2D). For WT ZapA, filamentous FtsZ sedimentation increases with increasing ZapA concentration until equimolar concentrations (Fig. 2C). At 0.5 μm WT ZapA sediments ∼16% of FtsZ filaments in solution compared with ∼86% at the physiologically relevant equimolar concentration of 4.8 μm (46). When saturated with ZapA (11.4 μm), ∼87% of FtsZ filaments are found in the pellet; this indicates that, at equimolar concentrations, WT ZapA can sufficiently sediment the majority of FtsZ filaments. As residues along the charged helix are altered (Fig. 1C, inset), clear differences emerge in the ability of the ZapA variants to pellet FtsZ, most notably at equimolar and saturated concentrations (Fig. 2C). For example, at equimolar concentrations N28A shows a 73% decrease in FtsZ sedimentation compared with WT ZapA (Fig. 2C). We also generated a D40A ZapA variant, but this construct did not express in E. coli cells, and we were not able to purify it. This result is similar to what Pacheco-Gómez et al. (40) reported with mutational analysis of residue 41 of E. coli ZapA, indicating that these residues may be critical for ZapA expression and proper protein folding in vivo.
FIGURE 2.
FtsZ sedimentation assay and the percent of FtsZ in the pelleted fractions. A, purified FtsZ filamented with 1 mm GTP in the absence of ZapA (bar, 250 nm; inset bar, 25 nm). B, FtsZ filaments bundled with purified ZapA (4.8 μm; bar, 100 nm). C, FtsZ (4.8 μm) filamented with 1 mm GTP in the presence of WT ZapA or ZapA variants (0.5–11.4 μm). Initial samples (I) were used to assess the total protein content in each reaction. Soluble (S) and pelleted (P) fractions were separated by centrifugation at 10,000 rpm for 15 min, and all three samples (I, S, and P) were analyzed by Coomassie-stained SDS-PAGE. Pelleted fractions contain FtsZ filaments that were bundled with ZapA. D, triplicate FtsZ sedimentation assays were performed for each ZapA variant, and Coomassie-stained SDS-PAGE results were analyzed by densitometry. The mean percentages of FtsZ in the pelleted fractions were plotted for each concentration of ZapA, where the error bars indicate ± S.E. (standard error of the mean). Reactions with equimolar ZapA (4.8 μm) and FtsZ (4.8 μm), lacking GTP, were run as a control for protein precipitation (not shown), and these values were subtracted from the sedimentation of each reaction.
To better visualize the differences observed in the sedimentation assays, we plotted the densitometric data as ZapA concentration versus the proportion of FtsZ filaments sedimented (Fig. 2D). The sedimentation profile of the D22A ZapA variant closely resembles WT ZapA. However, clear differences are seen in the ability of the other ZapA variants to bundle and sediment FtsZ filaments (Fig. 2D). R24A ZapA exhibits a clear shift when compared with WT ZapA, indicating that a higher concentration of this variant is required to initiate FtsZ sedimentation. D33A ZapA reaches maximal FtsZ filament pelleting at equimolar concentrations, similar to WT ZapA; however, the maximum proportion of FtsZ pelleted here is much lower than for WT. In contrast, ZapA variants N28A, D32A, D33K, and R46A show differences in both the amount of ZapA protein required to initiate sedimentation of FtsZ filaments and maximal FtsZ filament sedimentation. The most pronounced differences compared with WT are seen with D33A and N28A ZapA variants. ZapA D33A reveals the lowest FtsZ sedimentation at the saturated concentrations, and N28A ZapA shows the lowest FtsZ sedimentation at equimolar concentrations.
Based on the data from the sedimentation assays (Fig. 2D), EC50 values were calculated that correspond to the concentration of ZapA needed to sediment 50% of FtsZ filaments in each reaction (Table 3). An increase in EC50 signifies that more variant ZapA is required to effectively sediment FtsZ compared with WT and therefore suggests these ZapA variants bundle FtsZ filaments less efficiently. For WT ZapA and ZapA D22A, similar EC50 values of 2.22 and 2.67 μm were determined, respectively. Higher EC50 values than WT were obtained for ZapA variants R24A, N28A, D32A, D33A, D33K, and R46A (Table 3).
TABLE 3.
Summary of interactions for ZapA variants with FtsZ filaments
| ZapA variant | EC50a | Max Sed.b | Filaments/bundlec | Cell lengthd |
|---|---|---|---|---|
| μm | μm | |||
| WT | 2.22 | 0.87 | 9.86 ± 0.76 | 2.65 ± 0.06 |
| D22A | 2.67 | 0.93 | 7.33 ± 0.49 | 2.81 ± 0.08 |
| R24A | 4.48 | 0.94 | 7.13 ± 0.76 | 2.96 ± 0.15 |
| N28A | 8.77 | 0.75 | 5.21 ± 0.19 | 3.77 ± 0.25 |
| D32A | 4.86 | 0.71 | 5.73 ± 0.29 | 3.74 ± 0.17 |
| D33A | 8.74 | 0.53 | 5.75 ± 0.38 | 3.66 ± 0.17 |
| D33K | 7.05 | 0.78 | 5.46 ± 0.29 | 3.70 ± 0.10 |
| R46A | 7.61 | 0.74 | NAe | 3.87 ± 0.20 |
a EC50, concentration of ZapA needed to sediment 50% of FtsZ in each reaction.
b Max Sed., maximum proportion of FtsZ pelleted by each ZapA variant in the sedimentation assays.
c Determined from transmission electron micrographs (means ± S.E.).
d Values were measured from transmission electron micrographs (means ± S.E.). The mean cell length of ΔzapA E. coli is 3.52 μm.
e NA, data not available because no bundles were observed in transmission electron micrographs.
ZapA Variants Demonstrate Reduced Bundling of FtsZ Filaments
To directly assess the effect of ZapA variants on FtsZ filament bundling, ZapA-FtsZ complexes were visualized by TEM (Fig. 3A). Visualization of bundling was performed at equimolar concentrations of ZapA and FtsZ, because this concentration revealed the greatest difference in FtsZ sedimentation between ZapA variants (Fig. 2D) and is also the most physiologically relevant (50). From these micrographs, the number of filaments within each bundle was determined (Fig. 3B and Table 3). A decrease in the number of FtsZ filaments per bundle would indicate a decreased ability of ZapA to bundle FtsZ. At equimolar concentrations (4.8 μm) WT ZapA-FtsZ filaments appear as long twisted bundles of laterally associated FtsZ filaments (Fig. 3A; WT), matching those described previously (14, 34, 46, 47) with an average of ∼10 filaments per bundle (Fig. 3B and Table 3). FtsZ filaments bundled by ZapA variants D22A and R24A appeared less organized than those bundled by WT ZapA and demonstrated an average of ∼7 filaments per bundle (Fig. 3B and Table 3). It should also be noted that bundles formed in the presence of R24A ZapA appeared to have fewer lateral associations and were less ordered (Fig. 3A). Electron micrographs also showed that ZapA N28A formed short twisted bundles of FtsZ filaments when compared with WT ZapA (Fig. 3A), whereas ZapA variants D32A, D33A, and D33K produced long, twisted bundles of FtsZ filaments similar to those formed by WT ZapA, although they still contained fewer (∼5) FtsZ filaments per bundle (Fig. 3B and Table 3). Surprisingly, when sedimentation reactions containing ZapA variant R46A were imaged, no bundled FtsZ filaments, and very few nonbundled FtsZ filaments, were observed (Fig. 3A). This result was reproducible over six replicate experiments (data not shown). Based on the sedimentation profile of ZapA R46A, which is similar to that of WT ZapA (Fig. 2D), this suggests that the R46A variant may affect FtsZ polymer assembly or disassembly in vitro.
FIGURE 3.
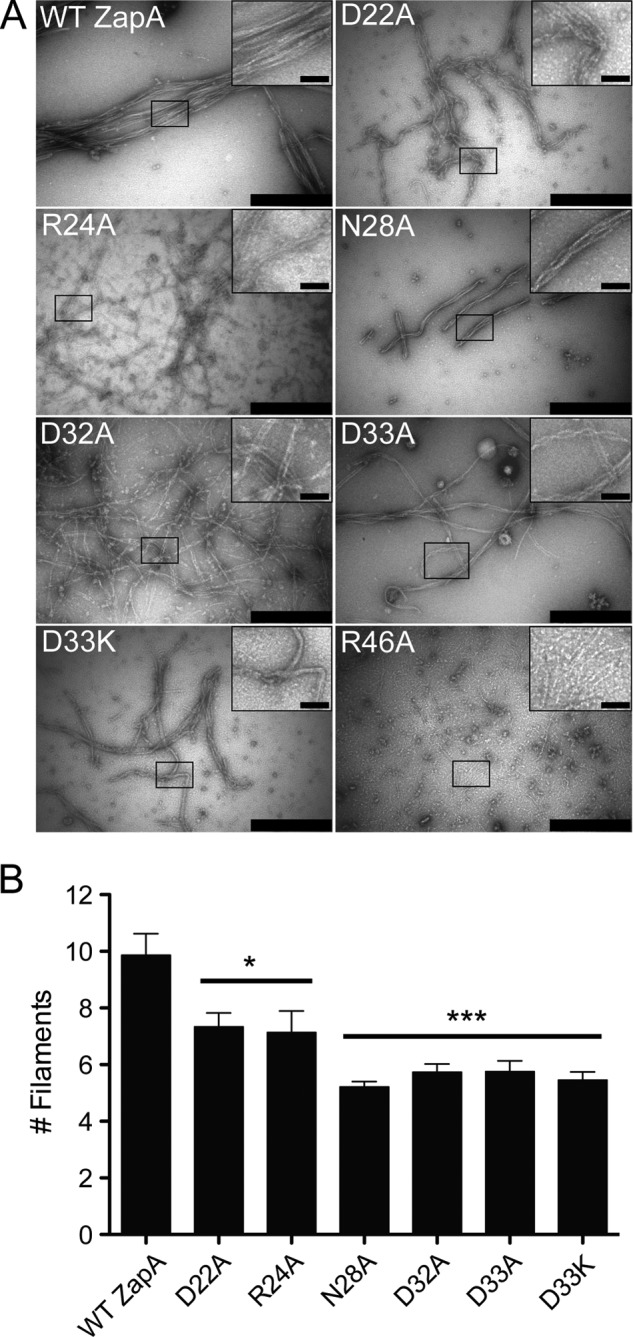
Transmission electron microscopy reveals differential bundling of FtsZ filaments by ZapA variants. A, equimolar concentrations of ZapA and FtsZ. FtsZ was filamented with 1 mm GTP and visualized by TEM (bar, 500 nm; inset bar, 50 nm). B, TEM images were analyzed, and the mean numbers of filaments per bundle ± S.E. were plotted for each ZapA variant (n > 50 bundles per ZapA variant); two-tailed t tests were used to compare ZapA variants to WT. *, p < 0.05; ***, p < 0.0001.
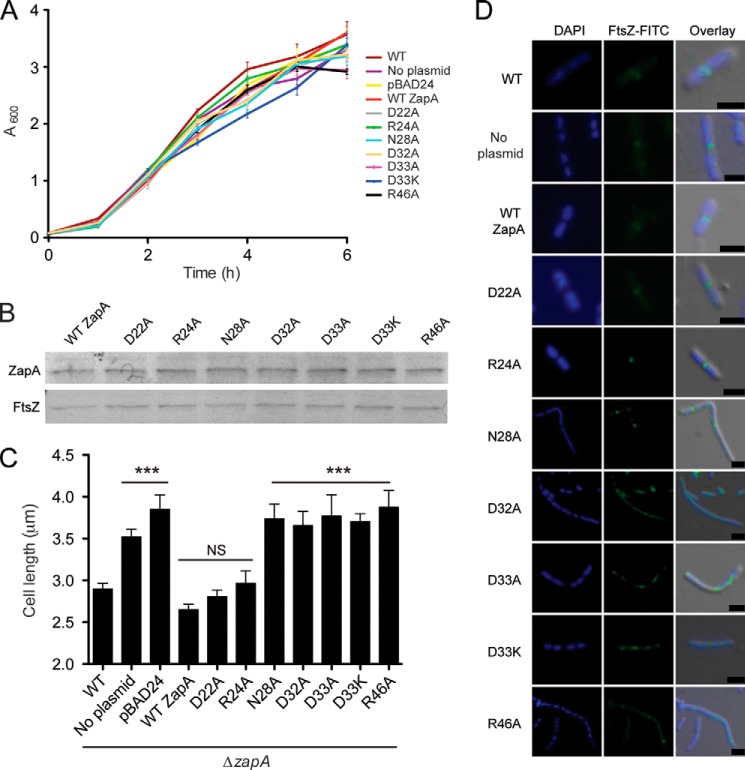
ZapA Variants That Demonstrate Reduced FtsZ Filament Bundling Are Unable to Generate Normal Cell Length in Vivo
To investigate the effect of the ZapA variants in vivo, a ZapA-deleted strain of E. coli (ΔzapA) was constructed. WT ZapA and ZapA variants were expressed in ΔzapA E. coli. No significant differences in growth were observed between any of these strains (Fig. 4A). Western blot analysis using an anti-His antibody confirmed that the His-tagged WT and ZapA variants were expressed at similar levels (Fig. 4B). The ΔzapA E. coli strain exhibited an elongated cell morphology as previously described (30), with a mean cell length of 3.52 μm (Fig. 4C). The WT ZapA variant restored a normal cell size with a mean cell length of 2.65 μm. As with the in vitro results, ZapA variants D22A and R24A restored cell length to that of WT ZapA, with mean cell lengths of 2.81 and 2.96 μm, respectively. However, ZapA variants N28A, D32A, D33A, D33K, and R46A did not restore ΔzapA cells to their normal length (Fig. 4C and Table 3). These results indicate that residues Asp22 and Arg24 are not critical for FtsZ bundling in vivo, whereas Asn28, Asp32, Asp33, and Arg46 are needed to maintain the bundling function of ZapA.
FIGURE 4.
Complementation of ΔzapA with WT ZapA and ZapA variants. E. coli ΔzapA cells were transformed with pBAD24 carrying zapA variants. A, growth was monitored over 6 h, and no significant differences were observed in cell density. B, Western blot analysis using an anti-His antibody confirmed that ZapA variants were expressed to the same levels during the complementation assays in ΔzapA cells (top panel) and Western blot analysis using an anti-FtsZ antibody (bottom panel) was performed as a loading control for comparison. C, cells were visualized by TEM and measured (n > 100 cells per E. coli strain). Mean cell length values ± S.E. were plotted and analyzed by two-tailed t tests as compared with WT cell lengths. NS, no significant difference; ***, p < 0.001. No significant differences in cell length were observed between WT cells and WT ZapA complemented cells and between ΔzapA and pBAD24 vector control cells. D, immunofluorescence was performed to detect differences in FtsZ localization and Z-ring formation among WT cells and ΔzapA cells expressing WT ZapA and ZapA variants. Scale, 1 μm.
The ability of this His-tagged ZapA protein to complement functionality in vivo is in contrast to a report by Mohammadi et al. (50) in which the addition of a His tag rendered ZapA nonfunctional in vivo. One notable difference between this construct and the one described previously is the cleavage site between the His tag and ZapA. The factor XA cleavage site used in this study contains fewer charged amino acids than the enterokinase cleavage site used by Mohammadi et al. (two versus five, respectively) (50). This could account for the differences seen in the in vivo and in vitro analyses of these proteins.
Immunofluorescence was also performed on these cells to monitor Z-ring formation and nucleoid segregation in ΔzapA cells carrying ZapA variants (Fig. 4D). WT E. coli cells divide normally, presenting a single condensed mid-cell localization of FtsZ and two segregated nucleoids destined for each of the daughter cells (Fig. 4D, top row). The ΔzapA cells differ from WT E. coli in that elongated cells show multiple localizations of FtsZ and multiple nucleoids. When ΔzapA cells were complemented with WT ZapA and ZapA variants, D22A and R24A closely resemble WT E. coli. These variants show normal Z-rings and efficient cell division, indicated by a maximum of two nucleoids in each dividing cell. However, when ΔzapA cells were complemented with ZapA variants N28A, D32A, D33A, D33K, and R46A, elongated E. coli cells show multiple Z-rings and multiple nucleoids. Additionally, Z-rings in these cells often appeared diffuse, perhaps indicating less FtsZ bundling in vivo. Alternately, the diffuse localization pattern of FtsZ may be the result of an inability of these ZapA variants to properly align FtsZ at mid-cell via interactions with ZapB (30). Although these results are consistent with inefficient cell division, the phenotype is resolved by the late exponential phase, suggesting a delay in bacterial cell division as seen previously by Galli and Gerdes (37).
DISCUSSION
ZapA is proposed to stabilize FtsZ filaments, aiding in Z-ring formation and/or positioning prior to bacterial cell division (30, 36, 38–40, 50). Although ZapA alone is not essential for cell division, it is hypothesized that collectively ZapA, ZapB, ZapC, and ZapD are required for FtsZ filament bundling and dynamics in E. coli (14–18, 20). Although the ZapA protein is widely conserved across bacterial species, it is not required for the lateral association of FtsZ filaments in some organisms (51). In E. coli and Bacillus subtilis, ZapA has been shown to be important for FtsZ bundling in vitro (27), and the absence of ZapA results in an elongated cell phenotype and abnormal septum formation in E. coli cells (14, 30).
Although nearly a decade has passed since the crystallographic structure of ZapA from P. aeruginosa was determined and its role in FtsZ bundling was proposed (38), questions remain about the specificity of ZapA-FtsZ interactions and the implication of these interactions on Z-ring architecture and dynamics. The structure of EcZapA should prove helpful in investigating these questions, given that E. coli has become the most common model for these studies. Although PaZapA has been used to probe the function and physiological role of ZapA in E. coli (40, 50), the details of the structure of EcZapA differ enough to impact the design and interpretation of ZapA structure-function studies. For example, a recent study by Pacheco-Gómez et al. (40) based their mutational analysis of the tetramerization domain of ZapA on the PaZapA structure, with the hypothesis that ZapA dimer/tetramer equilibrium is important for proper FtsZ bundling. Although they succeeded in making a properly folded ZapA dimer, Ile83 packs in a very different fashion in EcZapA than the equivalent residue in PaZapA, meaning that detailed predictions of the consequences of altering this residue are likely to be inaccurate. Interestingly, the I83E dimer variant of EcZapA interacted with FtsZ but did not form FtsZ bundled filaments in vitro (40). Given (i) the extensive stabilizing interactions within the EcZapA tetramer (Fig. 1D) and (ii) the predominant tetrameric configuration reported for WT ZapA by sedimentation velocity experiments (40), it is unlikely that native EcZapA dimers are present in sufficient levels to bundle FtsZ, as suggested for PaZapA (38). Therefore, EcZapA likely functions as a tetramer. If the N-terminal domains at both ends of the tetramer need to engage with FtsZ filaments to bundle them, the large observed differences in the orientation of these domains (Fig. 1, D and E) suggest differences in FtsZ bundling between these two species.
Here, the structure of EcZapA (Fig. 1) was used to better understand the role of ZapA in bundling and stabilizing FtsZ prior to bacterial cell division. Galli and Gerdes (36) recently showed that a 26-amino acid region of ZapA from E. coli was involved in ZapA-FtsZ interactions. In EcZapA, this region forms a charged α-helix (Fig. 1, A and C). Because this globular head of ZapA is not well conserved compared with the C-terminal tetramerization domain, and ZapA-FtsZ interactions vary in importance and extent between species (27), it is possible that this region may be critical for ZapA-FtsZ interactions in E. coli. Site-directed mutagenesis was used to create point mutations to alter specific amino acids along this charged α-helix. The functional implications of these point mutations at each residue are described in Fig. 5. ZapA variants at residues Asp22 and Arg24 (Fig. 5, green) show only minor deviations from WT ZapA-FtsZ interactions. ZapA R24A does not cause a significant change in FtsZ sedimentation at saturating concentrations (Fig. 2D), and this ZapA variant complemented the ΔzapA strain with the same efficiency as WT ZapA (Fig. 4). These results suggest that ZapA R24A interacts with FtsZ filaments. However, upon visualizing FtsZ filaments bundled by ZapA R24A, it became clear that the ZapA-FtsZ interactions were somewhat altered, because bundles appear as disorganized tangles of filaments as opposed to the laterally organized structures seen with WT ZapA (compare Fig. 3A WT versus R24A). Because the R24A ZapA variant appears to interact with FtsZ in vitro and in vivo, there are two possible explanations for this difference. First, ZapA R24A may have an altered functionality made possible by the removal of a charged residue involved in electrostatic interactions with FtsZ. Second, it is possible the full complementation of the ΔzapA phenotype in vivo is a result of the overlapping function of Zap proteins in E. coli (51).
FIGURE 5.
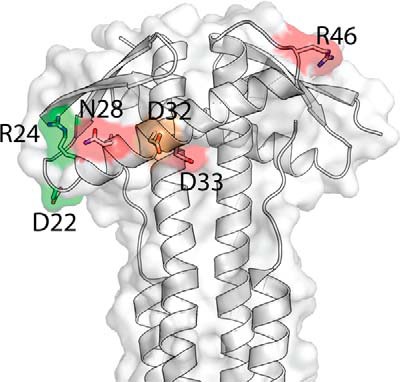
Mutant phenotypes mapped onto the EcZapA structure. Orientation as in Fig. 1C, but here colored according to the effect of point mutations at each residue. Residues in green have a relatively weak (though measurable) effect on the EC50 (EC50 < 5 μm), the number of filaments per bundle (>7) and the cell length (<3 μm). Residues highlighted in red, in contrast, have strong effects on the EC50 (EC50 > 5 μm), the number of filaments per bundle (<7), and the cell length (>3 μm). The residue highlighted in orange has a weak affect on the EC50 (EC50 <5) but nevertheless strongly affects both the number of filaments per bundle (<7) and the cell length (>3 μm).
ZapA variants with point mutations in the charged α-helix region at residues Asn28, Asp33, and Arg46 (Fig. 5, red) required much more ZapA to effectively bundle FtsZ filaments (Fig. 2D), demonstrated fewer FtsZ filaments per bundle (Fig. 3B and Table 3), and increased cell length in complementation assays (Fig. 4 and Table 3). In sedimentation assays, all of these ZapA variants demonstrated a significant decrease in FtsZ sedimentation at equimolar (p < 0.02) and saturated (p < 0.05) concentrations (Fig. 2C), and we linked this decrease in sedimentation to fewer FtsZ filaments per bundle (Fig. 3). It is also worth noting that these bundles appear to have adopted an inherent twisted, helical-like conformation, especially when observed as bundles of four FtsZ filaments. It cannot be determined whether this shift in conformation is a result of altered ZapA binding or whether it is a conformation typically seen with lower order FtsZ bundles. For ZapA variant R46A, we note that in the sedimentation assay FtsZ sediments significantly when ZapA is present at a saturating concentration (11.4 μm) (Fig. 2C). This contradicts the TEM results, where no significant bundling was observed (Fig. 3A). We hypothesize that this is due to a loss of bundling functionality, where ZapA may still be binding FtsZ filaments but in an altered way that does not allow the formation of organized, regularly spaced bundles. This may have functional implications for residue Arg46 in the coordination of ZapA in the FtsZ-ZapA interaction.
Interestingly, the D32A ZapA variant (Fig. 5, orange) presented only a weak effect on the measured EC50 values but strongly affected both the number of FtsZ filaments per bundle and the complemented cell length, suggesting that this residue may be important for enhancing Z-ring stability. Together, our results strongly suggest that the surface-exposed, charged residues on the EcZapA N-terminal α-helix mediate ZapA-FtsZ interactions to facilitate FtsZ filament bundling and Z-ring stability in dividing bacterial cells. Although this area of ZapA had been previously implicated in facilitating the ZapA-FtsZ interaction, this is the first report describing the structure of EcZapA and the effects of altering specific residues in this region on the interaction with FtsZ.
Acknowledgments
We thank Chris Whitfield and Deborah Stewart Khursigara for critical reading of the manuscript and editorial assistance. X-ray data for EcZapA was collected at Canadian Light Source by Shaun Labiuk.
This work was funded by Natural Sciences and Engineering Research Council of Canada Discovery Grants 371639 (to C. M. K.) and 327280 (to M. S. K.).
The atomic coordinates and structure factors (code 4P1M) have been deposited in the Protein Data Bank (http://wwpdb.org/).
- EcZapA
- E. coli ZapA
- PaZapA
- P. aeruginosa ZapA
- r.m.s.d.
- root mean square deviation
- TEM
- transmission electron microscopy.
REFERENCES
- 1. Ward J. E., Jr., Lutkenhaus J. (1985) Overproduction of FtsZ induces minicell formation in E. coli. Cell 42, 941–949 [DOI] [PubMed] [Google Scholar]
- 2. Bi E. F., Lutkenhaus J. (1991) FtsZ ring structure associated with division in Escherichia coli. Nature 354, 161–164 [DOI] [PubMed] [Google Scholar]
- 3. Mukherjee A., Dai K., Lutkenhaus J. (1993) Escherichia coli cell division protein FtsZ is a guanine nucleotide binding protein. Proc. Natl. Acad. Sci. U.S.A. 90, 1053–1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vicente M., Errington J. (1996) Structure, function and controls in microbial division. Mol. Microbiol. 20, 1–7 [DOI] [PubMed] [Google Scholar]
- 5. Bramhill D., Thompson C. M. (1994) GTP-dependent polymerization of Escherichia coli FtsZ protein to form tubules. Proc. Natl. Acad. Sci. U.S.A. 91, 5813–5817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mukherjee A., Lutkenhaus J. (1998) Dynamic assembly of FtsZ regulated by GTP hydrolysis. EMBO J. 17, 462–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Buddelmeijer N., Beckwith J. (2002) Assembly of cell division proteins at the E. coli cell center. Curr. Opin. Microbiol. 5, 553–557 [DOI] [PubMed] [Google Scholar]
- 8. Goehring N. W., Beckwith J. (2005) Diverse paths to midcell: assembly of the bacterial cell division machinery. Curr. Biol. 15, R514–R526 [DOI] [PubMed] [Google Scholar]
- 9. Natale P., Pazos M., Vicente M. (2013) The Escherichia coli divisome: born to divide. Environ. Microbiol. 15, 3169–3182 [DOI] [PubMed] [Google Scholar]
- 10. Wang X., Possoz C., Sherratt D. J. (2005) Dancing around the divisome: asymmetric chromosome segregation in Escherichia coli. Genes Dev. 19, 2367–2377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Aarsman M. E., Piette A., Fraipont C., Vinkenvleugel T. M., Nguyen-Distèche M., den Blaauwen T. (2005) Maturation of the Escherichia coli divisome occurs in two steps. Mol. Microbiol. 55, 1631–1645 [DOI] [PubMed] [Google Scholar]
- 12. de Boer P. A. (2010) Advances in understanding E. coli cell fission. Curr. Opin. Microbiol. 13, 730–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vicente M., Rico A. I. (2006) The order of the ring: assembly of Escherichia coli cell division components. Mol. Microbiol. 61, 5–8 [DOI] [PubMed] [Google Scholar]
- 14. Gueiros-Filho F. J., Losick R. (2002) A widely conserved bacterial cell division protein that promotes assembly of the tubulin-like protein FtsZ. Genes Dev. 16, 2544–2556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ebersbach G., Galli E., Møller-Jensen J., Löwe J., Gerdes K. (2008) Novel coiled-coil cell division factor ZapB stimulates Z ring assembly and cell division. Mol. Microbiol. 68, 720–735 [DOI] [PubMed] [Google Scholar]
- 16. Hale C. A., Shiomi D., Liu B., Bernhardt T. G., Margolin W., Niki H., de Boer P. A. (2011) Identification of Escherichia coli ZapC (YcbW) as a component of the division apparatus that binds and bundles FtsZ polymers. J. Bacteriol. 193, 1393–1404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Durand-Heredia J. M., Yu H. H., De Carlo S., Lesser C. F., Janakiraman A. (2011) Identification and characterization of ZapC, a stabilizer of the FtsZ ring in Escherichia coli. J. Bacteriol. 193, 1405–1413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Durand-Heredia J., Rivkin E., Fan G., Morales J., Janakiraman A. (2012) Identification of ZapD as a cell division factor that promotes the assembly of FtsZ in Escherichia coli. J. Bacteriol. 194, 3189–3198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rico A. I., Krupka M., Vicente M. (2013) In the beginning, Escherichia coli assembled the proto-ring: an initial phase of division. J. Biol. Chem. 288, 20830–20836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Huang K.-H., Durand-Heredia J., Janakiraman A. (2013) FtsZ ring stability: of bundles, tubules, crosslinks, and curves. J. Bacteriol. 195, 1859–1868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Popp D., Iwasa M., Narita A., Erickson H. P., Maéda Y. (2009) FtsZ condensates: an in vitro electron microscopy study. Biopolymers. 91, 340–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Osawa M., Anderson D. E., Erickson H. P. (2009) Curved FtsZ protofilaments generate bending forces on liposome membranes. EMBO J. 28, 3476–3484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rivas G., López A., Mingorance J., Ferrándiz M. J., Zorrilla S., Minton A. P., Vicente M., Andreu J. M. (2000) Magnesium-induced linear self-association of the FtsZ bacterial cell division protein monomer: the primary steps for FtsZ assembly. J. Biol. Chem. 275, 11740–11749 [DOI] [PubMed] [Google Scholar]
- 24. Löwe J., Amos L. A. (1999) Tubulin-like protofilaments in Ca2+-induced FtsZ sheets. EMBO J. 18, 2364–2371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Erickson H. P., Taylor D. W., Taylor K. A., Bramhill D. (1996) Bacterial cell division protein FtsZ assembles into protofilament sheets and minirings, structural homologs of tubulin polymers. Proc. Natl. Acad. Sci. U.S.A. 93, 519–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Niu L., Yu J. (2008) Investigating intracellular dynamics of FtsZ cytoskeleton with photoactivation single-molecule tracking. Biophys. J. 95, 2009–2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Anderson D. E., Gueiros-Filho F. J., Erickson H. P. (2004) Assembly dynamics of FtsZ rings in Bacillus subtilis and Escherichia coli and effects of FtsZ-regulating proteins. J. Bacteriol. 186, 5775–5781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Thanedar S., Margolin W. (2004) FtsZ exhibits rapid movement and oscillation waves in helix-like patterns in Escherichia coli. Curr. Biol. 14, 1167–1173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li Z., Trimble M. J., Brun Y. V., Jensen G. J. (2007) The structure of FtsZ filaments in vivo suggests a force-generating role in cell division. EMBO J. 26, 4694–4708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Buss J., Coltharp C., Huang T., Pohlmeyer C., Wang S.-C., Hatem C., Xiao J. (2013) In vivo organization of the FtsZ-ring by ZapA and ZapB revealed by quantitative super-resolution microscopy. Mol. Microbiol. 89, 1099–1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Strauss M. P., Liew A. T., Turnbull L., Whitchurch C. B., Monahan L. G., Harry E. J. (2012) 3D-SIM super resolution microscopy reveals a bead-like arrangement for FtsZ and the division machinery: implications for triggering cytokinesis. PLoS Biol. 10, e1001389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fu G., Huang T., Buss J., Coltharp C., Hensel Z., Xiao J. (2010) In vivo structure of the E. coli FtsZ-ring revealed by photoactivated localization microscopy (PALM). PLoS One 5, e12682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Biteen J. S., Goley E. D., Shapiro L., Moerner W. E. (2012) Three-dimensional super-resolution imaging of the midplane protein FtsZ in live Caulobacter crescentus cells using astigmatism. Chemphyschem. 13, 1007–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Holden S. J., Pengo T., Meibom K. L., Fernandez Fernandez C., Collier J., Manley S. (2014) High throughput 3D super-resolution microscopy reveals Caulobacter crescentus in vivo Z-ring organization. Proc. Natl. Acad. Sci. U.S.A. 111, 4566–4571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Monahan L. G., Robinson A., Harry E. J. (2009) Lateral FtsZ association and the assembly of the cytokinetic Z ring in bacteria. Mol. Microbiol. 74, 1004–1017 [DOI] [PubMed] [Google Scholar]
- 36. Galli E., Gerdes K. (2012) FtsZ-ZapA-ZapB interactome of Escherichia coli. J. Bacteriol. 194, 292–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Galli E., Gerdes K. (2010) Spatial resolution of two bacterial cell division proteins: ZapA recruits ZapB to the inner face of the Z-ring. Mol. Microbiol. 76, 1514–1526 [DOI] [PubMed] [Google Scholar]
- 38. Low H. H., Moncrieffe M. C., Löwe J. (2004) The crystal structure of ZapA and its modulation of FtsZ polymerisation. J. Mol. Biol. 341, 839–852 [DOI] [PubMed] [Google Scholar]
- 39. Small E., Marrington R., Rodger A., Scott D. J., Sloan K., Roper D., Dafforn T. R., Addinall S. G. (2007) FtsZ polymer-bundling by the Escherichia coli ZapA orthologue, YgfE, involves a conformational change in bound GTP. J. Mol. Biol. 369, 210–221 [DOI] [PubMed] [Google Scholar]
- 40. Pacheco-Gómez R., Cheng X., Hicks M. R., Smith C. J., Roper D. I., Addinall S., Rodger A., Dafforn T. R. (2013) Tetramerization of ZapA is required for FtsZ bundling. Biochem. J. 449, 795–802 [DOI] [PubMed] [Google Scholar]
- 41. Larkin M. A., Blackshields G., Brown N. P., Chenna R., McGettigan P. A., McWilliam H., Valentin F., Wallace I. M., Wilm A., Lopez R., Thompson J. D., Gibson T. J., Higgins D. G. (2007) Clustal W and Clustal X version 2.0. Bioinformatics. 23, 2947–2948 [DOI] [PubMed] [Google Scholar]
- 42. Datsenko K. A., Wanner B. L. (2000) One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. U.S.A. 97, 6640–6645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Datta S., Costantino N., Court D. L. (2006) A set of recombineering plasmids for gram-negative bacteria. Gene. 379, 109–115 [DOI] [PubMed] [Google Scholar]
- 44. Kabsch W. (2010) XDS. Acta Crystallogr. D Biol. Crystallogr. 66, 125–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. McCoy A. J., Grosse-Kunstleve R. W., Adams P. D., Winn M. D., Storoni L. C., Read R. J. (2007) Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Adams P. D., Grosse-Kunstleve R. W., Hung L.-W., Ioerger T. R., McCoy A. J., Moriarty N. W., Read R. J., Sacchettini J. C., Sauter N. K., Terwilliger T. C. (2002) PHENIX: building new software for automated crystallographic structure determination. Acta Crystallogr. D Biol. Crystallogr. 58, 1948–1954 [DOI] [PubMed] [Google Scholar]
- 47. Emsley P., Cowtan K. (2004) Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 [DOI] [PubMed] [Google Scholar]
- 48. Schneider C. A., Rasband W. S., Eliceiri K. W. (2012) NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hiraga S., Ichinose C., Niki H., Yamazoe M. (1998) Cell cycle-dependent duplication and bidirectional migration of SeqA-associated DNA-protein complexes in E. coli. Mol. Cell. 1, 381–387 [DOI] [PubMed] [Google Scholar]
- 50. Mohammadi T., Ploeger G. E., Verheul J., Comvalius A. D., Martos A., Alfonso C., van Marle J., Rivas G., den Blaauwen T. (2009) The GTPase activity of Escherichia coli FtsZ determines the magnitude of the FtsZ polymer bundling by ZapA in vitro. Biochemistry 48, 11056–11066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Dajkovic A., Pichoff S., Lutkenhaus J., Wirtz D. (2010) Cross-linking FtsZ polymers into coherent Z rings. Mol. Microbiol. 78, 651–668 [DOI] [PubMed] [Google Scholar]