Background: ZnT2 regulates zinc export from mammary cells.
Results: Prolactin stimulates ZnT2 ubiquitination, targeting ZnT2 to vesicles and activating zinc accumulation to transiently enhance zinc secretion dependent upon Lys4/Lys6, after which ZnT2 is degraded.
Conclusion: Prolactin is a critical regulator of transient ZnT2-mediated zinc secretion.
Significance: This work provides insight into post-translational hormonal mechanisms that regulate zinc transport.
Keywords: Mammary Gland, Prolactin, Protein Degradation, Secretion, Ubiquitination, Zinc Transporter, ZnT2
Abstract
The zinc transporter ZnT2 imports zinc into secretory vesicles and regulates zinc export from the mammary epithelial cell. Mutations in ZnT2 substantially impair zinc secretion into milk. The lactogenic hormone prolactin (PRL) transcriptionally increases ZnT2 expression through the Jak2/STAT5 signaling pathway, increasing zinc accumulation in secretory vesicles and zinc secretion. Herein, we report that PRL post-translationally stimulated ZnT2 ubiquitination, which altered ZnT2 trafficking and augmented vesicular zinc accumulation and secretion from mammary epithelial cells in a transient manner. Ubiquitination then down-regulated zinc secretion by stimulating degradation of ZnT2. Mutagenesis of two N-terminal lysine residues (K4R and K6R) inhibited ZnT2 ubiquitination, vesicular zinc accumulation and secretion, and protein degradation. These findings establish that PRL post-translationally regulates ZnT2-mediated zinc secretion in a multifactorial manner, first by enhancing zinc accumulation in vesicles to transiently enhance zinc secretion and then by activating ubiquitin-dependent ZnT2 degradation. This provides insight into novel mechanisms through which ZnT2 and zinc transport is tightly regulated in mammary epithelial cells.
Introduction
The zinc transporter ZnT2 (zinc transporter 2) is expressed in mammary epithelial cells (MECs)2 and is critical for regulating zinc secretion from the mammary gland into milk. The importance of ZnT2 in this process is evidenced by four different mutations in the gene that encodes ZnT2 (SLC30A2) that result in ∼75% less zinc being secreted into milk in lactating women (1–3), resulting in severe zinc deficiency in their nursing infants. ZnT2 is a zinc transporter that imports zinc into secretory vesicles (4, 5). Our previous studies determined that the lactogenic hormone prolactin (PRL) regulates ZnT2. PRL is a polypeptide hormone that regulates mammary gland differentiation (6), the maintenance of a secretory phenotype (7, 8), and acute secretory mechanisms (9, 10). PRL signaling occurs in response to binding to its cognate receptor (PRLr) on the MEC to stimulate various signaling cascades, including Jak2/STAT5 (11, 12), MAPK (13), PI3K/PKB (14), and ERK1/2 (15). PRL-mediated activation of the Jak2/STAT5 signaling pathway transcriptionally increases ZnT2 expression (16). In addition, PRL stimulates zinc accumulation into vesicles to efflux zinc (17). However, the mechanisms through which PRL regulates ZnT2-mediated zinc accumulation and secretion are not well understood.
PRL stimulates ubiquitination (18). Modification of receptors and transporters by ubiquitin is emerging as an important regulatory mechanism in response to extracellular signals and changing nutrient availability. Recent evidence illustrates that ubiquitin serves as a sorting signal to regulate protein trafficking through the secretory compartment (reviewed in Ref. 19) and is a major regulatory molecule in vesicle-mediated transport in neurons (20). More commonly, ubiquitination targets many proteins for proteosomal degradation (21). Specifically, ubiquitin-dependent internalization and degradation of nutrient transporters, such as GAP1 (amino acid transport), FUR4 (uracil transport), MAL6 (maltose transport), and GAL2 (galactose transport) (22), have been identified in yeast. Ubiquitination and degradation of the zinc transporters ZRT1 (23, 24) and ZIP4 (25) modulate zinc transport in yeast and mammalian cells, respectively. This suggests that PRL-mediated ubiquitination may play a role in post-translationally regulating zinc transport in MECs. Indeed, in the present study, we demonstrated that PRL activates the ubiquitination of ZnT2, stimulating ZnT2 trafficking and zinc accumulation in vesicles and secretion, followed by protein degradation. Importantly, we determined that two N-terminal lysine residues (Lys4 and Lys6) are required for PRL-induced ubiquitination and regulation of ZnT2.
EXPERIMENTAL PROCEDURES
Plasmid Constructs
A C-terminal tandem hemagglutinin (HA)-tagged ZnT2 plasmid was generated as described previously (1). The site-directed mutations (K4R-HA, K6R-HA, and K4R/H6R-HA) were constructed using a QuikChange® II XL site-directed mutagenesis kit (Stratagene, La Jolla, CA) according to the manufacturer's protocol. A commercially available human ubiquitin construct (Addgene; Cambridge, MA) was tagged at the C terminus with Myc by PCR. A C terminus Myc-tagged human ubiquitin dominant negative construct (Myc-Ub K48R) was generated using a QuikChange® II XL site-directed mutagenesis kit (Stratagene) according to the manufacturer's protocol. The site-directed mutation, orientation and fidelity of the inserts, and incorporation of the epitope tags were confirmed by directed sequencing (Pennsylvania State University Nucleic Acid Facility).
Cell Culture and Transient Transfection
Mouse MECs (HC11) were a gift from Dr. Jeffery Rosen (Baylor College of Medicine, Houston, TX) and used with permission of Dr. Bernd Groner (Institute for Biomedical Research, Frankfurt, Germany). Cells were routinely maintained in RPMI 1640 supplemented with 10% fetal bovine serum, insulin (5 μg/ml), epidermal growth factor (EGF; 10 ng/ml), and gentamycin. Cells were treated with either a combination of PRL (1 μg/ml) and cortisol (2 μm) or cortisol (2 μm) alone as a control, in serum-free RPMI 1640. HC11 cells were transfected with 0.8 μg (24-well plates), 4 μg (6-well plates), or 24 μg (10-cm2 dishes) of wild-type or mutant alleles of ZnT2 plasmid in serum- and antibiotic-free growth medium using Lipofectamine 2000 reagent (Invitrogen) at a DNA/transfection reagent ratio of 1:2.5 according to the manufacturer's specifications. Transfection medium was replaced 4–6 h later with antibiotic-free growth medium, and cells were cultured for an additional 24 h prior to experiments.
Immunoblot Analysis
Cells were washed in PBS and scraped into lysis buffer containing protease inhibitors, and the total membrane fraction was isolated as described previously (1). The protein concentration of the total membrane fraction was determined by the Bradford assay, and electrophoresis and immunoblotting were performed as described previously (1). Proteins were visualized by enhanced chemiluminescence (SuperSignal Femto, Pierce) following exposure to autoradiography film, and relative band density and molecular mass relative to standard molecular mass markers (Amersham Biosciences) were assessed using the Gel Quantification System (Carestream Health, Rochester, NY). Alternatively, proteins were detected using secondary antibodies labeled with IRDyes (LI-COR Biosciences, Lincoln, NE) and visualized using the Odyssey infrared imaging system (LI-COR Biosciences). As a loading control, the same membranes were stripped and reprobed with anti-β-actin antibody. In some experiments, cells were pretreated with cycloheximide (100 μg/ml) added to the medium to inhibit protein synthesis prior to treatment with PRL. In other experiments, proteasome inhibitor (MG132) or lysosome inhibitor (chloroquine) was added to culture medium as described.
Confocal Microscopy
Cells were transfected with ZnT2-HA or mutant K4R/K6R-HA for 24 h as described above. After transfection, cells were treated with PRL and cortisol or with cortisol alone for 2 h as described above. Cells were fixed in 4% (w/v) phosphate-buffered paraformaldehyde (pH 7.4) for 10 min, washed in PBS, and permeabilized with 0.2% Triton X-100 (Sigma-Aldrich) in PBS for 10 min. Coverslips were blocked with 5% goat serum, 1% bovine serum albumin in PBS for 20 min, followed by incubation with anti-HA Alexa 488-conjugated anti-mouse IgG (1 μg/ml; Invitrogen) for 45 min. Cells were washed with PBS and incubated with anti-Rab3A antibody (1 μg/ml; Abcam) for 45 min. Cells were washed, and nuclei were stained with TOPRO Nuclear Stain 647 (1 μm; Invitrogen) for 30 min. Following staining, cells were washed with PBS, mounted in ProLong Gold (Invitrogen), and sealed with nail polish. Cells were imaged using an Olympus FV1000 microscope with PlanApo ×100 oil lens, numerical aperture 1.42. Digital images were captured sequentially (FV10-ASW version 4.5, Olympus, Center Valley, PA) to eliminate potential interferences between fluorochromes; images were saved as .tif files to maintain image quality.
Visual Assessment of Intracellular Zinc Pools
Cells were transfected with ZnT2-HA or mutant K4R/K6R-HA for 24 h as described above. After transfection, cells were pretreated with ZnSO4 (10 μm for 1 h) and then treated with PRL and cortisol or with cortisol alone for 2 h. To visualize labile zinc pools, cells were incubated with FluoZin-3 AM (2 μm; Invitrogen) at 37 °C for 1 h, as described previously (26). Following incubation, cells were rinsed twice with PBS and washed in PBS for 30 min at 25 °C with constant shaking, and live cells were imaged as described above.
Cytoplasmic Zinc Assay
To determine the effect of ZnT2 attenuation on cytosolic zinc pools, cells were cultured in antibiotic-free growth medium in 24-well plates until 90–95% confluent. Cells were transfected with thymidine kinase promoter-linked Renilla luciferase vector (internal control, 0.05 μg) and either pGL3 empty vector (0.8 μg) plus 4× metal-responsive element (MRE)-pGL3 (a luciferase reporter containing four MREs from the mouse metallothionein 1A promoter upstream of the firefly luciferase open reading frame (Dr. Colin Duckett, University of Michigan Medical School, Ann Arbor, MI) or ZnT2 siRNA plus 4×MRE-pGL3 for 24 h before experiments. Luminescence was measured as described previously (26), and data were expressed as relative light units (ratio of firefly/Renilla luciferase activity).
Immunoprecipitation Experiments
To determine whether ZnT2-HA is ubiquitinated in response to PRL stimulation, cells were generated to express ZnT2-HA and/or Myc-Ub and then treated with PRL and cortisol for the indicated times. Cells were scraped into ice-cold PBS and pelleted by centrifugation and then lysed in radioimmune precipitation assay buffer (150 mm NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, 50 mm Tris-HCl, pH 8.0, plus protease inhibitors) for 30 min at 4 °C with rotation. Samples were centrifuged for 10 min at 15,000 × g, and the protein concentration of the lysates was determined by the Bradford assay. The cell lysates were preincubated with anti-HA-agarose (Pierce) overnight at 4 °C with rotation, followed by washing three times with radioimmune precipitation assay buffer before the proteins were eluted by boiling at 95 °C for 5 min in elution buffer. The eluates were run on 4–20% SDS-polyacrylamide gradient gels and transferred to nitrocellulose membranes, and the extent of ZnT2-HA ubiquitination was assessed by immunoblotting with anti-Myc antibody (Cell Signaling Technology) followed by horseradish peroxidase-conjugated secondary antibodies. Meanwhile, total cell lysates were immunoblotted either with anti-HA antibody or with anti-β-actin antibody followed by horseradish peroxidase-conjugated secondary antibodies. Proteins were visualized by enhanced chemiluminescence (SuperSignal Femto, Pierce) following exposure to autoradiography film.
Detection of ZnT2 by Cell Surface Biotinylation
To determine the amount of ZnT2 on the cell surface, cells transfected with ZnT2-HA or K4R/K6R-HA were pretreated with cycloheximide (100 μg/ml) and held at 20 °C for 2 h. Cells were then treated with PRL and cortisol or with cortisol alone for up to 8 h. N-hydroxysulfosuccinimide biotin (Pierce) was used to label cell surface proteins to detect HA-tagged ZnT2 or K4R/K6R at the cell membrane as described previously (4). Biotinylated proteins were eluted by heating to 95 °C in Laemmli buffer containing DTT (100 mm) and immunoblotted with anti-HA antibody and detected with secondary antibody labeled with IRdyes as described above.
ZnT2 Attenuation
Cells were plated in antibiotic-free OPTI-MEM in 6-well plates and cultured until ∼50% confluent. Cells were transfected with 100 pmol of ZnT2-specific small interfering RNA (5′- CCAUCUGCCUGGUGUUCAU-3′; Sigma-Aldrich) or mismatched control small interfering RNA (5′-CCGCGUCCUUCCUUAUGUAGGAAUU-3′; Invitrogen) using Lipofectamine 2000 at an oligonucleotide/transfection reagent ratio of 25:1 for 24 h before experiments.
65Zn Secretion Assay
To determine the effect of PRL stimulation on zinc secretion, HC11 cells (either expressing endogenous levels of ZnT2 or transfected to express ZnT2-HA or K4R/K6R-HA as described above) were radiolabeled in serum-free medium containing ZnSO4 (1 μm) and 65Zn (0.1 μCi; PerkinElmer Life Sciences) for 3 h at 37 °C and then washed briefly with cold PBS containing 1 mm EDTA to remove exofacial bound zinc. Cells were cultured in serum-free medium containing diethylenetriamine pentaacetic acid (50 μm) and PRL and/or cortisol for up to 24 h, and the amount of 65Zn exported into the culture medium was quantified in a γ-counter. Cellular protein concentration was determined by a Bradford assay, and measurements were normalized to total protein concentration.
Statistical Analysis
Results are presented as mean ± S.D. A minimum of at least two independent experiments were conducted with sample sizes as indicated. Statistical comparisons were performed using Student's t test (protein abundance and luciferase activity) or area under the curve (AUC; zinc secretion) (Prism Graph Pad, Berkeley, CA), and a significant difference was demonstrated at p < 0.05.
RESULTS
PRL Transiently Stimulates ZnT2-mediated Zinc Secretion from MECs
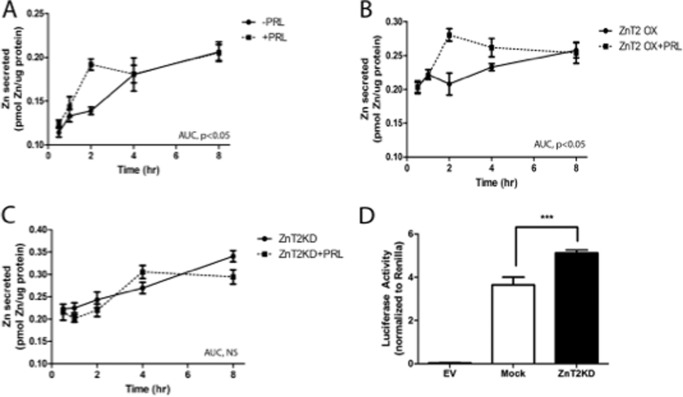
We first established the effects of PRL stimulation on zinc secretion in MECs preloaded with 65Zn. Our data demonstrated that PRL treatment significantly increased zinc secretion ∼2-fold (0.1802 ± 0.004 AUC units) in an acute and transient manner compared with untreated cells (0.0955 ± 0.003 AUC units, p < 0.05) (Fig. 1A). Next, we overexpressed ZnT2 and measured zinc secretion in response to PRL treatment (Fig. 1B). We found that PRL treatment in ZnT2-overexpressing cells significantly increased zinc secretion (0.2838 ± 0.006 AUC units) compared with untreated ZnT2-overexpressing cells (0.1074 ± 0.010 AUC units, p < 0.05). Moreover, ZnT2 overexpression augmented the effect of PRL treatment on zinc secretion compared with mock-transfected, PRL-treated cells (p < 0.01). To confirm that this transient PRL-mediated increase in zinc secretion was driven by ZnT2, we transfected cells with ZnT2 siRNA and measured zinc secretion in response to PRL treatment (Fig. 1C). We found that PRL treatment had no effect on zinc secretion in ZnT2-attenuated cells (ZnT2KD, 0.1467 ± 0.004 AUC units; ZnT2KD + PRL, 0.1354 ± 0.007 AUC units). During the course of these experiments, we noted that ZnT2-attenuated cells exported more zinc than ZnT2-expressing cells. This probably reflects a cytoprotective response due to the accumulation of zinc in the cytoplasm (Fig. 1D). We previously showed that ZnT4 exports zinc from MECs (26). Studies to co-attenuate ZnT2 and ZnT4 resulted in cell death (data not shown), indicating that ZnT4 is one mechanism through which zinc is exported in ZnT2-null cells. Nevertheless, our data collectively indicate that the transient increase in zinc secretion in response to PRL is mediated through ZnT2.
FIGURE 1.
ZnT2 was required for PRL-stimulated acute zinc secretion in MECs. Cells expressing endogenous levels of ZnT2 (A), cells overexpressing ZnT2 (B), or ZnT2-attenuated cells (C) were preincubated in serum-free medium containing 1.0 μm ZnSO4 and 0.1 μCi of 65Zn for 3 h. Medium was replaced with serum-free medium containing diethylenetriamine pentaacetic acid (50 μm) with or without PRL, and 65Zn was measured for up to 8 h. Values represent mean pmol of zinc/μg of protein ± S.D. (n = 4 samples/time point). Analysis of AUC indicates a significant difference of PRL treatment in cells expressing endogenous levels of ZnT2 (A) and overexpressing ZnT2 (B), p < 0.05. No effect of PRL in ZnT2-attenuated cells was detected (NS). Experiments were repeated three times. D, cells were transfected with thymidine kinase promoter-linked Renilla luciferase vector (internal control) and 4×MRE-pGL3 and either pGL3 empty vector (EV), scrambled siRNA (Mock), or ZnT2 siRNA. Luminescence was measured as an index of changes in cytoplasmic zinc pools. Data represent mean luminescence ± S.D. (error bars) (n = 4 samples/time point). *, significant effect of ZnT2KD on luciferase activity, p < 0.05. Experiments were repeated two times.
PRL Induces Ubiquitination of ZnT2
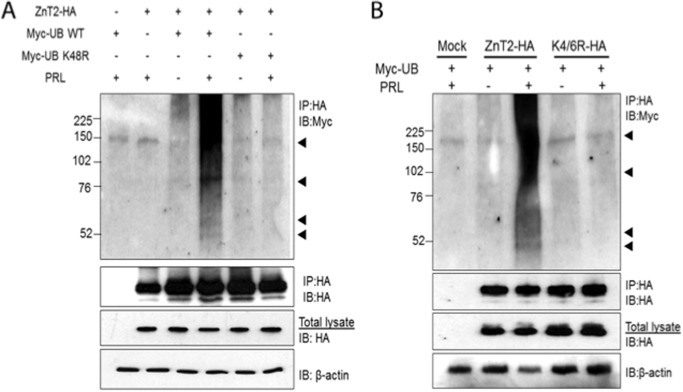
PRL stimulates ubiquitination (27), and ubiquitin serves as a sorting signal to regulate protein trafficking through the secretory compartment (reviewed in Ref. 22). Therefore, we next tested the hypothesis that ZnT2 is ubiquitinated in response to PRL. We detected the presence of ubiquitin in ZnT2-HA immunoprecipitates isolated from PRL-treated cells (Fig. 2A). Importantly, these ubiquitinated forms of ZnT2-HA were not observed in untreated cells. To confirm that PRL mediated the ubiquitination of ZnT2, we generated a dominant negative ubiquitin mutant in which lysine 48 was substituted with an arginine (Myc-Ub K48R). As shown in Fig. 2A, co-expression of ZnT2-HA with the dominant negative mutant Myc-Ub K48R eliminated the presence of ubiquitinated forms of ZnT2. Collectively, these data indicate that PRL stimulates the ubiquitination of ZnT2. Lysine residues on target proteins serve as the ubiquitin attachment sites (28), and bioinformatic analysis (UbPred) of ZnT2 identified several lysine residues in the N terminus (Lys4, Lys6, and Lys57) that may serve as potential targets for ubiquitination. Preliminary studies found no effect of K57R (data not shown). We replaced Lys4 and Lys6 with arginine to test the hypothesis that the Lys4/6 domain is a site for PRL-stimulated ubiquitination. ZnT2-HA, K4R/K6R-HA, and Myc-tagged ubiquitin (Myc-Ub WT) were co-expressed in cultured MECs and then stimulated with PRL. Anti-HA antibodies were used to immunoprecipitate ZnT2-HA, which was then immunoblotted for ubiquitin using anti-Myc antibody. Our data indicate that substitution of both lysine residues with arginine (K4R/K6R) resulted in a complete loss of ubiquitinated ZnT2 that is normally detected in PRL-treated cells (Fig. 2B), indicating that the Lys4/Lys6 domain serves as a ubiquitination motif in response to PRL.
FIGURE 2.
PRL stimulated ZnT2 ubiquitination. A, cells were transfected to express ZnT2-HA and Myc-ubiquitin (Myc-UB WT) alone or in combination or to co-express ZnT2-HA and Myc-ubiquitin K48R (Myc-UB K48R). Following a 24-h recovery, cells were pretreated with CHX to inhibit protein synthesis and with MG132 to inhibit proteasomal degradation and then treated for an additional 8 h in medium (−) or medium containing PRL (+). Cell lysates were passed over anti-HA affinity matrix (IP:HA), and the retained fraction was separated by 4–20% SDS-polyacrylamide gradient gels. Membranes were immunoblotted with anti-Myc antibody (IP:HA and IB:Myc) and stripped and reprobed for anti-HA antibody (IP:HA and IB:HA) as a loading control. Total cell lysates from the same samples were used to detect total abundance of ZnT2-HA protein and β-actin. Experiments were repeated more than three times. B, cells were transfected to express ZnT2-HA and Myc-ubiquitin (Myc-UB) alone or in combination or to co-express ZnT2-HA and K4R/K6R-HA. Following a 24-h recovery, cells were pretreated with CHX to inhibit protein synthesis and with MG132 to inhibit proteasomal degradation and then treated for an additional 8 h in medium (−) or medium containing PRL (+). Cell lysates were passed over anti-HA affinity matrix (IP:HA), and the retained fraction was separated by 4–20% SDS-polyacrylamide gradient gels. Membranes were immunoblotted with anti-Myc antibody (IP:HA and IB:Myc) and stripped and reprobed for anti-HA antibody (IP:HA and IB:HA) as a loading control. Total cell lysates from the same samples were used to detect total abundance of ZnT2-HA protein and β-actin. Experiments were repeated more than three times.
The Lys4/Lys6 Ubiquitination Motif Is Critical for Transient PRL-mediated Stimulation of Zinc Secretion
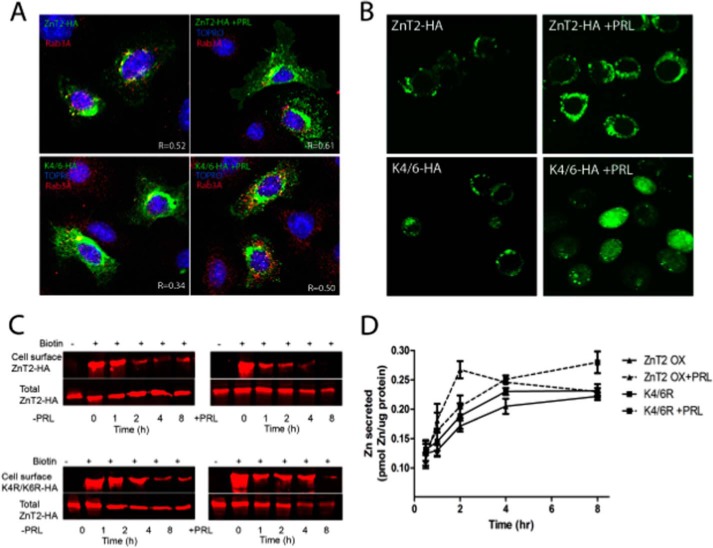
To test the hypothesis that the Lys4/Lys6 ubiquitination motif is important for the transient stimulation of ZnT2-mediated zinc secretion, cells were transfected to express ZnT2-HA or K4R/K6R-HA and treated with PRL for 2 h (peak zinc secretion). Using confocal imaging, we noted that under baseline conditions, the subcellular localization of ZnT2-HA was tightly restricted to a perinuclear compartment (probably the Golgi apparatus), was partially co-localized with the exocytotic vesicle marker Rab3A (Pearson's coefficient, 0.46 ± 0.06), and upon stimulation with PRL, relocalized in association with the exocytotic vesicle marker Rab3A (Pearson's coefficient, 0.61 ± 0.06) (Fig. 3A). In contrast, although K4R/K6R-HA was also predominantly localized to a perinuclear localization, it was less associated with Rab3A under unstimulated conditions (Pearson's coefficient, 0.31 ± 0.06), and when Rab3A-containing vesicles were relocalized in response to PRL, K4R/K6R-HA was not redistributed in parallel to the same degree (Pearson's coefficient, 0.5 ± 0.05). This suggests that in response to PRL, ZnT2 is ubiquitinated, which targets its entrance into the secretory system; however, our data also indicate that ubiquitination is not the only PRL-mediated signal. We next used FluoZin-3 as a reporter of labile zinc pools in MECs. These studies show that whereas FluoZin-3 fluorescence was vesicularized in cells expressing ZnT2-HA when stimulated with PRL, fluorescence accumulated in the cytoplasm in cells expressing K4R/K6R-HA (Fig. 3B). This suggests that PRL-mediated ubiquitination is essential for zinc accumulation in vesicles. We next used cell surface biotinylation to capture ZnT2 at the cell surface over time (Fig. 3C). These studies show that both wild-type and mutant ZnT2 are detected at the cell surface, suggesting that the movement of ZnT2-containing vesicles to the cell surface is not dependent upon ZnT2 ubiquitination. However, we noted that whereas PRL-stimulation transiently retained ZnT2 at the cell surface, the time frame in which mutant ZnT2 remained at the cell surface was extended. Finally, we measured zinc secretion using 65Zn. These studies show that whereas PRL transiently increased zinc secretion in cells expressing ZnT2-HA, the response to PRL was eliminated in cells expressing K4R/K6R-HA (Fig. 3D). Collectively, our data establish that the Lys4/Lys6 ubiquitination motif facilitates the targeting of ZnT2 to secretory vesicles to increase vesicular zinc accumulation, permitting the transient efflux of zinc in response to PRL. Moreover, our data suggest that the defect in cells expressing the K4R/K6R-HA mutant is in targeting ZnT2 to exocytotic vesicles for secretion but that the Lys4/Lys6 motif does not play a role in trafficking ZnT2-containing vesicles to the cell surface.
FIGURE 3.
Inability to ubiquitinate ZnT2 in response to PRL impaired ZnT2 trafficking, zinc accumulation, and secretion. A, representative confocal images of ZnT2-HA (green) and K4R/K6R-HA (green) and Rab3A (red) in MECs treated with (+PRL) or without prolactin (−PRL) for 2 h. Nucleus was stained with TOPRO and pseudocolored blue. Pearson's coefficient (R) was determined by correlation analysis using an Olympus FluoView viewer. Shown are values (−1 (no overlap of pixels) and +1 (100% overlap)) from five fields of view from two independent experiments. B, representative confocal images of FluoZin-3 (green) in cells expressing ZnT2-HA or K4R/K6R-HA in MECs treated with or without prolactin for 2 h. C, representative immunoblots of ZnT2-HA and K4R/K6R-HA detected at the cell surface by cell surface biotinylation (+) treated with and without prolactin over 8 h. Infrared images were normalized across immunoblots according to the manufacturer's instructions. D, zinc secretion from MECs expressing ZnT2-HA or K4R/K6R-HA treated with or without PRL. Data represent mean pmol of zinc/μg of protein ± S.D. (error bars) (n = 4 samples/time point). Experiments were repeated two times.
PRL Induces Proteasome-dependent Degradation of ZnT2
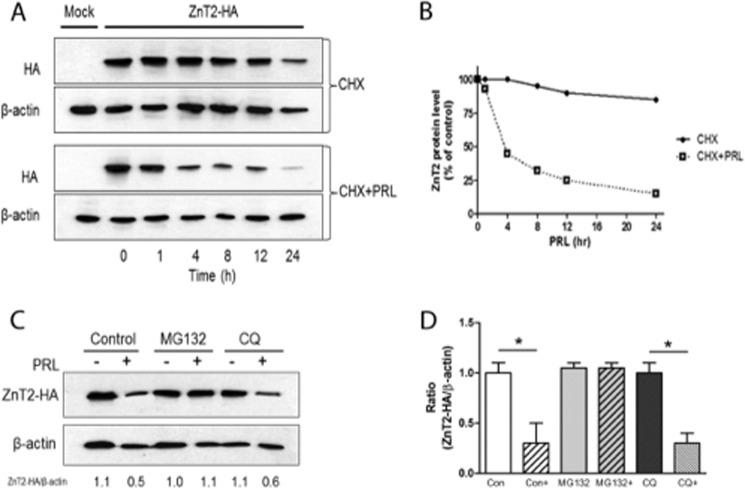
Following acute stimulation in response to PRL, we noted that cell surface ZnT2 and zinc secretion was rapidly attenuated. To test the hypothesis that PRL stimulates ubiquitin-mediated degradation of ZnT2 (29), we determined the effects of PRL treatment on ZnT2 abundance. Cells were pretreated with cycloheximide (CHX) to inhibit new protein synthesis, and changes in the total amount of ZnT2-HA protein in response to PRL treatment was determined (Fig. 4A). Consistent with the results in Fig. 3C, ZnT2 appeared relatively stable throughout 24 h. In contrast, PRL stimulation reduced ZnT2-HA stability by ∼55% in 4 h, and it continued to decline over time (Fig. 4B), collectively indicating that PRL stimulated the degradation of ZnT2. Pretreatment with the proteasome inhibitor MG132 completely blocked the PRL-stimulated degradation of ZnT2-HA, whereas pretreatment with the lysosome inhibitor chloroquine had no effect (Fig. 4C). These findings suggest that PRL-stimulated ZnT2 degradation is mediated through the proteasome pathway.
FIGURE 4.
PRL induced degradation of ZnT2. A, representative immunoblot of ZnT2-HA detected in the total membrane fraction of cells transfected with pcDNA3.1 (Mock) or cells expressing ZnT2-HA. Cells were treated with cycloheximide (100 μg/ml) alone (CHX) to inhibit new protein synthesis or in combination with PRL (CHX + PRL) for the indicated times. Total membrane fractions from the cells were separated by SDS-PAGE, and membranes were immunoblotted with HA antibody and then stripped and reprobed for β-actin as a loading control. B, ZnT2 protein abundance in response to PRL stimulation. Band intensity of ZnT2-HA was normalized to β-actin and expressed as a percentage of untreated cells. Data represent the mean percentage of ZnT2 protein level at time 0 ± S.D.; the experiment was repeated twice. C, representative immunoblot of ZnT2-HA detected in the total membrane fraction of cells transfected with ZnT2-HA. Cells were pretreated with cycloheximide (100 μg/ml) in the presence of MG132 (proteasome inhibitor) or chloroquine (CQ; lysosome inhibitor). Cells were then treated for an additional 8 h in medium (−) or medium containing PRL (+) in the presence of the above inhibitors. Total membrane fractions from the cells were separated by SDS-PAGE, and membranes were immunoblotted with anti-HA antibody and stripped and reprobed for β-actin as a loading control. Experiments were repeated more than three times. D, data represent the mean ratio of ZnT2/β-actin ± S.E. (error bars) from untreated cells (Con), untreated cells stimulated with PRL (Con+), cells pretreated with MG132 and stimulated with PRL (MG132+), or cells pretreated with chloroquine (CQ) and stimulated with PRL (CQ+) from two independent experiments. *, significant effect of PRL treatment on the ratio of ZnT2/β-actin, p < 0.05.
The Lys4/Lys6 Ubiquitination Motif Is Important for PRL-stimulated Degradation of ZnT2
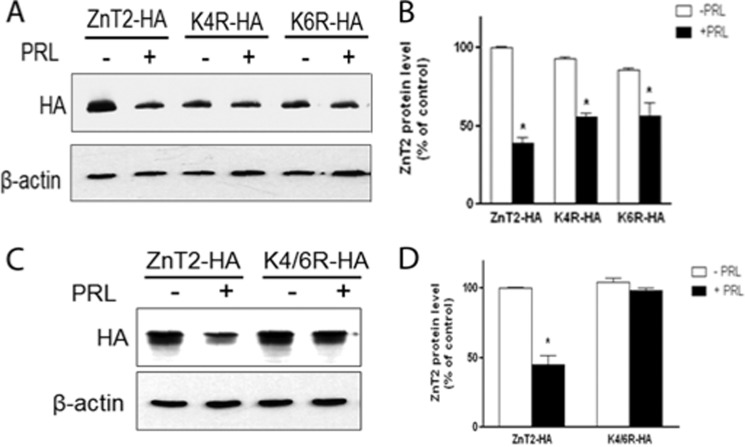
Because lysine residues on target proteins serve as the ubiquitin attachment sites that are required for proteasomal degradation (28), we replaced each lysine residue individually or in combination with arginine and then tested the ability of PRL to stimulate ZnT2 degradation (Fig. 5). We noted that PRL stimulation substantially degraded wild-type ZnT2 (reduced by ∼60% relative to untreated cells (Fig. 5, A and B). However, PRL-dependent degradation of ZnT2 was partially abolished in cells expressing either the K4R or K6R mutant (reduced by ∼40% relative to untreated cells; Fig. 5, A and B), whereas substitution of both lysine residues (K4R/K6R) rendered ZnT2 resistant to PRL-induced degradation (Fig. 5, C and D). This indicates that Lys4 and Lys6 act synergistically to mediate PRL-stimulated ZnT2 ubiquitination and degradation.
FIGURE 5.
PRL-stimulated ZnT2 degradation requires two lysine residues in the N terminus. A, representative immunoblot of ZnT2-HA in cells expressing ZnT2-HA, Lys4-HA, or Lys6-HA treated with PRL (+) compared with control (−) cells. Transfected cells were pretreated with cycloheximide and then treated with or without PRL for an 8 h, and the levels of each mutant protein were detected by anti-HA antibody. C, representative immunoblot of ZnT2-HA in cells expressing ZnT2-HA or K4R/K6R-HA treated with PRL (+) compared with control (−) cells. Transfected cells were pretreated with cychloheximide and then treated with or without PRL for 8 h, and the levels of each mutant protein were detected with anti-HA antibody. B and D, band intensity of ZnT2-HA normalized to β-actin expressed as a percentage of untreated cells. Data represent the mean percentage of ZnT2 protein level relative to untreated cells expressing ZnT2-HA ± S.D. (error bars) from two independent experiments. *, significant effect of PRL treatment on ZnT2 protein level, p < 0.05.
DISCUSSION
Regulation of ZnT2 function is a critical component of zinc secretion from the mammary gland into milk. We previously found that PRL increases ZnT2 transcription (16). Herein, we report that once expressed, PRL post-translationally stimulates ZnT2 ubiquitination, which targets ZnT2 to exocytotic vesicles for zinc accumulation to acutely augment zinc secretion. Ubiquitin can serve as a non-traditional sorting signal to regulate protein trafficking from the trans-Golgi apparatus into the secretory compartment (reviewed in Ref. 22). Herein, we provide compelling evidence that ubiquitination is important in targeting ZnT2 for incorporation into secretory vesicles in response to PRL. Localization in secretory vesicles may be important for ZnT2-mediated zinc transport because cells expressing the Lys4/Lys6 mutant form of ZnT2 were not able to accumulate zinc in response to PRL. For example, ZnT2 may require a proton gradient (30), which is key to the establishment of the secretory system (31), to facilitate exchange for zinc, as has been shown for ZnT5 (32), which is further supported by the fact that retention of ZnT2 at the cell surface did not augment zinc secretion. Although our studies strongly suggest that ubiquitination is critical for targeting ZnT2 to exocytotic vesicles for zinc accumulation, we cannot rule out the possibility that the inability to endocytose ZnT2 from the cell surface interferes with vesicular zinc accumulation, thus attenuating zinc secretion. Future studies to characterize how ZnT2 transports zinc are warranted.
PRL-mediated signals that activate ZnT2 ubiquitination remain to be understood. PRL stimulates numerous phosphorylation cascades, such as Jak2, STAT5, and MEK pathways in several tissues, including MECs (33–35), which mediate the non-genomic, rapid actions of PRL on nutrient transporters. Studies have shown that PRL-mediated, Jak2-induced tyrosine phosphorylation of Na+-K+-2Cl− co-transporters (33) transiently regulates chloride transport in MECs. In addition, PRL-mediated PI3K signaling also exerts acute effects on intestinal calcium transport (36), potentially through the phosphorylation of target protein, such as claudins, and the calcium transporters TRPV6 or PMCA1b. Bioinformatic analysis predicts that ZnT2 contains numerous potential phosphorylation sites at serine (Ser61, Ser216, and Ser296), tyrosine (Tyr75, Tyr171, Tyr247, and Tyr249), and threonine (Thr288) residues; however, further studies are required to determine whether ZnT2 is phosphorylated in response to extracellular cues and how PRL regulates ubiquitination mechanisms.
It is interesting that ubiquitination first increased zinc secretion and then activated degradation in a temporal manner. One possibility is that ubiquitination of either Lys4 or Lys6 may be important for targeting and augmenting zinc accumulation, whereas polyubiquitination at both Lys4 and Lys6 may be required for degradation. Multiple sites for ubiquitin ligation are often required to target a substrate for degradation (37–39), which is consistent with our data showing that only the substitution of both lysine residues (K4R/K6R) was entirely resistant to PRL-induced degradation. Although we observed a dramatic reduction in the detection of ubiquitinated forms in cells transfected to express the K4R/K6R mutant, it should be noted that mutation of single lysine residues was partially resistant to PRL-induced degradation. These data may suggest that these residues might be used as alternative ubiquitination sites, thereby partially down-regulating zinc secretion. Following ubiquitin binding, “internalization signals” within the cytoplasmic domains of membrane proteins are revealed, which stimulates protein internalization (40, 41). These signals include tyrosine-based (YXXØ (where X represents any residue and Ø is a large hydrophobic residue)) (42, 43) and dileucine-based motifs (44, 45). These signals promote internalization by interacting directly with adaptor protein complexes, such as AP2, that recruit proteins into clathrin-coated pits. Examination of the primary amino acid sequence of ZnT2 reveals two dileucine-based motifs, one of which is found directly upstream at Leu9. This suggests that ZnT2 ubiquitination at Lys4 and Lys6 may induce a conformational change and expose an internalization signal immediately downstream to stimulate protein internalization. Alternatively, the ubiquitin molecule itself can signal for internalization (40, 41). Further studies are needed to explore the role of ubiquitin-mediated internalization and degradation signals in ZnT2.
The ubiquitin-mediated degradation system is emerging as an important mechanism for regulating zinc homeostasis by rapidly regulating the function of zinc transporters. The inability to tightly control zinc transport rapidly leads to cellular dysfunction and death (46). For example, zinc exposure ubiquitinates and degrades the yeast zinc uptake transporter ZRT1 (23). Similarly, ZIP4 is quickly ubiquitinated and degraded in response to high zinc levels (47), suggesting that ubiquitination and degradation of zinc transporters may be a critical regulatory mechanism controlling zinc homeostasis. To our knowledge, our study is the first to identify a ubiquitin-mediated mechanism that is responsible for transiently increasing zinc efflux, contributing to our general understanding of zinc regulation. Ubiquitin-mediated regulation is not unique to zinc homeostasis. Ubiquitination of other membrane-bound ion transporters plays an important role in controlling ion transport in response to extracellular signals, such as micronutrient bioavailability and hormones. The iron-regulated transporter 1 (IRT1) in plants is monoubiquitinated, which internalizes IRT1 and regulates iron uptake (48). The manganese transporter SMF1 in yeast is phosphorylated, ubiquitinated, and internalized as well (49). In mammals, the key iron regulatory hormone hepcidin reduces intestinal iron absorption through ubiquitin-dependent proteasome degradation of the iron transporter DMT1 (50). In addition, hepcidin limits iron export by binding to the iron exporter ferroportin (51), inducing its Jak2-mediated tyrosine phosphorylation (52), ubiquitination, internalization, and degradation (51, 53, 54). Thus, ubiquitination may be a critical mechanism to tightly regulate ion homeostasis in numerous biological systems.
In summary, our study indicates that PRL stimulates the ubiquitination of two lysine residues in the N terminus of ZnT2, targeting ZnT2 to secretory vesicles, accumulating zinc, and initially augmenting zinc secretion, after which ZnT2 is degraded. Defects in the ability to activate ubiquitination may either augment or abrogate stimulated zinc efflux from MECs and thus compromise mammary gland function and zinc secretion into milk. Collectively, this provides a novel understanding of not only zinc homeostasis but also the complex role PRL plays in regulating lactogenic mechanisms and important molecular clues as to how PRL tightly regulates micronutrient transport into milk to provide optimal nutrition to the nursing infant.
Acknowledgments
We gratefully acknowledge the members of the Kelleher laboratory for generous input and constructive comments.
This work was supported, in whole or in part, by National Institutes of Health Grant R01 HD058614 (to S. L. K.).
- MEC
- mammary epithelial cell
- PRL
- prolactin
- MRE
- metal-responsive element
- AUC
- area under the curve
- Ub
- ubiquitin
- CHX
- cycloheximide.
REFERENCES
- 1. Chowanadisai W., Lönnerdal B., Kelleher S. L. (2006) Identification of a mutation in SLC30A2 (ZnT-2) in women with low milk zinc concentration that results in transient neonatal zinc deficiency. J. Biol. Chem. 281, 39699–39707 [DOI] [PubMed] [Google Scholar]
- 2. Lasry I., Seo Y. A., Ityel H., Shalva N., Pode-Shakked B., Glaser F., Berman B., Berezovsky I., Goncearenco A., Klar A., Levy J., Anikster Y., Kelleher S. L., Assaraf Y. G. (2012) A dominant negative heterozygous G87R mutation in the zinc transporter, ZnT-2 (SLC30A2), results in transient neonatal zinc deficiency. J. Biol. Chem. 287, 29348–29361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Itsumura N., Inamo Y., Okazaki F., Teranishi F., Narita H., Kambe T., Kodama H. (2013) Compound heterozygous mutations in SLC30A2/ZnT2 results in low milk zinc concentrations: a novel mechanism for zinc deficiency in a breast-fed infant. PloS One 8, e64045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lopez V., Kelleher S. L. (2009) Zinc transporter-2 (ZnT2) variants are localized to distinct subcellular compartments and functionally transport zinc. Biochem. J. 422, 43–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Seo Y. A., Kelleher S. L. (2010) Functional analysis of two single nucleotide polymorphisms in SLC30A2 (ZnT2): implications for mammary gland function and breast disease in women. Physiol. Genomics 42A, 219–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Freeman M. E., Kanyicska B., Lerant A., Nagy G. (2000) Prolactin: structure, function, and regulation of secretion. Physiol. Rev. 80, 1523–1631 [DOI] [PubMed] [Google Scholar]
- 7. Ball R. K., Friis R. R., Schoenenberger C. A., Doppler W., Groner B. (1988) Prolactin regulation of β-casein gene expression and of a cytosolic 120-kd protein in a cloned mouse mammary epithelial cell line. EMBO J. 7, 2089–2095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McManaman J. L., Hanson L., Neville M. C., Wright R. M. (2000) Lactogenic hormones regulate xanthine oxidoreductase and β-casein levels in mammary epithelial cells by distinct mechanisms. Arch. Biochem. Biophys. 373, 318–327 [DOI] [PubMed] [Google Scholar]
- 9. Lkhider M., Pétridou B., Aubourg A., Ollivier-Bousquet M. (2001) Prolactin signalling to milk protein secretion but not to gene expression depends on the integrity of the Golgi region. J. Cell Sci. 114, 1883–1891 [DOI] [PubMed] [Google Scholar]
- 10. Ollivier-Bousquet M. (1978) Early effects of prolactin on lactating rabbit mammary gland. Ultrastructural changes and stimulation of casein secretion. Cell Tissue Res. 187, 25–43 [DOI] [PubMed] [Google Scholar]
- 11. Winklehner-Jennewein P., Geymayer S., Lechner J., Welte T., Hansson L., Geley S., Doppler W. (1998) A distal enhancer region in the human β-casein gene mediates the response to prolactin and glucocorticoid hormones. Gene 217, 127–139 [DOI] [PubMed] [Google Scholar]
- 12. Jahn G. A., Daniel N., Jolivet G., Belair L., Bole-Feysot C., Kelly P. A., Djiane J. (1997) In vivo study of prolactin (PRL) intracellular signalling during lactogenesis in the rat: JAK/STAT pathway is activated by PRL in the mammary gland but not in the liver. Biol. Reprod. 57, 894–900 [DOI] [PubMed] [Google Scholar]
- 13. Goupille O., Barnier J. V., Guibert B., Paly J., Djiane J. (2000) Effect of PRL on MAPK activation: negative regulatory role of the C-terminal part of the PRL receptor. Mol. Cell Endocrinol. 159, 133–146 [DOI] [PubMed] [Google Scholar]
- 14. Bole-Feysot C., Goffin V., Edery M., Binart N., Kelly P. A. (1998) Prolactin (PRL) and its receptor: actions, signal transduction pathways and phenotypes observed in PRL receptor knockout mice. Endocr. Rev. 19, 225–268 [DOI] [PubMed] [Google Scholar]
- 15. Gutzman J. H., Nikolai S. E., Rugowski D. E., Watters J. J., Schuler L. A. (2005) Prolactin and estrogen enhance the activity of activating protein 1 in breast cancer cells: role of extracellularly regulated kinase 1/2-mediated signals to c-fos. Mol. Endocrinol. 19, 1765–1778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Qian L., Lopez V., Seo Y. A., Kelleher S. L. (2009) Prolactin regulates ZNT2 expression through the JAK2/STAT5 signaling pathway in mammary cells. Am. J. Physiol. Cell Physiol. 297, C369–C377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kelleher S. L., Velasquez V., Croxford T. P., McCormick N. H., Lopez V., MacDavid J. (2012) Mapping the zinc-transporting system in mammary cells: molecular analysis reveals a phenotype-dependent zinc-transporting network during lactation. J. Cell. Physiol. 227, 1761–1770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Varghese B., Barriere H., Carbone C. J., Banerjee A., Swaminathan G., Plotnikov A., Xu P., Peng J., Goffin V., Lukacs G. L., Fuchs S. Y. (2008) Polyubiquitination of prolactin receptor stimulates its internalization, postinternalization sorting, and degradation via the lysosomal pathway. Mol. Cell Biol. 28, 5275–5287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hicke L., Dunn R. (2003) Regulation of Membrane Protein Transport by Ubiquitin and Ubiquitin-binding Proteins. Annu. Rev. Cell Dev. Biol. 19, 141–172 [DOI] [PubMed] [Google Scholar]
- 20. Hegde A. N., DiAntonio A. (2002) Ubiquitin and the synapse. Nat. Rev. Neurosci. 3, 854–861 [DOI] [PubMed] [Google Scholar]
- 21. Amm I., Sommer T., Wolf D. (2014) Protein quality control and elimination of protein waste: the role of the ubiquitin-proteasome system. Biochim. Biophys. Acta 1843, 182–196 [DOI] [PubMed] [Google Scholar]
- 22. Hicke L. (1997) Ubiquitin-dependent internalization and down-regulation of plasma membrane proteins. FASEB J. 11, 1215–1226 [DOI] [PubMed] [Google Scholar]
- 23. Gitan R. S., Eide D. J. (2000) Zinc-regulated ubiquitin conjugation signals endocytosis of the yeast ZRT1 zinc transporter. Biochem. J. 346, 329–336 [PMC free article] [PubMed] [Google Scholar]
- 24. Gitan R. S., Luo H., Rodgers J., Broderius M., Eide D. (1998) Zinc-induced inactivation of the yeast ZRT1 zinc transporter occurs through endocytosis and vacuolar degradation. J. Biol. Chem. 273, 28617–28624 [DOI] [PubMed] [Google Scholar]
- 25. Mao X., Kim B. E., Wang F., Eide D. J., Petris M. J. (2007) A histidine-rich cluster mediates the ubiquitination and degradation of the human zinc transporter, hZIP4, and protects against zinc cytotoxicity. J. Biol. Chem. 282, 6992–7000 [DOI] [PubMed] [Google Scholar]
- 26. McCormick N. H., Kelleher S. L. (2012) ZnT4 provides zinc to zinc-dependent proteins in the trans-Golgi network critical for cell function and Zn export in mammary epithelial cells. Am. J. Physiol. Cell Physiol. 303, C291–C297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Swaminathan G., Varghese B., Thangavel C., Carbone C. J., Plotnikov A., Kumar K. G. S., Jablonski E. M., Clevenger C. V., Goffin V., Deng L., Frank S. J., Fuchs S. Y. (2008) Prolactin stimulates ubiquitination, initial internalization, and degradation of its receptor via catalytic activation of Janus kinase 2. J. Endocrinol. 196, R1–R7 [DOI] [PubMed] [Google Scholar]
- 28. Hershko A., Ciechanover A. (1998) The ubiquitin system. Annu. Rev. Biochem. 67, 425–479 [DOI] [PubMed] [Google Scholar]
- 29. Isasa M., Zuin A., Crosas B. (2012) Integration of multiple ubiquitin signals in proteasome regulation. Methods Mol. Biol. 910, 337–370 [DOI] [PubMed] [Google Scholar]
- 30. Palmiter R. D., Cole T. B., Findley S. D. (1996) ZnT-2, a mammalian protein that confers resistance to zinc by facilitating vesicular sequestration. EMBO J. 15, 1784–1791 [PMC free article] [PubMed] [Google Scholar]
- 31. Moore H.-P. H., Andresen J. M., Eaton B. A., Grabe M., Haugwitz M., Wu M. M., Machen T. E. (2002) Biosynthesis and secretion of pituitary hormones: dynamics and regulation. Arch. Physiol. Biochem. 110, 16–25 [DOI] [PubMed] [Google Scholar]
- 32. Ohana E., Hoch E., Keasar C., Kambe T., Yifrach O., Hershfinkel M., Sekler I. (2009) Identification of the Zn2+ binding site and mode of operation of a mammalian Zn2+ transporter. J. Biol. Chem. 284, 17677–17686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Selvaraj N. G., Omi E., Gibori G., Rao M. C. (2000) Janus kinase 2 (JAK2) regulates prolactin-mediated chloride transport in mouse mammary epithelial cells through tyrosine phosphorylation of Na+-K+-2Cl− cotransporter. Mol. Endocrinol. 14, 2054–2065 [DOI] [PubMed] [Google Scholar]
- 34. al-Sakkaf K. A., Dobson P. R., Brown B. L. (1997) Prolactin induced tyrosine phosphorylation of p59fyn may mediate phosphatidylinositol 3-kinase activation in Nb2 cells. J. Mol. Endocrinol. 19, 347–350 [DOI] [PubMed] [Google Scholar]
- 35. Piccoletti R., Maroni P., Bendinelli P., Bernelli-Zazzera A. (1994) Rapid stimulation of mitogen-activated protein kinase of rat liver by prolactin. Biochem. J. 303, 429–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jantarajit W., Thongon N., Pandaranandaka J., Teerapornpuntakit J., Krishnamra N., Charoenphandhu N. (2007) Prolactin-stimulated transepithelial calcium transport in duodenum and Caco-2 monolayer are mediated by the phosphoinositide 3-kinase pathway. Am. J. Physiol. Endocrinol. Metab. 293, E372–E384 [DOI] [PubMed] [Google Scholar]
- 37. Baldi L., Brown K., Franzoso G., Siebenlist U. (1996) Critical role for lysines 21 and 22 in signal-induced, ubiquitin-mediated proteolysis of I κ B-α. J. Biol. Chem. 271, 376–379 [DOI] [PubMed] [Google Scholar]
- 38. Chen Z., Hagler J., Palombella V. J., Melandri F., Scherer D., Ballard D., Maniatis T. (1995) Signal-induced site-specific phosphorylation targets I κ B α to the ubiquitin-proteasome pathway. Genes Dev. 9, 1586–1597 [DOI] [PubMed] [Google Scholar]
- 39. Treier M., Staszewski L. M., Bohmann D. (1994) Ubiquitin-dependent c-Jun degradation in vivo is mediated by the δ domain. Cell 78, 787–798 [DOI] [PubMed] [Google Scholar]
- 40. Mellman I. (1996) Endocytosis and molecular sorting. Annu. Rev. Cell Dev. Biol. 12, 575–625 [DOI] [PubMed] [Google Scholar]
- 41. Trowbridge I. S. (1991) Endocytosis and signals for internalization. Curr. Opin. Cell Biol. 3, 634–641 [DOI] [PubMed] [Google Scholar]
- 42. Marks M. S., Ohno H., Kirchnausen T., Bonracino J. S. (1997) Protein sorting by tyrosine-based signals: adapting to the Ys and wherefores. Trends Cell Biol. 7, 124–128 [DOI] [PubMed] [Google Scholar]
- 43. Owen D. J., Evans P. R. (1998) A structural explanation for the recognition of tyrosine-based endocytotic signals. Science 282, 1327–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dietrich J., Hou X., Wegener A. M., Pedersen L. O., Odum N., Geisler C. (1996) Molecular characterization of the di-leucine-based internalization motif of the T cell receptor. J. Biol. Chem. 271, 11441–11448 [DOI] [PubMed] [Google Scholar]
- 45. Pond L., Kuhn L. A., Teyton L., Schutze M. P., Tainer J. A., Jackson M. R., Peterson P. A. (1995) A role for acidic residues in di-leucine motif-based targeting to the endocytic pathway. J. Biol. Chem. 270, 19989–19997 [DOI] [PubMed] [Google Scholar]
- 46. Plum L. M., Rink L., Haase H. (2010) The essential toxin: impact of zinc on human health. Int. J. Environ. Res. Public Health 7, 1342–1365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wang F., Dufner-Beattie J., Kim B. E., Petris M. J., Andrews G., Eide D. J. (2004) Zinc-stimulated endocytosis controls activity of the mouse ZIP1 and ZIP3 zinc uptake transporters. J. Biol. Chem. 279, 24631–24639 [DOI] [PubMed] [Google Scholar]
- 48. Barberon M., Zelazny E., Robert S., Conéjéro G., Curie C., Friml J., Vert G. (2011) Monoubiquitin-dependent endocytosis of the iron-regulated transporter 1 (IRT1) transporter controls iron uptake in plants. Proc. Natl. Acad. Sci. U.S.A. 108, E450–E458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nikko E., Sullivan J. A., Pelham H. R. (2008) Arrestin-like proteins mediate ubiquitination and endocytosis of the yeast metal transporter Smf1. EMBO Rep. 9, 1216–1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Brasse-Lagnel C., Karim Z., Letteron P., Bekri S., Bado A., Beaumont C. (2011) Intestinal DMT1 cotransporter is down-regulated by hepcidin via proteasome internalization and degradation. Gastroenterology 140, 1261–1271.e1 [DOI] [PubMed] [Google Scholar]
- 51. De Domenico I., Nemeth E., Nelson J. M., Phillips J. D., Ajioka R. S., Kay M. S., Kushner J. P., Ganz T., Ward D. M., Kaplan J. (2008) The hepcidin-binding site on ferroportin is evolutionarily conserved. Cell Metab. 8, 146–156 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 52. De Domenico I., Lo E., Ward D. M., Kaplan J. (2009) Hepcidin-induced internalization of ferroportin requires binding and cooperative interaction with Jak2. Proc. Natl. Acad. Sci. U.S.A. 106, 3800–3805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Nemeth E., Tuttle M. S., Powelson J., Vaughn M. B., Donovan A., Ward D. M., Ganz T., Kaplan J. (2004) Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 306, 2090–2093 [DOI] [PubMed] [Google Scholar]
- 54. Delaby C., Pilard N., Gonc̃alves A. S., Beaumont C., Canonne-Hergaux F. (2005) Presence of the iron exporter ferroportin at the plasma membrane of macrophages is enhanced by iron loading and down-regulated by hepcidin. Blood 106, 3979–3984 [DOI] [PubMed] [Google Scholar]