Background: Exposure to uncommon pets such as a Siberian hamster has been described as a new risk factor for the development of allergic disease.
Results: For the first time, we have cloned and characterized an allergen from Siberian hamster.
Conclusion: The recombinant allergen shows a similar pattern of IgE binding and biological activity compared with salivary gland extract.
Significance: The recombinant form will be useful for clinical applications.
Keywords: Allergen, Allergy, Asthma, Immunoglobulin E (IgE), Inflammation, Hamster, Lipocalin, Pet Allergy
Abstract
The most frequent pet allergy is to cat and dog, but in recent years, it has become increasingly popular to have other pets, and the risk of exposure to new allergens is more prevalent. The list of new pets includes hamsters, and one of the most popular hamsters is the Siberian hamster (Phodopus sungorus). The aim of this study was the characterization and cloning of the major allergen from this hamster. The study of its allergenicity and cross-reactivity could improve the specific diagnosis and treatment for hamster-allergic patients. Thirteen Siberian hamster-allergic patients were recruited at the outpatient clinic. Protein extracts were prepared from the hair, urine, and salivary glands of four hamster species (European, golden, Siberian, and Roborovski). IgE-binding proteins were detected by immunoblotting and identified by mass spectrometry. The recombinant protein was produced in Escherichia coli and then purified by metal chelate affinity chromatography. The allergenic properties of the recombinant protein were tested by ELISA and immunoblotting, and biological activity was tested according to capacity for basophil activation. Three IgE-binding proteins were identified in extracts obtained from Siberian hamster hair, urine, and salivary glands. All proteins corresponded to the same protein, which was identified as a lipocalin. This lipocalin had no cross-reactivity with common and golden hamsters. The recombinant allergen was cloned and purified, showing similar IgE reactivity in vitro to Siberian hamster protein extracts. Also, the recombinant allergen was capable of producing biological activation in vivo. The major Siberian hamster allergen was cloned, and allergenic properties were characterized, providing a new tool for specific diagnosis of allergy to Siberian hamster.
Introduction
Exposure to domestic animals has been described as a potential risk factor for the development of asthma and allergic disease (1). Although the most frequent pet allergy is to cat and dog, in recent years, it has become increasingly popular to have other animals as pets, and the risk of exposure to unknown allergens is increasing (2). The list of new pets includes rodents (hamsters, guinea pigs, chinchillas, gerbils, etc.), other mammals, exotic birds, and reptiles (3). Two of the most popular new pets in most recent years are the Siberian (Phodopus sungorus) and Roborovski (Phodopus roborovskii) hamsters, as these are more sociable than other hamsters. The Siberian hamster (SH) has a similar appearance to common hamsters such as the European (Cricetus cricetus) and golden (Mesocricetus auratus) hamsters but belongs to a different genus. Although a growing number of patients present to outpatient clinic with symptoms of SH allergy, there is little information about the allergens involved. The allergic symptoms caused by SH varies from upper and lower respiratory tract symptoms (4) to anaphylaxis after bites (5–7). In these reports, IgE-binding bands ranging from 18 to 32 kDa were described as the responsible allergens, although the specific allergens were not identified.
Torres et al. (7) characterized three IgE-binding bands found in SH hair, urine, and salivary glands. These bands have molecular masses of ∼18, 21, and 23 kDa. Two of the IgE-binding bands have the same N-terminal sequence, and all of them were shown to have identical internal peptides when analyzed by mass spectrometry, suggesting that the 23-kDa IgE-binding band is a unique protein and the latter two IgE-binding bands could be truncated forms that result from degradation of the former. The allergen was identified as an odorant-binding protein belonging to the lipocalin family, showing no cross-reactivity with allergens from other mammalian species. This allergen was named Pho s 21kD (Allergome). There are no studies on cross-reactivity between SH and others hamster species. This absence makes diagnosis difficult because there is currently no standardized commercial extract available for SH. Although avoidance therapy is the best measure to prevent allergic reactions to SH, characterization of the major SH allergen, which will make it possible to study its allergenicity and cross-reactivity, will be crucial in the effort to improve specific diagnosis of patients and to develop new therapies such as attenuated allergy vaccines designed to treat symptoms.
EXPERIMENTAL PROCEDURES
Patient Sera
Sera obtained from 13 patients and a serum pool from SH-allergic patients with specific IgE to SH were selected at the Hospital Fundación Jimenez Díaz in Madrid, Spain. SH allergy was diagnosed on the basis of a history of symptoms compatible with exposure to SH and a positive skin prick test to SH extracts (hair, urine, and salivary glands) following the technique described by Dreborg and Foucard (8) and according to European Academy of Allergy and Clinical Immunology guidelines (9) using an ALK-LANCET needle (ALK, Hørsholm, Denmark). The solutions for the skin prick test contained protein extract (10 μg/ml) in 10 mm phosphate, 75 mm NaCl, and 50% glycerol. A panel of animal allergens (cat, dog, horse, and European hamster; ALK-Abelló S.A., Madrid, Spain) was tested in all patients using a skin prick test. Specific IgE antibodies to animal allergens were determined using the CAP System fluorescent enzyme immunoassay (Pharmacia Diagnostics AB, Uppsala, Sweden). Specific IgE to SH extracts was determined by ELISA and immunoblot techniques. In addition, sera from five non-atopic patients were used as negative controls. Other pet allergies were diagnosed in patients with a clear history of adverse reactions suggestive of IgE-mediated allergy, along with positive skin prick tests. The study was approved by the hospital ethics committee, and all patients gave their written consent to participate in the study.
Preparation of Hamster Extracts
All hamsters were purchased in a local pet shop. All extracts were derived from the hair, urine, and salivary glands of the different hamster species: European hamster (C. cricetus), golden hamster (M. auratus), Roborovski hamster (P. roborovskii), and SH (P. sungorus, also known as the Djungarian hamster). Hair extracts were obtained by shaving the animals. The hair (10%, w/v) was homogenized in PBS, and, after centrifugation, dialysis, and further lyophilization, hair protein extracts were obtained. Urine was obtained by keeping the animals in a metabolic cage for 3 days. The hamsters' urine was dialyzed against PBS and then lyophilized to obtain urine protein extracts. The salivary glands were obtained by surgical dissection performed by a veterinarian on an SH killed previously. The submaxillary glands were harvested immediately after death and conserved in RNAlater (Qiagen, Hilden, Germany) for RNA extraction. After the salivary glands were homogenized, the same procedures as described above were followed to obtain the extract (salivary glands extract). The procedure used to isolate the salivary glands of the other hamsters (European, golden, and Roborovski) was the same as the one described above. Total protein content was determined by the Coomassie Plus (Bradford) assay (Pierce). Animal handling met the European guidelines for experimental animals.
Isolation of cDNA Encoding Pho s 21kD
Total RNA was extracted from submaxillary glands using the PureLink RNA mini kit (Invitrogen). cDNA was obtained using SuperScript III reverse transcriptase (Invitrogen). The cDNA was amplified using Taq polymerase (Biomedal, Seville, Spain) and specific primers whose design was based on the published N-terminal amino acid sequence (7): forward, 5′-TATCGGGATCCGAA(C/T)GA(C/T)TA(C/T)GCIGA(A/G)(C/T)TIGA(A/G)GG-3′; and reverse, 5′-CTTGGTACCTTTTTTTTTTTTTTT-3′). The PCR amplification product was cloned into pBluescript SK+ for sequence analysis. The excised allergen gene was cloned into the expression vector T7/Cascade pMAB36 (Biomedal) using primers obtained for the open reading frame coding of the mature protein (forward, 5′-AGCATGCTCAATGACTATGCGGAGC-3′; and reverse, 5′-ATTAAGCTTAGTGATGGTGATGGTGATGACCAGAGCCTTTAGGACAAGTATC-3′).
Expression and Purification of Recombinant Pho s 21kD
cDNA coding for Pho s 21kD was expressed in E. coli cells (strain BL21(DE3)4S2, Biomedal) as recombinant protein with a C-terminal hexahistidine tag. Bacteria were induced at an absorbance of 0.75 at 600 nm using 1 mm sodium salicylate (Alpha Aesar GmbH & Co. KG, Karlsruhe, Germany) and 10 mm 3-methyl benzoate (Acros Organics, Geel, Belgium) overnight at 20 °C. Bacteria were collected by centrifugation and resuspended in lysis buffer (20 mm NaH2PO4, 150 mm NaCl, 10 mm MgCl2, and 50 units/ml DNase I, pH 7.4). Cells were lysed using a PANDA 2K NS1001L laboratory homogenizer (GEA Niro Soavi S.P.A., Parma, Italy) at 800–900 bar. The expressed protein was purified by metal chelate affinity using HIS-Select nickel affinity gel (Sigma) according to the manufacturer's instructions. Fractions containing recombinant allergen were pooled, dialyzed against ammonium bicarbonate, lyophilized, and resuspended at 1 mg/ml. The purity of the recombinant proteins was analyzed by SDS-PAGE, and identity was confirmed by mass spectrometry as described previously (10).
SDS-PAGE, Immunoblot Analysis, and Inhibition Assays
SDS-PAGE, immunoblotting, and inhibition assays were carried out as described previously (11). Polyacrylamide concentrations of 14% (w/v) and 5% (w/v) were used for separating and stacking gels, respectively. Protein was applied at 50 μg/lane. The samples were mixed with 0.1 m Tris, pH 6.8, containing 4% (w/v) SDS, 20% (w/v) glycerol, 10% (w/v) β-mercaptoethanol, and 0.02% (w/v) bromphenol blue. To ensure proper protein separation and visualization, the gels were stained with Coomassie Brilliant Blue (Sigma). Membranes were blocked with PBS containing 0.1% Tween 20, 3% skimmed milk powder, and 3% BSA for 2 h at room temperature and then incubated overnight at 4 °C with the patient sera (or serum pool) diluted 1:10 in 20 mm Tris-buffered saline, pH 7.5, containing 0.1% Tween 20 and 3% BSA. To perform immunoblot inhibition assays, the serum pool was incubated with 100 μg/ml hamster extracts or 50 μg/ml recombinant Pho (rPho)3 s 21kD for 4 h at room temperature.
ELISA
For ELISAs, 96-well flat bottom plates were used (Immulon 4HBX, Thermo Scientific). The plates were coated in duplicate overnight at 4 °C with 20 μg SH extract/well or 0.5 μg of Pho s 21kD/well. ELISAs were carried out as described previously (11).
Protein Identification and Characterization by Mass Spectrometry
The proteins were identified by MS and/or MS/MS as described previously (10). This procedure was performed in the Proteomics Facility of the Universidad Complutense de Madrid (a member of the ProteoRed-ISCIII Network).
BasoTest Assays
A basophil activation test was performed with the BasoFlowEx test kit (Exbio). Evaluation of basophil degranulation was performed by flow cytometry following the manufacturer's directions and as described previously (12). Cells were sensitized with purified SH extract and recombinant allergen at concentrations ranging from 0.1 to 10 μg/ml of heparinized whole blood. To estimate activated basophils, cells from heparinized whole blood were stained with monoclonal phycoerythrin-conjugated anti-human CD203c and FITC-conjugated anti-human CD63 and used for flow cytometry analysis of basophil activation in a FACSCanto II flow cytometer (BD Biosciences). To analyze a sufficient number of basophils, 100,000 events were acquire per sample. FL2 channel and side scatter were employed to gate basophils that expressed a high density of surface CD203c. Subsequently within this gate, the percentage of activated basophils is those coexpressing CD63 (FL1 channel). A basophil activation test was considered positive when an increase in at least 6% of CD63-positive basophils was measured for at least one dilution of allergen in comparison with the basal activation level measured in the negative control.
RESULTS
Clinical Features of SH-allergic Patients
The clinical characteristics of patients are summarized in Table 1. Thirteen patients were included, with a mean age of 27.2 years (±10–46 years); 9 were female (70%), and four were male (30%). All patients had at least one SH in their homes, and all of them removed their hamster following medical advice. Ten patients (77%) presented with rhinoconjunctivitis and asthma, two patients (15%) presented with only rhinoconjunctivitis, and only one patient (7%) presented with anaphylaxis after an SH bite. In 52% of the patients, the average latency of onset of symptoms following the start of exposure to SH was 4–8 weeks. It was noted that nine patients (70%) were sensitized only to SH through skin testing (hair, salivary gland, and urine extracts), whereas four patients (30%) were sensitized to SH and Roborovski hamster. A clear clinical improvement was seen in up to 42% of patients after removing SH from their homes. The improvement started after the fourth week in 42% of patients and after the eighth week in 38% of patients. While the hamsters were in their homes, 62% of the patients were being treated with long-acting β2-inhaled steroids along with oral antihistamines and salbutamol as rescue medication. The patient who experienced anaphylaxis after being bitten by the animal required medical attention in the emergency department, where the patient received adrenaline, parenteral antihistamine, and corticosteroids. Table 2 depicts specific IgE to other animal allergens. Of note, four of the 13 patients included were sensitized to lipocalins from cat, dog, or horse, and five were sensitized to albumins from these animals.
TABLE 1.
Clinical features of patients with allergy to SH
| Patient | Age (years) | Sex | Symptoms | Skin prick test |
Other pet allergy | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| SH |
Other hamsters (hair extract) |
|||||||||
| Hair | Salivary glands | Urine | European | Golden | Roborowski | |||||
| 1 | 46 | Female | Rhinoconjunctivitis + asthma | + | + | + | − | − | + | Dog |
| 2 | 43 | Female | Rhinoconjunctivitis + asthma | + | + | + | − | − | − | Dog |
| 3 | 27 | Female | Rhinoconjunctivitis | + | − | − | − | − | − | Cat |
| 4 | 42 | Female | Rhinoconjunctivitis + asthma | + | + | + | − | − | − | Dog |
| 5 | 10 | Female | Rhinoconjunctivitis + asthma | + | + | + | − | − | − | Dog |
| 6 | 43 | Female | Rhinoconjunctivitis + asthma | + | + | + | − | − | − | Cat |
| 7 | 33 | Female | Anaphylaxis | + | + | + | + | − | − | Cat |
| 8 | 12 | Male | Rhinoconjunctivitis + asthma | + | + | + | − | − | − | Rabbit |
| 9 | 12 | Female | Rhinoconjunctivitis | + | + | + | − | − | − | Cat |
| 10 | 41 | Female | Rhinoconjunctivitis + asthma | + | + | + | − | − | − | |
| 11 | 39 | Female | Rhinoconjunctivitis + asthma | + | + | + | − | − | + | Cat |
| 12 | 39 | Female | Rhinoconjunctivitis + asthma | + | + | + | + | + | + | Cat |
| 13 | 18 | Male | Rhinoconjunctivitis + asthma | + | + | + | + | + | + | Cat |
TABLE 2.
Specific IgE CAP System fluorescent enzyme immunoassay for the most important animal allergens
Values are expressed as allergen-specific kilo-units/liter. Cat allergens are Fel d 1 (uteroglobin), Fel d 2 (serum albumin), Fel d 4 (lipocalin). Dog allergens are Can f 1 (lipocalin), Can f 2 (lipocalin), Can f 3 (serum albumin), and Can f 5 (prostatic dog kallikrein). The horse allergen is Equ c 1 (lipocalin).
| Patient | Previous pet exposure at home | Fel d 1 | Fel d 2 | Fel d 4 | Can f 1 | Can f 2 | Can f 3 | Can f 5 | Equ c 1 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Dog | 0.01 | 0.02 | 0.04 | 0.02 | 0.01 | 0.01 | 0.03 | 0.02 |
| 2 | Dog | 0.04 | 0.08 | 0.05 | 2.37 | 0.04 | 1.04 | 0.05 | 0.02 |
| 3 | Cat | 1.04 | 2.04 | 0.02 | 0.07 | 0.01 | 0.12 | 0.08 | 0.01 |
| 4 | Dog | 0.03 | 0.01 | 0.03 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| 5 | Dog | 0.40 | 1.53 | 4.53 | >100 | 0.14 | 3.38 | 0.90 | 18.7 |
| 6 | Cat | 0.02 | 0.02 | 0.08 | 0.01 | 0.02 | 0.02 | 0.02 | 0.02 |
| 7 | Cat | 2.35 | 3.30 | 0.07 | 0.01 | 0.02 | 1.10 | 0.01 | 0.02 |
| 8 | Rabbit | 1.10 | 0.05 | 0.04 | 0.03 | 0.02 | 0.02 | 0.01 | 0.6 |
| 9 | Cat | 1.30 | 0.05 | 0.06 | 0.03 | 0.02 | 0.03 | 0.04 | 0.20 |
| 10 | None | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 |
| 11 | Cat | 1.94 | 0.00 | 0.01 | 0.00 | 0.00 | 0.02 | 0.00 | 0.01 |
| 12 | Cat | 0.01 | 0.01 | 0.01 | 0.02 | 0.02 | 0.01 | 0.00 | 0.01 |
| 13 | Cat | 4.73 | 2.40 | 0.45 | 0.22 | 0.12 | 0.04 | 0.77 | 4.46 |
Immunoblotting and Cross-reactivity
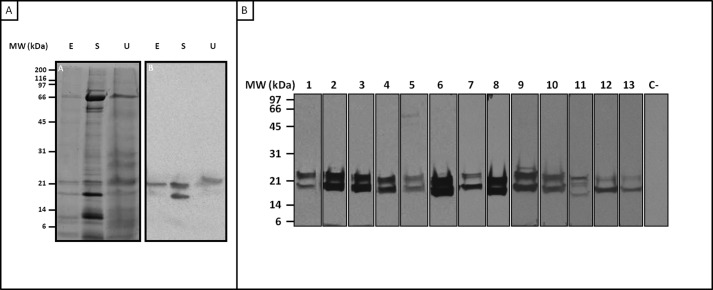
SDS-PAGE of SH extracts showed multiple protein bands with molecular masses ranging from 4 to 150 kDa (Fig. 1A, panel A). Incubation with the serum pool revealed bands with molecular masses of 18, 21, and 23 kDa in hair, salivary gland, and urine extracts, respectively (Fig. 1A, panel B). IgE immunoblotting with patient sera using GSE revealed three IgE-binding bands at 18, 21, and 23 kDa in all patients (Fig. 1B), as described previously (7).
FIGURE 1.
A, SDS-PAGE (panel A) and immunoblotting with a serum pool from SH-allergic patients (panel B) of SH extracts under reducing conditions. E, hair extract; S, salivary glands; U, urine. B, IgE-binding bands in SH salivary gland extract determined by immunoblotting. Lanes 1–13 represent sera from 13 patients. Lane C− represents a negative control of the serum pool from non-atopic patients.
To study cross-reactivity between SH and other hamsters, immunoblot inhibition assays were performed using GSE from SH in solid phase. The serum pool from five SH-allergic patients was incubated with GSE from European, golden, and Roborovski hamsters. Pre-absorption of the serum pool with different GSE resulted in no inhibition of IgE binding (Fig. 2), demonstrating no cross-reactivity between SH and golden and common hamsters. However, there was a partial inhibition between extracts from SH and Roborovski hamsters, showing a possible cross-reactivity.
FIGURE 2.
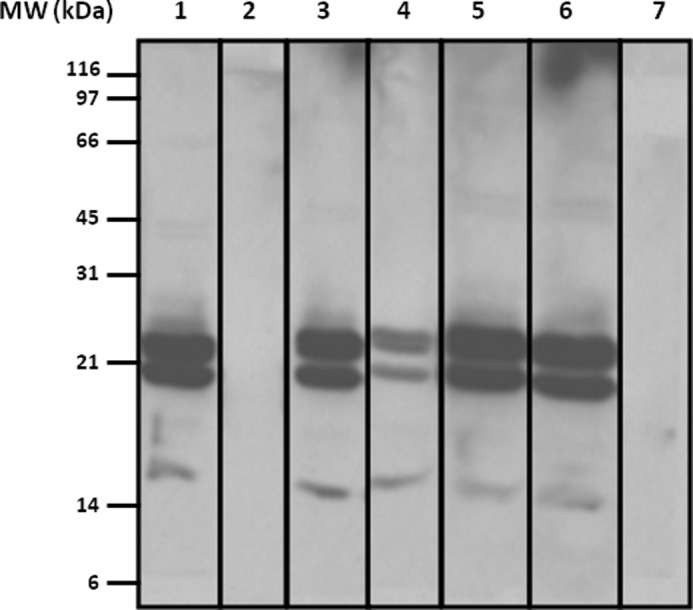
Cross-reactivity between SH and other hamsters. Immunoblot inhibition analysis of SH salivary gland extract (20 μg) with a serum pool from SH-allergic patients. Lane 1, serum pool from SH-allergic patients inhibited with European hamster salivary gland extract; lane 2, serum pool from SH-allergic patients inhibited with SH salivary gland extract; lane 3, serum pool from SH-allergic patients inhibited with golden hamster salivary gland extract; lane 4, serum pool from SH-allergic patients inhibited with Roborovski hamster salivary gland extract; lane 5, serum pool from SH-allergic patients inhibited with BSA; lane 6, a serum pool from SH-allergic patients (non-inhibited); lane 7, serum pool from non-atopic patients.
Cloning of Recombinant Allergen
rPho s 21kD sequence length was 453 bp, encoding for a protein of 151 amino acids. The sequence has been deposited in GenBankTM (accession number KF148615). In Fig. 3, the amino acids sequence of rPho s 21kD is aligned and compared with similar mammalian proteins present in protein databases. The amino acid sequence of rPho s 21kD was clearly assigned to the lipocalin protein family and specifically to the odorant-binding and aphrodisin-like proteins. The lipocalin protein signature GXW was found at positions 8–10 of rPho s 21kD.
FIGURE 3.
Alignment of amino acid sequences of Pho s 21kD (accession number gi 530376029) with different lipocalin-like proteins from rodents (aphrodisin-like (Cricetulus griseus), gi 354502895; odorant-binding protein 3 (OBP3; Myodes glareolus), gi 289474246; aphrodisin (C. cricetus), gi 1168469; and aphrodisin (M. auratus), gi 20177826. Only differences with respect to Pho s 21kD (P. sungorus) are shown.
Allergenicity of rPho s 21kD
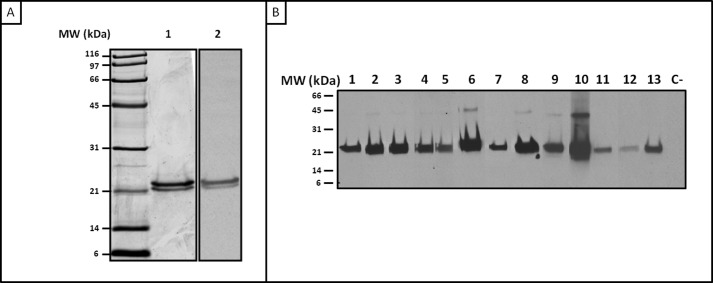
Recombinant proteins were expressed in E. coli with a C-terminal hexahistidine tag and purified as described under “Experimental Procedures.” Two bands were observed by both SDS-PAGE and immunoblot assay at 23 and 21 kDa (Fig. 4A), analyzed by MS/MS, and identified as rPho s 21kD (data not shown). Furthermore, immunoblotting with individual sera was performed using rPho s 21kD. All patients recognized the two bands of rPho s 21kD (Fig. 4B).
FIGURE 4.
A, SDS-PAGE (lane 1) and immunoblotting with a serum pool from SH-allergic patients (lane 2) of rPho s 21kD. B, IgE reactivity to recombinant rPho s 21kD (immunoblot with sera from SH-allergic patients). Lanes 1–13 represent sera from 13 patients. Lane C− represents a negative control of a serum pool from non-atopic patients.
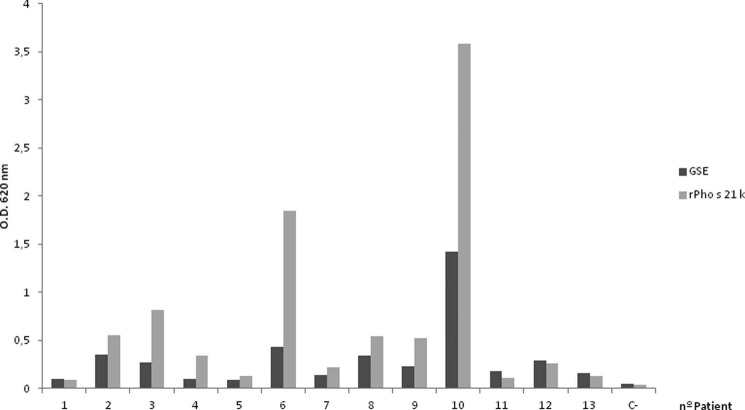
To evaluate rPho s 21kD sensitization of patients, ELISA experiments were performed on sera from 13 patients (Fig. 5). A high correlation was observed between the levels of specific IgE of GSE and rPho S 21kD, suggesting that rPho s 21kD could be an efficient tool in in vitro tests to diagnose SH allergy. Control immunoblot and ELISA experiments with a serum pool from five non-atopic patients did not show IgE-binding measures.
FIGURE 5.
IgE ELISA analysis of specific IgE reactivity to rPho s 21kD versus GSH. IgE reactivity was considered positive when the absorbance at 620 nm was more than or equal to the absorbance at 620 nm of the negative control × 2 (0.098). All tests were performed in duplicate.
Cross-reactivity of rPho s 21kD with Other Hamster Extracts
To study the cross-reactivity of rPho s 21kD with other hamster extracts, IgE ELISA inhibition assay was performed using rPho s 21kD in solid phase. A serum pool from five SH-allergic patients was incubated with GSE from European, golden, and Roborovski hamsters. Pre-absorption of the serum pool with different GSH resulted in no inhibition of IgE binding with golden and common hamsters, demonstrating no cross-reactivity with these hamsters (Fig. 6). Also, seen using GSE, there was a partial inhibition between rPho s 21kD and Roborovski hamsters, showing a possible cross-reactivity.
FIGURE 6.
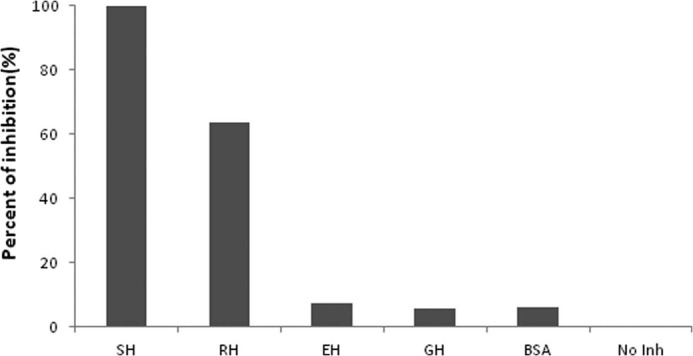
IgE ELISA inhibition assay. Purified rPho s 21kD was used at solid phase, and the serum pool was preincubated with GSE from European hamster (EH), golden hamster (GH), Roborovski hamster (RH), and BSA. No Inh represents an assay with a non-inhibited serum pool. All tests were performed in duplicate.
BasoTest Assays
To confirm the ability of rPho s 21kD to induce a response at the cellular level, a basophil activation test was performed using increasing concentrations of GSE from SH and recombinant proteins in two patients (patients 2 and 12). Activation was 92% (patient 2) and 11% (patient 12) at 10 μg/ml GSE. Using the recombinant form at this concentration, basophil activation was 46% (patient 2) and 10.5% (patient 12). Positive controls were 68% (patient 2) and 26% (patient 12).Negative controls were 3% (patient 2) and 0.24% (patient 12). No activation response was observed with blood from a non-atopic patient, used as a negative control.
DISCUSSION
There are few articles published on hamster allergy. Only the albumin of these animals has been characterized as an allergen (Cri c 4 and Mes a 4; Allergome). In this study, we have reported the first allergenic lipocalin described in any species of hamster. This allergen has been found in SH hair, urine, and salivary glands. This lipocalin has no cross-reactivity with common and golden hamsters, although it does exhibit partial inhibition between extracts from SH and Roborovski hamsters (Fig. 2). This fact could explain why patients with SH allergy are monosensitized to this hamster but not to others. In our study, four patients (30%) who were allergic to SH also presented positive skin tests with the hair, saliva, and urine of Roborovski hamsters. The inhibition assays proved that there was a partial inhibition between extracts from both hamsters, which makes us think there is cross-reactivity between the two species. This could be explained by their phylogenetic proximity because both hamsters belong to the genus Phodopus, also known as the genus of the short-tailed dwarf hamsters.
Pho s 21kD has a high homology (positive between 80 and 91%) to lipocalin-like proteins (aphrodisins) from other hamsters (Fig. 3), but these proteins have not been described as allergens yet. Phylogenetically, the closest allergenic lipocalins described are cloned from guinea pig, with an identity of 38–42% (13).
Five patients had an albumin sensitization to dog or cat by Specific IgE CAP System (Table 2), but only one patient (patient 5) had an IgE-binding band of ∼66 kDa as revealed by immunoblot assay (Fig. 1B). The rest of the patients did not recognize albumins, and thus, there is probably no cross-reactivity between albumins from cat or dog and SH. Although serum albumin shows a high homology between species (14), a remarkable difference in intensity of IgE reactivities with various albumins was observed, indicating the presence of different IgE epitopes (15). Nevertheless, sensitization to allergens from other sources (in this case, from other animals) could predispose patients to sensitization to allergens from other sources such as lipocalins in the case of hamsters (16).
In our study, the major allergen was a lipocalin that is present in hair, urine, or salivary glands. The allergen has an apparent molecular mass of 21 kDa and shows a partial cross-reactivity to the Roborovski hamster according to in vitro studies. As with serum albumin, only four patients had specific IgE to dog, cat, or horse lipocalins, suggesting that Pho s 21kD has no cross-reactivity with these proteins. These data are consistent with the lack of cross-reactivity with other animals as described for Pho s 21kD (7).
SDS-PAGE of purified rPho s 21kD showed a double band in the case of the recombinant form and three in the case of the native protein (Fig. 4A). Because glycosylation modifications were discarded by the expression system, the presence of various bands is most probably due to other post-translational modifications, and the bands with apparent molecular masses of 18 and 21 kDa could be degradation fragments from the mature protein. All these bands were identified as Pho s 21kD by MS analysis and were confirmed by specific recognition using the sera from allergic patients (Fig. 4B). ELISAs with sera from allergic patients demonstrated that rPho s 21kD has a similar IgE reactivity to salivary gland extract (Fig. 5).
The positive basophil activation test with rPho s 21kD and GSE demonstrated the biological activity, confirming that specific IgE is able to elicit basophil degranulation in patients who are allergic to SH. Therefore, we conclude that rPho s 21kD shows a similar pattern of IgE binding and biological activity to GSE. In clinical practice, rPho s 21kD may be very helpful in diagnosing sensitization and may be even more useful in developing a method for specific diagnosis of SH allergy.
Acknowledgments
We thank Oliver Shaw for assistance in editing the English version of the manuscript. Mass spectrometry analyses were carried out in the Proteomics Facility of the Universidad Complutense de Madrid, Parque Científico de Madrid, a member of the ProteoRed-ISCIII Network.
This work was supported by RETIC Red de Investigación de Reacciones Adversas a Alérgenos y Fármacos Grant RD12/0013/0013 and Allergopharma (Merck Serono).
- rPho
- recombinant Pho.
REFERENCES
- 1. Custovic A., Simpson A., Woodcock A. (1998) Importance of indoor allergens in the induction of allergy and elicitation of allergic disease. Allergy 53, 115–120 [DOI] [PubMed] [Google Scholar]
- 2. Pecquet C. (2012) New pets and allergies. Eur. J. Dermatol. 22, 14–22 [DOI] [PubMed] [Google Scholar]
- 3. Díaz-Perales A., González-de-Olano D., Pérez-Gordo M., Pastor-Vargas C. (2013) Allergy to uncommon pets: new allergies but the same allergens. Front. Immunol. 4, 492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bertó J. M., Peláez A., Fernández E., Lombardero M., Ferrer M. (2002) Siberian hamster: a new indoor source of allergic sensitization and respiratory disease. Allergy 57, 155–159 [PubMed] [Google Scholar]
- 5. Niitsuma T., Tsuji A., Nukaga M., Izawa A., Okita M., Maruoka N., Morita S., Tsuyuguchi M. (2003) Two cases of anaphylaxis after dwarf hamster bites. Allergy 58, 1081. [DOI] [PubMed] [Google Scholar]
- 6. Lim D. L., Chan R. M., Wen H., Van Bever H. P., Chua K. Y. (2004) Anaphylaxis after hamster bites–identification of a novel allergen. Clin. Exp. Allergy 34, 1122–1123 [DOI] [PubMed] [Google Scholar]
- 7. Torres J. A., Pastor-Vargas C., de las Heras M., Vivanco F., Cuesta J., Sastre J. (2012) An odorant-binding protein as a new allergen from Siberian hamster (Phodopus sungorus). Int. Arch. Allergy Immunol. 157, 109–112 [DOI] [PubMed] [Google Scholar]
- 8. Dreborg S., Foucard T. (1983) Allergy to apple, carrot and potato in children with birch pollen allergy. Allergy 38, 167–172 [DOI] [PubMed] [Google Scholar]
- 9. Sub-Committee on Skin Tests of the European Academy of Allergology and Clinical Immunology. (1989) Skin tests used in type I allergy testing Position paper. Allergy 44, Suppl. 10, 1–59 [PubMed] [Google Scholar]
- 10. Pastor C., Cuesta-Herranz J., Cases B., Pérez-Gordo M., Figueredo E., de las Heras M., Vivanco F. (2009) Identification of major allergens in watermelon. Int. Arch. Allergy Immunol. 149, 291–298 [DOI] [PubMed] [Google Scholar]
- 11. Cases B., Pastor-Vargas C., Dones F. G., Perez-Gordo M., Maroto A. S., de las Heras M., Vivanco F., Cuesta-Herranz J. (2010) Watermelon profilin: characterization of a major allergen as a model for plant-derived food profilins. Int. Arch. Allergy Immunol. 153, 215–222 [DOI] [PubMed] [Google Scholar]
- 12. Manso L., Polo B., Fernández-Nieto M., Sastre L. B., del Pozo V., Sastre J. (2010) Basophil activation test in a case of systemic hypersensitivity reaction to infliximab with good tolerance to another anti-TNF-α agent (adalimumab). J. Investig. Allergol. Clin. Immunol. 20, 537–538 [PubMed] [Google Scholar]
- 13. Hilger C., Swiontek K., Kler S., Diederich C., Lehners C., Vogel L., Vieths S., Hentges F. (2011) Evaluation of two new recombinant guinea-pig lipocalins, Cav p 2 and Cav p 3, in the diagnosis of guinea-pig allergy. Clin. Exp. Allergy 41, 899–908 [DOI] [PubMed] [Google Scholar]
- 14. Chruszcz M., Mikolajczak K., Mank N., Majorek K. A., Porebski P. J., Minor W. (2013) Serum albumins–unusual allergens. Biochim. Biophys. Acta 1830, 5375–5381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Goubran Botros H., Gregoire C., Rabillon J., David B., Dandeu J. P. (1996) Cross-antigenicity of horse serum albumin with dog and cat albumins: study of three short peptides with significant inhibitory activity towards specific human IgE and IgG antibodies. Immunology 88, 340–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liccardi G., Passalacqua G., Salzillo A., Piccolo A., Falagiani P., Russo M., Canonic G. W., D'Amato G. (2011) Is sensitization to furry animals an independent allergic phenotype in nonoccupationally exposed individuals? J. Investig. Allergol. Clin. Immunol. 21, 137–141 [PubMed] [Google Scholar]