Background: Protein phosphatases exist as multisubunit complexes.
Results: Two protein kinases in endogenous brain protein phosphatase 1I were found to regulate its activation in opposing directions through inhibitor 2 phosphorylation.
Conclusion: These kinases support a signaling cascade that regulates protein phosphatase 1I activation in global cerebral ischemia.
Significance: Understanding the signaling pathways regulating the activity of protein phosphatases is critical to elucidating their physiological and pathological roles.
Keywords: Cell Signaling, Enzyme, Enzyme Mechanism, Ischemia, Protein Phosphorylation, Inhibitor 2
Abstract
Protein phosphatase 1I (PP-1I) is a major endogenous form of protein phosphatase 1 (PP-1) that consists of the core catalytic subunit PP-1c and the regulatory subunit inhibitor 2 (I-2). Phosphorylation of the Thr-72 residue of I-2 is required for activation of PP-1I. We studied the effects of two protein kinases identified previously in purified brain PP-1I by mass spectrometry, Cdc25C-associated kinase 1 (C-TAK1) and PFTAIRE (PFTK1) kinase, for their ability to regulate PP-1I. Purified C-TAK1 phosphorylated I-2 in reconstituted PP-1I (PP-1c·I-2) on Ser-71, which resulted in partial inhibition of its ATP-dependent phosphatase activity and inhibited subsequent phosphorylation of Thr-72 by the exogenous activating kinase GSK-3. In contrast, purified PFTK1 phosphorylated I-2 at Ser-86, a site known to potentiate Thr-72 phosphorylation and activation of PP-1I phosphatase activity by GSK-3. These findings indicate that brain PP-1I associates with and is regulated by the associated protein kinases C-TAK1 and PFTK1. Multisite phosphorylation of the I-2 regulatory subunit of PP-1I leads to activation or inactivation of PP-1I through bidirectional modulation of Thr-72 phosphorylation, the critical activating residue of I-2.
Introduction
Protein phosphatase 1 (PP-1)3 is a ubiquitous multifunctional serine/threonine protein phosphatase. It regulates many neuronal proteins critical to ischemic cell death and protection, including NMDA- and AMPA-type glutamate receptors, voltage-gated Ca2+ and Na+ channels, metabolic enzymes, and components of the apoptotic cell death pathway (1–3). Brain PP-1 exists as hetero-oligomers of the catalytic subunit (PP-1c) associated with targeting proteins, inhibitory regulatory subunits, and/or substrates that confer spatiotemporal and substrate specificity (4, 5). A major form of PP-1 found in the brain is ATP/Mg2+-dependent protein phosphatase 1 (6, 7), also known as protein phosphatase 1I (PP-1I). The detailed mechanisms of PP-1I activation and regulation are currently unclear.
PP-1I consists of PP-1c and the regulatory subunit inhibitor 2 (I-2) that interact to form an inactive complex (8). Both subunits are expressed ubiquitously in the brain (9, 10). PP-1I activation is complex and poorly understood. The endogenous kinases that mediate PP-1I activation and regulation in the brain are unknown, but various protein kinases, including GSK-3, cyclin-dependent kinases, and ERK1, can phosphorylate Thr-72 of I-2 in vitro to activate the reconstituted enzyme complex (11, 12). ATP/Mg2+-dependent phosphorylation and activation of PP-1I is believed to involve relief of inhibition of PP-1c by I-2 via a conformational change in the complex (13).
The identification of endogenous protein kinases that regulate PP-1I phosphatase activity is critical to understand the role of PP-1 in various signal transduction pathways involved in both physiological and pathological processes. For example, we have shown previously that PP-1I is activated in vivo in a pig model of global cerebral ischemia and reperfusion and that the activated enzyme complex copurifies with two endogenous protein kinases, Cdc25C-associated kinase 1 (C-TAK1) and PFTAIRE kinase (PFTK1) (14). Here we show that these copurifying kinases have opposing actions on PP-1I activation and therefore may play a role in increasing phosphatase activity following global ischemia and reperfusion.
EXPERIMENTAL PROCEDURES
Materials
ATP, phosphorylase b, tautomycin, bovine serum albumin, and TBB (4,5,6,7-tetrabromobenzotriazole) were from Sigma-Aldrich (St. Louis, MO). Retinoblastoma protein (Rb) was from Millipore (Billerica, MA). D4476 was from Tocris (Bristol, UK). [γ32P]ATP and nickel-nitrilotriacetic acid-Sepharose were from GE Healthcare (Piscataway, NJ). Purified recombinant human GSK-3β and C-TAK1 were from Upstate (Lake Placid, NY), and casein kinase 1 (CK1) and casein kinase 2 (CK2) were from New England Biolabs (Ipswich, MA). Roscovitine, 6-bromoindirubin-3′-oxime, cdk-5, and cdk-5 substrate, prepared as described previously (15), were provided by Dr. L. Meijer (Roscoff, France).
Enzymes and Substrates
Native PP-1I was purified from freshly harvested pig brain as described previously (14). Recombinant human phosphorylase kinase, PP-1c, and I-2 were overexpressed in BL21 (DE3) Escherichia coli (Invitrogen) as N-terminal His6 proteins using the pTrcHis-Topo vector (Invitrogen) and purified by chromatography on nickel-nitrilotriacetic acid-Sepharose. Human PFTK1 was expressed heterologously in HEK cells by transient transfection. The cDNA of full-length human PFTK1 (GenBankTM accession no. AF119833) was inserted into the mammalian expression vector pcDNA3.1(-minus])/Myc-His (Invitrogen) for expression of PFTK1 with a C-terminal myc epitope in HEK 293FT cells (Invitrogen). These cells were grown on 75-cm2 polycarbonate tissue culture plates in DMEM supplemented with 10% (v/v) fetal bovine serum, 0.1 mm non-essential amino acids, 6 mm l-glutamine, 1 mm sodium pyruvate, 100 units/ml penicillin, 100 μg/ml streptomycin, and 500 μg/ml Geneticin (Invitrogen) at 37 °C in a humidified atmosphere of 95% air and 5% CO2. Transient transfection of pcDNA3.1(−)/Myc-His-PFTK1 was performed using Lipofectamine 2000 (Invitrogen) according to the instructions of the manufacturer, and transfected 293FT cells were lysed with lysis buffer (50 mm Tris (pH 7.5), 150 mm NaCl, 1 mm EDTA, and complete EDTA-free protease inhibitor mixture (Pierce)). PFTK1 was then immunoprecipitated, and the immune complex was used for kinase assays with purified PP-1I and I-2 as substrates. Immunoprecipitation was performed using protein G Dynabeads and 5 μg of myc antibody (Invitrogen) or IgG as a control (Pierce).
Preparation of PP-1I
Native PP-1I was purified as a holoenzyme from freshly harvested pig rostral brain cytosol as described previously (14). PP-1I devoid of activating kinase was reconstituted by incubating purified recombinant PP-1c (300 μg) and I-2 (200 μg) in 50 mm imidazole-Cl (pH 7.2), 0.2 mm EGTA, and 0.1% (v/v) 2-mercaptoethanol at 30 °C for 30 min, followed by chromatography through Superdex 200 (16). Fractions with PP-1c or PP-1I activity, assayed as described in Ref. 12, were pooled and concentrated.
Site-directed Mutagenesis
The full-length human I-2 coding sequence (GenBankTM accession no. NM_006241) was inserted into the pTrcHis vector (Invitrogen) for bacterial expression. Six I-2 mutants (T72A, S86A, S121A, S129A, T184A, and T192A) were generated by PCR using a mutagenesis kit (Stratagene, La Jolla, CA).
Phosphorylation and Phosphatase Assays
[32P]Phosphorylase a was prepared by phosphorylation of phosphorylase b using phosphorylase kinase. PP-1I was assayed for its ability to dephosphorylate [32P]phosphorylase a following activation by preincubation with ATP/Mg2+ as described previously (14). For in vitro phosphorylation assays, recombinant I-2 (2 μg) or Rb (2.5 μm) was incubated with 50 mm imidazole (pH 7.4), 0.2 mm EGTA, 0.1% (v/v) 2-mercaptoethanol, 10 mm MgCl2, 100 μm ATP, and 10 μCi [γ-32P]ATP (PerkinElmer Life Sciences) plus C-TAK1 (0.02 μg), GSK-3β (0.02 μg), C-TAK1 and GSK-3β, PFTK1 immunoprecipitate (0.2 mg of Dynabeads/reaction), CK1 (0.2 units/reaction), or CK2 (0.4 units/reaction) and incubated for 1 h at 30 °C. The inhibitors D4476 and TBB were used at 3 μm. Reactions were stopped by addition of 20 μl of SDS-PAGE sample buffer, and proteins were separated by SDS-PAGE and visualized by autoradiography and protein staining (SYPRO or Coomassie Blue, Invitrogen) as described previously (17, 18). Phosphorylation was expressed as a ratio to control band density from autoradiograms and normalized to protein staining. Autoradiograms and gels were scanned on a flatbed gel scanner (Typhoon Trio, GE Healthcare) and analyzed using National Institutes of Health ImageJ software.
Mass Spectrometry
The PP-1I complex was purified from pig brain, and the individual components were identified by mass spectrometry as described previously (14). For identification of phosphorylation site(s) by mass spectrometry, I-2 phosphorylated by C-TAK1 was separated by SDS-PAGE, stained with Sypro Ruby, excised from the gel, and proteolyzed with trypsin. Then the released tryptic peptides were subjected to phospho-amino acid analysis as described previously (14). Peptides were analyzed by LC electrospray ionization and ion trap MS/MS using an 1100 series LC coupled to an XCT Plus ion trap mass spectrometer (Agilent Technologies, Palo Alto, CA) at the Weill Cornell Medical College Protein Core Facility. Mass spectra were acquired in positive ion mode with automated data-dependent MS/MS on the four most intense ions from precursor MS scans. Analysis of MS/MS spectra was performed by protein database searching with Spectrum Mill bioinformatics software (Agilent Technologies).
Miscellaneous Methods
Protein concentration was determined by the method of Bradford using bovine serum albumin as a standard (19).
Data Analysis
Activity assays and phosphorylation levels included three to four experiments per group. Statistical analyses were performed by two-tailed Student's t test or by one-way analysis of variance followed by Tukey's post hoc test for multiple comparisons using GraphPad Prism version 5 software with a threshold for significance set at p < 0.05. Data, where applicable, are shown as means ± S.E. or S.D. as indicated. Asterisks indicate values significantly different from control groups: *, p < 0.05; **, p < 0.01; ***, p < 0.001.
RESULTS
Activation of PP-1I
PP-1I purified from fresh pig brain was used to identify endogenous components of the PP-1I complex and their regulation of phosphorylase a phosphatase activity. Purified PP-1I was activated markedly by preincubation with ATP/Mg2+ (Fig. 1A), indicating the presence of a copurifying PP-1I-activating kinase. Specific inhibitors of GSK-3 (6-bromoindirubin-3′-oxime, IC50 of 5 nm) or cdk-5 (roscovitine, IC50 of 200 nm), exogenous kinases known to phosphorylate and activate I-2 in vitro (11, 12), did not inhibit the activation of purified brain PP-1I by ATP/Mg2+ (data not shown). Therefore, we focused our attention on the ability of two protein kinases shown previously to copurify with brain PP-1I (14) to regulate PP-1I.
FIGURE 1.
ATP-dependent activation of native PP-1I. A, native PP-1I purified 1120-fold from pig brain (14) was incubated without (■) or with (●) ATP/Mg2+ for 5 min prior to initiation of the phosphatase assay by the addition of [32P]phosphorylase a. Incubation with ATP/Mg2+ increased phosphatase activity ∼5-fold. B, chromatography of purified PP-1I on Superdex 200 showing elution at Mr ∼160,000. PP-1I was assayed using [32P]phosphorylase a as a substrate following preincubation with ATP/Mg2+ and GSK-3β. C, SDS/PAGE analysis of brain PP-1I showing copurification of six major proteins (n = 3).
In addition to the core catalytic subunit PP-1c and the regulatory subunit I-2, purified brain PP-1I contains four major additional proteins, including the two kinases C-TAK1 (81 kDa) and PFTK1 (52 kDa) (Fig. 1, B and C) (14). C-TAK1 is a component of the MAP kinase scaffolding complex and has been identified as a kinase phosphorylating Cdc25, consistent with its ubiquitous expression in the brain (20–22). Similarly, PFTK1 is a cdc2-related kinase highly expressed in the brain (23, 24).
Regulation of Reconstituted PP-1I by C-TAK1 and PFTK1
To determine the effects of these kinases on PP-1I activity, PP-1I devoid of activating kinase was reconstituted from purified recombinant PP-1c and I-2 (Fig. 2, A–D). The reconstituted PP-1I eluted with an apparent molecular mass of 70 kDa by gel filtration (Fig. 2C). C-TAK1 and immunoprecipitated PFTK1 phosphorylated I-2 (Fig. 3A). However, neither C-TAK1 nor PFTK1 directly activated reconstituted PP-1I, whereas it was activated by preincubation with the positive control kinase GSK-3β (Fig. 3, B and C). Interestingly, prior phosphorylation of reconstituted PP-1I by C-TAK1 significantly reduced subsequent activation by GSK-3β (Fig. 3B), indicating that C-TAK1 acts as an inhibitory kinase. Taken together, these results indicate that neither of the copurifying kinases, C-TAK1 and PFTK1, is an endogenous activating kinase. Therefore, these kinases likely phosphorylate I-2 on residues distinct from Thr-72.
FIGURE 2.
Reconstitution of core PP-1I from purified recombinant PP-1c and I-2. A, chromatography of recombinant PP-1αcat on Superdex 200 showing elution at Mr 40. PP-1 activity was assayed using [32P]phosphorylase a as a substrate. B, SDS/PAGE analysis of PP-1c eluted from Superdex 200. C, chromatography of reconstituted PP-1I on Superdex 200 showing elution at Mr 75. PP-1I was assayed using [32P]phosphorylase a as a substrate following preincubation with ATP/Mg2+ and GSK-3β. D, SDS/PAGE analysis of reconstituted PP-1I eluted from Superdex 200 showing coelution of PP-1c and I-2 at Mr ∼70,000 (n = 3).
FIGURE 3.
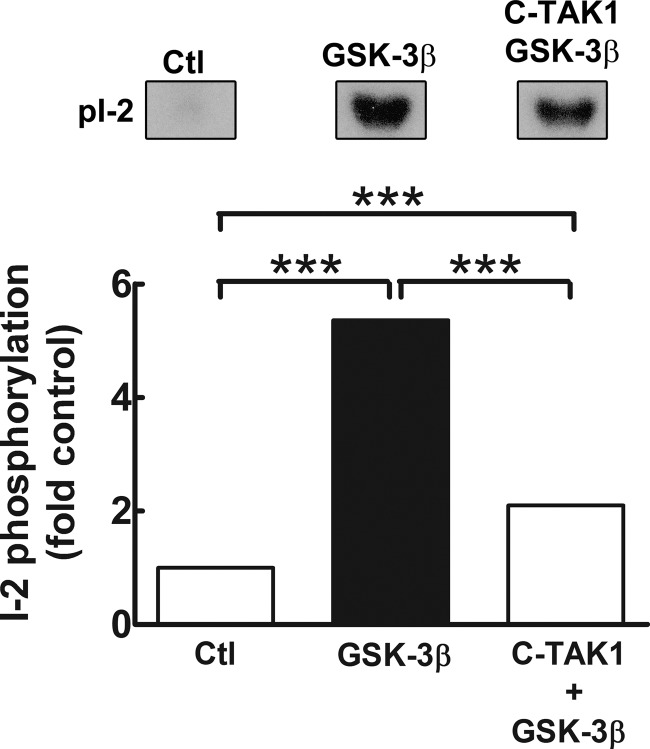
Reconstituted PP-1I is not activated by C-TAK1 or PFTK1. Recombinant I-2 was incubated with Mg2+/[γ-32P]ATP (control (Ctl) and control IgG immunoprecipitate (Ctl IP)) or with Mg2+/[γ-32P]ATP plus C-TAK1 (C-TAK1) or PFTK1 (PFTK1 IP), all including 100 nm tautomycin, for 30 min at 30 °C. Reactants were separated by SDS/PAGE, and the gels were stained, destained, dried, and subjected to autoradiography. Representative autoradiogram (A, top panel) and quantitative densitometric analysis (A, bottom panel) of I-2 phosphorylation (pI-2) (relative to background phosphorylation by control or IgG immunoprecipitate) are shown. Data are mean ± S.E. (n = 3). ***, p < 0.001 versus control (Student's t test). B, reconstituted PP-1I (0.2 μg) assayed in the presence of Mg2+/ATP alone (Ctl) or Mg2+/ATP plus GSK-3β (0.01 μg), C-TAK1 (0.02 μg), or GSK-3β and C-TAK1. C, reconstituted PP-1I (0.2 μg) assayed for phosphatase activity in the presence of Mg2+/ATP (control), Mg2+/ATP plus IgG IP (control), Mg2+/ATP plus GSK-3 β (0.01 μg), or PFTK1 immunoprecipitate. C-TAK1 or PFTK1 did not activate reconstituted PP-1I. However, prior phosphorylation of I-2 by C-TAK1 reduced subsequent activation of PP-1I by GSK-3 β (Β). Data are mean ± S.E. (n = 3). ***, p < 0.001 versus control (one-way analysis of variance).
Identification of the C-TAK1 Phosphorylation Site
The phosphorylated tryptic peptide fragment corresponding to amino acid residues IDEPSTPYHSMMGDDEDACSDTEATEAMAPDILAR plus a phosphoryl group (m/z = 1313.53+) was identified in I-2 phosphorylated by C-TAK1. Analysis of the MS/MS spectra of the phospho-I-2 fragmentation pattern showed the presence of two subfragments (y272+, m/z = 1477.7; y322+, m/z = 1790.1) corresponding to HSMMGDDEDACSDTEATEAMAPDILAR and p(STPY)HSMMGDDEDACSDTEATEAMAPDILAR, respectively, indicating that the site of phosphorylation is localized within STPY (Fig. 4). Phospho-amino acid analysis of I-2 phosphorylated by C-TAK1 identified only phosphoserine (data not shown). Therefore, the site in I-2 phosphorylated by C-TAK1 is Ser-71. Because C-TAK1 reduced subsequent activation by GSK-3β (Fig. 3B) of reconstituted PP-1I, we tested whether this corresponded to phosphorylation of I-2 Thr-72. Phosphorylation of I-2 by C-TAK1 at Ser-71 reduced subsequent phosphorylation of Thr-72 by GSK-3 (Fig. 5). These results indicate that C-TAK1 functions as an inhibitory protein kinase by phosphorylating an adjacent residue, therefore reducing PP-1I activation via Thr-72 phosphorylation.
FIGURE 4.
MS/MS spectra of the tryptic phosphopeptide from I-2 phosphorylated by C-TAK1. I-2 was phosphorylated with C-TAK1, subjected to SDS/PAGE, excised, and digested with trypsin. Liberated peptides were then analyzed by LC electrospray ionization MS/MS. MS/MS spectra of a phosphopeptide (m/z = 1313.53+) were obtained and analyzed. b and y ions are marked in the spectra. The amino acid sequence of the phosphopeptide is shown at the top. Subfragment ion y272+ (p(STPY) HSMMGDDEDACSDTEATEAMAPDILAR, m/z = 1790.1) was identified in the phosphopeptide preparation, indicating that the site phosphorylated by C-TAK1 is within STPY. The phosphorylated residue was confirmed as Ser-71 by phospho-amino acid analysis. C*, acrylamide-modified cysteine.
FIGURE 5.
Phosphorylation of I-2 by C-TAK1 inhibits subsequent phosphorylation by GSK-3β. I-2 (2 μg) was incubated with Mg2+/[γ-32P]ATP (control (Ctl), top left panel), Mg2+/[γ-32P]ATP plus GSK-3β (0.02 μg) (top center panel), or Mg2+/ATP plus C-TAK1 (0.02 μg) for 30 min and then with Mg2+/[γ-32P]ATP plus GSK-3β (0.02 μg) (top right panel). Reactants were separated by SDS/PAGE, and the gels were stained with Coomassie Blue, destained, dried, and subjected to autoradiography (top panels) and quantitative analysis (bottom panel). Prior phosphorylation of I-2 by C-TAK1 reduced subsequent phosphorylation by GSK-3β. Data are mean ± S.E. (n = 3). ***, p < 0.001 versus control (one-way analysis of variance).
Identification of the PFTK1 Phosphorylation Site
Phosphorylation of I-2 by isolated PFTK1 occurred at low stoichiometry (Fig. 6A). Therefore, serial alanine scanning mutagenesis was necessary to determine the site of I-2 phosphorylated by PFTK1. A NetPhosK 1.0 search with a threshold of 0.45 identified 13 putative sites for phosphorylation by CDKs among the 33 Ser/Thr residues in the 204-amino acid human I-2 sequence (25). Six possible sites were selected for further evaluation as potential PFTK1 phosphorylation sites by site-directed mutagenesis on the basis of NetPhosK high predictive scores (Thr-72, Ser-121, Ser-129, Thr-184, and Thr-192) or prior identification as I-2 phosphorylation sites in the Protein Knowledge Base (Ser-86, Thr-72, and Ser-121; UniProtKB IPP2_HUMAN P41236). Of the six residues selected, there was no significant difference in phosphorylation by PFTK1 upon mutation of five sites (Thr-72, Ser-121, Ser-129, Thr-184, and Thr-192) to alanine, indicating that these are not PFTK1 phosphorylation sites (Fig. 6B). However, the S86A mutation reduced I-2 phosphorylation by PFTK1 by 58 ± 2.5% compared with wild-type I-2, establishing this as a major site of phosphorylation by PFTK1 (Fig. 6C, left). In agreement with our finding that Thr-72 is not a PFTK1 phosphorylation site, PFTK1 did not activate reconstituted PP-1I (Fig. 3C). The PFTK1 phosphorylation site Ser-86 is also phosphorylated by either CK1 or CK2 (26–29). To rule out Ser-86 phosphorylation by contaminating CK1 or CK2 in the immunoprecipitated PFTK1 preparation, the CK1 inhibitor D4476 (30) or the CK2 inhibitor TBB (31) was included in kinase assays. The S86A mutation decreased phosphorylation of I-2 by both purified CK1 and CK2 as expected (Fig. 6C, center and right). D4476 (3 μm) significantly inhibited I-2 phosphorylation by CK1 but did not affect phosphorylation of I-2 by PFTK1 (Fig. 6D). Similarly, TBB (3 μm) significantly inhibited I-2 phosphorylation by CK2 but did not affect phosphorylation of I-2 by PFTK1 (Fig. 6D). Therefore, phosphorylation of I-2 by PFTK1 was not due to contamination of the PFTK1 immunoprecipitate with CK1 or CK2. Furthermore, although both CK1 and CK2 were present in HEK 293FT cell lysate, they were not detected in the PFTK1 immunoprecipitate by immunoblotting (data not shown). Together, these results indicate that PFTK1 phosphorylates I-2 at Ser-86. Phosphorylation of I-2 Ser-86 by CK2 has been shown previously to potentiate activation of PP-1I by the activating kinase GSK-3β (26, 29). On the basis of these studies, PFTK1 should activate PP-1I indirectly by facilitating Thr-72 phosphorylation. However, because in vitro phosphorylation studies are limited, we cannot rule out that minor sites other than Ser-86 also contribute functionally to PP-1I modulation by PFTK1.
FIGURE 6.
PFTK1 phosphorylates I-2 at Ser-86. A, stoichiometry of the phosphorylation of I-2 and Rb (positive control) by PFTK1. PFTK1 phosphorylated I-2 to one-fourth the stoichiometry of Rb phosphorylation. Data are mean ± S.E. (n = 3). B, recombinant I-2 WT and five Ser/Thr → Ala mutants were incubated with Mg2+/[γ-32P]ATP and PFTK1 for 1 h at 30 °C. The I-2 reactants were separated by SDS/PAGE, and the gels were stained with Sypro protein stain and subjected to autoradiography. Phosphorylation was calculated as a percentage of WT band density from autoradiograms and normalized to Sypro protein staining. Densitometric analysis of I-2 WT and mutant phosphorylation by PFTK1 are shown. Data are mean ± S.E. (n = 2). C, representative autoradiograms (top panel) and quantitative densitometric analysis (bottom panel) from in vitro phosphorylation assays of I-2 WT and S86A mutant I-2 phosphorylated by PFTK1, CK1, or CK2. IP, immunoprecipitate. D, phosphorylation of I-2 WT by PFTK1, CK1, or CK2 in the presence of the CK1 inhibitor D4476 (D) or the CK2 inhibitor TBB (T). N, no inhibitor. Data are mean ± S.E. (n = 4). ***, p < 0.001 versus WT or without inhibitor (Student's t test).
DISCUSSION
Native PP-1I isolated from fresh pig brain consists of the catalytic subunit PP-1c, the inhibitory regulatory protein I-2, and several potential regulatory proteins. To preserve the integrity of the native PP-1I complex, it must be purified from the brain immediately following death because of rapid post-mortem loss of activity (Ref. 14 and data not shown). The finding that purified PP-1I is activated by incubation with ATP/Mg2+ indicates the presence of a copurifying activating kinase. Six major proteins have been identified in purified PP-1I, two of which are protein kinases (14). Here we report that the two copurifying kinases, C-TAK1 and PFTK1, phosphorylate I-2 at sites that can modulate phosphorylation of the activating residue Thr-72. This is consistent with a role for this multisite phosphorylation in the regulation of endogenous brain PP-1I in global cerebral ischemia.
Neither of the protein kinases identified by proteomic analysis of purified brain PP-1I phosphorylated I-2 at Thr-72 or activated reconstituted PP-1I. Because reconstituted PP-1I was activatable by GSK-3β, the activating kinase must be present in the native complex at a lower molar ratio, or the complex is so labile that it was not detected in our proteomic analysis (14). Future studies involving alternative approaches to analyzing the PP-1I holoenzyme will be required to identify the endogenous copurifying kinase.
The activating kinase for brain PP-1I has been proposed to be GSK-3 (6). However, the endogenous activating kinase present in purified pig brain PP-1I is not GSK-3 because the specific GSK-3 inhibitor 6-bromoindirubin-3′-oxime did not prevent PP-1I activation. This is supported by an analysis of Thr-72 phosphorylation in HeLa cells in which the kinase appeared to be a cyclin-dependent kinase (32). However, the specific cdk-5 inhibitor roscovitine also did not prevent PP-1I activation. MAP kinase has been implicated in growth factor-induced activation of PP-1I (12), but has not been implicated as the activating kinase in brain, and was not identified in the PP-1I complex by mass spectrometry.
The two protein kinases that copurify with PP-1I, C-TAK1 and PFTK1 (14), had opposing effects on the modulation of PP-1I activity. C-TAK1 acts as an inhibitory kinase by phosphorylating I-2 at a novel site (Ser-71) to reduce subsequent phosphorylation of the adjacent Thr-72, probably through steric and electrostatic hindrance by the phosphoryl group. In contrast, PFTK1 phosphorylates I-2 at Ser-86, a site known to potentiate PP-1I activation (26, 29). Therefore, the PP-1I complex includes kinases capable of bidirectional modulation of PP-1I activity through phosphorylation of the regulatory subunit I-2. A role for these sites in PP-1I regulation is consistent with the previous finding that endogenous I-2 is phosphorylated on at least one residue other than Thr-72 between residues 70 and 90 by a kinase other than GSK-3 (28). On the basis of the expression of PP-1I and its regulatory components in the brain and their copurification in brain PP-1I, both kinases could play a role in regulating endogenous PP-1I activity. In the basal state, C-TAK1 phosphorylation of Ser-71 would keep PP-1I activity low. Activation of PFTK1, possibly through a cyclin pathway (23), leading to phosphorylation of Ser-86 would increase PP-1I activity.
Endogenous PP-1I activation is complex and has not been verified in in vivo studies. In vitro studies show that phosphorylation of Thr-72 on I-2 converts PP1c to its active conformation. When active, PP1c dephosphorylates I-2 before it can dephosphorylate exogenous substrates (33–38). This complex activation mechanism is highly conserved across species (39, 40), suggesting a physiological role. The presence of PP1c and I-2 in our purified holoenzyme and the necessity for Mg2+/ATP for activation indicates that endogenous PP-1I is most likely regulated in a similar manner. However, it is important to point out that direct evidence for phosphorylation of Thr-72 of I-2 in vivo is limited. Analysis of rabbit skeletal muscle I-2 by fast atom bombardment mass spectrometry revealed only serine phosphorylation (41). This has been corroborated by immunoprecipitation of I-2 extracts also showing that 90–95% of phosphorylation was on seryl residues (42). This is consistent with the transient nature of Thr-72 phosphorylation in the mechanism of PP-1I activation.
Previous work has implicated PP-1 in the pathobiochemistry of cerebral ischemia. For example, we have shown previously that PP-1I activity increases following global cerebral ischemia/reperfusion in vivo (14). Moreover, PP-1 controls critical neuroprotective and cell death pathways in the brain (43). In PP-1I purified from control and ischemic brain, the amounts of C-TAK1 and PFTK1 were decreased or increased, respectively (14). This is consistent with multisite phosphorylation changes in I-2 leading to increased PP-1I activation following global cerebral ischemia. Because C-TAK1 inhibits PP-1I activation, although PFTK1 facilitates PP-1I activation, these kinases could contribute to a signaling cascade that results in increased PP-1I activity following global cerebral ischemia.
PP-1c interacts with and dephosphorylates critical components of the apoptotic cell death pathway, including Rb, Bcl-2, Bcl-XL, Bcl-w, and Bad, implicating PP-1 in the control of cell death (44, 45). 14-3-3 prevents Bad, an essential proapoptotic factor, from binding to and inactivating the antiapoptotic factor Bcl-XL in a phosphorylation-dependent manner. The finding that 14-3-3 interacts with purified (14) and reconstituted PP-1I provides a mechanism for targeting of PP-1I to cell death effectors (14) and implicates PP-1I in the dephosphorylation of Bad, a key step in the initiation of apoptosis. C-TAK1 is a ubiquitously expressed serine/threonine kinase involved in various cellular functions through generation of 14-3-3 binding sites (46). Therefore, in addition to inhibition of PP-1I phosphatase activity through Ser-71 phosphorylation, a mechanism involving PP-1I, 14-3-3, and C-TAK1 could also regulate activation of PP-1I in ischemia. Phosphorylation of I-2 Ser-86 could be an important positive regulatory step involved in the synergistic activation of PP-1I by PFTK1 and an activating kinase. The resulting increase in PP-1 activity would activate apoptosis following global cerebral ischemia through dephosphorylation of cell death regulators such as Bad and Rb (45, 47) (Fig. 7). Further studies are warranted to address the role of these kinases in the regulation of PP-1I activity following global cerebral ischemia.
FIGURE 7.

Schematic models of PP-1I activation relative to I-2 phosphorylation. I-2 inhibition (inactive) of PP-1c is relieved when Thr-72 of I-2 is phosphorylated (active). Prior phosphorylation of Ser-71 reduces phosphorylation of Thr-72 (low) and, therefore, reduces PP-1I activity. In contrast, prior phosphorylation of Ser-86 results in increased phosphorylation of Thr-72 (high) and, therefore, increases PP-1I activity. Under ischemic conditions, with increased PFTK1 activity and reduced CTAK1 activity, both of these phosphorylation events would lead to enhanced phosphorylation of I-2 Thr-72 (ischemia) and PP-1I activity (very high). Stoichiometry of phosphorylation is proportional to symbol size.
Acknowledgments
We thank Dr. H. Y. L. Tung (New York, NY) for contributions, including the mass spectrometry analysis, and Dr. L. Meijer (Roscoff, France) for cdk-5, cdk-5 substrate, 6-bromoindirubin-3′-oxime, and roscovitine.
This work was supported, in whole or in part, by National Institutes of Health Grant NS 56315. This work was also supported by the Weill Cornell Medical College Department of Anesthesiology.
- PP
- protein phosphatase
- PP-1c
- protein phosphatase 1 catalytic subunit
- I-2
- inhibitor 2
- TBB
- 4,5,6,7-tetrabromobenzotriazole
- Rb
- retinoblastoma protein.
REFERENCES
- 1. Cohen P. T. (2002) Protein phosphatase 1: targeted in many directions. J. Cell Sci. 115, 241–256 [DOI] [PubMed] [Google Scholar]
- 2. Ceulemans H., Bollen M. (2004) Functional diversity of protein phosphatase-1, a cellular economizer and reset button. Physiol. Rev. 84, 1–39 [DOI] [PubMed] [Google Scholar]
- 3. Munton R. P., Vizi S., Mansuy I. M. (2004) The role of protein phosphatase-1 in the modulation of synaptic and structural plasticity. FEBS Lett. 567, 121–128 [DOI] [PubMed] [Google Scholar]
- 4. Peti W., Nairn A. C., Page R. (2013) Structural basis for protein phosphatase 1 regulation and specificity. FEBS J. 280, 596–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bollen M., Peti W., Ragusa M. J., Beullens M. (2010) The extended PP1 toolkit: designed to create specificity. Trends Biochem. Sci. 35, 450–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yang S. D., Fong Y. L. (1985) Identification and characterization of an ATP. Mg-dependent protein phosphatase from pig brain. J. Biol. Chem. 260, 13464–13470 [PubMed] [Google Scholar]
- 7. Yang S. D. (1986) Identification of the ATP. Mg-dependent protein phosphatase activator (FA) as a myelin basic protein kinase in the brain. J. Biol. Chem. 261, 11786–11791 [PubMed] [Google Scholar]
- 8. Resink T. J., Hemmings B. A., Tung H. Y., Cohen P. (1983) Characterisation of a reconstituted Mg-ATP-dependent protein phosphatase. Eur. J. Biochem. 133, 455–461 [DOI] [PubMed] [Google Scholar]
- 9. da Cruz e Silva E. F., Fox C. A., Ouimet C. C., Gustafson E., Watson S. J., Greengard P. (1995) Differential expression of protein phosphatase 1 isoforms in mammalian brain. J. Neurosci. 15, 3375–3389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tanimukai H., Grundke-Iqbal I., Iqbal K. (2004) Inhibitors of protein phosphatase-2A: topography and subcellular localization. Brain Res. Mol. Brain Res. 126, 146–156 [DOI] [PubMed] [Google Scholar]
- 11. Hemmings B. A., Resink T. J., Cohen P. (1982) Reconstitution of a Mg-ATP-dependent protein phosphatase and its activation through a phosphorylation mechanism. FEBS Lett. 150, 319–324 [DOI] [PubMed] [Google Scholar]
- 12. Wang Q. M., Guan K. L., Roach P. J., DePaoli-Roach A. A. (1995) Phosphorylation and activation of the ATP-Mg-dependent protein phosphatase by the mitogen-activated protein kinase. J. Biol. Chem. 270, 18352–18358 [DOI] [PubMed] [Google Scholar]
- 13. Hurley T. D., Yang J., Zhang L., Goodwin K. D., Zou Q., Cortese M., Dunker A. K., DePaoli-Roach A. A. (2007) Structural basis for regulation of protein phosphatase 1 by inhibitor-2. J. Biol. Chem. 282, 28874–28883 [DOI] [PubMed] [Google Scholar]
- 14. Platholi J., Heerdt P. M., Lim Tung H. Y., Hemmings H. C., Jr. (2008) Activation of brain protein phosphatase-1(I) following cardiac arrest and resuscitation involving an interaction with 14-3-3 γ. J. Neurochem. 105, 2029–2038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Meijer L., Skaltsounis A. L., Magiatis P., Polychronopoulos P., Knockaert M., Leost M., Ryan X. P., Vonica C. A., Brivanlou A., Dajani R., Crovace C., Tarricone C., Musacchio A., Roe S. M., Pearl L., Greengard P. (2003) GSK-3-selective inhibitors derived from Tyrian purple indirubins. Chem. Biol. 10, 1255–1266 [DOI] [PubMed] [Google Scholar]
- 16. Tung H. Y., Cohen P. (1984) The protein phosphatases involved in cellular regulation: comparison of native and reconstituted Mg-ATP-dependent protein phosphatases from rabbit skeletal muscle. Eur. J. Biochem. 145, 57–64 [DOI] [PubMed] [Google Scholar]
- 17. Laemmli U. K. (1970) Cleavage of structural proteins during the assembly of the head of the bacteriophage T4. Nature 227, 680–685 [DOI] [PubMed] [Google Scholar]
- 18. Berggren K., Chernokalskaya E., Steinberg T. H., Kemper C., Lopez M. F., Diwu Z., Haugland R. P., Patton W. F. (2000) Background-free, high sensitivity staining of proteins in one- and two-dimensional sodium dodecyl sulfate-polyacrylamide gels using a luminescent ruthenium complex. Electrophoresis 21, 2509–2521 [DOI] [PubMed] [Google Scholar]
- 19. Bradford M. M. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254 [DOI] [PubMed] [Google Scholar]
- 20. Müller J., Ory S., Copeland T., Piwnica-Worms H., Morrison D. K. (2001) C-TAK1 regulates Ras signaling by phosphorylating the MAPK scaffold, KSR1. Mol. Cell 8, 983–993 [DOI] [PubMed] [Google Scholar]
- 21. Peng C. Y., Graves P. R., Ogg S., Thoma R. S., Byrnes M. J., 3rd, Wu Z., Stephenson M. T., Piwnica-Worms H. (1998) C-TAK1 protein kinase phosphorylates human Cdc25C on serine 216 and promotes 14-3-3 binding. Cell Growth & Differ. 9, 197–208 [PubMed] [Google Scholar]
- 22. Ogg S., Gabrielli B., Piwnica-Worms H. (1994) Purification of a serine kinase that associates with and phosphorylates human Cdc25C on serine 216. J. Biol. Chem. 269, 30461–30469 [PubMed] [Google Scholar]
- 23. Besset V., Rhee K., Wolgemuth D. J. (1998) The identification and characterization of expression of Pftaire-1, a novel Cdk family member, suggest its function in the mouse testis and nervous system. Mol. Reprod. Dev. 50, 18–29 [DOI] [PubMed] [Google Scholar]
- 24. Lazzaro M. A., Albert P. R., Julien J. P. (1997) A novel cdc2-related protein kinase expressed in the nervous system. J. Neurochem. 69, 348–364 [DOI] [PubMed] [Google Scholar]
- 25. Blom N., Sicheritz-Pontén T., Gupta R., Gammeltoft S., Brunak S. (2004) Prediction of post- translational glycosylation and phosphorylation of proteins from the amino acid sequence. Proteomics 4, 1633–1649 [DOI] [PubMed] [Google Scholar]
- 26. DePaoli-Roach A. A. (1984) Synergistic phosphorylation and activation of ATP- Mg-dependent phosphoprotein phosphatase by F A/GSK-3 and casein kinase II (PC0.7). J. Biol. Chem. 259, 12144–12152 [PubMed] [Google Scholar]
- 27. Holmes C. F., Kuret J., Chisholm A. A., Cohen P. (1986) Identification of the sites on rabbit skeletal muscle protein phosphatase inhibitor-2 phosphorylated by casein kinase-II. Biochim. Biophys. Acta 870, 408–416 [DOI] [PubMed] [Google Scholar]
- 28. Agostinis P., Marin O., James P., Hendrix P., Merlevede W., Vandenheede J. R., Pinna L. A. (1992) Phosphorylation of the phosphatase modulator subunit (inhibitor-2) by casein kinase-1: identification of the phosphorylation sites. FEBS Lett. 305, 121–124 [DOI] [PubMed] [Google Scholar]
- 29. Park I. K., Roach P., Bondor J., Fox S. P., DePaoli-Roach A. A. (1994) Molecular mechanism of the synergistic phosphorylation of phosphatase inhibitor-2: cloning, expression, and site-directed mutagenesis of inhibitor-2. J. Biol. Chem. 269, 944–954 [PubMed] [Google Scholar]
- 30. Rena G., Bain J., Elliott M., Cohen P. (2004) D4476, a cell-permeant inhibitor of CK1, suppresses the site-specific phosphorylation and nuclear exclusion of FOXO1a. EMBO Rep. 5, 60–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sarno S., Reddy H., Meggio F., Ruzzene M., Davies S. P., Donella-Deana A., Shugar D., Pinna L. A. (2001) Selectivity of 4,5,6,7-tetrabromobenzotriazole, an ATP site-directed inhibitor of protein kinase CK2 (casein kinase-2). FEBS Lett. 496, 44–48 [DOI] [PubMed] [Google Scholar]
- 32. Leach C., Shenolikar S., Brautigan D. L. (2003) Phosphorylation of phosphatase inhibitor-2 at centrosomes during mitosis. J. Biol. Chem. 278, 26015–26020 [DOI] [PubMed] [Google Scholar]
- 33. Jurgensen S., Shacter E., Huang C. Y., Chock P. B., Yang S. D., Vandenheede J. R., Merlevede W. (1984) On the mechanism of activation of the ATP X Mg(II)-dependent phosphoprotein phosphatase by kinase FA. J. Biol. Chem. 259, 5864–5870 [PubMed] [Google Scholar]
- 34. Price D. J., Li H. C. (1985) Activation of bovine heart ATP-Mg2+-dependent phosphoprotein phosphatase: isolation of a phosphoenyzme intermediate and its conversion to the active form via a Mg2+-dependent autodephosphorylation reaction. Biochem. Biophys. Res. Commun. 128, 1203–1210 [DOI] [PubMed] [Google Scholar]
- 35. Villa-Moruzzi E., Ballou L. M., Fischer E. H. (1984) Phosphorylase phosphatase: interconversion of active and inactive forms. J. Biol. Chem. 259, 5857–5863 [PubMed] [Google Scholar]
- 36. Vandenheede J. R., Yang S. D., Merlevede W., Jurgensen S., Chock P. B. (1985) Kinase FA- mediated regulation of rabbit skeletal muscle protein phosphatase. Reversible phosphorylation of the modulator subunit. J. Biol. Chem. 260, 10512–10516 [PubMed] [Google Scholar]
- 37. Li H. C., Price D. J., Tabarini D. (1985) On the mechanism of regulation of type I phosphoprotein phosphatase from bovine heart. Regulation by a novel intracyclic activation-deactivation mechanism via transient phosphorylation of the regulatory subunit by phosphatase-1 kinase (FA). J. Biol. Chem. 260, 6416–6426 [PubMed] [Google Scholar]
- 38. Ballou L. M., Fischer E. H. (1986) in Control by Phosphorylation: The Enzymes (Boyer P. D., Kreb E. G., eds) p. 331, Academic Press, Orlando, FL [Google Scholar]
- 39. Orgad S., Dudai Y., Cohen P. (1987) The protein phosphatases of Drosophila melanogaster and their inhibitors. Eur. J. Biochem. 164, 31–38 [DOI] [PubMed] [Google Scholar]
- 40. Pondaven P., Cohen P. (1987) Identification of protein phosphatases-1 and 2A and inhibitor-2 in oocytes of the starfish Asterias rubens and Marthasterias glacialis. Eur. J. Biochem. 167, 135–140 [DOI] [PubMed] [Google Scholar]
- 41. Holmes C. F., Tonks N. K., Major H., Cohen P. (1987) Analysis of the in vivo phosphorylation state of protein phosphatase inhibitor-2 from rabbit skeletal muscle by fast-atom bombardment mass spectrometry. Biochim. Biophys. Acta 929, 208–219 [DOI] [PubMed] [Google Scholar]
- 42. Lawrence J. C., Jr., Hiken J., Burnette B., DePaoli-Roach A. A. (1988) Phosphorylation of phosphoprotein phosphatase inhibitor-2 (I-2) in rat fat cells. Biochem. Biophys. Res. Commun. 150, 197–203 [DOI] [PubMed] [Google Scholar]
- 43. Hédou G. F., Koshibu K., Farinelli M., Kilic E., Gee C. E., Kilic U., Baumgärtel K., Hermann D. M., Mansuy I. M. (2008) Protein phosphatase 1-dependent bidirectional synaptic plasticity controls ischemic recovery in the adult brain. J. Neurosci. 28, 154–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ayllón V., Cayla X., García A., Fleischer A., Rebollo A. (2002) The anti- apoptotic molecules Bcl-xL and Bcl-w target protein phosphatase 1α to Bad. Eur. J. Immunol. 32, 1847–1855 [DOI] [PubMed] [Google Scholar]
- 45. Wang R. H., Liu C. W., Avramis V. I., Berndt N. (2001) Protein phosphatase 1α-mediated stimulation of apoptosis is associated with dephosphorylation of the retinoblastoma protein. Oncogene 20, 6111–6122 [DOI] [PubMed] [Google Scholar]
- 46. Müller J., Ritt D. A., Copeland T. D., Morrison D. K. (2003) Functional analysis of C-TAK1 substrate binding and identification of PKP2 as a new C-TAK1 substrate. EMBO J. 22, 4431–4442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ayllón V., Martínez A. C., García A., Cayla X., Rebollo A. (2000) Protein phosphatase 1α is a Ras-activated Bad phosphatase that regulates interleukin-2 deprivation-induced apoptosis. EMBO J. 19, 2237–2246 [DOI] [PMC free article] [PubMed] [Google Scholar]