Background: Mitotic progression is regulated by reversible protein phosphorylation involving kinases and phosphatases.
Results: Pnuts functions as a master regulator of mitosis by modulating PP1; Pnuts expression peaks in mitosis and is degraded at mitotic exit.
Conclusion: This study reveals the function and regulation of a new and essential mitotic regulator.
Significance: This study improves our understanding of M-phase regulation.
Keywords: Cell Cycle, Mitosis, Phosphatase, Phosphoprotein Phosphatase 1 (PP1), Xenopus, APC/C, Pnuts
Abstract
Mitotic progression is regulated largely through dynamic and reversible protein phosphorylation that is modulated by opposing actions of protein kinases and phosphatases. In this study, we show that phosphatase 1 nuclear targeting subunit (Pnuts) functions as a master regulator of mitosis by modulating protein phosphatase 1 (PP1). Overexpression of Pnuts in Xenopus egg extracts inhibited both mitotic and meiotic exit. Immunodepletion of Pnuts from egg extracts revealed its essential functions in mitotic entry and maintenance. The level of Pnuts oscillates during the cell cycle and peaks in mitosis. Pnuts destruction during M-phase exit is mediated by the anaphase-promoting complex/cyclosome (APC/C)-targeted ubiquitination and proteolysis, and conserved destruction motifs of Pnuts. Disruption of Pnuts degradation delayed M-phase exit, suggesting it as an important mechanism to permit M-phase exit.
Introduction
As a fundamental process of life, cell division in M-phase is tightly regulated and evolutionarily conserved. Mitotic defects are often associated with human diseases, particularly cancer, whereas antimitotic agents are among the most successful drugs for cancer therapy (1). Mitotic entry, progression, and exit involve extensive cellular reorganization that is programmed and regulated through sophisticated molecular mechanisms. A multitude of existing studies stress the importance of mitotic phosphorylation that occurs on hundreds of substrate proteins in a spatially and temporally defined manner. Consistently, a group of Ser/Thr kinases has been well recognized as central regulators of mitosis (2). The maturation promoting factor (MPF), composed of cyclin-dependent kinase 1 (Cdk1)4 and its activator, cyclin B, is regarded as the principal mitotic kinase. The activity of Cdk1 is responsible for mitotic phosphorylation of a broad spectrum of substrates that control virtually all aspects of M-phase progression. Accordingly, regulated activation and deactivation of Cdk1 have been well characterized as molecular events that dictate M-phase entry and exit (3). To a lesser extent, Plk1, Aurora A, Aurora B, and several other mitotic kinases have also been shown to function as important M-phase regulators. Disrupting the function of these kinases leads to various defects in mitotic progression (4).
As all reversible phosphorylation events are regulated by counteracting protein kinases and protein phosphatases, there is a clear rationale for an essential regulatory role played by protein phosphatases in M-phase. However, in contrast to that of protein kinases, the specific involvement of phosphatases in M-phase regulation only recently came to light (5–7). It has been shown in budding yeast that the dual specificity phosphatase Cdc14 plays a critical role in promoting mitotic exit through dephosphorylation of Cdk1 substrates (8). However, the mitotic function of Cdc14 in budding yeast does not seem to be fully conserved in higher organisms, suggesting the existence of alternative Cdk1-counteracting phosphatases, particularly protein phosphatase 1 (PP1) and PP2A (6, 7, 9). PP1 and PP2A are the major forms of serine/threonine phosphatases in animal cells, and inhibition of these phosphatases has been long known to alter mitotic progression. Cellular PP1 and PP2A rarely exist in free forms; instead, these catalytic subunits associate with numerous regulatory subunits that control their phosphatase activity, substrate specificity, and cellular localization (10, 11). Although the specific function of PP1 and PP2A in M-phase regulation is largely unknown, an elegant example has been provided through characterization of the PP2A-B55δ complex as a mitotic regulator. Depletion of B55δ led to defects in dephosphorylation of Cdk1 substrates and mitotic exit, suggesting that this phosphatase complex directly or indirectly antagonizes Cdk1 (12–14). Inhibition of the phosphatase activity of PP2A-B55δ is essential for M-phase entry and maintenance and was later attributed to Ensa and Arpp-19, and their mitotic phosphorylation by Greatwall kinase (12, 13, 15, 16). In addition to regulation of Cdk1 substrates, PP1 and PP2A have also been shown to antagonize the action of Plk1, Aurora, and other mitotic kinases (6, 7). For example, it has been shown that PP1 opposes the mitotic function of Aurora B at various subcellular sites, leading to delicate control of the spatial gradient of Aurora B substrate phosphorylation (17–19). Obviously, the vast content and complex pattern of protein phosphorylation in mitosis can be much better understood with identification of various phosphatase complexes involved in M-phase progression and delineation of molecular mechanisms through which these mitotic phosphatases are regulated.
Phosphatase 1 nuclear targeting subunit 1 (Pnuts), also known as PPP1R10, was described as one of the nuclear regulators of PP1 that are responsible for the nuclear retention of PP1 (20). It was suggested that Pnuts possesses RNA binding activity and may be involved in RNA processing (21). Moreover, Pnuts was biochemically identified within a 200-kDa protein complex that also contains PP1, Tox4, and WDR83. The function of this protein complex is unclear, but it was suggested to regulate chromatin structure and RNA polymerase II phosphorylation (22). Pnuts was found to bind DNA adducts generated by cisplatin and other DNA-damaging agents (23). Consistently, Pnuts is involved in regulation of the DNA damage response and maintenance of telomere stability (24–26). The suggested function of Pnuts also includes modulation of tumor suppressor genes, such as retinoblastoma (Rb) and Phosphatase and tensin homolog (Pten), and at least in the case of Rb, Pnuts functions through inhibition of PP1 (27–29). Finally, Pnuts enhances in vitro chromatin decondensation in a PP1-dependent manner, and expression of a PP1 binding-deficient mutant form of Pnuts in cells caused defects in chromatin decondensation at telophase (30).
As several protein phosphatase complexes emerged as a new class of cell cycle regulators, we sought to reveal a potential role of Pnuts in cell cycle regulation. In this study, we discovered that Pnuts functions as an essential regulator of M-phase entry, maintenance, and exit. The cell cycle-dependent accumulation and degradation of Pnuts are tightly regulated and critical for the biochemical progression of M-phase.
EXPERIMENTAL PROCEDURES
Antibodies
Commercial antibodies used in this study include: Cdc27 antibody purchased from BD Transduction Laboratories; PP1 and Pnuts antibodies purchased from Bethyl Laboratories (Montgomery, TX); histone H3, phospho-H3 Ser-10, Cdc20, and phospho-CDK substrate antibodies from Cell Signaling Technology (Beverly, MA); MBP antibody from New England Biolabs (Ipswich, MA); GST antibody from Sigma; and ubiquitin and β-actin antibody from Abcam (Cambridge, MA). Rabbit polyclonal antibodies to Xenopus Pnuts were generated against the N-terminal sequence of Pnuts.
Immunoblotting
Samples were harvested in Laemmli sample buffer (Bio-Rad), resolved by SDS-PAGE, and then electrotransferred to PVDF membranes (Millipore, Billerica, MA). Membranes were blocked with 5% nonfat dry milk in 1× TBST (10 mm Tris-HCl, pH 7.5, 150 mm NaCl, 0.05% Tween 20) for 1 h, incubated with primary antibodies for 2 h, washed three times in 1× TBST, incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies (Sigma) for 1 h, washed three times, and then detected using an enhanced chemiluminescence (ECL) substrate kit (Pierce).
Immunodepletion
Immunodepletion was performed in Xenopus egg extracts as described previously (31). Briefly, anti-mouse or -rabbit magnetic beads (New England Biolabs) were washed three times with a washing buffer (50 mm HEPES, pH 7.5, 150 mm NaCl, 1 mm DTT, and 0.5% Tween 20) and then incubated with antibodies for 2 h at room temperature. Beads conjugated to the antibody were washed and then added into Xenopus egg extracts. After incubation for 30 min, the beads were removed with a magnet, and the remaining extract was collected for experiments.
Protein Expression and Purification
The Xenopus Pnuts gene was cloned from a Xenopus oocyte cDNA library, as described previously (32), using the following targeting sequence for primers (atggggtcagggcc; ttatggcagtggtgg). The gene was then inserted into the pGEX4T-1 vector with an N-terminal GST tag or the pMAL-parallel II plasmid with an N-terminal MBP tag. Pnuts mutants used in this study were produced by site-directed mutagenesis, and the mutations were confirmed by sequencing. These proteins were then expressed in BL21 bacterial cells and purified with glutathione or amylose beads. Recombinant Emi2 was provided by Dr. J. Liu (Cal Poly Pomona).
Protein Pulldown
For reisolation of MBP- or GST-tagged proteins from Xenopus egg extracts, proteins bound to either amylose or glutathione beads were added to egg extract and incubated at room temperature. The beads were separated from the extract with low speed centrifugation, washed three times, and then resolved by SDS-PAGE and analyzed by immunoblotting.
Phosphatase Assay
The MBP-tagged N terminus (amino acids 1–27) of histone H3, a gift from Dr. M. L. Goldberg, was expressed in BL21 cells and purified with amylose resin. Purified histone H3 peptide was phosphorylated with Aurora A kinase (a gift from Dr. M. Y. Tsai) in kinase buffer (20 mm HEPES, pH 7.5, 2 mm DTT, 10 mm MgCl2, 0.1 mm EGTA, 100 μm ATP) for 30 min at 30 °C. Following the kinase reaction, the protein bound to beads was washed with modified extract buffer (1 m KCl, 11 mm MgCl2, 100 mm HEPES, pH 7.7, 500 mm sucrose, and 5 mm EGTA, pH 7.7) and eluted with 10 mm maltose in modified extract buffer. For the PP1 phosphatase assay, prephosphorylated histone H3 peptide was incubated with PP1 (New England Biolabs) in phosphatase buffer (New England Biolabs) with and without Pnuts protein at room temperature for the time indicated. Small aliquots were removed at the indicated time points and diluted 1:10 in Laemmli sample buffer, resolved by SDS-PAGE, and detected by immunoblotting.
Xenopus Egg Extracts
Cytostatic factor (CSF) extracts were freshly prepared as described previously (31). Eggs were dejellied with 2% cysteine in 1× extract buffer (1 m KCl, 10 mm MgCl2, 100 mm HEPES, pH 7.7, and 500 mm sucrose), washed four times with 1× extract buffer, and then washed once with 1× modified extract buffer (1 m KCl, 11 mm MgCl2, 100 mm HEPES, pH 7.7, 500 mm sucrose, and 5 mm EGTA, pH 7.7). Eggs were packed in centrifuge tubes with low speed centrifugation and then crushed by centrifugation at 10,000 × g at 4 °C for 10 min. The cytoplasmic layer was further separated by centrifugation at 10,000 × g for 15 min at 4 °C. For cycling extracts, eggs were rinsed with distilled water and then soaked in water for 10 min before being dejellied with 2% cysteine in 1× extract buffer. They were washed five times in 0.2× Marc's Modified Ringers buffer (100 mm NaCl, 2 mm KCl, 1 mm MgCl2, 2 mm CaCl2, 0.1 mm EDTA, 10 mm HEPES, and KOH to pH 7.8). The Ca2+ ionophore, A23187, was added to 10 ng/ml until the animal poles rotated. The eggs were then washed with 0.2× Marc's Modified Ringers 10 times and 1× extract buffer four times. Eggs were packed by low speed centrifugation. The eggs were crushed 35 min after the addition of A23187 by centrifugation at 10,000 × g at 4 °C. The cytoplasmic layer was transferred to new tubes, and energy mix (7.5 mm creatine phosphate, 1 mm ATP, 1 MgCl2) was added. The cytoplasmic layer was further separated by centrifugation at 10,000 × g for 15 min at 4 °C.
RESULTS
Pnuts Overexpression Suppresses Both Meiotic and Mitotic Exit
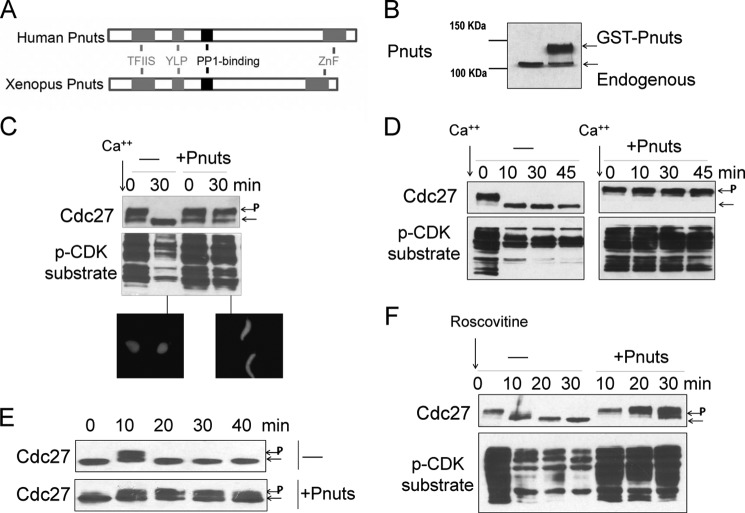
We assessed the role of Pnuts in M-phase regulation using Xenopus egg extract, an in vitro model of cell cycle progression that has been widely used to study mitotic kinases and phosphatases (33, 34). We cloned the Xenopus homolog of Pnuts from an oocyte cDNA library. As shown in Fig. 1A, Xenopus Pnuts is well conserved to the human homolog, containing the same set of functional domains, including the middle RVX(F/W)P motif that binds PP1 phosphatase (20, 21, 27), the YLP motif that associates with TRF2 and modulates telomere stability (25), the N-terminal RNA-binding motif that is potentially related to its function in transcriptional control (21, 35, 36), and the C-terminal zinc finger motif that has not been functionally characterized. To examine the role of Pnuts in M-phase exit, recombinant Pnuts proteins with GST or MBP tag were purified and added to Xenopus extracts at severalfold over endogenous Pnuts level (Figs. 1B and 3B). The cell cycle stage was determined by the mitotic phosphorylation of Cdc27 that can be judged by retarded mobility in gel, phosphorylation of other mitotic substrates that can be recognized using a phospho-CDK substrate antibody, and the morphology of sperm nuclei incubated in the extracts. CSF extract that is naturally arrested in metaphase was released by the addition of calcium. Although the control extract entered interphase following the addition of calcium, Pnuts-supplemented extract remained in M-phase (Fig. 1C). A similar defect of M-phase exit was observed upon the addition of Pnuts in extracts without presupplementation of sperm nuclei (Fig. 1D). The release of CSF extract better recapitulates the condition of meiotic exit than mitotic exit (37). However, increasing Pnuts level in cycling egg extract also extended mitosis (Fig. 1E). When added to CSF extract, roscovitine inhibits Cdk1, allowing its substrates to be dephosphorylated by counteracting phosphatases and the M-phase extract to be released into interphase (38). Increased expression of Pnuts delayed roscovitine-induced M-phase exit (Fig. 1F), suggesting that it may prevent dephosphorylation of mitotic phosphoproteins. Collectively, these results characterize Pnuts as an inhibitor of M-phase exit.
FIGURE 1.
Pnuts regulates M-phase exit. A, schematic representation of human and Xenopus Pnuts proteins showing domains conserved from Xenopus to human. TFIIS, transcription elongation factor II-like domain; YLP, telomeric repeat binding factor 2-binding motif; ZnF, zinc finger domain. B, the addition of purified recombinant Xenopus Pnuts in Xenopus egg extracts. The relative amount of endogenous and exogenous Pnuts is shown by immunoblotting using an anti-Pnuts antibody. C, calcium (40 nm) was added to CSF Xenopus egg extracts with or without exogenous Pnuts as in panel B. Samples were taken at the indicated time points and immunoblotted for Cdc27 and Phospho-CDK (p-CDK) substrates. Phosphorylated Cdc27 is indicated by P. Extracts were supplemented with sperm nuclei and monitored for the morphology of sperm nuclei, stained with DAPI. D, calcium was added to CSF Xenopus egg extracts with or without exogenous Pnuts to induce M-phase exit. Samples were taken at the indicated time points and immunoblotted for Cdc27 and Phospho-CDK substrates. E, cycling extracts in the absence or presence of exogenous Pnuts were examined for Cdc27 phosphorylation. F, the CDK inhibitor roscovitine (0.5 mm) was added to CSF extracts in the absence or presence of exogenous Pnuts and incubated at room temperature for 30 min. Extract samples were taken at the indicated time points and immunoblotted for Cdc27 and phospho-CDK substrates.
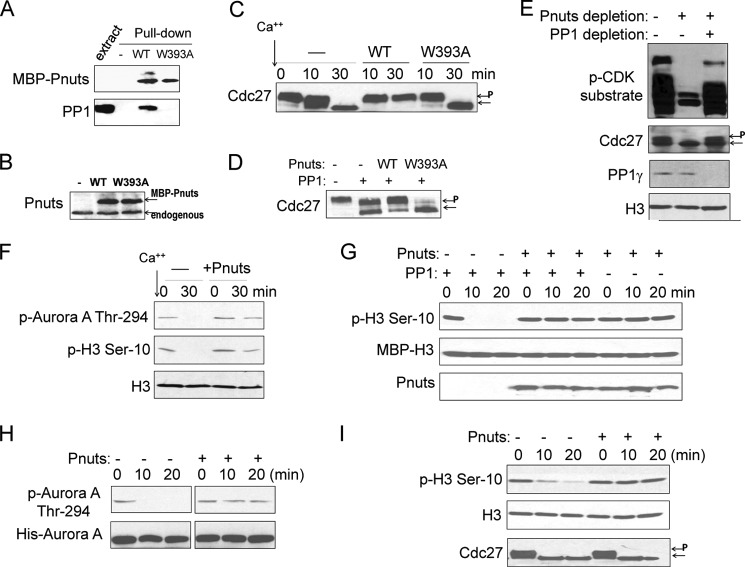
FIGURE 3.
Pnuts regulates M-phase through PP1. A, wild-type, but not W393A mutant, Pnuts binds PP1. MBP-Pnuts bound to amylose resin was incubated in Xenopus egg extracts and then reisolated as described under “Experimental Procedures.” The input extract and pulldown products were examined by immunoblotting for MBP and PP1. B, supplementation of WT or W393A MBP-Pnuts in Xenopus egg extracts. Extract samples were immunoblotted using anti-Pnuts antibody. C, as in Fig. 1C, calcium was added into CSF extracts to induce M-phase exit, with or without WT or W393A Pnuts. Extract samples were harvested after 30 min of incubation and analyzed by immunoblotting. Phosphorylated Cdc27 is indicated by P. D, PP1 (New England Biolabs, 0.42 unit/μl) was added into CSF extracts to induce M-phase exit, with or without WT or W393A Pnuts. Extract samples were harvested after 30 min of incubation and analyzed by immunoblotting. E, CSF extracts were mock-treated (−), depleted of Pnuts as in Fig. 2A, or co-depleted of Pnuts and PP1γ, and incubated at room temperature for 30 min. Immunoblotting of phospho-CDK (p-CDK) substrates, Cdc27, and PP1 is shown. F, CSF extracts with or without supplementation of MBP-Pnuts were treated with calcium. Extract samples were collected at the indicated time points and analyzed by immunoblotting for phospho-Aurora A (p-Aurora A), phospho-H3 (p-H3), and H3. G, the MBP-H3-S10 substrate was generated and prephosphorylated as described under “Experimental Procedures.” PP1 was used to dephosphorylate the substrate in vitro, with or without the addition of Pnuts protein. Immunoblots of phopho-H3 Ser-10, MBP, and Pnuts are shown. H, purified WT Aurora A was added to interphase extracts with or without MBP-Pnuts and incubated at room temperature. Samples were taken at the indicated time points and immunoblotted using phospho-Aurora A and His tag antibodies. I, CSF extracts were diluted (1:5) in the PP1-containing buffer (New England Biolabs) with or without Pnuts. Samples were collected at the indicated time points and analyzed by immunoblotting using phospho-H3, H3, and Cdc27 antibodies.
Pnuts Expression Is Essential for Mitotic Maintenance and Entry
Next we sought to investigate the functional importance of endogenous Pnuts in M-phase. As Pnuts up-regulation in mitosis blocks mitotic exit, we hypothesized that depletion of the endogenous Pnuts in mitosis will affect the length and quality of mitosis. We generated specific antibodies that are capable of removing endogenous Pnuts from CSF extracts. Interestingly, extracts depleted of Pnuts failed to maintain M-phase (Fig. 2, A and B), which can be restored with the add-back of recombinant Pnuts (Fig. 2, A and C). These results therefore support the idea that Pnuts expression is necessary for the maintenance of mitosis. To directly investigate whether Pnuts plays a role in regulation of mitotic entry, we immunodepleted Pnuts in cycling extracts while they were in interphase and observed that the resulting extracts failed to enter mitosis (Fig. 2D).
FIGURE 2.
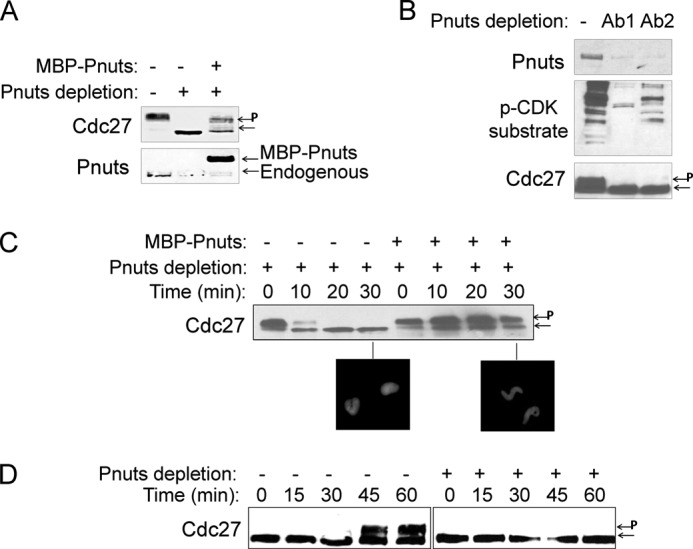
Pnuts is an essential M-phase regulator. A, Pnuts was immunodepleted from CSF extracts as described under “Experimental Procedures.” Recombinant Pnuts was used to restore Pnuts expression in the depleted extract. Extracts were incubated at room temperature for 30 min and immunoblotted for Pnuts and Cdc27. Phosphorylated Cdc27 is indicated by P. B, Pnuts was immunodepleted from CSF extracts using two antibodies (Ab1 and Ab2). Extracts were incubated at room temperature for 30 min and immunoblotted for Pnuts, Cdc27, and phospho-CDK (p-CDK) substrates. C, as in panel A, Pnuts was immunodepleted from CSF extracts with or without adding back MBP-Pnuts. Extract samples were harvested at the indicated time points and analyzed by immunoblotting and the morphology of sperm nuclei. D, cycling extracts were mock-treated (−), or immunodepleted of Pnuts (+), and immunoblotted for Cdc27.
Pnuts Regulates Mitosis through PP1
It has been shown that PP1 is critically involved in M-phase regulation (5–7, 38, 39), and Pnuts was discovered as a regulatory subunit that, at least in some cases, inhibits PP1 activity (21, 27, 30). We therefore reasoned that Pnuts may regulate M-phase progression through PP1. We first asked whether PP1 binding is necessary for Pnuts to regulate M-phase. As shown in Fig. 3A, a W393A mutant form of Pnuts does not interact with PP1 in Xenopus egg extracts, consistent with a previous study in human cells (21). Importantly, unlike WT Pnuts, W393A Pnuts at a comparable concentration (Fig. 3B) was unable to block calcium-induced release of CSF extract (Fig. 3C). Similarly, mitotic exit can be induced in CSF extracts by the addition of exogenous PP1, whereas the addition of WT, but not W393A, Pnuts prevented PP1-induced M-phase exit (Fig. 3D). Moreover, mitotic release caused by Pnuts depletion can be rescued if PP1 is co-depleted (Fig. 3E). These lines of evidence indicated that the mitotic function of Pnuts is largely conferred through modulation of PP1.
It has been shown that PP1 dephosphorylates histone H3, Aurora A, Aurora B, and other mitotic factors (5–7). To shed more light on how Pnuts may regulate mitotic progression through PP1, we examined the dephosphorylation of H3 and Aurora A and observed sustained phosphorylation of both substrates in extracts supplemented with Pnuts (Fig. 3F). We further confirmed that Pnuts efficiently inhibited PP1 in an in vitro phosphatase assay using histone H3 prephosphorylated at Ser-10 as substrate (Fig. 3G), indicating that Pnuts directly inhibits PP1-dependent substrate dephosphorylation. Similarly, dephosphorylation of Aurora A in extracts was prevented with the addition of Pnuts (Fig. 3H). Interestingly, although Pnuts suppressed dephosphorylation of H3, dephosphorylation of Cdc27 in the same extract was not inhibited by Pnuts (Fig. 3I). Notably, a previous study showed that Cdc27 dephosphorylation is PP2A-dependent (40).
Pnuts Expression Is Elevated in M-phase
Like cyclin B and some other cell cycle regulators whose expression oscillates during the cell cycle, we found Pnuts to be expressed at a higher level in M-phase in Xenopus egg extracts (Fig. 4A). In a cycling extract, high expression of Pnuts was observed when the extract entered mitosis, and the expression level of Pnuts decreased when the extract underwent mitotic exit (Fig. 4B). Consistently, the level of Pnuts decreased quickly during the release of the CSF extract into interphase (Fig. 4C). These results observed during both mitotic and meiotic exit prompted us to investigate how Pnuts expression was down-regulated in the process of M-phase exit. Interestingly, both the endogenous and the exogenous forms of Pnuts exhibited an identical pattern of reduction in protein level (Fig. 4C), suggesting that the reduction is attributed to a change in the rate of protein degradation rather than protein synthesis. Indeed, exogenous proteins incubated in either mitotic or interphase extracts revealed greatly reduced protein stability of Pnuts in interphase (Fig. 4D).
FIGURE 4.
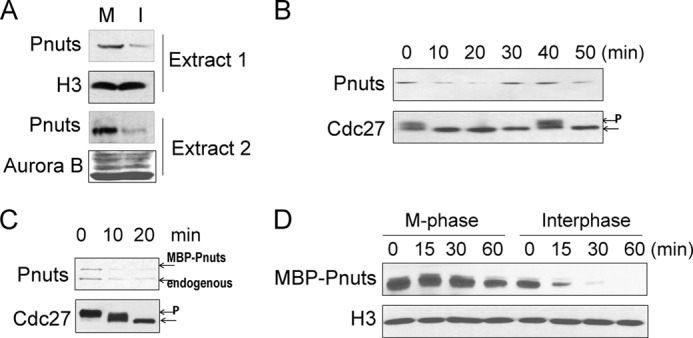
Cell cycle-dependent regulation of Pnuts expression. A, immunoblotting of Pnuts in M-phase (M, CSF extract) or interphase (I, released from the CSF extract) in two independent sets of extracts. H3 and Aurora B are shown as loading controls. B, cycling extract samples were taken at the indicated time points and then analyzed by immunoblotting for Pnuts expression and Cdc27 phosphorylation during the cell cycle. Phosphorylated Cdc27 is indicated by P. C, MBP-Pnuts was added to M-phase extract along with calcium to induce M-phase exit, and extract samples were taken at the indicated time points and analyzed for Cdc27 and Pnuts. D, MBP-Pnuts was added to CSF extract (M-phase) or interphase extract (released from the same CSF extract) and incubated as indicated. Extracts were then analyzed by immunoblotting using MBP tag and H3 antibodies.
Pnuts Expression Is Regulated by APC/C-dependent Ubiquitination and Degradation
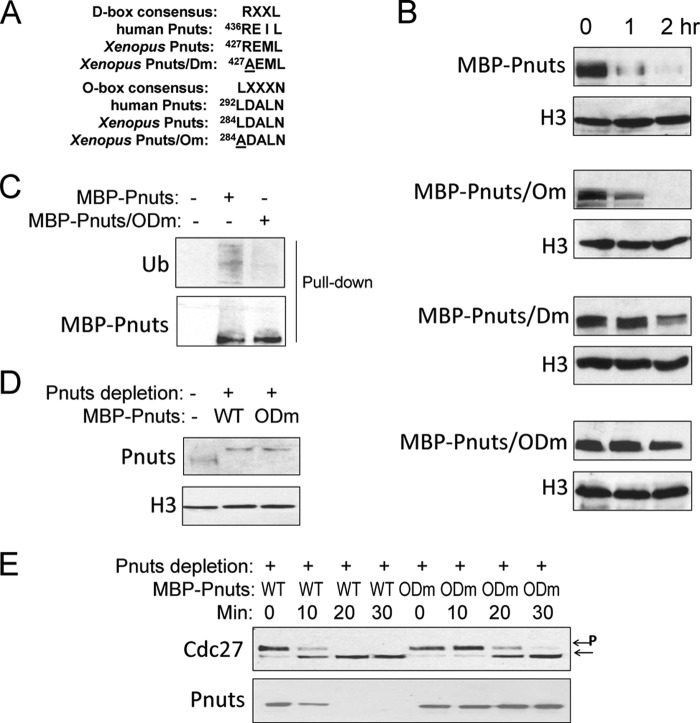
The anaphase-promoting complex/cyclosome (APC/C) is known to ubiquitinate cyclin B and many other mitotic proteins, thereby targeting them for the proteasome-dependent destruction (41, 42). When we examined the proteins that were pulled down from egg extract with MBP-Pnuts, we detected Cdc27 and Cdc20, which are components of the APC/C complex (Fig. 5A). In metaphase-arrested CSF extracts, the association of Pnuts with Cdc27 and Cdc20 was significantly reduced, presumably consistent with the high expression level of Pnuts in M-phase. The involvement of the proteasome in Pnuts regulation was confirmed using a proteasome inhibitor, MG132. Although MBP-Pnuts added into interphase extracts exhibited much lower stability when compared with that added into M-phase extracts, such difference was no longer evident with the addition of MG132 (Fig. 5B), which apparently increased the stability of Pnuts in interphase extracts (Fig. 5, B and C). Pulldown of Pnuts from extracts also revealed that it undergoes ubiquitination, which was suppressed in the presence of Emi2, a well characterized inhibitor of APC/C (43) (Fig. 5D).
FIGURE 5.
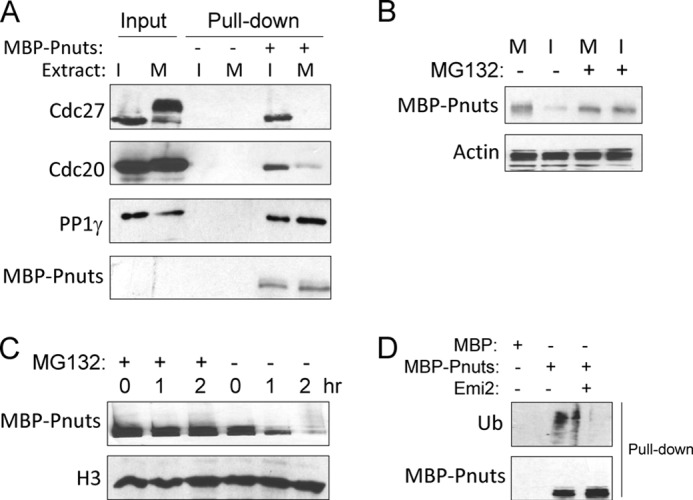
Regulation of Pnuts stability through APC/C-mediated ubiquitination and proteolysis. A, MBP-Pnuts (+) or control MBP (−) was reisolated from either interphase (I) or M-phase (M) extracts, as described under “Experimental Procedures,” and analyzed by immunoblotting. The input contains 10% of the extracts used. B, MBP-Pnuts was added into M-phase or interphase extracts and incubated for 1 h, with or without proteasome inhibitor MG132. Immunoblotting of MBP and actin is shown. C, MBP-Pnuts and MG132 were added into interphase extracts and incubated over time, as indicated. Extract samples were then analyzed by immunoblotting or MBP and H3. D, ubiquitination (Ub) of Pnuts was measured by immunoblotting of MBP-Pnuts reisolated from extract with or without the APC/C inhibitor Emi2, using anti-ubiquitin and anti-MBP antibodies.
Pnuts Is Regulated through Conserved Destruction Box Motifs
APC/C targets cyclin B and other substrates in a sequence-specific manner, and several recognition motifs have been characterized, including the most common destruction box (D-box), KEN box, and Orc1-destructing box (O-box) (42, 44). We found that Pnuts contains a D-box and an O-box, both of which are well conserved from Xenopus to human (Fig. 6A). Changing the first amino acid in either the D-box or the O-box to an alanine increased the stability of Pnuts in interphase extracts (Fig. 6B). The D-box mutant (Dm) exhibited a more profound effect when compared with the O-box mutant (Om), whereas a double mutation (ODm) greatly stabilized Pnuts and reduced its ubiquitination (Fig. 6, B and C). Given the function of Pnuts reported in this study and the pattern of its expression during the cell cycle, it is plausible that its proteolysis constitutes one of the key events underlying mitotic exit. To this end, we depleted endogenous Pnuts in M-phase extracts and reconstituted the extracts with either WT or ODm Pnuts to approximately the endogenous level (Fig. 6D). When compared with WT Pnuts, ODm Pnuts delayed the release of the extracts to interphase induced by Ca2+ (Fig. 6E).
FIGURE 6.
Pnuts stability is regulated through conserved destruction box motifs. A, alignment of the D- and O-box sequences of human and Xenopus Pnuts and the mutations made to them in this study. B, Pnuts was mutated in the O-box (Om), D-box (Dm), or both boxes (ODm), as in panel A. The resulting mutants, along with WT Pnuts, were added into interphase extracts, incubated over time, and measured by immunoblotting for their stability. C, as in Fig. 5D, ubiquitination (Ub) of WT or ODm Pnuts was measured by immunoblotting. D and E, endogenous Pnuts was immunodepleted from CSF extracts, which were then supplemented with either WT or ODm Pnuts. The extracts were then treated with Ca2+, collected at the indicated time points, and analyzed by immunoblotting for Cdc27 and Pnuts. Phosphorylated Cdc27 is indicated by P.
DISCUSSION
Pnuts Is a Quantitative Regulator of the Cell Cycle
Our results showed that the expression of Pnuts oscillates during the cell cycle and peaks in M-phase. The accumulation of Pnuts is required for M-phase maintenance as its depletion caused M-phase exit. On the other hand, Pnuts is down-regulated during M-phase exit, and supplementation of exogenous Pnuts efficiently blocked M-phase exit. It has been shown in a previous study that PP2B/calcineurin is required for meiotic exit, but not mitotic exit (45). By comparison, we observed the same role of Pnuts in extracts released from either mitosis or meiosis. Consistent with its role in M-phase maintenance, Pnuts is required for M-phase entry, and its depletion in interphase extracts prevented M-phase entry. The function of Pnuts in mitosis is seemingly analogous to a “locking” mechanism. Once the cell is committed to M-phase entry, Pnuts is up-regulated to ensure mitotic entry, maintain M-phase, and prevent premature M-phase exit, whereas its down-regulation facilitates M-phase exit. Replacing endogenous Pnuts with a non-degradable mutant delayed M-phase exit. The role of Pnuts and its pattern of expression during the cell cycle may contribute to the irreversibility and ultrasensitivity of cell cycle transition through M-phase. A previous study suggested that Pnuts may be involved in the regulation of chromosome condensation (30), a necessary step of M-phase exit. However, Xenopus egg extracts are depleted of chromatin DNA, and the role of Pnuts in M-phase progression was seen consistently in Xenopus egg extracts with or without the supplementation of sperm chromatin, suggesting that Pnuts is an essential component of the biochemical machinery that controls cell cycle progression. It has been shown that human Pnuts localizes in the nucleus in interphase and is excluded from mitotic chromosomes in M-phase (30). This pattern of localization appears somewhat reminiscent of Greatwall kinase and other mitotic regulators (46, 47), but the exact implication to the function or regulation of Pnuts remains to be clarified.
Cell Cycle-dependent Regulation of Pnuts
Regulated proteolysis plays an important role in carving cell cycle transitions. In particular, a number of E3 ubiquitin ligases have been shown to mediate the ubiquitination and proteasome-dependent degradation of key cell cycle regulators. For example, cyclin B is targeted by the APC/C ubiquitin ligase, leading to its degradation and inactivation of mitotic Cdk1. Interestingly, in this study, we found that oscillation of Pnuts expression during the cell cycle is regulated through APC/C-mediated ubiquitination and proteasome-dependent degradation. Moreover, it has been shown that APC/C targets its various substrates in a sequence-specific manner. Pnuts contains two evolutionarily conserved motifs that are predicted targets of the APC/C, including the D-box that is responsible for cyclin B degradation and the O-box that is present in Orc1 (42, 44). We confirmed that both the D-box and the O-box are involved in the regulation of Pnuts ubiquitination and stability. Thus, these results identified a new target of APC/C and delineated an important mechanism of cell cycle regulation through ubiquitin-mediated proteolysis. The critical nature of Pnuts degradation during M-phase exit was demonstrated as replacing endogenous Pnuts with a mutant form defective in APC/C-mediated degradation delayed M-phase exit.
Regulation of Mitotic Phosphorylation by the PP1 and Pnuts Module
We confirmed in this study that the function of Pnuts in cell cycle regulation is largely conferred through PP1, which finding contributes to the emerging picture of mitotic regulation through protein phosphatases. It is well established that the complex pattern of phospho-regulation plays a central role in governing M-phase progression and that PP1 and PP2A are mitotic regulators whose inhibition led to various mitotic defects (6, 7). However, revealing how the functions of mitotic phosphatases are specifically regulated still stands as a key challenge. PP1 has been implicated in multiple aspects of M-phase entry and exit as both a negative and a positive regulator. For example, H3, Aurora A, Aurora B, Nek2, Plk1, Cdc25, and numerous other mitotic regulators have been characterized as substrates of PP1, through which PP1 can promote both mitotic entry and exit (5–7, 38, 39). Here we showed that up-regulation of Pnuts in M-phase represents an important mechanism employed by the cell to modulate PP1 activity and maintain mitosis. Despite the essential role of Pnuts throughout mitotic progression, Pnuts only associated with a very small portion of PP1, suggesting specific modulation of only a subset of PP1 substrates. It should also be noted that, in addition to Pnuts, various other molecular mechanisms are likely to be included to govern the function of PP1, given that PP1 is a major Ser/Thr phosphatase whose activity needs to be regulated in a spatiotemporally defined manner. Continuous research efforts in revealing the detailed function and regulation of specific M-phase phosphatase subunits will undoubtedly yield a more complete understanding of phospho-regulation of the cell cycle.
Acknowledgments
We thank Drs. James Maller (University of Colorado at Denver), Junjun Liu (Cal Poly Pomona), Michael Goldberg (Cornell University), and James Wahl and Ming-Ying Tsai (University of Nebraska Medical Center) for reagents.
This work was supported, in whole or in part, by National Institutes of Health Grants 1R01CA172574 and 5P20GM103489 (to A. P.).
- Cdk1
- cyclin-dependent kinase 1
- Pnuts
- phosphatase 1 nuclear targeting subunit 1
- PP
- protein phosphatase
- APC/C
- anaphase-promoting complex/cyclosome
- MBP
- myelin basic protein
- CSF
- cytostatic factor
- D-box
- destruction box
- O-box
- Orc1-destructing box
- Dm
- D-box mutant
- Om
- O-box mutant
- ODm
- double O-box/D-box mutant.
REFERENCES
- 1. Janssen A., Medema R. H. (2011) Mitosis as an anti-cancer target. Oncogene 30, 2799–2809 [DOI] [PubMed] [Google Scholar]
- 2. Nigg E. A. (2001) Mitotic kinases as regulators of cell division and its checkpoints. Nat. Rev. Mol. Cell Biol. 2, 21–32 [DOI] [PubMed] [Google Scholar]
- 3. Malumbres M., Harlow E., Hunt T., Hunter T., Lahti J. M., Manning G., Morgan D. O., Tsai L. H., Wolgemuth D. J. (2009) Cyclin-dependent kinases: a family portrait. Nat. Cell Biol. 11, 1275–1276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lens S. M., Voest E. E., Medema R. H. (2010) Shared and separate functions of Polo-like kinases and Aurora kinases in cancer. Nat. Rev. Cancer 10, 825–841 [DOI] [PubMed] [Google Scholar]
- 5. Mochida S., Hunt T. (2012) Protein phosphatases and their regulation in the control of mitosis. EMBO Rep. 13, 197–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bollen M., Gerlich D. W., Lesage B. (2009) Mitotic phosphatases: from entry guards to exit guides. Trends Cell Biol. 19, 531–541 [DOI] [PubMed] [Google Scholar]
- 7. Wurzenberger C., Gerlich D. W. (2011) Phosphatases:providing safe passage through mitotic exit. Nat. Rev. Mol. Cell Biol. 12, 469–482 [DOI] [PubMed] [Google Scholar]
- 8. Stegmeier F., Amon A. (2004) Closing mitosis: the functions of the Cdc14 phosphatase and its regulation. Annu. Rev. Genet. 38, 203–232 [DOI] [PubMed] [Google Scholar]
- 9. Mocciaro A., Schiebel E. (2010) Cdc14: a highly conserved family of phosphatases with non-conserved functions? J. Cell Sci. 123, 2867–2876 [DOI] [PubMed] [Google Scholar]
- 10. Moorhead G. B., Trinkle-Mulcahy L., Ulke-Lemée A. (2007) Emerging roles of nuclear protein phosphatases. Nat. Rev. Mol. Cell Biol. 8, 234–244 [DOI] [PubMed] [Google Scholar]
- 11. Peng A., Maller J. L. (2010) Serine/threonine phosphatases in the DNA damage response and cancer. Oncogene 29, 5977–5988 [DOI] [PubMed] [Google Scholar]
- 12. Castilho P. V., Williams B. C., Mochida S., Zhao Y., Goldberg M. L. (2009) The M phase kinase Greatwall (Gwl) promotes inactivation of PP2A/B55δ, a phosphatase directed against CDK phosphosites. Mol. Biol. Cell 20, 4777–4789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mochida S., Ikeo S., Gannon J., Hunt T. (2009) Regulated activity of PP2A-B55δ is crucial for controlling entry into and exit from mitosis in Xenopus egg extracts. EMBO J. 28, 2777–2785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Manchado E., Guillamot M., de Cárcer G., Eguren M., Trickey M., García-Higuera I., Moreno S., Yamano H., Cañamero M., Malumbres M. (2010) Targeting mitotic exit leads to tumor regression in vivo: modulation by Cdk1, Mastl, and the PP2A/B55α,δ phosphatase. Cancer Cell 18, 641–654 [DOI] [PubMed] [Google Scholar]
- 15. Gharbi-Ayachi A., Labbé J. C., Burgess A., Vigneron S., Strub J. M., Brioudes E., Van-Dorsselaer A., Castro A., Lorca T. (2010) The substrate of Greatwall kinase, Arpp19, controls mitosis by inhibiting protein phosphatase 2A. Science 330, 1673–1677 [DOI] [PubMed] [Google Scholar]
- 16. Mochida S., Maslen S. L., Skehel M., Hunt T. (2010) Greatwall phosphorylates an inhibitor of protein phosphatase 2A that is essential for mitosis. Science 330, 1670–1673 [DOI] [PubMed] [Google Scholar]
- 17. Emanuele M. J., Lan W., Jwa M., Miller S. A., Chan C. S., Stukenberg P. T. (2008) Aurora B kinase and protein phosphatase 1 have opposing roles in modulating kinetochore assembly. J. Cell Biol. 181, 241–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fuller B. G., Lampson M. A., Foley E. A., Rosasco-Nitcher S., Le K. V., Tobelmann P., Brautigan D. L., Stukenberg P. T., Kapoor T. M. (2008) Midzone activation of Aurora B in anaphase produces an intracellular phosphorylation gradient. Nature 453, 1132–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tsai M. Y., Wiese C., Cao K., Martin O., Donovan P., Ruderman J., Prigent C., Zheng Y. (2003) A Ran signalling pathway mediated by the mitotic kinase Aurora A in spindle assembly. Nat. Cell Biol. 5, 242–248 [DOI] [PubMed] [Google Scholar]
- 20. Allen P. B., Kwon Y. G., Nairn A. C., Greengard P. (1998) Isolation and characterization of PNUTS, a putative protein phosphatase 1 nuclear targeting subunit. J. Biol. Chem. 273, 4089–4095 [DOI] [PubMed] [Google Scholar]
- 21. Kim Y. M., Watanabe T., Allen P. B., Kim Y. M., Lee S. J., Greengard P., Nairn A. C., Kwon Y. G. (2003) PNUTS, a protein phosphatase 1 (PP1) nuclear targeting subunit: characterization of its PP1- and RNA-binding domains and regulation by phosphorylation. J. Biol. Chem. 278, 13819–13828 [DOI] [PubMed] [Google Scholar]
- 22. Lee J. H., You J., Dobrota E., Skalnik D. G. (2010) Identification and characterization of a novel human PP1 phosphatase complex. J. Biol. Chem. 285, 24466–24476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bounaix Morand du Puch C., Barbier E., Kraut A., Couté Y., Fuchs J., Buhot A., Livache T., Sève M., Favier A., Douki T., Gasparutto D., Sauvaigo S., Breton J. (2011) TOX4 and its binding partners recognize DNA adducts generated by platinum anticancer drugs. Arch. Biochem. Biophys. 507, 296–303 [DOI] [PubMed] [Google Scholar]
- 24. Landsverk H. B., Mora-Bermúdez F., Landsverk O. J., Hasvold G., Naderi S., Bakke O., Ellenberg J., Collas P., Syljuåsen R. G., Küntziger T. (2010) The protein phosphatase 1 regulator PNUTS is a new component of the DNA damage response. EMBO Rep. 11, 868–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kim H., Lee O. H., Xin H., Chen L. Y., Qin J., Chae H. K., Lin S. Y., Safari A., Liu D., Songyang Z. (2009) TRF2 functions as a protein hub and regulates telomere maintenance by recognizing specific peptide motifs. Nat. Struct. Mol. Biol. 16, 372–379 [DOI] [PubMed] [Google Scholar]
- 26. Boon R. A., Iekushi K., Lechner S., Seeger T., Fischer A., Heydt S., Kaluza D., Tréguer K., Carmona G., Bonauer A., Horrevoets A. J., Didier N., Girmatsion Z., Biliczki P., Ehrlich J. R., Katus H. A., Müller O. J., Potente M., Zeiher A. M., Hermeking H., Dimmeler S. (2013) MicroRNA-34a regulates cardiac ageing and function. Nature 495, 107–110 [DOI] [PubMed] [Google Scholar]
- 27. Udho E., Tedesco V. C., Zygmunt A., Krucher N. A. (2002) PNUTS (phosphatase nuclear targeting subunit) inhibits retinoblastoma-directed PP1 activity. Biochem. Biophys. Res. Commun. 297, 463–467 [DOI] [PubMed] [Google Scholar]
- 28. Krucher N. A., Rubin E., Tedesco V. C., Roberts M. H., Sherry T. C., De Leon G. (2006) Dephosphorylation of Rb (Thr-821) in response to cell stress. Exp. Cell Res. 312, 2757–2763 [DOI] [PubMed] [Google Scholar]
- 29. Kavela S., Shinde S. R., Ratheesh R., Viswakalyan K., Bashyam M. D., Gowrishankar S., Vamsy M., Pattnaik S., Rao S., Sastry R. A., Srinivasulu M., Chen J., Maddika S. (2013) PNUTS functions as a proto-oncogene by sequestering PTEN. Cancer Res. 73, 205–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Landsverk H. B., Kirkhus M., Bollen M., Küntziger T., Collas P. (2005) PNUTS enhances in vitro chromosome decondensation in a PP1-dependent manner. Biochem. J. 390, 709–717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Peng A., Wang L., Fisher L. A. (2011) Greatwall and Polo-like kinase 1 coordinate to promote checkpoint recovery. J Biol. Chem. 286, 28996–29004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Peng A., Lewellyn A. L., Schiemann W. P., Maller J. L. (2010) Repo-Man controls a protein phosphatase 1-dependent threshold for DNA damage checkpoint activation. Curr. Biol. 20, 387–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Crane R. F., Ruderman J. V. (2006) Using Xenopus oocyte extracts to study signal transduction. Methods Mol. Biol. 322, 435–443 [DOI] [PubMed] [Google Scholar]
- 34. Philpott A., Yew P. R. (2005) The Xenopus cell cycle: an overview. Methods Mol. Biol. 296, 95–112 [DOI] [PubMed] [Google Scholar]
- 35. Lee S. J., Lee J. K., Maeng Y. S., Kim Y. M., Kwon Y. G. (2009) Langerhans cell protein 1 (LCP1) binds to PNUTS in the nucleus: implications for this complex in transcriptional regulation. Exp. Mol. Med. 41, 189–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jerebtsova M., Klotchenko S. A., Artamonova T. O., Ammosova T., Washington K., Egorov V. V., Shaldzhyan A. A., Sergeeva M. V., Zatulovskiy E. A., Temkina O. A., Petukhov M. G., Vasin A. V., Khodorkovskii M. A., Orlov Y. N., Nekhai S. (2011) Mass spectrometry and biochemical analysis of RNA polymerase II: targeting by protein phosphatase-1. Mol. Cell. Biochem. 347, 79–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tunquist B. J., Maller J. L. (2003) Under arrest: cytostatic factor (CSF)-mediated metaphase arrest in vertebrate eggs. Genes Dev. 17, 683–710 [DOI] [PubMed] [Google Scholar]
- 38. Wu J. Q., Guo J. Y., Tang W., Yang C. S., Freel C. D., Chen C., Nairn A. C., Kornbluth S. (2009) PP1-mediated dephosphorylation of phosphoproteins at mitotic exit is controlled by inhibitor-1 and PP1 phosphorylation. Nat. Cell Biol. 11, 644–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Margolis S. S., Walsh S., Weiser D. C., Yoshida M., Shenolikar S., Kornbluth S. (2003) PP1 control of M phase entry exerted through 14-3-3-regulated Cdc25 dephosphorylation. Embo J. 22, 5734–5745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Torres J. Z., Ban K. H., Jackson P. K. (2010) A specific form of phospho protein phosphatase 2 regulates anaphase-promoting complex/cyclosome association with spindle poles. Mol. Biol. Cell 21, 897–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sullivan M., Morgan D. O. (2007) Finishing mitosis, one step at a time. Nat. Rev. Mol. Cell Biol. 8, 894–903 [DOI] [PubMed] [Google Scholar]
- 42. Castro A., Bernis C., Vigneron S., Labbé J. C., Lorca T. (2005) The anaphase-promoting complex: a key factor in the regulation of cell cycle. Oncogene 24, 314–325 [DOI] [PubMed] [Google Scholar]
- 43. Liu J., Grimison B., Maller J. L. (2007) New insight into metaphase arrest by cytostatic factor: from establishment to release. Oncogene 26, 1286–1289 [DOI] [PubMed] [Google Scholar]
- 44. Araki M., Yu H., Asano M. (2005) A novel motif governs APC-dependent degradation of Drosophila ORC1 in vivo. Genes Dev. 19, 2458–2465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mochida S., Hunt T. (2007) Calcineurin is required to release Xenopus egg extracts from meiotic M phase. Nature 449, 336–340 [DOI] [PubMed] [Google Scholar]
- 46. Voets E., Wolthuis R. M. (2010) MASTL is the human orthologue of Greatwall kinase that facilitates mitotic entry, anaphase and cytokinesis. Cell Cycle 9, 3591–3601 [DOI] [PubMed] [Google Scholar]
- 47. Burgess A., Vigneron S., Brioudes E., Labbé J. C., Lorca T., Castro A. (2010) Loss of human Greatwall results in G2 arrest and multiple mitotic defects due to deregulation of the cyclin B-Cdc2/PP2A balance. Proc. Natl. Acad. Sci. U. S. A. 107, 12564–12569 [DOI] [PMC free article] [PubMed] [Google Scholar]