Background: The role of the blood-brain barrier for cholesterol homeostasis in the brain is not known.
Results: Significant influx of cholesterol into the brain and increased efflux of 24(S)-hydroxycholesterol were observed in mice with a defect blood-brain barrier.
Conclusion: A defect blood-brain barrier increases permeability for steroid flux in both directions.
Significance: Elucidation of the role of the blood-brain barrier for brain cholesterol turnover.
Keywords: Brain Metabolism, Cholesterol Regulation, Hydroxylase, Neurodegeneration, Sterol
Abstract
The presence of the blood-brain barrier (BBB) is critical for cholesterol metabolism in the brain, preventing uptake of lipoprotein-bound cholesterol from the circulation. The metabolic consequences of a leaking BBB for cholesterol metabolism have not been studied previously. Here we used a pericyte-deficient mouse model, Pdgfbret/ret, shown to have increased permeability of the BBB to a range of low-molecular mass and high-molecular mass tracers. There was a significant accumulation of plant sterols in the brains of the Pdgfbret/ret mice. By dietary treatment with 0.3% deuterium-labeled cholesterol, we could demonstrate a significant flux of cholesterol from the circulation into the brains of the mutant mice roughly corresponding to about half of the measured turnover of cholesterol in the brain. We expected the cholesterol flux into the brain to cause a down-regulation of cholesterol synthesis. Instead, cholesterol synthesis was increased by about 60%. The levels of 24(S)-hydroxycholesterol (24S-OHC) were significantly reduced in the brains of the pericyte-deficient mice but increased in the circulation. After treatment with 1% cholesterol in diet, the difference in cholesterol synthesis between mutants and controls disappeared. The findings are consistent with increased leakage of 24S-OHC from the brain into the circulation in the pericyte-deficient mice. This oxysterol is an efficient suppressor of cholesterol synthesis, and the results are consistent with a regulatory role of 24S-OHC in the brain. To our knowledge, this is the first demonstration that a defective BBB may lead to increased flux of a lipophilic compound out from the brain. The relevance of the findings for the human situation is discussed.
Introduction
The blood-brain barrier (BBB)2 is a combination of physical barriers, enzymes, and transporters. At the level of endothelial cells, neuronal cells, glial cells, and pericytes are of regulatory importance. Pericytes seem to be of particular importance for the permeability of the BBB (1, 2). It was recently shown that pericyte deficiency increases the permeability of BBB to water and to a range of low- and high-molecular mass tracers (2). The increased permeability was found to be dependent upon endothelial transcytosis. It was also recently reported that lack of the cholesterol transporter ApoE or the presence of the isoform ApoE4 leads to BBB dysfunction (3). The latter dysfunction is mediated by activation of a cyclophilin A pathway in pericytes.
The BBB is of particular importance for cholesterol metabolism in the brain (for reviews, see Refs. 4 and 5). This barrier effectively prevents uptake of lipoprotein-bound cholesterol from the circulation, and de novo synthesis is considered to be responsible for practically all cholesterol in this organ. The independence of the isolated pool of cholesterol in the brain is likely to reflect a high need for constancy in the cholesterol content of membrane and myelin, a constancy that would be difficult to keep if brain cholesterol had been exchangeable with lipoprotein cholesterol in the circulation. The brain does not seem to respond to many of the regulatory mechanisms operating in maintaining extracerebral cholesterol homeostasis. The half-life of the bulk of cholesterol in the brain has been estimated to be about 5 years and 4 months in humans and mice, respectively (4, 5). In contrast, the half-life of cholesterol in human circulation is only a few days.
In contrast to cholesterol itself, some side-chain hydroxylated metabolites of cholesterol are able to pass the blood-brain barrier (6). It has been shown that about two-thirds of the synthesis of cholesterol in the brain is balanced by neuronal formation of 24(S)-hydroxycholesterol (24S-OHC) that is fluxing across the BBB into the circulation (7, 8). Another side-chain hydroxylated oxysterol, 27-hydroxycholesterol (27-OHC), is taken up by the brain and converted into a steroid acid, 7α-hydroxy-3-oxo-4-cholestenoic acid, that is fluxing out from the brain into the circulation (9). In view of the potent effect of 27-OHC on a number of systems that are of importance in connection with neurodegeneration (for a review, see Ref. 10), the latter pathway may be regarded as a detoxification. Both 24S-OHC and 27-OHC are efficient inhibitors of cholesterol synthesis in vitro (11, 12). Recent studies with transgenic mouse models are consistent with the possibility that both the flux of 27-OHC into the brain and the size of the pool of 24S-OHC in the brain are of regulatory importance for cholesterol synthesis (13).
Very little is known about the consequences of a disrupted BBB on cholesterol homeostasis in the brain. Increased permeability of the BBB may lead to a significant flux of cholesterol into the brain and/or loss of cholesterol from the brain into the circulation. With respect to the side-chain oxidized oxysterols, a disrupted BBB would be expected to increase the flux of 27-OHC into the brain and possibly also the flux of 24S-OHC out from the brain.
In the present work, we have studied the consequences of pericyte deficiency for brain cholesterol homeostasis in a mouse model, Pdgfbret/ret. This mouse model has been shown to have increased permeability of the BBB to water and a range of both low- and high-molecular mass tracers. These mice have severe glomerular and retinal defects but have a normal life span (14). The leaking BBB in these mice would be expected to lead to an influx of cholesterol from the circulation with consequences for cholesterol homeostasis in the brain. It is shown here that Pdgfbret/ret mice have a slightly increased synthesis of cholesterol in the brain and that at least part of this increase is likely to be due to increased loss of intracerebral 24S-OHC.
EXPERIMENTAL PROCEDURES
Animals
The studies were performed with transgenic mice (Pdgfbret/ret) lacking the PDGF-B retention motif and their littermate controls (Pdgfbret/+), backcrossed at least seven generations against C57Bl/6 and identified by PCR genotyping. The heterozygote controls are shown to have normal BBB function (2). Only male mice were used with an age of about 10 weeks (8–12 weeks). Three types of diets were used in the study: chow diet, 1% cholesterol diet, and chow diet labeled with 0.3% cholesterol-d6. All of the mice were sacrificed between 8 and 10 a.m.
Ethical Considerations
All experimental procedures in this study were in compliance with the National Institutes of Health Guide for Care and Use of Laboratory Animals and the European Communities Council Directive of 24 November 1986 (86/609/EEC) and approved by the Northern Stockholm Research Animal Ethics Committee.
Lipid Extraction and Analysis
Lipids were extracted according to the Folch method. Different volumes of Folch solution (chloroform/methanol, 2:1 (v/v)) were added to half of a brain and other different organs according to their weights. After 24 h at room temperature, extracts were transferred to new vials and evaporated under argon. After evaporation, the extracts were redissolved in appropriate volumes of Folch and stored at −20 °C until required.
Sterols and oxysterols were measured by gas chromatography-mass spectrometry (GC-MS) using deuterium-labeled internal standards as described previously (15, 16). 24(R)- and 24(S)-hydroxycholesterols were analyzed as their Girard P hydrazone derivatives by liquid chromatography-mass spectrometry (LC-MSn) as described (17). The two isomers separate chromatographically on the reversed phase LC column.
RNA Preparation and Real-time PCR
Total RNA was prepared using TRIzol reagent (Invitrogen) according to the manufacturer's protocol. RNA (1 μg) was transcribed into cDNA using a high-capacity cDNA reverse transcription kit (Applied Biosystems, Carlsbad, CA). The cDNA obtained was diluted 1:10 in RNase-free H2O. Real-time PCR was performed with 5 μl of cDNA and 12.5 μl of SYBR Green Mastermix (Applied Biosystems). The forward and reverse primers are shown in Table 1. All values were normalized to hypoxanthine phosphoribosyltransferase mRNA concentrations for hepatic analyses.
TABLE 1.
Forward and reverse primers used to measure the relative expression of different genes
| Gene | Sequence (5′–3′) | Concentration |
|---|---|---|
| nm | ||
| Hprt forward | GGT GAA AAG GAC CTC TCG AAG TG | 200 |
| Hprt reverse | ATA GTC AAG GGC ATA TCC AAC AAC A | 200 |
| Hmgcr forward | CCG GCA ACA ACA AGA TCT GTG | 200 |
| Hmgcr reverse | ATG TAC AGG ATG GCG ATG CA | 200 |
| Hmgcs1 forward | CTC TGT CTA TGG TTC CCT GGC T | 200 |
| Hmgcs1 reverse | TCC AAT CCT CTT CCC TGC C | 200 |
| Abca1 forward | CCC AGA GCA AAA AGC GAC TC | 200 |
| Abca1 reverse | GGT CAT CAT CAC TTT GGT CCT TG | 200 |
| Abcg1 forward | GCTGAAGAGGACTCCGCCT | 200 |
| Abcg1 reverse | GAGGATGCAGAACTGGGTGAG | 200 |
| Srebp1c forward | GGAGCCATGGATTGCACATT | 200 |
| Srebp1c reverse | GGCCCGGGAAGTCACTGT | 200 |
| Srebp2 forward | GCG TTC TGG AGA CCA TGG A | 700 |
| Srebp2 reverse | ACA AAG TTG CTC TGA AAA CAA ATC | 700 |
| ApoE forward | GCA GGC GGA GAT CTT CCA | 200 |
| ApoE reverse | CCA CTG GCG ATG CAT GTC | 200 |
| Cyp7b1 forward | CCT CTT TCC TCC ACT CAT ACA CAA | 200 |
| Cyp7b1 reverse | GAA CCG ATC GAA CCT AAA TTC CTT | 200 |
| Cyp27 forward | GCC TTG CAC AAG GAA GTG ACT | 200 |
| Cyp27 reverse | CGC AGG GTC TCC TTA ATC ACA | 200 |
| Fas forward | GGCATCATTGGGCACTCCTT | 200 |
| Fas reverse | GCTGCAAGCACAGCCTCTCT | 200 |
| Cyp46 forward | AACCATCTGGCATTCACAGTGA | 200 |
| Cyp46 reverse | GGAACCGACAACCTCATCCA | 200 |
Western Blots
Microsomes prepared from brains of the Pdgfbret/ret mice and their controls were subjected to electrophoresis (20–25 μg/lane) on a 10% SDS or BisTris polyacrylamide gel and transferred to nitrocellulose membranes. The membranes were incubated for 2 h at room temperature in blocking buffer (5% milk in phosphate-buffered saline, 0.05% Tween) followed by incubation overnight at +4 °C with an anti-CYP46 antibody (a generous gift from Prof. D. Russell, University of Texas Southwestern Medical Center, Dallas, TX) or an antibody directed toward Abca1 (anti-Abca1 antibody ab 18180 from Abcam). Goat anti-rabbit IgG or goat anti-mouse IgG coupled with horseradish peroxidase (Pierce) was used as a secondary antibody with incubation at room temperature for 2 h. The membranes were incubated in Super Signal West Dura extended duration substrate (product number 34075 (Pierce) according to the manufacturer's instructions. The signal (around 50 kDa for CYP46 and 250 kDa for ABCA1) was determined using Universal hood II equipment (Bio-Rad).
Experiments with Deuterated Water and Calculation of Fractional Synthetic Rate
The mice were exposed to drinking water containing 10% deuterium water for a period of 11 days before sacrifice (18). In addition to measurements of deuterium content in brain cholesterol, the deuterium content was measured in body fluid (serum) of the mice as described (18). From the deuterium enrichment in cholesterol and body water, the fractional synthetic rate could be calculated (18).
Statistics
Gene expression data are expressed as mean ± range as described by Livak et al. (19). Sterol determinations are expressed as means ± S.E. Statistical comparisons were performed using the unpaired Student's t test.
RESULTS
Experiments with Deuterium-labeled Cholesterol
Fig. 1A shows the enrichment of deuterium in serum cholesterol and in cholesterol isolated from different organs of a mutant (Pdgfbret/ret) mouse and a control (Pdgfbret/+) mouse treated with 0.3% cholesterol-d6 in the diet for 20 days. The enrichment of deuterium was found to be about 45% in serum with very little difference between the mutant and the control. The situation was about the same in cholesterol isolated from liver, lung, and adrenals. A barrier function would be expected to give lower enrichment of deuterium in the cholesterol pool, and in accordance with this, lower enrichment was found in testis cholesterol, and markedly lower enrichment was found in brain cholesterol. If the barrier function is defective, higher enrichment of deuterium would be expected in the cholesterol isolated from the mutant than from the control. In accordance with this, slightly higher enrichment of deuterium was found in the testis from the mutant (probably due to a defective testis-blood barrier). Markedly higher enrichment was found in cholesterol isolated from the brains of the mutant as compared with the control, suggesting a serious defect in the blood-brain barrier. The data shown in Fig. 1A are from one single experiment and are representative of two independent experiments.
FIGURE 1.
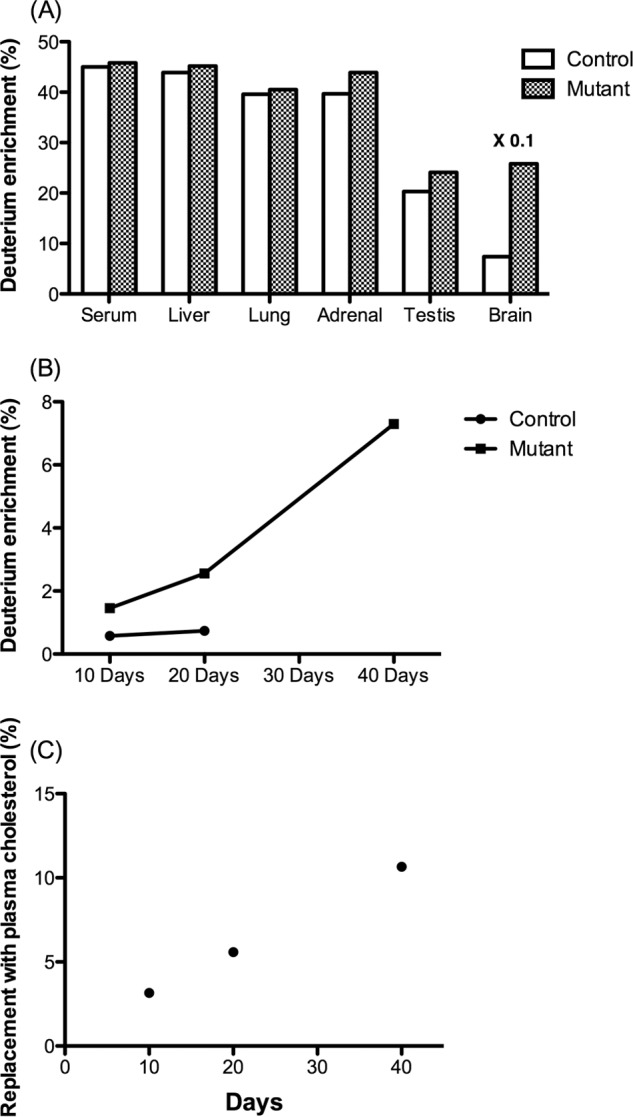
A, enrichment of cholesterol-d6 in cholesterol isolated from serum and different organs in a control mouse and a mutant mouse treated with 0.3% cholesterol-d6 in the diet for 20 days. B, enrichment of cholesterol-d6 in cholesterol isolated from the brains of three mutants and three control mice treated with 0.3% cholesterol-d6 for 10, 20, or 40 days, respectively. C, replacement of brain cholesterol with serum cholesterol was calculated at the different time points with use of the percentage incorporation of deuterium in plasma cholesterol. Data are from GC-MS analysis.
Because it is almost impossible to remove all of the blood from the brain, it is likely that a substantial part of the small enrichment of deuterium in cholesterol isolated from the brains of the above mice is due to contamination with blood containing cholesterol-d6. A flux of cholesterol-d6 into the brain would be expected to lead to a time-dependent increase in the accumulation of isotope in the brain, whereas this is not the case with the cholesterol-d6 present due to contamination with blood. In order to estimate the rate of the flux from the circulation into the brain, we compared the accumulation of cholesterol-d6 in the brain after 10 and 20 days of dietary treatment with cholesterol-d6. The enrichment of deuterium in brain cholesterol in the control increased from 0.6 to 0.7% between days 10 and 20. The corresponding figures for the mutant were 1.5 and 2.6%, respectively (Fig. 1B). In an additional experiment, we treated a mutant mouse with 0.3% cholesterol-d6 for 40 days. The enrichment of deuterium in brain cholesterol was 7.3%. In the first two experiments, the enrichment of deuterium in circulating cholesterol was about 50%, and in the latter experiment, it was about 70%. Fig. 1C shows the enrichment of deuterium in the brain cholesterol that could be expected if the enrichment of deuterium in circulating cholesterol had been 100%. The higher content of deuterium in circulating cholesterol in the long term experiments (40 days) than in the short term experiments (10 and 20 days) may be a consequence of a slow exchange of endogenous cholesterol in different extracerebal pools with exogenous cholesterol-d6.
The data shown in Fig. 1, B and C, are from three separate independent experiments with one mutant and one control mouse in each. The very high costs of the isotope prevented us from performing additional experiments allowing statistical evaluations. However, the linear enrichment of isotope with time and the marked difference between the controls and the mutants allow safe conclusions also in the absence of statistical evaluation.
It is concluded that most or all cholesterol-d6 present in brain cholesterol in the control is likely to be due to a contamination with blood cholesterol. Thus, there is no significant flux of cholesterol-d6 into the brain in the controls. In the mutant, however, there is a significant flux from the circulation into the brain between day 10 and 20 of the treatment. This enrichment was 1.1% under the conditions employed. Because the enrichment of serum cholesterol was 45% rather than 100%, the data are consistent with a flux of cholesterol from the circulation into the brain corresponding to replacement of about 2.2% of the total cholesterol pool with plasma cholesterol during the 10 days of treatment. The difference in replacement of brain cholesterol between day 10 and day 40 was calculated to be about 8.6%. The time course of the accumulation of cholesterol-d6 suggests that there is an increasing leakage of cholesterol into the brain with time. Fig. 2B shows the percentage of the replacement of brain cholesterol by plasma cholesterol in the mutant mice at different time points. By day 40, it is estimated that more than 10% of total brain cholesterol in these mice originates from the plasma.
FIGURE 2.
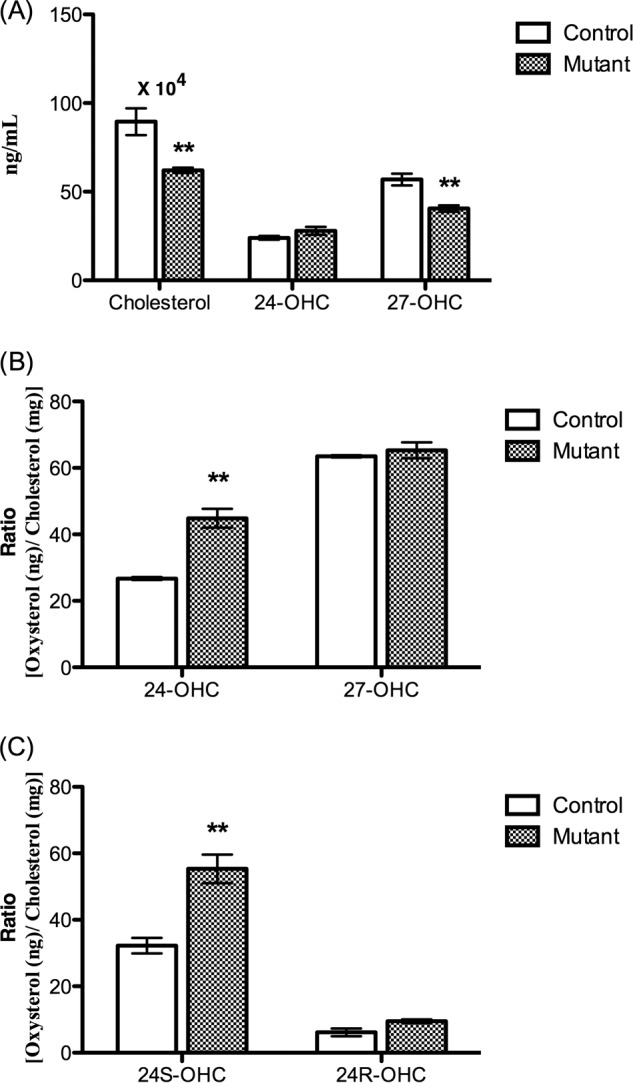
A and B, levels of cholesterol, 24(S)-hydroxycholesterol, and 27-hydroxycholesterol in serum from Pdgfbret/ret mice and their controls (absolute levels) (A) and cholesterol-correlated levels of the oxysterols (B) (data from GC-MS analysis). C, cholesterol-correlated levels of 24(R)- and 24(S)-hydroxycholesterol in serum from three control mice and three Pdgfbret/ret mice (age 10 weeks, n = 3). Data are from LC-MSn analysis. Error bars, S.E.
The incorporation of cholesterol-d6 was also measured in cholesterol in different parts of the brain in the long term experiment (40 days of feeding with 0.3% cholesterol-d6). The incorporation in cholesterol was as follows: hippocampus, 9.4%; cortex, 8.3%; cerebellum, 3.7%; corpus callosum, 6.0%; pituitary, 47%. It should be noted that, in contrast to the other parts of the brain, the pituitary is not protected by the blood-brain barrier.
Cholesterol and Oxysterol Levels in Circulation of the Pdgfbret/ret Mice
Fig. 2A summarizes the levels of cholesterol and the oxysterols 24-OHC and 27-OHC in the circulation of the Pdgfret/ret mice and their controls. The cholesterol levels as well as the levels of 27-hydroxycholesterol were significantly lower in the mutated mice. The levels of 24-OHC were slightly higher. In view of the fact that the oxysterols are transported by cholesterol-containing lipoproteins in the circulation (20), the ratio between the oxysterols and cholesterol should be used in a comparison. As shown in Fig. 2B, the cholesterol-correlated levels of 24-OHC in serum were significantly higher in the mutated mice than in the controls. The cholesterol-correlated levels of 27-OHC were similar in the mutants and the controls.
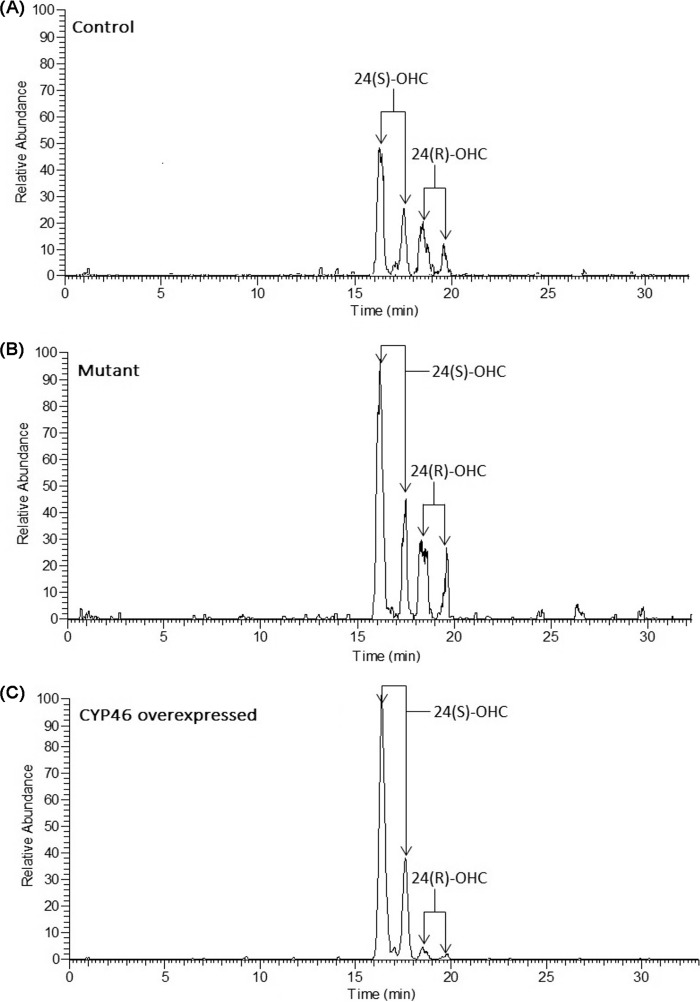
In humans, all of the 24-hydroxycholesterol present in the circulation originates from the brain, and it is only the 24(S)-stereoisomer that is formed (21). In mice, almost half of the 24-hydroxycholesterol present in the circulation originates from an organ(s) or tissues other than the brain (22). We have found that 24(S)- is the only stereoisomer of 24-OHC present in the mouse brain, whereas the circulation contains a mixture of the 24(S)- and the 24(R)-isomer.3 Most of the 24(S)-isomer in the circulation must originate from the brain. It is difficult to separate the 24(S)- and 24(R)-isomers by gas chromatography-mass spectrometry of the trimethylsilyl ether derivatives. An LC-MSn method has recently been developed capable of separating the two isomers after oxidation with cholesterol oxidase and conversion into Girard hydrazones (17). We analyzed serum from three control mice and three Pdgfret/ret mice with this method. Fig. 3 shows typical chromatograms obtained. As shown, both 24(R)- and 24(S)-epimers give two peaks corresponding to the syn- and anti-conformers of the derivative (Fig. 4). For reasons of comparison, we show the chromatogram obtained in the analysis of serum from a mouse with overexpressed CYP46. In this mouse, the situation is similar to the human situation, with 24S-OHC greatly dominating. The levels of 24S-OHC increased from 32 ± 2 ng/mg cholesterol in the control mice to 55 ± 4 ng/mg cholesterol in mutant mice (p < 0.01). The levels of 24(R)-hydroxycholesterol increased from 6.1 ± 1.1 ng/mg cholesterol in the control mouse to 9.4 ± 0.5 ng/mg cholesterol in the mutant mouse (p > 0.05). Fig. 2C shows the cholesterol-correlated levels of 24(S)- and 24(R)-hydroxycholesterol in the circulation of the control mice and the Pdgfret/ret mice.
FIGURE 3.
Typical reconstructed ion chromatograms (RIC) obtained in the analysis of 24(R)- and 24(S)-hydroxycholesterols in serum from a control mouse (A), a Pdgfbret/ret mouse (B), and a CYP46 transgenic mouse (40) (C) using LC-MSn after derivatization to Girard P hydrazones (see “Experimental Procedures”). The reconstructed ion chromatograms were generated from multiple reaction-monitoring transitions specific for 24-OHC isomers (m/z 534 → 455 → 353).
FIGURE 4.
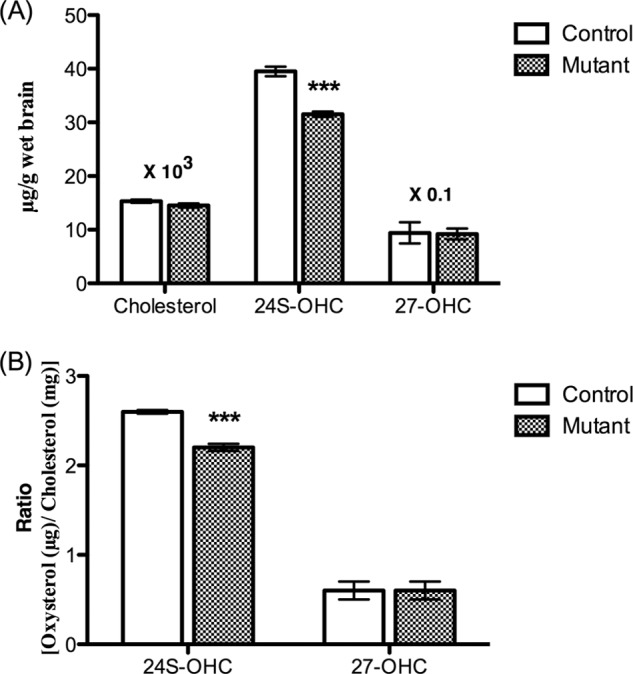
Levels of cholesterol and oxysterols in the brains of Pdgfbret/ret mice and their controls (age 10 weeks, n = 6) related to brain weight (A) and oxysterols related to cholesterol (B). Data are from GC-MS analysis. Error bars, S.E.
The above data are consistent with a higher flux of 24(S)-OHC from the brain into the circulation in the mutated mice. Part of the increase in 24(S)- and 24(R)-hydroxycholesterol may, however, be due to increased production of these oxysteroids in extracerebral tissues. Oxysterols formed extracerebrally in mice treated with cholesterol-d6 can be expected to contain deuterium to about the same extent as circulating cholesterol. In contrast, the cerebral formation of 24S-OHC would be expected to contain very little deuterium (7% in the experiment in which a mutant mouse was treated with cholesterol-d6 for 40 days). The content of deuterium in 24(S)-hydroxycholesterol in the circulation of the above mouse was found to be 18%. After correction for the content of deuterium in the brain cholesterol (7%) and the fact that the enrichment of deuterium in circulating cholesterol was about 70% rather than 100%, it can be concluded that 15–20% of the 24(S)-hydroxycholesterol in the circulation originates from extracerebral sources. The content of deuterium in 24R-hydroxycholesterol was found to be 90%. After correction for the enrichment of deuterium in the cholesterol in the circulation (about 70%), it can be concluded that all of the 24(R)-hydroxycholesterol in the circulation originates from extracerebral sources.
For reasons of comparison, we also measured the deuterium content in 27-OHC in the circulation of the control mouse and the mutant mouse treated with cholesterol-d6 for 40 days. The deuterium content was 37 and 33%, respectively. Because one of the six deuterium atoms in cholesterol-d6 is lost in connection with its conversion into 27-OHC, the product will contain five rather than six atoms of deuterium. It has been shown that there is a significant isotope effect in connection with replacement of one deuterium in the steroid side chain of cholesterol-d6 (23). Thus, the deuterium content in d5-labeled 27-OHC is lower than had been the case in the absence of an isotope effect.
Cholesterol and Oxysterol Levels in the Brains of Pdgfbret/ret Mice
Fig. 4 summarizes the levels of cholesterol and the oxysterols in the brains of the Pdgfret/ret mice. The cholesterol levels (related to brain weight) were found to be similar to those in the brains of the mutant mice (Fig. 4A). The situation was similar when the levels were related to protein (results not shown). The level of 24(S)-OHC was significantly lower in the brains of the mutant mice than in the controls, and this was the situation also when correlated to protein or cholesterol (Fig. 4B shows the cholesterol-related levels). The levels of 27-OHC were not different in the brains of the mutants and the controls, regardless of whether related to brain weight, protein, or cholesterol.
Levels of Plant Sterols in the Brains of the Pdgfbret/ret Mice
Plant sterols may accumulate to some extent in the brain, probably as a consequence of the fact that in contrast to cholesterol, these steroids cannot be eliminated by 24(S)-hydroxylation (24). A defective blood-brain barrier would be expected to lead to an increase in such accumulation. As shown in Fig. 5, there was a significant accumulation of both campesterol and sitosterol in the brains of the mutated mice. The degree of accumulation of sitosterol was lower than that of campesterol.
FIGURE 5.
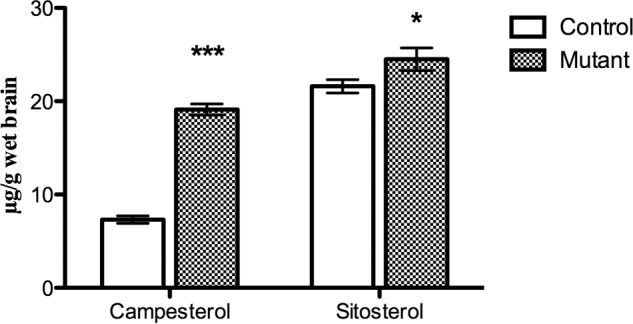
Levels of plant sterols in the brain of the Pdgfbret/ret mice and the controls (age 10 weeks, n = 6). Data are from GC-MS analysis. Error bars, S.E.
Levels of Cholesterol Precursors in the Brains of Pdgfbret/ret Mice
The ratio between cholesterol precursors and cholesterol is generally used as a marker for cholesterol synthesis. As shown in Fig. 6A, the cholesterol-related levels of the cholesterol precursors desmosterol, testicular meiosis-activating sterol (T-MAS), follicular fluid meiosis-activating sterol (FF-MAS), and 7-dehydrocholesterol (7-DHS) were significantly higher in the brains of the mutated mice than in the controls, suggesting an increased rate of cholesterol synthesis.
FIGURE 6.
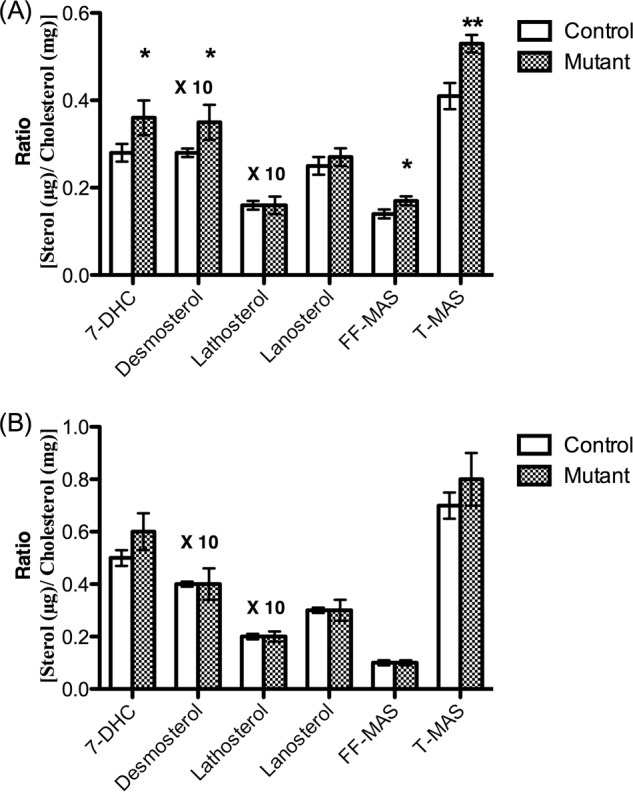
Cholesterol-correlated levels of cholesterol precursors in the brains of the Pdgfbret/ret mice and their controls (n = 6) on regular chow diet (A) and on 1% cholesterol diet (n = 4) (B). Data are from GC-MS analysis. FF-MAS, follicular fluid meiosis-activating sterol, 4,4-dimethyl-5α-cholesta-8,14,24-trien-3β-ol; T-MAS, testicular meiosis-activating sterol, 4,4-dimethyl-5α-cholesta-8,24-dien-3β-ol; 7-DHC, 7-dehydrocholesterol. Error bars, S.E.
Expression of Key Genes in Cholesterol Homeostasis
The increased levels of cholesterol precursors in the brain suggest an increased rate of synthesis of cholesterol. Such increased synthesis could be expected to be combined with increased levels of HMG-CoA reductase (Hmgcr) and/or HMG-CoA synthase (Hmgcs1). The mRNA levels of HMG-CoA synthase were slightly but significantly higher in the mutants as compared with the controls (Fig. 7A). The mRNA level of Srebp2 was not significantly increased, and this was the case also with Cyp46. Expression of two LXR-regulated genes, Cyp7b1 and Abca1, were significantly increased in the mutant. Other LXR-regulated genes (Fas, Abcg1, and Apoe) were not affected by the mutation.
FIGURE 7.
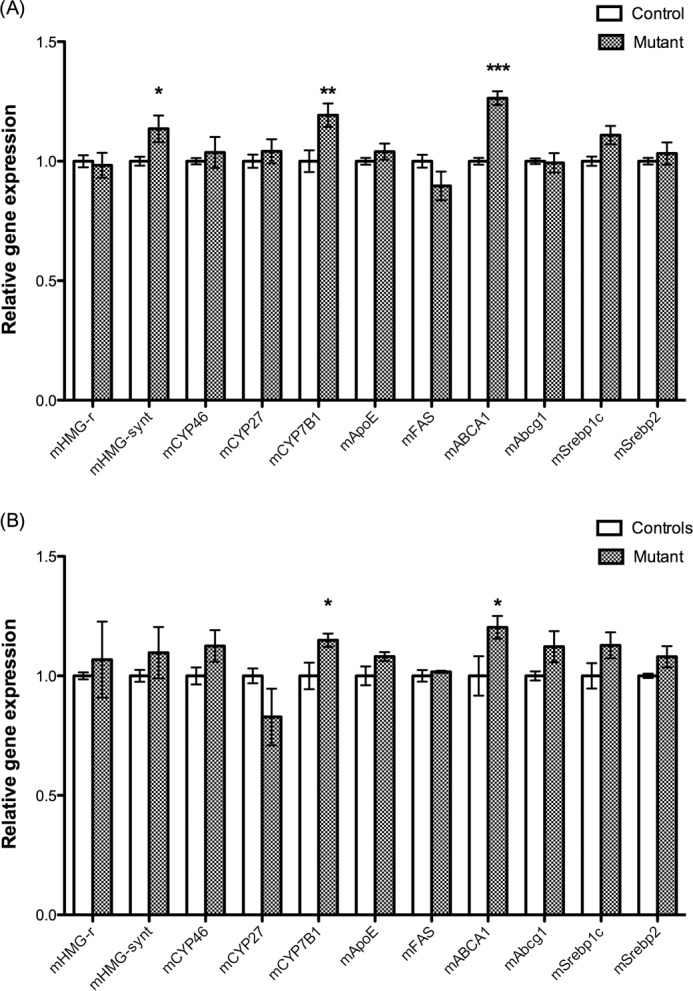
A, expression of key genes in cholesterols homeostasis in the untreated Pdgfbret/ret mice and their controls (n = 6). B, expression of the same genes in the Pdgfbret/ret mice (n = 4) treated with 1% cholesterol and their controls (n = 4) (B). Error bars, S.E.
Western Blot of the Cholesterol 24(S)-Hydroxylase (Cyp46) and Abca1
The gene encoding the cholesterol 24(S)-hydroxylase was not up-regulated in the mutant mice, but in view of the importance of this enzyme for cholesterol homeostasis, we also measured the protein content of this hydroxylase. The protein content in brain microsomes from the mutant mice and the controls was not significantly different. The ratio between cyp46 and β-actin protein was thus 0.040 ± 0.005 in the analysis of microsomes from the controls and 0.038 ± 0.009 in the analysis of microsomes from the mutants (n = 6 ± 6, p > 0.05). The LXR target gene Abca1 was up-regulated by about 20% (Fig. 7); thus, we measured the corresponding protein levels by Western blots. The ratio between Abca1 and β-actin protein was 0.18 ± 0.02 in the analysis of microsomes from the controls and 0.33 ± 0.02 in the corresponding analysis of the mutants (n = 6 ± 6, p < 0.002).
Evidence for Increased Cholesterol Synthesis in the Brains of the Pdgfbret/ret Mice
The increased levels of cholesterol precursor in the brains of the mutant mice and the higher mRNA levels of HMG-CoA synthase suggest a higher rate of synthesis in these mice. In order to confirm this, we utilized a technique based on incorporation of deuterium into brain cholesterol from deuterated water (18). Mice were exposed to 10% D2O in deuterated water for 11 days before sacrifice. The incorporation of deuterium was found to be 6.3 ± 0.3% in the brain cholesterol from the control mice (n = 6) and 9.0 ± 0.4% in the brain cholesterol from the mutants (p < 0.001, Student's t test). From this incorporation of deuterium and the enrichment of deuterium measured in the body water, the fractional synthetic rate was calculated to be 0.29 ± 0.03%/24 h in the controls and 0.47 ± 0.05%/24 h in the mutants (p < 0.05, Student's t test).
Effects of Treating the Pdgfbret/ret Mice and Their Controls with 1% Cholesterol in Diet
If the blood-brain barrier is leaking, one would expect a higher influx of cholesterol into the brain after treatment of the mice with cholesterol. Such an increased influx would be expected to reduce cholesterol synthesis in the brain. As shown in Fig. 6B, a dietary load of 1% cholesterol during 10 days normalized the levels of the cholesterol precursors. Also, the mRNA levels of HMG-CoA synthase were normalized as a consequence of the cholesterol load (Fig. 7B). The level of 24-OHC in the brain was still significantly lower in the brains of the mutated mice as compared with the controls (results not shown). For reasons of comparison, we also measured the mRNA levels of HMG-CoA synthase in the livers of the mutant and the control mice treated with cholesterol. The cholesterol treatment caused a reduction by about 88% in the livers of both the mutant and the control mice (results not shown).
DISCUSSION
The Pdgfbret/ret mouse model was used here to study the consequences of a leaking blood-brain barrier for cholesterol homeostasis in the brain. It should be emphasized that the model is complicated and that also extracerebral cholesterol homeostasis is affected. Pericyte deficiency leads to increased vascular permeability not only in the brain but also in the liver (25). In preliminary studies, we have shown that the Pdgfbret/ret mice have an abnormal lipoprotein pattern in the circulation with low levels of HDL. Most probably this is due to increased leakage of this lipoprotein from the microvessels. There is a tendency toward increased cholesterol content in extracerebral organs, possibly because of increased leakage of lipoprotein-bound cholesterol from the microvessels.3
We had expected the leaking blood-brain barrier to result in an increased flux of lipoprotein-bound cholesterol from the circulation into the brain. In accordance with this, we found that enrichment of the cholesterol in the circulation with deuterium led to a modest accumulation of deuterium-labeled cholesterol in the brain. The enrichment of deuterium in brain cholesterol during the 40 days of treatment corresponds to a daily replacement of about 0.25% of the pool in the mutants. Given our observation that the fractional synthetic rate of brain cholesterol was found to be 0.47%/24 h in the mutant mouse, it is evident that the flux of cholesterol into the brain is about half of the synthesis.
The slight increase in enrichment of cholesterol-d6 in the brain of the control, from 0.6 to 0.7% during day 10 and day 20 of the treatment, is not significant. If there is a flux of cholesterol from the circulation into the brain under normal conditions, this flux must be very low. This finding is in accord with previous in vivo studies with different animal models (21, 26–28) and in one study with humans (29).
Evidence has been presented that endothelial cells contain a low density lipoprotein (LDL) receptor (30). It has also been shown that this receptor is able to mediate transcytosis of LDL across an in vitro model of the blood-brain barrier with cultured endothelial cells on one side of a filter and astrocytes on the opposite side (31). Some results also suggest a role of caveolae in the transcellular transport of LDL across the brain endothelium (31). Thus, it is evident that there is a potential for receptor-mediated transcytosis across brain endothelial cells. The present and previous experiments suggest, however, that such a transcytosis is not of importance under normal in vivo conditions. Pericytes have been shown to inhibit transcytosis of different low- and high-molecular traces across the blood-brain barrier (2), and the results of the present study suggest that this is likely to be the case also for transcytosis of LDL and LDL-cholesterol across the BBB.
The increased flux of cholesterol from the circulation into the brain of the mutant mice would be expected to lead to a down-regulation of cholesterol synthesis. Surprisingly, the opposite was found. Cholesterol synthesis in the brains of untreated Pdgfbret/ret mice was thus found to be increased as judged from three different observations: 1) increased levels of cholesterol precursors, 2) slightly increased mRNA levels of HMG-CoA synthase, and 3) increased fractional synthetic rate of brain cholesterol, as demonstrated with the D2O technique. It is evident that the increased flux of cholesterol itself into the brain of the mutant is not able to down-regulate cholesterol synthesis. Some other factor(s) related to the leaking BBB may cause a stimulation of brain cholesterol synthesis.
A flux of lipoprotein-bound cholesterol from the circulation into the brain must be dependent upon its plasma concentration. The fact that the levels of cholesterol were reduced in the circulation of the untreated mutant mice may be part of the explanation for the relatively modest flux of cholesterol into the brains of these mice. The finding that treatment of the mutants with dietary cholesterol normalized cholesterol synthesis in relation to the controls is consistent with an increased flux of cholesterol into the brains of the Pdgfbret/ret mice when the levels of cholesterol increase in the circulation.
The increased cholesterol synthesis in the brain of the untreated Pdgfbret/ret mouse is likely to be a compensation for a loss of either cholesterol itself or a regulating metabolite. The concentration of cholesterol itself in the brains of the untreated Pdgfbret/ret mice was not significantly changed. The level of 24-OHC, the dominating oxysterol in the brain, was, however, significantly reduced in relation to tissue weight, cholesterol, and protein. The reduction was not due to reduced activity of the cholesterol 24(S)-hydroxylase because the mRNA levels and protein levels corresponding to this enzyme were unaffected by the mutation. 24-OHC is an efficient suppressor of cholesterol synthesis in vitro (11, 12), and we recently showed that reduced levels of 24-OHC in the brain as a consequence of increased metabolism result in increased cholesterol synthesis (13). Increased flux of 24-OHC from the brain into the circulation would be expected to lead to increased levels in the circulation. Utilizing data from the experiment with cholesterol-d6, we could demonstrate that a least a substantial part of the increased level of 24-OHC in the circulation of the mutant mice is due to an increased flux of this oxysterol from the brain.
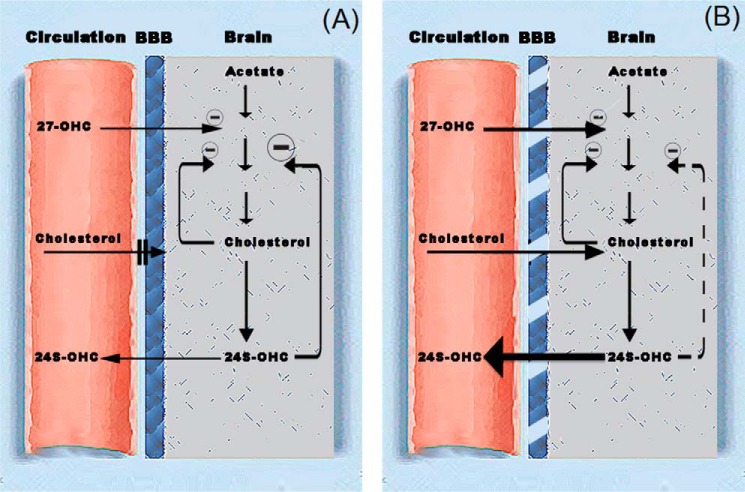
In a previous work from our laboratory, we presented a theoretical model for cholesterol homeostasis in the brain that is shown in Fig. 8. This model is consistent with previous in vitro results (11, 12) and in vivo results based on mouse models with increased or decreased levels of 24-OHC and 27-OHC (13). In this model, cholesterol synthesis is suppressed by the levels of 24-OHC and to some extent also by the flux of 27-OHC from the circulation into the brain. In Fig. 8B, the situation in the brain of the Pdgfbret/ret mouse is shown. The flux of the suppressor 24-OHC from the brain into the circulation is increased, leading to reduced inhibition of cholesterol synthesis. This effect is counteracted by the flux of cholesterol from the circulation into the brain. In the untreated Pdgfbret/ret mouse, the stimulatory effect of the reduced level of 24-OHC appears to be more important than the inhibitory effect of the flux of cholesterol. After treatment with dietary cholesterol, increasing the flux of cholesterol into the brain and increasing the suppression of cholesterol synthesis, the overall effect of the leaking blood-brain barrier was a normalization of the rate of cholesterol synthesis.
FIGURE 8.
Theoretical model for the mechanism of regulation of cholesterol homeostasis in the brain.
The mechanism behind the normal flux of 24-OHC from the brain to the circulation has not been clarified in detail. The presence of a hydroxyl group in the steroid side chain leads to reordering of phospholipids in biomembranes, allowing a flux of such steroids across the membranes orders of magnitude faster than cholesterol (32, 33). This fact, in combination with a very high concentration gradient between the brain and the circulation, about 100-fold (20), is likely to reduce the need for active transport of 24-OHC across biomembranes. Evidence has been presented from in vitro studies that the scavenger receptor class B, type I, is of some importance for the efflux of 24S-OHC from cultured endothelial cells (34). According to in vivo studies with mouse models, also the transporter Abcg1 and Abcg4 may be of some importance for efflux of side-chain oxidized oxysterols from the brain (35).
It should be noted that the mRNA levels of scavenger receptor B1 (Srb1) and abcg1 were similar in the brains of the mutant mice and the control mice. Both the mRNA levels and protein levels corresponding to Abca1 were, however, slightly increased in the brains of the mutants. In this regard, it is of interest that one important function of Abca1 in the brain is likely to be a transport of cholesterol from astrocytes to neuronal cells (36, 37). Given the increased flux of 24OH from the neuronal cells in the present mouse model, there is a need for a higher efflux of cholesterol from the astrocytes to the neuronal cells. From this point of view, the up-regulation of Abca1 in our mouse model may reflect a compensatory mechanism. Up-regulation of Abca1 may be a consequence of LXR activation. However, several other LXR-dependent genes were not up-regulated in the brains of the mutant mice, and we are unable to identify the factors behind up-regulation of Abca1.
Plant sterols, to some extent, accumulate in the brain, and the possibility has been excluded that the presence of these steroids in the brain is due to a contamination of blood (23). Part of this accumulation is likely to be a consequence of the fact that they cannot be eliminated by the same mechanism as cholesterol by 24(S)-hydroxylation (23). Increased permeability of the BBB would be expected to lead to higher levels of the plant sterols in the brains of the Pdgfbret/ret mice than in the controls. In accordance with this, the levels of the two plant sterols campesterol and sitosterol were significantly higher than in the controls. The degree of accumulation of campesterol is markedly higher than that of sitosterol. High accumulated levels of these two plant sterols in the mouse brain has been observed also in previous work (23).
There is a continuous flux of the oxysterol 27-OHC from the circulation into the brain, and this flux has been shown to increase in patients with a defective blood-brain barrier (38). In view of this, higher levels of 27-OHC would be expected in the brains of the mutated mice as compared with the controls. This was not found, however. Part of the explanation may be that, similar to 24-OHC, 27-OHC may also leak out from the brain into the circulation in the Pdgfbret/ret mouse. Most 27-OHC present in the brain originates from the circulation (6). The brain contains some sterol 27-hydroxlase, however, and thus there may be some production of 27-OHC also in this organ. 27-OHC formed in the brain of a mouse treated with cholesterol-d6 would not be expected to contain deuterium. The finding that the ratio between unlabeled and labeled 27-OHC in the circulation of the mutant mouse treated with cholesterol-d6 was slightly higher than the corresponding ratio in 27-OHC in the corresponding control mouse is consistent with some flux of 27-OHC from the brain into the circulation. Another possible explanation for the failure to find increased levels of 27-OHC in the brains of the mutant mice may be increased metabolism. The oxysterol 7α-hydroxylase CYP7B1 is the most important enzyme for the metabolism of 27-OHC. The mRNA levels corresponding to this enzyme were increased in the brains of the mutant mice, a finding consistent with the hypothesis of increased metabolism.
The relevance of the present results for the human situation is difficult to evaluate. Very recently, a mutation in the human PDGF receptor B gene was described, causing calcification of basal ganglia and cognitive defects (39). Also, the present mouse model has been shown to have calcifications in basal ganglia (39). Thus far, no disturbances in cholesterol homeostasis have been described in the patients with a mutation in the human PDGF receptor B gene. In a population of patients with a defective blood brain barrier secondary to different diseases, no significant increase in levels of 24-OHC was observed, however (38). Thus, it is possible that these clinical conditions are not associated with the same severe effects on the blood-brain barrier as in the present mouse model.
To summarize, we have shown that pericyte deficiency leads to increased flux of cholesterol and plant sterols from the circulation into a mouse brain. Unless the cholesterol levels in the circulation are increased, this flux is not sufficient to down-regulate cholesterol synthesis in the brain. The leaking blood-brain barrier leads to loss of the regulating oxysterol 24S-OHC from the brain into the circulation, resulting in increased cholesterol synthesis. This stimulatory effect can, however, be counteracted by increasing the concentration of cholesterol in the circulation, which is likely to lead to higher flux into the brain.
Acknowledgments
We thank Zeina Ali for help with sample collection. The skillful assistance of Inger Moberg is gratefully acknowledged.
This work was supported, in whole or in part, by National Institutes of Health Grant GM 62282. This work was also supported by grants from the Swedish Research Council and Brain Power. Work in Swansea was supported by Biotechnology and Biological Sciences Research Council Grants BB/I001735/1 and BBH0010181.
A. A. Saeed, G. Genové, T. Li, D. Lütjohann, M. Olin, N. Mast, I. A. Pikuleva, P. Crick, Y. Wang, W. Griffiths, C. Betsholtz, and I. Björkhem, unpublished observations.
- BBB
- blood-brain barrier
- 24-OHC
- 24-hydroxycholesterol
- 24S-OHC
- 24(S)-hydroxycholesterol
- 27-OHC
- 27-hydroxycholesterol
- BisTris
- 2-[bis(2-hydroxyethyl)amino]-2-(hydroxymethyl)propane-1,3-diol.
REFERENCES
- 1. Armulik A., Genové G., Betsholtz C. (2011) Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev. Cell 21, 193–215 [DOI] [PubMed] [Google Scholar]
- 2. Armulik A., Genové G., Mäe M., Nisancioglu M. H., Wallgard E., Niaudet C., He L., Norlin J., Lindblom P., Strittmatter K., Johansson B. R., Betsholtz C. (2010) Pericytes regulate the blood-brain barrier. Nature 468, 557–561 [DOI] [PubMed] [Google Scholar]
- 3. Bell R. D., Winkler E. A., Singh I., Sagare A. P., Deane R., Wu Z., Holtzman D. M., Betsholtz C., Armulik A., Sallstrom J., Berk B. C., Zlokovic B. V. (2012) Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature 485, 512–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Björkhem I., Meaney S. (2004) Brain cholesterol: long secret life behind a barrier. Arterioscler. Thromb. Vasc. Biol. 24, 806–815 [DOI] [PubMed] [Google Scholar]
- 5. Dietschy J. M., Turley S. D. (2001) Cholesterol metabolism in the brain. Curr. Opin. Lipidol. 12, 105–112 [DOI] [PubMed] [Google Scholar]
- 6. Björkhem I. (2006) Crossing the barrier: oxysterols as cholesterol transporters and metabolic modulators in the brain. J. Intern. Med. 260, 493–508 [DOI] [PubMed] [Google Scholar]
- 7. Björkhem I., Lütjohann D., Breuer O., Sakinis A., Wennmalm A. (1997) Importance of a novel oxidative mechanism for elimination of brain cholesterol. Turnover of cholesterol and 24(S)-hydroxycholesterol in rat brain as measured with 18O2 techniques in vivo and in vitro. J. Biol. Chem. 272, 30178–30184 [DOI] [PubMed] [Google Scholar]
- 8. Xie C., Lund E. G., Turley S. D., Russell D. W., Dietschy J. M. (2003) Quantitation of two pathways for cholesterol excretion from the brain in normal mice and mice with neurodegeneration. J. Lipid Res. 44, 1780–1789 [DOI] [PubMed] [Google Scholar]
- 9. Meaney S., Heverin M., Panzenboeck U., Ekström L., Axelsson M., Andersson U., Diczfalusy U., Pikuleva I., Wahren J., Sattler W., Björkhem I. (2007) Novel route for elimination of brain oxysterols across the blood-brain barrier: conversion into 7α-hydroxy-3-oxo-4-cholestenoic acid. J. Lipid Res. 48, 944–951 [DOI] [PubMed] [Google Scholar]
- 10. Björkhem I., Cedazo-Minguez A., Leoni V., Meaney S. (2009) Oxysterols and neurodegenerative diseases. Mol. Aspects Med. 30, 171–179 [DOI] [PubMed] [Google Scholar]
- 11. Wang Y., Muneton S., Sjövall J., Jovanovic J. N., Griffiths W. J. (2008) The effect of 24S-hydroxycholesterol on cholesterol homeostasis in neurons: quantitative changes to the cortical neuron proteome. J. Proteome Res. 7, 1606–1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schroepfer G. J., Jr. (2000) Oxysterols: modulators of cholesterol metabolism and other processes. Physiol. Rev. 80, 361–554 [DOI] [PubMed] [Google Scholar]
- 13. Ali Z., Heverin M., Olin M., Acimovic J., Lövgren-Sandblom A., Shafaati M., Båvner A., Meiner V., Leitersdorf E., Björkhem I. (2013) On the regulatory role of side-chain hydroxylated oxysterols in the brain. Lessons from CYP27A1 transgenic and Cyp27a1(−/−) mice. J. Lipid Res. 54, 1033–1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lindblom P., Gerhardt H., Liebner S., Abramsson A., Enge M., Hellstrom M., Backstrom G., Fredriksson S., Landegren U., Nystrom H. C., Bergstrom G., Dejana E., Ostman A., Lindahl P., Betsholtz C. (2003) Endothelial PDGF-B retention is required for proper investment of pericytes in the microvessel wall. Genes Dev. 17, 1835–1840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Acimovic J., Lövgren-Sandblom A., Monostory K., Rozman D., Golicnik M., Lutjohann D., Björkhem I. (2009) Combined gas chromatographic/mass spectrometric analysis of cholesterol precursors and plant sterols in cultured cells. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 877, 2081–2086 [DOI] [PubMed] [Google Scholar]
- 16. Dzeletovic S., Breuer O., Lund E., Diczfalusy U. (1995) Determination of cholesterol oxidation products in human plasma by isotope dilution-mass spectrometry. Anal. Biochem. 225, 73–80 [DOI] [PubMed] [Google Scholar]
- 17. Griffiths W. J., Crick P. J., Wang Y., Ogundare M., Tuschl K., Morris A. A., Bigger B. W., Clayton P. T., Wang Y. (2013) Analytical strategies for characterization of oxysterol lipidomes: liver X receptor ligands in plasma. Free Radic. Biol. Med. 59, 69–84 [DOI] [PubMed] [Google Scholar]
- 18. Diraison F., Pachiaudi C., Beylot M. (1996) In vivo measurement of plasma cholesterol and fatty acid synthesis with deuterated water: determination of the average number of deuterium atoms incorporated. Metab. Clin. Exp. 45, 817–821 [DOI] [PubMed] [Google Scholar]
- 19. Livak K. J., Schmittgen T. D. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−ΔΔC(T)) method. Methods 25, 402–408 [DOI] [PubMed] [Google Scholar]
- 20. Babiker A., Diczfalusy U. (1998) Transport of side-chain oxidized oxysterols in the human circulation. Biochim. Biophys. Acta 1392, 333–339 [DOI] [PubMed] [Google Scholar]
- 21. Lütjohann D., Breuer O., Ahlborg G., Nennesmo I., Sidén A., Diczfalusy U., Björkhem I. (1996) Cholesterol homeostasis in human brain: evidence for an age-dependent flux of 24S-hydroxycholesterol from the brain into the circulation. Proc. Natl. Acad. Sci. U.S.A. 93, 9799–9804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Meaney S., Lütjohann D., Diczfalusy U., Björkhem I. (2000) Formation of oxysterols from different pools of cholesterol as studied by stable isotope technique: cerebral origin of most circulating 24S-hydroxycholesterol in rats, but not in mice. Biochim. Biophys. Acta 1486, 293–298 [DOI] [PubMed] [Google Scholar]
- 23. Lund E., Breuer O., Björkhem I. (1992) Evidence that 24- and 27-hydroxylation are not involved in the cholesterol-induced down-regulation of hydroxymethylglutaryl-CoA reductase in mouse liver. J. Biol. Chem. 267, 25092–25097 [PubMed] [Google Scholar]
- 24. Vanmierlo T., Weingärtner O., van der Pol S., Husche C., Kerksiek A., Friedrichs S., Sijbrands E., Steinbusch H., Grimm M., Hartmann T., Laufs U., Böhm M., de Vries H. E., Mulder M., Lütjohann D. (2012) Dietary intake of plant sterols stably increases plant sterol levels in the murine brain. J. Lipid Res. 53, 726–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Raines S. M., Richards O. C., Schneider L. R., Schueler K. L., Rabaglia M. E., Oler A. T., Stapleton D. S., Genové G., Dawson J. A., Betsholtz C., Attie A. D. (2011) Loss of PDGF-B activity increases hepatic vascular permeability and enhances insulin sensitivity. Am. J. Physiol. Endocrinol. Metab. 301, E517–E526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Turley S. D., Burns D. K., Rosenfeld C. R., Dietschy J. M. (1996) Brain does not utilize low density lipoprotein-cholesterol during fetal and neonatal development in the sheep. J. Lipid Res. 37, 1953–1961 [PubMed] [Google Scholar]
- 27. Jurevics H., Morell P. (1995) Cholesterol for synthesis of myelin is made locally, not imported into brain. J. Neurochem. 64, 895–901 [DOI] [PubMed] [Google Scholar]
- 28. Lütjohann D., Stroick M., Bertsch T., Kühl S., Lindenthal B., Thelen K., Andersson U., Björkhem I., Bergmann K. K., Fassbender K. (2004) High doses of simvastatin, pravastatin, and cholesterol reduce brain cholesterol synthesis in guinea pigs. Steroids 69, 431–438 [DOI] [PubMed] [Google Scholar]
- 29. Chobanian A. V., Hollander W. (1962) Body cholesterol metabolism in man. I. The equilibration of serum and tissue cholesterol. J. Clin. Invest. 41, 1732–1737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Méresse S., Delbart C., Fruchart J. C., Cecchelli R. (1989) Low-density lipoprotein receptor on endothelium of brain capillaries. J. Neurochem. 53, 340–345 [DOI] [PubMed] [Google Scholar]
- 31. Dehouck B., Fenart L., Dehouck M. P., Pierce A., Torpier G., Cecchelli R. (1997) A new function for the LDL receptor: transcytosis of LDL across the blood-brain barrier. J. Cell Biol. 138, 877–889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lange Y., Ye J., Strebel F. (1995) Movement of 25-hydroxycholesterol from the plasma membrane to the rough endoplasmic reticulum in cultured hepatoma cells. J. Lipid Res. 36, 1092–1097 [PubMed] [Google Scholar]
- 33. Meaney S., Bodin K., Diczfalusy U., Björkhem I. (2002) On the rate of translocation in vitro and kinetics in vivo of the major oxysterols in human circulation: critical importance of the position of the oxygen function. J. Lipid Res. 43, 2130–2135 [DOI] [PubMed] [Google Scholar]
- 34. Panzenboeck U., Balazs Z., Sovic A., Hrzenjak A., Levak-Frank S., Wintersperger A., Malle E., Sattler W. (2002) ABCA1 and scavenger receptor class B, type I, are modulators of reverse sterol transport at an in vitro blood-brain barrier constituted of porcine brain capillary endothelial cells. J. Biol. Chem. 277, 42781–42789 [DOI] [PubMed] [Google Scholar]
- 35. Bojanic D. D., Tarr P. T., Gale G. D., Smith D. J., Bok D., Chen B., Nusinowitz S., Lövgren-Sandblom A., Björkhem I., Edwards P. A. (2010) Differential expression and function of ABCG1 and ABCG4 during development and aging. J. Lipid Res. 51, 169–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pfrieger F. W. (2003) Outsourcing in the brain: do neurons depend on cholesterol delivery by astrocytes? BioEssays 25, 72–78 [DOI] [PubMed] [Google Scholar]
- 37. Abildayeva K., Jansen P. J., Hirsch-Reinshagen V., Bloks V. W., Bakker A. H., Ramaekers F. C., de Vente J., Groen A. K., Wellington C. L., Kuipers F., Mulder M. (2006) 24(S)-Hydroxycholesterol participates in a liver X receptor-controlled pathway in astrocytes that regulates apolipoprotein E-mediated cholesterol efflux. J. Biol. Chem. 281, 12799–12808 [DOI] [PubMed] [Google Scholar]
- 38. Leoni V., Masterman T., Patel P., Meaney S., Diczfalusy U., Björkhem I. (2003) Side chain oxidized oxysterols in cerebrospinal fluid and the integrity of blood-brain and blood-cerebrospinal fluid barriers. J. Lipid Res. 44, 793–799 [DOI] [PubMed] [Google Scholar]
- 39. Keller A., Westenberger A., Sobrido M. J., García-Murias M., Domingo A., Sears R. L., Lemos R. R., Ordoñez-Ugalde A., Nicolas G., da Cunha J. E., Rushing E. J., Hugelshofer M., Wurnig M. C., Kaech A., Reimann R., Lohmann K., Dobričić V., Carracedo A., Petrović I., Miyasaki J. M., Abakumova I., Mäe M. A., Raschperger E., Zatz M., Zschiedrich K., Klepper J., Spiteri E., Prieto J. M., Navas I., Preuss M., Dering C., Janković M., Paucar M., Svenningsson P., Saliminejad K., Khorshid H. R., Novaković I., Aguzzi A., Boss A., Le Ber I., Defer G., Hannequin D., Kostić V. S., Campion D., Geschwind D. H., Coppola G., Betsholtz C., Klein C., Oliveira J. R. (2013) Mutations in the gene encoding PDGF-B cause brain calcifications in humans and mice. Nat. Genet. 45, 1077–1082 [DOI] [PubMed] [Google Scholar]
- 40. Shafaati M., Olin M., Båvner A., Pettersson H., Rozell B., Meaney S., Parini P., Björkhem I. (2011) Enhanced production of 24S-hydroxycholesterol is not sufficient to drive liver X receptor target genes in vivo. J. Intern. Med. 270, 377–387 [DOI] [PubMed] [Google Scholar]