Abstract
Nearly 40,000 Americans are newly infected with Human Immunodeficiency Virus (HIV) each year. Recently, studies have demonstrated associations between group-level characteristics and the prevalence and incidence of HIV/Acquired Immune Deficiency Syndrome (AIDS) and other sexually transmitted diseases. Two mechanisms previously posited to explain these associations are neighborhood effects on risk behaviors and social or institutional policies. In this paper, we hypothesize that adversity at the population level, such as neighborhood poverty, also influences HIV risk through stress-mediated aberrations in immunological susceptibility by reviewing existing data examining each of these pathways. In particular, we review the evidence showing that: (1) Neighborhood ecologic stressors influence neighborhood-and individual-levels of mental health, psychosocial stress, and HIV/AIDS risk, (2) Individual-level psychosocial stressors influence progression from HIV to AIDS through stress-related hormonal changes, and (3) Individual-level psychosocial stressors influence HIV acquisition via stress-related reactivation of latent herpesviruses, specifically EBV and HSV-2. Our review indicates that further studies are needed to examine the joint pathways linking neighborhood-level sources of psychosocial stress, stress-related reactivation of HSV-2 and EBV, and increased acquisition rates of HIV. We suggest using a multi-level framework for targeting HIV prevention efforts that address not only behavioral risk factors, but structural, political, and institutional factors associated with neighborhood disadvantage, levels of psychosocial stress, and prevention or treatment of HSV-2 and EBV.
Keywords: HIV, Neighborhood, Psychosocial stress, HSV-2, EBV
Introduction
Approximately 40,000 Americans are newly infected with human immunodeficiency virus (HIV) each year (Centers for Disease Control and Prevention 2006). Extensive research on risk factors for incident HIV infection has primarily focused on identifying individual-level, and to some extent network-level, determinants of HIV risk. These individual-level risk factors include but are not limited to: socioeconomic status, gender, race/ethnicity, culture, genetic markers, drug use, risk-networks, and high risk sexual behaviors (Astemborski et al. 1994; Kottiri et al. 2002; Latkin et al. 1995; Latkin 1995; Saracco et al. 1989; Zapka et al. 1993). While these insights are important, several recent studies have demonstrated relationships at the neighborhood-level, such as the association between ecologic stressors with incidence of HIV/Acquired Immune Deficiency Syndrome (AIDS) and other sexually transmitted diseases (STDs), even after controlling for individual-level risk behaviors, such as sexual behavior and socioeconomic status (Brugal et al. 2003; Cohen et al. 2000; Zierler et al. 2000).
Ecologic stressors (e.g. stressors operating at the neighborhood or census level) include determinants such as concentrated disadvantage, unequal income distribution, residential segregation, institutionalized racism, and poor quality of the built environment. These factors represent compositional measures (i.e. an aggregate or average of the individual-level characteristics) or contextual measures (i.e. group-level attribute(s) for which there is no individual-level equivalent). For example, concentrated disadvantage is a compositional factor that is comprised of an average of individual-measures of personal income, employment status, occupational level, and educational attainment. Income distribution, on the other hand, is a contextual factor to which there is no individual equivalent measure, such as high income disparity among individuals residing in a given neighborhood. Unequal income distribution can lead to increased inter-individual tension, violence, decreased social trust, loss of social capital and decreased investment in material and institutional/health resources for the community. Policies that limit the availability of HIV prevention and treatment services are an example of a contextual determinant that has been shown to increase risk of HIV transmission within a community, independent of individual behavior or characteristics (Rhodes et al. 2005; Takahashi et al. 2001; Wallace et al. 1994).
Another contextual factor is residential segregation, which represents the separation of racial/ethnic or socioeconomic groups into different geographic areas. Segregation can lead to restricted socioeconomic attainment because it determines access to educational and employment opportunities, health-related resources and enforces social HIV risk networks/behaviors. Similarly, institutionalized racism is a contextual factor that could increase risk of HIV transmission within populations by limiting access to care and treatment as well as other resources (Anonymous 2006; Foster 2007; Zamboni and Crawford 2007). The quality of the built environment is also a contextual factor. Built environment characteristics include the amount of litter, crime, vandalism, and abandoned buildings in an area. Such adverse built environmental features may lead to locations and circumstances that foster participation in HIV risk behaviors.
Galea et al. (2003), suggest that in areas characterized by adverse characteristics (e.g., poverty, substantial social disorder and lack of safety) people engage in disproportionately more risk behaviors, which influences the risk of transmission of HIV and of STDs (Galea et al. 2003). There is evidence supporting a relationship between these types of ecologic stressors and HIV and STDs (Cohen et al. 2000; Friedman et al. 2005; Zierler et al. 2000). For example, Cohen et al. (2000) found a significant relationship between poor quality built environment features such as broken windows and higher gonorrhea rates in census block groups in New Orleans, even after adjusting for other relevant covariates like demographic composition, age distribution, and neighborhood poverty levels (Cohen et al. 2000). Friedman et al. (2005), showed that metropolitan areas with higher levels of income inequality also have higher rates of HIV prevalence (Friedman et al. 2005). Importantly, these associations are often maintained after controlling for individual-level risk factors (Cohen et al. 2000; Zierler et al. 2000). For example, Zierler et al. (2000), found that after controlling for sex, race/ethnicity and individual socioecoconomic status, the lowest incidence of AIDS occurred among white women in the least impoverished neighborhoods (Zierler et al. 2000).
One way in which ecologic stressors may influence behavioral risk factors for HIV is through detrimental effects on mental health. Numerous studies have suggested an association between ecologic stressors, such as neighborhood disadvantage and poor mental health outcomes, including depression and anxiety- all of which may impact HIV risk behaviors (Aneshensel and Sucoff 1996; Hill et al. 2005; Kubzansky et al. 2005; Latkin and Curry 2003; Ross and Mirowsky 2001; Silver et al. 2002). Mental health has been shown to increase HIV/AIDS risk through changes in sexual and drug use risk behaviors (Blumberg and Dickey 2003; Sterk et al. 2006; Stevens et al. 2003; Stiffman et al. 1992). Factors such as poverty, substantial social disorder and lack of safety may lead to an increase in risk behaviors that enhance transmission of HIV and of STDs (Galea et al. 2003).
Although risk behaviors are likely to represent a key component of the effects of ecologic stressors on HIV/AIDS risk, we review the literature that supports a biological mechanism that may also explain the association between ecologic stressors and risk of HIV/AIDS. We hypothesize that neighborhood stressors influence likelihood of HIV acquisition through stress-related aberrations in immunological susceptibility among populations exposed to HIV (see Fig. 1). Indeed, there is abundant evidence that chronic stressors directly influence the immune system, including enhanced susceptibility to viral and bacterial infections among individuals exposed during human challenge trials and vaccination (Biondi and Zannino 1997; Bonneau et al. 2007; Miller et al. 2004; Morag et al. 1999). If ecologic stressors, such as concentrated disadvantage and segregation, determine the distribution of psychological stress in a community, then it is likely that stress-related changes in immune function may also vary by neighborhood context. As a result, the rate of acquisition and progression of HIV upon exposure, which is affected by immune function, would also vary by neighborhood characteristics. In particular, we review the evidence showing that: (1) Neighborhood stressors influence mental health, psychosocial stress, and HIV/AIDS risk, (2) Individual-level stressors enhance progression from HIV to AIDS through stress-related hormonal changes, and (3) Individual-level stressors influence HIV acquisition via stress-related reactivation of latent herpesviruses, specifically EBV and HSV-2.
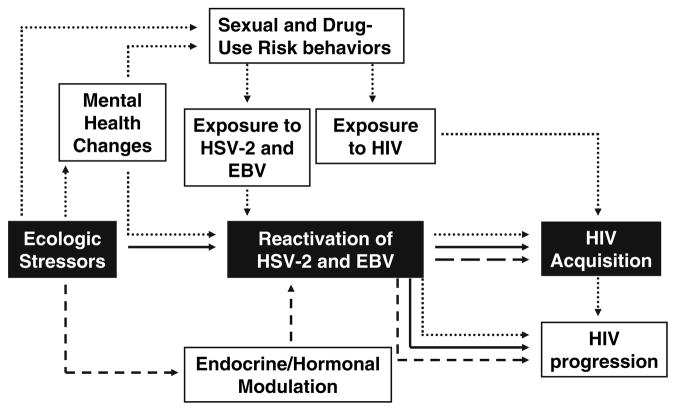
Fig. 1.
Pathways between ecological stressors, mental health, risk behaviors, EBV, HSV-1, and HIV. EBV, Epstein Barr Virus; HSV-2, Herpes Simplex Virus Type-2; HIV, Human Immunodeficiency Virus. Path 1 (
 ): Ecologic stressors may lead to mental health changes which cause increased sexual and drug-use risk behaviors and consequent increased exposure to HSV-2 and EBV, future reactivation of HSV-2 and EBV and subsequent increased risk of HIV acquisition and progression. Path 2 (
): Ecologic stressors may lead to mental health changes which cause increased sexual and drug-use risk behaviors and consequent increased exposure to HSV-2 and EBV, future reactivation of HSV-2 and EBV and subsequent increased risk of HIV acquisition and progression. Path 2 (
 ): Ecologic stressors may cause increased reactivation of HSV-2 and EBV directly, which subsequently increases risk for HIV acquisition and progression. Path 3 (
): Ecologic stressors may cause increased reactivation of HSV-2 and EBV directly, which subsequently increases risk for HIV acquisition and progression. Path 3 (
 ): Ecologic stressors may cause endocrine/hormonal changes which lead to reactivation of HSV-2 and EBV and subsequent increased risk for HIV acquisition and progression
): Ecologic stressors may cause endocrine/hormonal changes which lead to reactivation of HSV-2 and EBV and subsequent increased risk for HIV acquisition and progression
Neighborhoods, Psychosocial Stress, and Disease
Neighborhood characteristics, such as poverty, crime, stability and unemployment have been shown to influence psychosocial stress (Boardman 2004; Steptoe and Feldman 2001). For example, a study conducted by Steptoe and Feldman (2001) demonstrated that litter in the streets, smells and fumes, noise, traffic, vandalism and other negative characteristics were associated with significantly higher levels of psychological distress, even after adjusting for individual-level factors such as age, sex, and socioeconomic status (Steptoe and Feldman 2001). In another study, neighborhood characteristic of residential stability, measured as the proportion of residents owning their own home and proportion of residents residing in same dwelling five years earlier, were examined in relation to levels of psychosocial stress (Boardman 2004). An interaction was identified between neighborhood instability and stress such that stress had a greater impact on self-rated health among those in less stable neighborhoods, compared to those in more stable neighborhoods (Boardman 2004). After controlling for individual-level characteristics such as age, sex, race, and socioeconomic status, variation in levels of stress across neighborhoods still explained a significant proportion of self-rated health outcomes (Boardman 2004). These studies suggest that ecologic stressors can influence psychosocial stress and furthermore, the extent of psychosocial stress experienced across neighborhoods may partially account for differences in health between neighborhoods.
Population based studies have shown large socioeconomic and race/ethnic disparities in several STDs, including HSV-2 and HIV (Espinoza et al. 2007; Fleming et al. 1997; McQuillan et al. 2004; Smith et al. 2000). Importantly, a variety of disease outcomes, including STDs, has also been identified at the neighborhood level among disadvantaged areas and race/ethnic groups (Chaix et al. 2007; Cohen et al. 2000; Cozier et al. 2007; Ross and Mirowsky 2001; Roca et at. 1995; Sampson et al. 2002; Wallace 1990). Several studies have found a higher prevalence of STDs in areas with greater socioeconomic deprivation (Brugal et al. 2003; Das et al. 2005; Monteiro et al. 2005; Winter et al. 2000; Zierler et al. 2000). For example, Das et al. (2005), showed that incidence rates of co-infection with Chlamydia and gonorrhea were 2.49 times as high for those living in areas with high material deprivation scores compared to those living in low or moderate levels of deprivation (Das et al. 2005). Similarly, Monteiro et al. (2005) found that genital herpes infections, genital warts, Chlamydia, and Gonorrhea, clustered in more deprived central urban areas of Leeds, UK, even after controlling for individual-level factors such as sex, age, ethnicity and socioeconomic position. The mediating factors that relate neighborhood deprivation and the distribution of sexually transmitted diseases in certain geographic areas were not specifically explored in these studies, but are likely to include high risk behaviors. It is also possible that stress-related immune susceptibility to infection may partially account for the relationship between neighborhood deprivation and STD prevalence, since disadvantage is related to higher-levels of psychosocial stress (Table 1.) (Boardman 2004; Boardman et al. 2001; Steptoe and Feldman 2001).
Table 1.
Summary of studies examining ecologic stressors, herpesvirus reactivation and HIV acquisition/progression
| Topic | Exposure | Outcomes | References |
|---|---|---|---|
| 1. Neighborhoods, ecological stressors and disease | |||
| Neighborhoods and stress | An increase in adverse neighborhood characteristics such as litter in the streets, smells and fumes, noise, traffic, vandalism and residential stability, social capital | Higher levels of psychosocial stress | Boardman 2004; Steptoe and Feldman 2001 |
| Neighborhoods and disease | A higher level of neighborhood disadvantage such as socioeconomic deprivation, disorder, residential instability, broken windows index (poorer housing quality, abandoned cars, graffiti, trash, public school deterioration), lower median housing value | Higher prevalence of drug use, incidence of ischemic heart disease, lower survival after myocardial infarction, heightened risk of hypertension, worse self-reported health, poorer physical functioning, higher number of chronic conditions and sexually transmitted diseases | Boardman et al. 2001; Brugal et al. 2003; Chaix et al. 2007; Cohen et al. 2000; Cozier et al. 2007; Das et al. 2005; Espinoza et al. 2007; Fleming et al. 1997; Monteiro et al. 2005; McQuillan et al. 2004; Roca et al. 1995; Ross and Mirowsky 2001; Sampson et al. 2002*; Smith et al. 2000*; Wallace 1990* Winter et al. 2000; Zierler et al. 2000 |
| 2. Individual level psychosocial stressors, progression from HIV to AIDS | |||
| Stress, immune function and progression from HIV to AIDS | Greater exposure to chronic and acute stressors | Heightened susceptibility to infection, attenuated immune response to vaccination, endocrine/hormonal alterations, immunosuppression, enhanced viral replication, increased acquisition of HIV, more rapid progression to AIDS and an increase in other HIV-related immunological parameters | Antoni et al. 1990*; Biondi 2001*; Biondi and Zannino 1997*; Carrico et al. 2005; Christeff et al. 1997; Clerici et al. 1997*; Cole et al. 1998; Glaser and Kiecolt-Glaser 1987*; Glaser and Kiecolt-Glaser 2005*; Leserman et al. 2002; Leserman 2003*; Maggi et al. 1994; Solomon 1991*; Norbiato et al. 1997* |
| 3. Individual level psychosocial stressors, reactivation of latent HSV-2 and EBV, HIV acquisition/progression | |||
| Stress and reactivation of herpesviruses | Greater exposure to chronic and acute stressors such as negative life events, chronic stress associated with caring for a family member with Alzheimer’s Disease, poor marital quality, academic stress, depression, loneliness, vigor and mood after housing relocation, stress disclosure, and status and lifestyle incongruity | Endocrine/hormonal alterations including an increase in stress hormones such as gluccocorticoids, increased herpesvirus replication and reactivation of herpesviruses | Glaser et al. 1991; Glaser et al. 1994; Glaser et al. 2005*; Glaser et al. 1985; Glaser et al. 1985; Herbert and Cohen 1993*; Kennedy 1996*; Kiecolt-Glaser et al. 1987a; Kiecolt-Glaser et al. 1987b; Kiecolt-Glaser et al. 2002*; Longo and Koehn 1993*; Lutgendorf et al. 1994; Lutgendorf et al. 2001; McDade et al. 2000, McDade 2002; Monteiro et al. 2005; Sainz et al. 2001* |
| HSV-2 and HIV acquisition/progression | Infection with/reactivation of HSV-2 | An increase in genital ulcer disease (GUD), enhancement of HIV-1 replication, increased HIV acquisition | Corey et al. 2004; Gray et al. 2001; Herbert and Cohen 1993*; Kaul et al. 2007; Kiecolt-Glaser et al. 2002*; Longo and Koehn 1993*; Sainz et al. 2001*; Rebbapragada et al. 2007 |
| EBV and HIV acquisition/progression | Infection with/reactivation of EBV | Enhancement of HIV-1 replication, transactivation of HIV-1, pathogenesis of HIV-1, increased HIV-1 acquisition | Carbonar et al. 1989; Esterling et al. 1992; Glaser et al. 2005*; Herbert and Cohen 1993*; Kiecolt-Glaser et al. 2002*; Klatzmann et al. 1984; Lin 1993; Margalith et al. 1990; Moriuchi et al. 2000; Moriuchi and Moriuchi 2003; Nerurkar et al. 1987; Rahman et al. 1991; Scala et al. 1993; Schattner et al. 1991; Tassan Din et al. 2007 |
HSV-2, Herpes Simplex-2 Virus; EBV, Epstein Barr Virus; HIV, Human Immunodeficiency Virus; AIDS, Acquired Immunodeficiency Syndrome
Review
Psychosocial Stress and HIV
Various measures of psychosocial stress have been shown to directly influence susceptibility to viral and bacterial infections, including the common cold, influenza, Toxo-plasma, Salmonella (Biondi 2001; Biondi and Zannino 1997). Chronic stress has also been shown to influence immune response to vaccination (Biondi 2001; Biondi and Zannino 1997). Given the strong link between stress, immune suppression, and enhanced susceptibility to multiple infections, it is likely that a susceptible host’s immunological condition at the time of HIV acquisition may moderate parameters such as viral replication and the ability of HIV to infect cells (Solomon et al. 1991). Although there are many studies examining the effect of psychosocial stress on progression to AIDS and other HIV-related immunological parameters (Antoni et al. 1990; Carrico et al. 2005; Solomon et al. 1991), there are fewer prospective studies that have attempted to directly asses the relationship between psychosocial stress and increased susceptibility to primary infection with HIV among exposed populations.
Stress and Progression from HIV to AIDS
It has been well established that stress enhances progression to HIV through several pathways (Antoni et al. 1990; Glaser et al. 1987; Solomon et al. 1991). One pathway is via the central nervous system (CNS) activation of the hypo-thalamic-pituitary-adrenal axis (HPA) and sympathetic nervous system (SNS) (Glaser and Kiecolt-Glaser 2005). Activation of the HPA and SNS modulate the release of hormones such as cortisol, epinephrine, and norepinephrine, which can lead to qualitative and quantitative changes in immune functioning (Glaser and Kiecolt-Glaser 2005). For example, Cole et al. found that norepinephrine accelerated HIV-1 replication by as much as 11-fold in peripheral blood mononuclear cells (Cole et al. 1998). A recent review conducted by Leserman (2003), outlines the mechanisms by which stress hormones impact HIV progression, such as increased cytokine production and enhanced viral replication (Leserman 2003). Others have suggested that excessive glucococorticoid levels along with decreased dehydroepiandrosterone influences cytokine production, increases HIV viral replication, and is associated with decreased CD4 cell counts (Christeff et al. 1997; Clerici et al. 1997; Maggi et al. 1994). These hormonal imbalances cause suppression of Th-1 cytokines which are associated with HIV progression (Clerici et al. 1997; Norbiato et al. 1997). Leserman et al. (2002), showed that a 3 μg/dl increase in cumulative average cortisol (equal to one standard deviation change) resulted in a 40% increased risk of progression to AIDS, using blood samples gathered from a cohort of HIV-positive men (Leserman et al. 2002). This study also examined the relationship between number of stressful life events and progression to AIDS, finding for each one point increase in cumulative average stressful life events, the risk of progressing to AIDS increased by 14% (Leserman et al. 2002).
Given the relationships between stress, immune suppression, and progression to AIDS, it is also plausible that stress could influence a susceptible host’s immunological condition at the time of HIV exposure and hence likelihood of acquisition of primary infection. Stress-related changes in immune function during exposure may moderate HIV susceptibility parameters such as viral replication and the ability of HIV to infect cells (Solomon et al. 1991).
Stress and Acquisition of HIV
A mechanism by which psychosocial stress may impact acquisition of HIV is through stress-mediated reactivation of prior herpesvirus infection. Data suggests that stress influences herpesvirus reactivation, including herpes simplex virus type-2 (HSV-2) and Epstein Barr virus (EBV) among generally health populations (Glaser et al. 2005; Longo and Koehn 1993; Sainz et al. 2001). Increased antibody response to herpesviruses has been considered one of the strongest and most consistent immunological markers of stress (Glaser et al. 2005; Herbert and Cohen 1993). The biological pathway by which stress may lead to an increased herpesvirus antibody response includes release of stress hormones, such as gluccocorticoids, which have been shown to increase viral replication (Glaser et al. 1994). Psychosocial, physical, and environmental stressors can trigger increased antibody response to latent herpesviruses, including HSV-2 and EBV (Kennedy 1996). Many psychosocial stressors have been found to significantly increase antibody titers to herpesviruses, including negative life events, chronic stress associated with caring for a family member with Alzheimer’s Disease, poor marital quality, academic stress, depression, loneliness, vigor and mood after housing relocation, stress disclosure, and status and lifestyle incongruity (Glaser et al. 1985; Glaser et al. 1994; Glaser et al. 1991; Glaser et al. 1985; Kiecolt-Glaser et al. 1987a; Kiecolt-Glaser et al. 1987b; Lutgendorf et al. 1994; Lutgendorf et al. 2001; McDade 2002; McDade et al. 2000). In addition to these individual-level stressors, neighborhood deprivation has also been correlated with herpesvirus reactivation as indicated by a higher prevalence of herpes genital ulcers among individuals living in more economically deprived urban areas (Monteiro et al. 2005).
Stress-related reactivation of HSV-2 and EBV may impact risk of HIV acquisition in several ways, including increased occurrence of HSV-2 related genital ulcer disease (GUD), enhancement of HIV-1 replication by infection with HSV-2 and/or EBV, transactivation of HIV-1 by EBV, and CD4 receptor binding similarities with EBV (Carbonari et al. 1989; Corey et al. 2004; Klatzmann et al. 1984; Lin 1993; Scala et al. 1993). Indeed, both epidemiological and laboratory findings support a role for ongoing HSV-2 reactivation as a risk factor for HIV-1 acquisition (Corey et al. 2004). In a large review of epidemiological studies, Corey et al. (2004) found a significant risk of HIV acquisition among individuals infected with HSV-2 in cohort, case-control and cross-sectional studies (Corey et al. 2004). It is important to note that this review identified a significantly elevated risk of HIV across studies, even after the studies had carefully controlled for sexual behaviors (Corey et al. 2004). Given the breadth of the study populations examined and consistent results, it is unlikely that residual confounding associated with sexual behaviors accounts for the association between HSV-2 and HIV-1 acquisition, suggesting that other mechanisms, possibly immunological susceptibility, may partially explain the observed relationships (Corey et al. 2004). In a recent study, HSV-2 +/HIV− women showed an increase in genital mucosal target cells populations that are known to enhance HIV infection, even in the absence of genital ulceration or HSV2 reactivation (Rebbapragada et al. 2007). Kaul et al. has reviewed the evidence for negative mucosal synergy between HIV and HSV-2 (Kaul et al. 2007). They conclude that the evidence supporting synergy between HSV-2 and HIV as factors driving acquisition and transmission is strong (Kaul et al. 2007). Importantly, research examining whether suppressing HSV-2 can prevent HIV acquisition is now underway (Kaul et al. 2007).
Infection with EBV has also been associated with enhanced HIV acquisition. For example, studies conducted among cohorts of homosexual men have reported that elevated EBV antibody titers were detected in blood samples obtained prior to HIV seroconversion (Margalith et al. 1990; Rahman et al. 1991; Schattner et al. 1991). One of these studies reported that 26.6% of HIV positive homosexual men had higher titers of EBV prior to sero-conversion as compared with only 2.5% of HIV negative homosexual men (Schattner et al. 1991). Another study tested EBV antibody levels for five weeks before and after HIV tests results were administered to a group of homosexual men (Esterling et al. 1992). This study found that those who were seropositive for HIV had higher levels of EBV-viral capsid antigen antibody titers than those that were seronegative at all times before and after result notification (Esterling et al. 1992). Of note, increased EBV antibody titers have been shown to be uncorrelated with number of partners or sexual behaviors among healthy HIV- negative homosexual men, suggesting that the virus is not simply a marker of increased number of sexual partnerships and may therefore have effects on immune susceptibility (Nerurkar et al. 1987).
There are biological mechanisms that may explain why stress-related reactivation of HSV-2 and EBV may influence HIV acquisition. The main biological mechanism by which infection and reactivation of HSV-2 has been suggested to contribute to HIV-1 acquisition is through clinical manifestation of GUD. HSV-2 has been demonstrated as the major cause of GUD in developed and developing countries (Corey et al. 2004). Mucosal disruption due to reactivation of HSV-2 leads to ulcers and concomitant infiltration of activated CD-4 cells may increase the number of target cells for HIV-1 infection (Corey et al. 2004). In the Rakai study by Gray et al., (2001), overall transmission probability of HIV-1 increased from 0.0011 without genital ulceration to 0.0041 with genital ulceration (Gray et al. 2001). There is also evidence supporting EBV as a cofactor in the pathophysiology of HIV infection. EBV DNA polymerase has been shown to influence the transactivation of HIV-1 which promotes HIV-1 replication and thus contributes to progression to AIDS (Lin 1993; Scala et al. 1993). Furthermore, infection with EBV may influence the expression of HIV because B cells transformed by EBV have the CD4 receptor to which HIV binds (Klatzmann et al. 1984). More recently, studies have suggested that increased susceptibility to HIV by EBV infection may be related to greater expression of CCR5 on CD4 lymphocytes (Moriuchi and Moriuchi 2003; Moriuchi et al. 2000; Tassan Din et al. 2007). In addition, HIV has been shown to infect and multiply within B cells infected with EBV (Carbonari et al. 1989). Thus, it is possible that infection with EBV assists in the acquisition and progression of HIV infection. Further research is needed to examine whether stress-related reactivation of HSV-2 and EBV as measured by an increase in serum antibody titers partially explains the relationship between ecologic stressors and acquisition of HIV, even after controlling for sexual risk behaviors, drug use, and other individual-level covariates.
Discussion
Our review provides evidence that: (1) ecologic stressors are associated with levels of psychosocial stress, (2) ecologic and individual-level stressors influence HSV-2 and EBV reactivation, and (3) stress-related reactivation of HSV-2 and EBV may increase HIV-1 acquisition and progression. As depicted in the figure pathways, we suggest that ecologic stressors may contribute to neighborhood differences in HIV acquisition by impacting immunological functioning and reactivation of HSV-2 and EBV infections in high risk groups and/or neighborhoods. The close link between psychosocial stress and immunological functioning may partly explain observed variability in HIV risk by neighborhood disadvantage, even after controlling for sexual risk behaviors. Further studies are needed to assess whether ecologic stressors directly influence reactivation of herpesviruses as shown in our pathway figure and to identify whether stress-related reactivation leads to increased HIV acquisition. It is also likely that stress-related reactivation of HSV-2 and EBV may modify the influence of drug and sex risk behaviors on HIV acquisition. For example, individuals with poor mental health, increased anxiety and stress, and a higher number of partners may be at greater risk of HSV-2 and EBV reactivation and therefore heightened susceptibility to HIV infection upon exposure. For these reasons, large prospective population based studies among diverse race/ethnic populations that include information on ecologic stressors, drug and sex risk behaviors, individual-level psychosocial stress measures, GUD assessments, HSV-2/EBV antibody levels, and incident HIV infection are needed to examine the pathways described here and shown in the Fig. 1.
If ecologic stressors influence immune function, reactivation of herpesviruses, and HIV acquisition, there are many potential points of intervention that might be considered. For example, decreasing ecologic stressors may reduce individual-levels of psychosocial stress and thereby reduce reactivation and risk of HIV acquisition. Interventions that lower an individual’s level of psychosocial stress may help reduce the per-contact probability of HIV-1 acquisition in high risk groups such as those that are at greater risk of reactivation of HSV-2 and EBV. Studies have shown that relaxation training reduced antibody titers to HSV and increased natural killer cell activity (Glaser et al. 2005). Another study found that EBV antibodies were reduced over the course of ten weeks in HIV-positive and HIV-negative subjects participating in a cognitive behavioral stress management program (Esterling et al. 1992).
Directly targeting political policies and addressing social justice issues that aim to mitigate ecologic stressors, such as concentrated disadvantage, residential segregation, institutionalized racism, and poor quality of the built environment may improve mental health and reduce psychosocial stress as well as HIV risk behaviors. For example, policies that increase the availability of mental health treatment services, community centers, and job opportunities in disadvantaged areas and among at-risk race/ethnic groups as well as improving the built environment by reducing litter, abandoned buildings, and crime, are all factors that may lead to reductions in psychosocial stress. Indeed, some have suggested that interventions addressing the underlying economic, racial, and social tensions through improving the built environment, employment opportunities, and other structural resources, are likely to reduce health disparities to a greater extent compared to programs that use more specific individual-level targeting of health/disease outcomes (Mechanic 2005). As applied to the argument here, reducing ecological stressors may not only attenuate reactivation of HSV-2 and EBV, but ultimately susceptibility to HIV and progression to AIDS.
Another important potential intervention pathway is neighborhood-level prevention and treatment of HSV-2 and EBV. Neighborhood-based interventions focused on behavioral risk-reduction such as condom use, STD education and/or increasing the availability of treatment have been shown to decrease the transmission and spread of herpesviruses (Patel and Rompalo 2005; Wald et al. 2005). There is also evidence to suggest that the development of a vaccine against HSV-2 and EBV would have an impact on HIV infection rates and treatment for herpesvirus has been shown to lower HIV-1 viral load (Nagot et al. 2007).
Taken together, these data suggest a multi-level framework for targeting prevention of HIV, ranging from the ecologic or neighborhood level to individual level risk factors including behaviors, mental health, psychosocial stress, and prevention or treatment of HSV-2 and EBV. Further longitudinal studies are needed to directly assess whether ecologic stressors influence stress-related reactivation of HSV-2 and EBV and ultimately influences neighborhood and group-level disparities in HIV acquisition.
References
- Aneshensel CS, Sucoff CA. The neighborhood context of adolescent mental health. Journal of Health and Social Behavior. 1996;37(4):293–310. [PubMed] [Google Scholar]
- Anonymous. Racism, poverty, sexism all play a role in epidemic’s spread among black women. New resource guide educates on problem. AIDS Alert. 2006;21(2):13–17. [PubMed] [Google Scholar]
- Antoni MH, Schneiderman N, Fletcher MA, Goldstein DA, Ironson G, Laperriere A. Psychoneuroimmunology and HIV-1. Journal of Consulting and Clinical Psychology. 1990;58(1):38–49. doi: 10.1037//0022-006x.58.1.38. [DOI] [PubMed] [Google Scholar]
- Astemborski J, Vlahov D, Warren D, Solomon L, Nelson KE. The trading of sex for drugs or money and HIV seropositivity among female intravenous drug users. American Journal of Public Health. 1994;84(3):382–387. doi: 10.2105/ajph.84.3.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biondi M. Psychoneuroimmunology. 3. Vol. 2. New York: Academic Press; 2001. Chapter. 39: Effects of Stress on Immune Functions: An Overview. [Google Scholar]
- Biondi M, Zannino LG. Psychological stress, neuroimmunomodulation, and susceptibility to infectious diseases in animals and man: a review. Psychotherapy and Psychosomatics. 1997;66(1):3–26. doi: 10.1159/000289101. [DOI] [PubMed] [Google Scholar]
- Blumberg SJ, Dickey WC. Prevalence of HIV risk behaviors, risk perceptions, and testing among US adults with mental disorders. Journal of Acquired Immune Deficiency Syndromes. 2003;32(1):77–79. doi: 10.1097/00126334-200301010-00011. [DOI] [PubMed] [Google Scholar]
- Boardman JD. Stress and physical health: the role of neighborhoods as mediating and moderating mechanisms. Social Science & Medicine. 2004;58(12):2473–2483. doi: 10.1016/j.socscimed.2003.09.029. [DOI] [PubMed] [Google Scholar]
- Boardman JD, Finch BK, Ellison CG, Williams DR, Jackson JS. Neighborhood disadvantage, stress, and drug use among adults. Journal of Health and Social Behavior. 2001;42(2):151–165. [PubMed] [Google Scholar]
- Bonneau RH, Padgett DA, Sheridan JF. Twenty years of psychoneuroimmunology and viral infections in Brain, Behavior, and Immunity. Brain, Behavior and Immunity. 2007;21(3):273–280. doi: 10.1016/j.bbi.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Brugal MT, Borrell C, Diaz-Quijano E, Pasarin MI, Garcia-Olalla P, Villalbi JR. Deprivation and AIDS in a southern European city: Different patterns across transmission group. European Journal of Public Health. 2003;13(3):259–261. doi: 10.1093/eurpub/13.3.259. [DOI] [PubMed] [Google Scholar]
- Carbonari M, Fiorilli M, Mezzaroma I, Cherchi M, Aiuti F. CD4 as the receptor for retroviruses of the HTLV family: immunopathogenetic implications. Advances in Experimental Medicine and Biology. 1989;257:3–7. doi: 10.1007/978-1-4684-5712-4_2. [DOI] [PubMed] [Google Scholar]
- Carrico AW, Antoni MH, Pereira DB, Fletcher MA, Klimas N, Lechner SC, et al. Cognitive behavioral stress management effects on mood, social support, and a marker of antiviral immunity are maintained up to 1 year in HIV-infected gay men. International Journal of Behavioral Medicine. 2005;12(4):218–226. doi: 10.1207/s15327558ijbm1204_2. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. HIV/AIDS Surveillance Report, 2005. 2006 Retrieved. from http://www.cdc.gov/hiv/topics/surveillance/resources/reports/
- Chaix B, Rosvall M, Merlo J. Neighborhood socioeconomic deprivation and residential instability: effects on incidence of ischemic heart disease and survival after myocardial infarction. Epidemiology. 2007;18(1):104–111. doi: 10.1097/01.ede.0000249573.22856.9a. [DOI] [PubMed] [Google Scholar]
- Christeff N, Gherbi N, Mammes O, Dalle MT, Gharakhanian S, Lortholary O, et al. Serum cortisol and DHEA concentrations during HIV infection. Psychoneuroendocrinology. 1997;22(Suppl 1):S11–S18. doi: 10.1016/s0306-4530(97)00015-2. [DOI] [PubMed] [Google Scholar]
- Clerici M, Trabattoni D, Piconi S, Fusi ML, Ruzzante S, Clerici C, et al. A possible role for the cortisol/anticortisols imbalance in the progression of human immunodeficiency virus. Psychoneuroendocrinology. 1997;22(Suppl 1):S27–S31. doi: 10.1016/s0306-4530(97)00019-x. [DOI] [PubMed] [Google Scholar]
- Cohen D, Spear S, Scribner R, Kissinger P, Mason K, Wildgen J. “Broken windows” and the risk of gonorrhea. American Journal of Public Health. 2000;90(2):230–236. doi: 10.2105/ajph.90.2.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW, Korin YD, Fahey JL, Zack JA. Norepinephrine accelerates HIV replication via protein kinase A-dependent effects on cytokine production. Journal of Immunology. 1998;161(2):610–616. [PubMed] [Google Scholar]
- Corey L, Wald A, Celum CL, Quinn TC. The effects of herpes simplex virus-2 on HIV-1 acquisition and transmission: a review of two overlapping epidemics. Journal of Acquired Immune Deficiency Syndromes. 2004;35(5):435–445. doi: 10.1097/00126334-200404150-00001. [DOI] [PubMed] [Google Scholar]
- Cozier YC, Palmer JR, Horton NJ, Fredman L, Wise LA, Rosenberg L. Relation between neighborhood median housing value and hypertension risk among black women in the United States. American Journal of Public Health. 2007;97(4):718–724. doi: 10.2105/AJPH.2005.074740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S, Sabin C, Wade A, Allan S. Sociodemography of genital co-infection with Neisseria gonorrhoeae and Chlamydia trachomatis in Coventry, UK. International Journal of STD & AIDS. 2005;16(4):318–322. doi: 10.1258/0956462053654320. [DOI] [PubMed] [Google Scholar]
- Espinoza L, Hall HI, Hardnett F, Selik RM, Ling Q, Lee LM. Characteristics of persons with heterosexually acquired HIV infection, United States 1999–2004. American Journal of public health. 2007;97(1):144–149. doi: 10.2105/AJPH.2005.077461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esterling BA, Antoni MH, Schneiderman N, Carver CS, LaPerriere A, Ironson G, et al. Psychosocial modulation of antibody to Epstein-Barr viral capsid antigen and human herpesvirus type-6 in HIV-1-infected and at-risk gay men. Psychosomatic Medicine. 1992;54(3):354–371. doi: 10.1097/00006842-199205000-00011. [DOI] [PubMed] [Google Scholar]
- Fleming DT, McQuillan GM, Johnson RE, Nahmias AJ, Aral SO, Lee FK, et al. Herpes simplex virus type 2 in the United States, 1976 to 1994. New England Journal of Medicine. 1997;337(16):1105–1111. doi: 10.1056/NEJM199710163371601. [DOI] [PubMed] [Google Scholar]
- Foster PH. Use of stigma, fear, and denial in development of a framework for prevention of HIV/AIDS in rural African American communities. Family Community Health. 2007;30(4):318–327. doi: 10.1097/01.FCH.0000290544.48576.01. [DOI] [PubMed] [Google Scholar]
- Friedman SR, Lieb S, Tempalski B, Cooper H, Keem M, Friedman R, et al. HIV among injection drug users in large US metropolitan areas, 1998. Journal of urban health : bulletin of the New York Academy of Medicine. 2005;82(3):434–445. doi: 10.1093/jurban/jti088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea S, Ahern J, Vlahov D. Contextual determinants of drug use risk behavior: a theoretic framework. Journal of urban health: bulletin of the New York Academy of Medicine. 2003;80(4 Suppl 3):iii50–iii58. doi: 10.1093/jurban/jtg082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser R, Kiecolt-Glaser J. Stress-associated depression in cellular immunity: implications for acquired immune deficiency syndrome (AIDS) Brain, Behavior and Immunity. 1987;1(2):107–112. doi: 10.1016/0889-1591(87)90013-4. [DOI] [PubMed] [Google Scholar]
- Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: implications for health. Nature reviews Immunology. 2005;5(3):243–251. doi: 10.1038/nri1571. [DOI] [PubMed] [Google Scholar]
- Glaser R, Kiecolt-Glaser JK, Speicher CE, Holliday JE. Stress, loneliness, and changes in herpesvirus latency. Journal of Behavioral Medicine. 1985;8(3):249–260. doi: 10.1007/BF00870312. [DOI] [PubMed] [Google Scholar]
- Glaser R, Padgett DA, Litsky ML, Baiocchi RA, Yang EV, Chen M, et al. Stress-associated changes in the steady-state expression of latent Epstein-Barr virus: implications for chronic fatigue syndrome and cancer. Brain, Behavior and Immunity. 2005;19(2):91–103. doi: 10.1016/j.bbi.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Glaser R, Pearl DK, Kiecolt-Glaser JK, Malarkey WB. Plasma cortisol levels and reactivation of latent Epstein-Barr virus in response to examination stress. Psychoneuroendocrinology. 1994;19(8):765–772. doi: 10.1016/0306-4530(94)90023-x. [DOI] [PubMed] [Google Scholar]
- Glaser R, Pearson GR, Jones JF, Hillhouse J, Kennedy S, Mao HY, et al. Stress-related activation of Epstein-Barr virus. Brain, Behavior and Immunity. 1991;5(2):219–232. doi: 10.1016/0889-1591(91)90018-6. [DOI] [PubMed] [Google Scholar]
- Glaser R, Strain EC, Tarr KL, Holliday JE, Donnerberg RL, Kiecolt-Glaser JK. Changes in Epstein-Barr virus antibody titers associated with aging. Proceedings of the Society for Experimental Biology and Medicine. Society for Experimental Biology and Medicine; New York, N.Y. 1985. pp. 352–355. [DOI] [PubMed] [Google Scholar]
- Gray RH, Wawer MJ, Brookmeyer R, Sewankambo NK, Serwadda D, Wabwire-Mangen F, et al. Probability of HIV-1 transmission per coital act in monogamous, heterosexual, HIV-1-discordant couples in Rakai, Uganda. Lancet. 2001;357(9263):1149–1153. doi: 10.1016/S0140-6736(00)04331-2. [DOI] [PubMed] [Google Scholar]
- Herbert TB, Cohen S. Stress and immunity in humans: a meta-analytic review. Psychosomatic Medicine. 1993;55(4):364–379. doi: 10.1097/00006842-199307000-00004. [DOI] [PubMed] [Google Scholar]
- Hill TD, Ross CE, Angel RJ. Neighborhood disorder, psychophysiological distress, and health. Journal of Health and Social Behavior. 2005;46(2):170–186. doi: 10.1177/002214650504600204. [DOI] [PubMed] [Google Scholar]
- Kaul R, Pettengell C, Sheth PM, Sunderji S, Biringer A, Macdonald K, et al. The genital tract immune milieu: An important determinant of HIV susceptibility and secondary transmission. Journal of Reproductive Immunology. 2007;77(1):32–40. doi: 10.1016/j.jri.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Kennedy S. Herpes virus infections and psychoneuroimmunology. In: Friedman H, Klein TW, Friedman AL, editors. Psychoneuroimmunology, stress, and infection. New York: CRC Press; 1996. pp. 231–242. [Google Scholar]
- Kiecolt-Glaser JK, Fisher LD, Ogrocki P, Stout JC, Speicher CE, Glaser R. Marital quality, marital disruption, and immune function. Psychosomatic Medicine. 1987a;49(1):13–34. doi: 10.1097/00006842-198701000-00002. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Glaser R, Shuttleworth EC, Dyer CS, Ogrocki P, Speicher CE. Chronic stress and immunity in family caregivers of Alzheimer’s disease victims. Psychosomatic Medicine. 1987b;49(5):523–535. doi: 10.1097/00006842-198709000-00008. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, McGuire L, Robles TF, Glaser R. Psychoneuroimmunology: psychological influences on immune function and health. Journal of Consulting and Clinical Psychology. 2002;70(3):537–547. doi: 10.1037//0022-006x.70.3.537. [DOI] [PubMed] [Google Scholar]
- Klatzmann D, Champagne E, Chamaret S, Gruest J, Guetard D, Hercend T, et al. T-lymphocyte T4 molecule behaves as the receptor for human retrovirus LAV. Nature. 1984;312(5996):767–768. doi: 10.1038/312767a0. [DOI] [PubMed] [Google Scholar]
- Kottiri BJ, Friedman SR, Neaigus A, Curtis R, Des Jarlais DC. Risk networks and racial/ethnic differences in the prevalence of HIV infection among injection drug users. Journal of Acquired Immune Deficiency Syndromes. 2002;30(1):95–104. doi: 10.1097/00042560-200205010-00013. [DOI] [PubMed] [Google Scholar]
- Kubzansky LD, Subramanian SV, Kawachi I, Fay ME, Soobader MJ, Berkman LF. Neighborhood contextual influences on depressive symptoms in the elderly. American Journal of Epidemiology. 2005;162(3):253–260. doi: 10.1093/aje/kwi185. [DOI] [PubMed] [Google Scholar]
- Latkin C, Mandell W, Oziemkowska M, Celentano D, Vlahov D, Ensminger M, et al. Using social network analysis to study patterns of drug use among urban drug users at high risk for HIV/AIDS. Drug and Alcohol Dependence. 1995;38(1):1–9. doi: 10.1016/0376-8716(94)01082-v. [DOI] [PubMed] [Google Scholar]
- Latkin CA. A personal network approach to AIDS prevention: an experimental peer group intervention for street-injecting drug users: the SAFE study. NIDA Research Monograph. 1995;151:181–195. [PubMed] [Google Scholar]
- Latkin CA, Curry AD. Stressful neighborhoods and depression: a prospective study of the impact of neighborhood disorder. Journal of Health and Social Behavior. 2003;44(1):34–44. [PubMed] [Google Scholar]
- Leserman J. HIV disease progression: depression, stress, and possible mechanisms. Biological Psychiatry. 2003;54(3):295–306. doi: 10.1016/s0006-3223(03)00323-8. [DOI] [PubMed] [Google Scholar]
- Leserman J, Petitto JM, Gu H, Gaynes BN, Barroso J, Golden RN, et al. Progression to AIDS, a clinical AIDS condition and mortality: psychosocial and physiological predictors. Psychological Medicine. 2002;32(6):1059–1073. doi: 10.1017/s0033291702005949. [DOI] [PubMed] [Google Scholar]
- Lin JC. The Epstein-Barr virus DNA polymerase transactivates the human immunodeficiency virus type 1 5′ long terminal repeat. Biochemical and Biophysical Research Communications. 1993;195(1):242–249. doi: 10.1006/bbrc.1993.2036. [DOI] [PubMed] [Google Scholar]
- Longo D, Koehn K. Psychosocial factors and recurrent genital herpes: a review of prediction and psychiatric treatment studies. International Journal of Psychiatry in Medicine. 1993;23(2):99–117. doi: 10.2190/L5MH-0TCW-1PKD-5BM0. [DOI] [PubMed] [Google Scholar]
- Lutgendorf SK, Antoni MH, Kumar M, Schneiderman N. Changes in cognitive coping strategies predict EBV-antibody titre change following a stressor disclosure induction. Journal of Psychosomatic Research. 1994;38(1):63–78. doi: 10.1016/0022-3999(94)90009-4. [DOI] [PubMed] [Google Scholar]
- Lutgendorf SK, Reimer TT, Harvey JH, Marks G, Hong SY, Hillis SL, et al. Effects of housing relocation on immunocompetence and psychosocial functioning in older adults. The Journals of Gerontology Series A, Biological Sciences and Medical Sciences. 2001;56(2):M97–M105. doi: 10.1093/gerona/56.2.m97. [DOI] [PubMed] [Google Scholar]
- Maggi E, Mazzetti M, Ravina A, Annunziato F, de Carli M, Piccinni MP, et al. Ability of HIV to promote a TH1 to TH0 shift and to replicate preferentially in TH2 and TH0 cells. Science. 1994;265(5169):244–248. doi: 10.1126/science.8023142. [DOI] [PubMed] [Google Scholar]
- Margalith M, Sarov B, Sarov I, Rinaldo C, Detels R, Phair J, et al. Serum IgG and IgA antibodies specific to Epstein-Barr virus capsid antigen in a longitudinal study of human immunodeficiency virus infection and disease progression in homosexual men. AIDS Research and Human Retroviruses. 1990;6(5):607–616. doi: 10.1089/aid.1990.6.607. [DOI] [PubMed] [Google Scholar]
- McDade TW. Status incongruity in Samoan youth: a biocultural analysis of culture change, stress, and immune function. Medical Anthropology Quarterly. 2002;16(2):123–150. doi: 10.1525/maq.2002.16.2.123. [DOI] [PubMed] [Google Scholar]
- McDade TW, Stallings JF, Worthman CM. Culture change and stress in Western Samoan youth: Methodological issues in the cross-cultural study of stress and immune function. American journal of human biology: the official journal of the Human Biology Council. 2000;12(6):792–802. doi: 10.1002/1520-6300(200011/12)12:6<792::AID-AJHB7>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- McQuillan GM, Kruszon-Moran D, Kottiri BJ, Curtin LR, Lucas JW, Kington RS. Racial and ethnic differences in the seroprevalence of 6 infectious diseases in the United States: data from NHANES III, 1988–1994. American Journal of Public Health. 2004;94(11):1952–1958. doi: 10.2105/ajph.94.11.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechanic D. Policy challenges in addressing racial disparities and improving population health. Health Affairs. 2005;24(2):335–338. doi: 10.1377/hlthaff.24.2.335. [DOI] [PubMed] [Google Scholar]
- Miller GE, Cohen S, Pressman S, Barkin A, Rabin BS, Treanor JJ. Psychological stress and antibody response to influenza vaccination: when is the critical period for stress, and how does it get inside the body? Psychosomatic Medicine. 2004;66(2):215–223. doi: 10.1097/01.psy.0000116718.54414.9e. [DOI] [PubMed] [Google Scholar]
- Monteiro EF, Lacey CJ, Merrick D. The interrelation of demographic and geospatial risk factors between four common sexually transmitted diseases. Sexually Transmitted Infections. 2005;81(1):41–46. doi: 10.1136/sti.2004.009431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morag M, Morag A, Reichenberg A, Lerer B, Yirmiya R. Psychological variables as predictors of rubella antibody titers and fatigue–a prospective, double blind study. Journal of Psychiatric Research. 1999;33(5):389–395. doi: 10.1016/s0022-3956(99)00010-2. [DOI] [PubMed] [Google Scholar]
- Moriuchi M, Moriuchi H. Increased susceptibility to HIV-1 of peripheral blood lymphocytes in acute infection with Epstein-Barr virus. Journal of Medical Virology. 2003;71(3):343–346. doi: 10.1002/jmv.10494. [DOI] [PubMed] [Google Scholar]
- Moriuchi M, Moriuchi H, Williams R, Straus SE. Herpes simplex virus infection induces replication of human immunodeficiency virus type 1. Virology. 2000;278(2):534–540. doi: 10.1006/viro.2000.0667. [DOI] [PubMed] [Google Scholar]
- Nagot N, Ouedraogo A, Foulongne V, Konate I, Weiss HA, Vergne L, et al. Reduction of HIV-1 RNA levels with therapy to suppress herpes simplex virus. New England Journal of Medicine. 2007;356(8):790–799. doi: 10.1056/NEJMoa062607. [DOI] [PubMed] [Google Scholar]
- Nerurkar LS, Biggar RJ, Goedert JJ, Wallen W, Becker P, West F, et al. Antiviral antibodies in the sera of homosexual men: correlation with their lifestyle and drug usage. Journal of Medical Virology. 1987;21(2):123–135. doi: 10.1002/jmv.1890210204. [DOI] [PubMed] [Google Scholar]
- Norbiato G, Bevilacqua M, Vago T. Glucocorticoids and the immune system in AIDS. Psychoneuroendocrinology. 1997;22(Suppl 1):S19–S25. doi: 10.1016/s0306-4530(97)00011-5. [DOI] [PubMed] [Google Scholar]
- Patel R, Rompalo A. Managing patients with genital herpes and their sexual partners. Infectious Disease Clinics of North America. 2005;19(2):427–438. x. doi: 10.1016/j.idc.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Rahman MA, Kingsley LA, Atchison RW, Belle S, Breinig MC, Ho M, et al. Reactivation of Epstein-Barr virus during early infection with human immunodeficiency virus. Journal of Clinical Microbiology. 1991;29(6):1215–1220. doi: 10.1128/jcm.29.6.1215-1220.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebbapragada A, Wachihi C, Pettengell C, Sunderji S, Huibner S, Jaoko W, et al. Negative mucosal synergy between Herpes simplex type 2 and HIV in the female genital tract. AIDS. 2007;21(5):589–598. doi: 10.1097/QAD.0b013e328012b896. [DOI] [PubMed] [Google Scholar]
- Roca J, Vlahov D, Borrell C, Jansa JM, Brugal T, Yazbeck H, et al. Geographic variation in HIV infection among injecting drug users with Barcelona. The International Journal of the Addictions. 1995;30(2):219–229. doi: 10.3109/10826089509060744. [DOI] [PubMed] [Google Scholar]
- Rhodes T, Singer M, Bourgois P, Friedman SR, Strathdee SA. The social structural production of HIV risk among injecting drug users. Social Science & Medicine. 2005;61(5):1026–1044. doi: 10.1016/j.socscimed.2004.12.024. [DOI] [PubMed] [Google Scholar]
- Ross CE, Mirowsky J. Neighborhood disadvantage, disorder, and health. Journal of Health and Social Behavior. 2001;42(3):258–276. [PubMed] [Google Scholar]
- Sainz B, Loutsch JM, Marquart ME, Hill JM. Stress-associated immunomodulation and herpes simplex virus infections. Medical Hypotheses. 2001;56(3):348–356. doi: 10.1054/mehy.2000.1219. [DOI] [PubMed] [Google Scholar]
- Sampson RJ, Morenoff JD, Gannon-Rowley T. Assessing neighborhood effects: Social processes and new directions in research. Annual Review of Sociology. 2002;28(1):473–478. [Google Scholar]
- Saracco A, Lazzarin A, Musicco M, Moroni M. Gender, relationship length and the risk of heterosexual HIV transmission. European Journal of Epidemiology. 1989;5(3):403–404. doi: 10.1007/BF00144846. [DOI] [PubMed] [Google Scholar]
- Scala G, Quinto I, Ruocco MR, Mallardo M, Ambrosino C, Squitieri B, et al. Epstein-Barr virus nuclear antigen 2 transactivates the long terminal repeat of human immunodeficiency virus type 1. Journal of Virology. 1993;67(5):2853–2861. doi: 10.1128/jvi.67.5.2853-2861.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schattner A, Hanuka N, Sarov B, Sarov I, Handzel Z, Bentwich Z. Sequential serological studies of homosexual men with and without HIV infection. Epstein-Barr virus activation preceding and following HIV seroconversion. Clinical and Experimental Immunology. 1991;85(2):209–213. doi: 10.1111/j.1365-2249.1991.tb05706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver E, Mulvey EP, Swanson JW. Neighborhood structural characteristics and mental disorder: Faris and Dunham revisited. Social Science & Medicine. 2002;55(8):1457–1470. doi: 10.1016/s0277-9536(01)00266-0. [DOI] [PubMed] [Google Scholar]
- Smith DK, Gwinn M, Selik RM, Miller KS, Dean-Gaitor H, Ma’at PI, et al. HIV/AIDS among African Americans: progress or progression? Aids. 2000;14(9):1237–1248. doi: 10.1097/00002030-200006160-00022. [DOI] [PubMed] [Google Scholar]
- Solomon GF, Kemeny ME, Temoshok L. Psychoneuroimmunological aspects of HIV. In: Ader R, Felton DL, Cohen N, editors. Psychoneuroimmunology. New York: Simon and Schuster; 1991. pp. 1081–1113. [Google Scholar]
- Steptoe A, Feldman PJ. Neighborhood problems as sources of chronic stress: development of a measure of neighborhood problems, and associations with socioeconomic status and health. Annals of behavioral medicine : a publication of the Society of Behavioral Medicine. 2001;23(3):177–185. doi: 10.1207/S15324796ABM2303_5. [DOI] [PubMed] [Google Scholar]
- Sterk CE, Theall KP, Elifson KW. The impact of emotional distress on HIV risk reduction among women. Substance Use & Misuse. 2006;41(2):157–173. doi: 10.1080/10826080500391639. [DOI] [PubMed] [Google Scholar]
- Stevens SJ, Murphy BS, McKnight K. Traumatic stress and gender differences in relationship to substance abuse, mental health, physical health, and HIV risk behavior in a sample of adolescents enrolled in drug treatment. Child Maltreatment. 2003;8(1):46–57. doi: 10.1177/1077559502239611. [DOI] [PubMed] [Google Scholar]
- Stiffman AR, Dore P, Earls F, Cunningham R. The influence of mental health problems on AIDS-related risk behaviors in young adults. The Journal of Nervous and Mental Disease. 1992;180(5):314–320. doi: 10.1097/00005053-199205000-00005. [DOI] [PubMed] [Google Scholar]
- Takahashi LM, Wiebe D, Rodriguez R. Navigating the time-space context of HIV and AIDS: daily routines and access to care. Social science & medicine. 2001;53(7):845–863. doi: 10.1016/s0277-9536(00)00363-4. [DOI] [PubMed] [Google Scholar]
- Tassan Din C, Vecchi A, Tambussi G, De Rossi A, Bestetti A, Biswas P. Virological responses in a patient with recent HIV-1 infection experiencing an EBV reactivation. The new microbiologica : Official journal of the Italian Society for Medical, Odontoiatric, and Clinical Microbiology (SIMMOC) 2007;30(3):283–285. [PubMed] [Google Scholar]
- Wald A, Langenberg AG, Krantz E, Douglas JM, Jr, Handsfield HH, DiCarlo RP, et al. The relationship between condom use and herpes simplex virus acquisition. Annals of Internal Medicine. 2005;143(10):707–713. doi: 10.7326/0003-4819-143-10-200511150-00007. [DOI] [PubMed] [Google Scholar]
- Wallace R. Urban desertification, public health and public order: ‘planned shrinkage’, violent death, substance abuse and AIDS in the Bronx. Social Science & Medicine. 1990;31(7):801–813. doi: 10.1016/0277-9536(90)90175-r. [DOI] [PubMed] [Google Scholar]
- Wallace R, Fullilove M, Fullilove R, Gould P, Wallace D. Will AIDS be contained within U.S. minority urban populations? Social Science & Medicine. 1994;39(8):1051–1062. doi: 10.1016/0277-9536(94)90376-x. [DOI] [PubMed] [Google Scholar]
- Winter AJ, Sriskandabalan P, Wade AA, Cummins C, Barker P. Sociodemography of genital Chlamydia trachomatis in Coventry, UK, 1992–6. Sexually Transmitted Infections. 2000;76(2):103–109. doi: 10.1136/sti.76.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamboni BD, Crawford I. Minority stress and sexual problems among African-American gay and bisexual men. Archives of Sexual Behavior. 2007;36(4):569–578. doi: 10.1007/s10508-006-9081-z. [DOI] [PubMed] [Google Scholar]
- Zapka JG, Stoddard AM, McCusker J. Social network, support and influence: relationships with drug use and protective AIDS behavior. AIDS education and prevention: Official publication of the International Society for AIDS Education. 1993;5(4):352–366. [PubMed] [Google Scholar]
- Zierler S, Krieger N, Tang Y, Coady W, Siegfried E, DeMaria A, et al. Economic deprivation and AIDS incidence in Massachusetts. American Journal of Public Health. 2000;90(7):1064–1073. doi: 10.2105/ajph.90.7.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]