OVERVIEW
In the era of targeted therapies, patients with gynecological malignancies have not yet been major beneficiaries of this new class of agents. This may reflect the fact that the main tumor types, ovarian, uterine and cervical cancers, are a highly heterogeneous group of cancers, with variable response to standard chemotherapies. This is also likely due to poor model development in which to study the diversity of these cancers. Cancer-derived cell lines fail to adequately recapitulate molecular hallmarks of specific cancer subsets and complex microenvironments, which may be critical for sensitivity to targeted therapies. Patient derived xenografts (PDX), using fresh human tumor without prior in vitro culture, combined with whole genome expression, gene copy number and sequencing analyses, could dramatically aid novel therapy development in gynecological malignancies. Gynecological tumors can be engrafted in immunodeficient mice with a high rate of success and within a reasonable time frame. The resulting PDX accurately recapitulate the patient’s tumour in histological, molecular and in vivo treatment response characteristics. Orthotopic PDX develop complications relevant for the clinic, such as ascites and bowel obstruction, providing opportunities for understanding the biology of these clinical problems. Thus, PDX have great promise for delivering improved understanding of gynecological malignancies, serve as better models for designing novel therapies and clinical trials and could underpin individualized, directed therapy for patients from whom PDX models have been established.
Keywords: ovarian cancer, cervical cancer, model systems
1. PATIENT-DERIVED XENOGRAFTS IN CANCER
Tumor cells have been studied in in vivo xenograft models in rodents for more than fifty years. Initially these were mainly leukemic models in hamsters looking at effects on immune response.1 Subsequently, solid tumor cell lines from tumor fragments cultured in vitro were successfully xenograft in immunodeficient nude mice.2 As in vitro cell line culture became more widespread, a large number of established cancer cell lines were transplanted into immunodeficient mice for the study of natural history and therapeutic responses. However, many of those cell lines have been cultured in vitro for decades and no longer represent the cancer type to which they are ascribed.3 The use of solid tumor fragments, without tumor digestion or prior in vitro culture, results in serial xenografts reflecting the patient’s tumor according to histologic, molecular and treatment outcome responses, although for the majority of those published ovarian cancer PDX, detailed characterization is not reported.4 The murine host provides an in vivo microenvironment in which human stroma and vasculature is replaced by murine counterparts with subsequent rounds of transplantation, often with very similar morphologic appearance.5 Host-derived immune cells are present in initial T1 grafts and most cannot be replaced in immunocompromised models, limiting the study of immune therapies in these models.
Despite the notable heterogeneity of gynecological malignancies, our treatments for this collection of mostly rare gynecologic diseases are applied in a very homogeneous fashion. For example, irrespective of histological subtype or known mutational status (e.g. BRCA1 or BRCA2), the first-line systemic treatment for all subtypes of EOC is a taxane in combination with a platinum agent - most commonly, paclitaxel plus carboplatin. This standard treatment has changed little in over a decade of targeted therapeutic development. Despite initial efficacy, the large majority of patients receiving this treatment for advanced EOC will ultimately experience progression of their cancer and die of complications. The development of novel therapies has largely failed to impact the clinic for patients with gynecological malignancies.
A frequently cited reason for this failure of targeted therapies is the lack of adequate models to recapitulate the diversity and heterogeneity of cancers in cell culture models. Cell lines and xenograft models derived from human ovarian tumors have contributed tremendously to our current understanding of EOC initiation and progression. Cell lines, used either in vitro, or as implanted xenografts in vivo, are highly selected for phenotypic properties that support growth on a solid, artificial structure (e.g. plastic). Thus, the alterations with repeated passages favor expression profiles that enhance growth on plastic support thus complicating the extrapolation of data to the original parent tumor.6, 7 Such methodology may also artificially eliminate subpopulations that are critical determinants of the treatment responsiveness of the source tumors. Indeed, it is clear that cell lines generated in this fashion, which may be suitable for focused mechanistic work, are poor surrogates of the source patient.
Xenograft models have overcome some of these limitations by subcutaneous, intraperitoneal, or retroperitoneal heterotransplantation of human tumor cells into nude or SCID mice.8–11 However, the relevance of ovarian tumors orthotopically grown in subcutaneous tissue or the renal capsule has been questioned. In addition, the majority of xenograft models utilize ovarian cancer cell lines, with the inherent limitations mentioned above, rather than primary tumors naïve to cell culture media and plastic. Several clinical trials have examined the efficacy of targeted therapies that were expected to be successful, based on animal and cell culture studies. However, therapies such as gefitinib, imatinib, and hormone modulators have been underwhelming.12–14 The need for better preclinical models is further exemplified by a phase II clinical trial of trastuzumab, a HER2 receptor antibody.15. Forty-one patients with recurrent or persistent ovarian or primary peritoneal carcinoma expressing moderate to high levels of membranous HER2 were treated with trastuzumab but only 7.3% (3 patients) responded. This study suggests that protein expression alone is insufficient to predict the clinical impact of a potential novel therapy in vivo.
The emergence of The Cancer Genome Atlas (TCGA) ovarian data set has highlighted the fact that many of the commonly used cell lines in ovarian cancer research, for example, do not harbor many of the similar genotypic and phenotypic features of ovarian cancer. This is not surprising, as cell lines are established from a clonal or oligoclonal lineage with selective pressure that varies from cancer tissue in situ. As such, the establishment of such lines from primary patient tissues may take up to a year and be successful less than 50% of the time, particularly for tumors bearing BRCA1/2 mutations.16–18 Cell lines will also be devoid of supportive stroma, which contribute to a substantial bulk of the tumors in gynecological malignancies. Novel signaling pathways that may be critical for tumor growth in vivo and are the subject of intense drug development, such as insulin-like growth factor (IGF) signaling and cMET/HGF signaling rely on stromal/mesenchymal niche paracrine production of growth factors to support growth, survival and metastasis.19–21
The establishment and use of patient derived xenografts (PDX) may be able to overcome the shortcomings. PDX are tumor xenograft models (Figure 1) that are established directly from patient tumor tissue. Typically, tumors are procured directly from the operating room during a primary debulking/staging surgery. Alternatively, tissue can also be obtained from biopsy material. Tumor tissue is prepared (e.g., slicing into fragments or mechanical mincing or chemical digestion) and inoculated or surgically implanted into immuno-compromised mice. Ascites can also be prepared for injection, however, as cells have undergone anoikis, may differ in some characteristics from the accompanying solid tumor. The anatomic site of injection can be orthotopic (e.g. intraperitoneal or inside the rodent ovarian bursa which encompasses the fallopian tube fimbriae, in the case of ovarian cancer) or non-orthotopic (e.g., subcutaneous tissue, renal capsule, mammary fat pad, other). Tumors typically engraft over the course of weeks to months. The initial engrafted tumor is referred to as Transplantation 1 (T1) or founder mouse (F1). Upon engraftment and evidence of expansive growth, the tumor from the founder is harvested in a sterile, viable fashion, and prepared for inoculation (fragment, mincing or digestion) into additional mice to expand the volume of tumor. Mice during the expansion phase are referred to as T2, F2 or simply expanders. Serial expansions can take place (T3, T4, etc.) and have been performed in some models systems for greater than 10 passages with genetic fidelity.22 However, the degree of fidelity across multiple models has not been comprehensively evaluated and confirmation of the genetics and histology is more important than expanding to an arbitrary number of passages.23
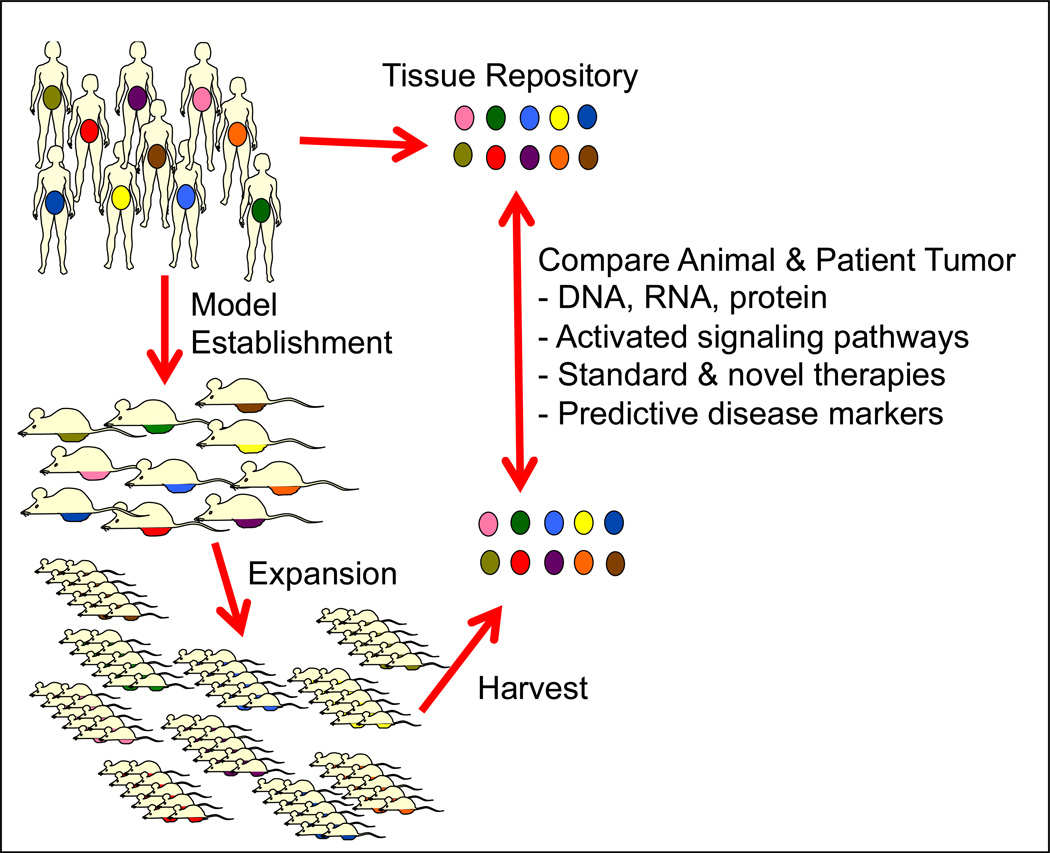
Figure 1. Model Establishment Schema.
The rationale for establishing PDX for understanding of gynecological malignancies is the assumption that each patient’s tumor is different from the others. This is represented by the varying colors. Aliquots of fresh tumors are immediately processed after surgical resection and injected into immunocompromised mice. Upon engraftment, tumors are resected and expanded in additional mice, once harvested; the tumors can be compared by a variety of molecular techniques to the source tumor specimens. By annotating specimens with clinical information from patients, correlations may be made including response to chemotherapy and survival time. Ultimately, tumors can be established to evaluating responses to treatment and potentially use these data to direct therapy in patients.
The unique presentation and clinical management of patients with gynecological malignancy is well suited to the generation patient-derived xenograft models. For example, patients with EOC often present with large volume disease and undergo a surgical debulking procedure to remove as much disease as possible prior to the administration of chemotherapy. This provides the opportunity to procure fresh tumor specimens for injecting into animals for model generation. As uterine cancer is also typically treated surgically prior to the administration of chemotherapy, the opportunity to develop PDX models from previously untreated tumors are similar. For cervix cancer, the fairly external location of disease also provides the opportunity to obtain substantial fresh tumor tissue prior to exposing the specimens to any treatment. Importantly, analysis of ovarian and endometrial cancers by the TCGA failed to identify clear subgroups for distinct therapeutic approaches, as is standard practice in breast cancer.24, 25 However, armed with the whole genome expression, mutation, and gene copy number data, the response of well-annotated PDX models to treatment may help uncover subgroups of patients expected to response to novel therapies (Figure 2). Here we summarize the use of PDX models to improve our understanding and treatment for gynecological malignancies.
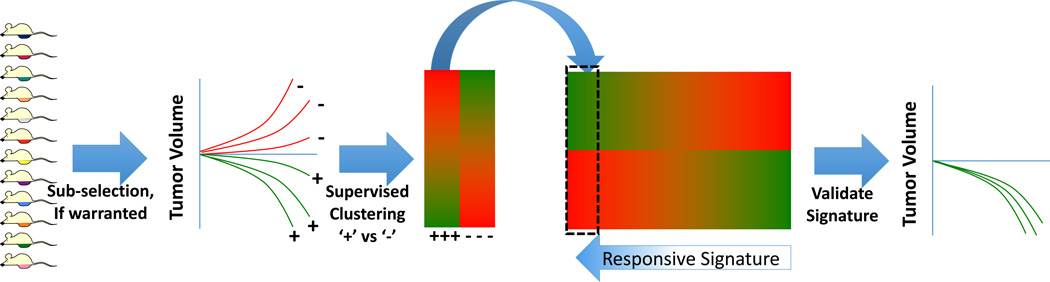
Figure 2. Drug Development Schema Using PDX Models.
Using unselected PDX models (or sub-selected based on a pertinent characteristic, such as platinum-resistance or BRCA2 mutation), models can be screened for tumor regression in response to the experimental therapy. Using DNA microarray or other high-density whole-genome data, models would then be clustered based on response (+= responders, − = non-responders). The ‘response signature’ could then be developed and validated against the remaining pool of PDX models.
2. OVARIAN CANCER PDX MODELS
2.1 Orthotopic models
A variety of subtypes of ovarian cancer have been xenografted previously but many publications lack detail about the histologic subtype from which the PDX were derived.4 A cohort of EOC was transplanted via the intra-peritoneal (i.p.) route using minced tumor, with an overall success rate of 85% (11 of 13 EOC xenografted).26 Of these, six out of six papillary serous OC transplanted successfully. High success rates were also observed in recent cohorts of EOC (74%, n= 150 EOC, minced tumor via the i.p. route)5 and high-grade serous ovarian cancer (HG-SOC) (tumor fragments via the intra-rodent ovarian bursa (IB route), 83% or 10 of 12 HG-SOC attempted).27 The resulting PDX display similar morphologic appearance and molecular characteristics as do the baseline patient tumors from which they were derived, for multiple serial transplantations.5, 26, 27 The use of SCID mice improved xenografting success rate compared with nu/nu mice (PMID 12477452)26 and the use of NOD-SCID-IL-2rγ mice may further increase that.27, 28
2.2 Non-orthotopic models
The use of non-orthotopic sites, such as subcutaneous (s.c.) tissue or sub-renal capsule, as sites of tumor transplantation have been well-explored, although the lack of a relevant microenvironment, for example, the hormonal milieu, may alter transplantation success or phenotype. Despite this concern, at least for advanced cancers such as HG-SOC, non-orthotopic transplantation appears to be of great utility. In a study of up to 41 serous OC, success rate of transplantation of OC cells which had undergone a digestion process (including depletion of CD45 hematopoietic cells) followed by inoculation into non-orthotopic sites (mammary fat pad and sub-renal capsule) compared with orthotopic sites (i.p. and IB) was equally high (95 and 73% vs. 100 and 83%).29 In a separate study, nine HG-SOC were transplanted via the IB route, six of seven tumors, which successfully xenografted via the IB route also transplanted via the s.c. routes (two tumors failed by both routes).27 Success rate is more likely to be dependent on the biologic and molecular characteristics of the individual tumor type, such that HG-SOC has one of the highest xenograft success rates and mucinous OC one of the lowest.5 For a tumor type with high transplantability, such as HG-SOC, the choice of site for transplantation e.g. i.p. or s.c. versus IB) could be guided by preference as to subsequent use of the xenografted mouse (such as desire for small tumours, which can be accurately measured or stable tumour phenotype over many serial transplantations (fragment-in, fragment-out approach).
2.3 Using Ovarian PDX models for understanding biology
Depending on the choice of mode of inoculation, different questions can be asked pertaining to the biology and natural history of different EOC. Site of tumor spread or distant metastasis has been noted to vary in keeping with patient outcome, for example, honing to the bowel.5 Models that have a predisposition to metastasize to the bowel have been identified and can be compared directly to non-bowel engrafting tumors from the isogenic tumors (i.e. same PDX model) or across multiple models with bowel invasion characteristics. These models may be helpful in understanding why EOC has such a predilection for bowel invasion and the subject of current investigations. PDX models may also be able to inform us about the behavior of individual patient tumors. Time to relapse in the corresponding patient reflects likelihood of initial transplantation success, suggesting that overall sensitivity of the EOC is similar in patient and mouse. In addition, patients in whom PDX models formed had an inferior survival to patients whose tumor tissue failed to engraft.5
2.4 Using Ovarian PDX models to understand resistance to chemotherapy
OC PDX have been utilized to study therapeutic responses, reviewed in Scott.4 Recently, in two separate PDX series, in vivo response of EOC PDX to platinum-based treatment has been shown to correlate with patient outcome.5, 27 A systematic assessment of platinum-response (with definitions for cisplatin sensitive vs. resistant vs. refractory PDX) was described in consecutive HG-SOC PDX.27 Long-term in vivo response (>100 d) correlated with platinum responsive disease in the patient, whereas PDX which were platinum resistant or refractory were more likely to be associated with a poor patient outcome. PDX which were sensitive to platinum were noted to harbor mutations in DNA repair genes (BRCA1 or BRCA2) and those which were refractory to platinum were noted to harbor oncogenes. Molecular profiling of PDX using the Foundation Medicine platform was consistent with this pattern and revealed other putative therapeutic targets for future study. PDX provide a tractable system in which the dependence of a tumour on a putative target can be assessed (of great importance given the new standard of ”platform” molecular profiling of patient tumours) and in which drug resistance can be driven and explored.27 Serial collection of circulating tumor (ct) DNA in plasma from mice, paired with PDX biopsies, allows the analysis of clonal evolution before and after specific choices of therapies. This will provide a wealth of information about the way in which EOC evolve under therapeutic pressure and ways to circumvent drug resistance.
2.5 Clinical Correlations and Applications
In order to replicate clinical care, PDX of a similar histologic (e.g. HG-SOC), phenotypic (e.g. platinum resistant) and molecular (e.g. BRCA1/2 mutant) type can be treated in a similar way to the patient from whom the PDX was derived (e.g. first-line platinum therapy followed by single agent PARP inhibitor upon relapse, or second-line platinum followed by maintenance PARPi), to allow the study of tumour evolution. Other molecular features of the baseline HG-SOC may reveal a cause for platinum or PARP inhibitor failure, or the cause of drug resistance may become more apparent upon harvest of recurrent PDX tumour. These studies may be critical for our understanding of optimal clinical trial design. PDX will be required for each major subset (phenotypic or molecular) of each major OC histology and the development of a range of appropriate cohorts of PDX, with relevant patient outcome data, are underway. Despite recent attempts to improve access to fresh tumor biopsies of recurrent EOC, paired tumor samples will remain difficult to obtain in the clinic and PDX will retain an advantage in this context (pre and post drug; comparison of the same PDX treated with a variety of drugs “head-to-head”; biopsies following multiple lines of therapy). PDX cohorts designed along these lines will prove invaluable for the identification of subsets of OC responsive to new therapeutics and for the study of modes of secondary drug resistance.
2.6 Challenges and Limitations
Given the necessary time to engraftment and expansion of PDX models, it is currently not feasible for using them to direct front-line therapy is the source patient and, thus remains a major limitation. However, they may still be useful at directing therapy at the time of recurrence (Figure 3), provided models could be tested for treatment response prior to recurrence, which allows a lead time of at least 20 months in EOC (PMID 19767092 and 16394300). Another major limitation of the use of PDX is the inability to study the role of the immune system in the treatment of ovarian cancer, due to the necessity of using immunodeficient mice. Thus, exciting new immune therapies need to be studied in transgenic or other models and ultimately the response in patients will be the defining paradigm. However, it is possible that human lymphocytic infiltrates in EOC transplanted into immunodeficient mice may be capable of instructing the murine stroma, which replaces the human stroma during the growth of EOC PDX (still to be determined). The effects of tumor stroma and tumor vasculature are likely to be important and will also be studied, using comparison of human versus murine stromal/vascular components in serial PDX (T1-3). Studies to date suggest that at least for HG-SOC, the established tumors obtained from women at the time of biopsy or surgery are sufficiently “wired” to behave in a similar manner in a mouse, as in the patient, suggesting that for treatments chosen to “match” the cancer, intrinsic molecular characteristics are more important than immune, stromal or vasculature capability in the mouse (or that these can be instructed by the tumor cells, even in the mouse). A major limitation to the use of PDX is the expense and complexity of detailed annotation and a large-scale in vivo treatment program.
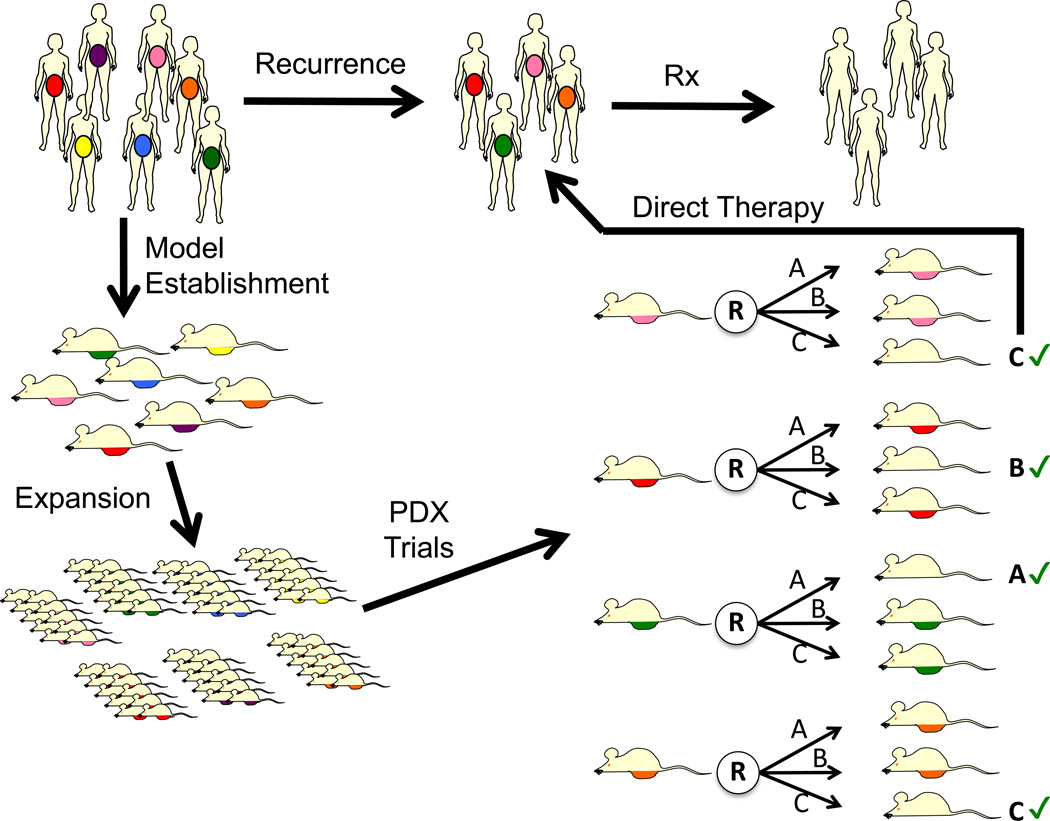
Figure 3. Individualized Therapy Directed by PDX Models.
Using PDX models engrafted and expanded from patients, each model can be tested pre-emptively with investigational and/or standard therapies (depicted as treatments A, B and C). Such investigations could be initiated, for example, while patients are in remission from primary treatment. At the time of recurrence, the therapy determined to be most effective by the patients own ‘Avatar’ (denoted with ‘check’ sign), could then be delivered. This would require all PDX models to undergo a PDX therapy trial prior to knowing if patient would recur.
3. CERVICAL PDX MODELS26
3.1 Rationale for pre-clinical models in cervical cancer
The incidence of cervical cancer in the developed world is falling, however, for those diagnosed with metastatic disease median overall survival remains short.30 Furthermore, standard of care combinations of platinum based chemotherapy and radiotherapy for the primary, potentially “curative” treatment of stage IB-IV cervical cancer have remained largely unchanged since the 1990s, as has outcome.31–34 In some respects, our understanding of the biological and molecular heterogeneity of this disease has lagged behind that of other cancers. There is an urgent unmet need to investigate new therapeutic strategies in both the newly diagnosed and recurrent patient populations. The explosion in the number of molecularly targeted agents available for study, coupled with the limited pool of patients (and resources) available for clinical trials, makes it essential that any trial we undertake is based on a sound biological rationale. This is particularly important in tumors, like cervical adenocarcinoma, that fall into the rare category (which includes nearly 35% of gynecologic cancers). High quality, relevant, translational and pre-clinical data are essential which not only identifies potential “druggable” targets but also explores efficacy and toxicity of new agents providing data on potential predictive biomarkers and imaging.
Overcoming treatment resistance and repopulation in patients undergoing chemo-radiotherapy for primary treatment of cervical cancer is critical if we are to improve long-term survival and “cure” for women diagnosed with cervical cancer. There is, therefore, an urgent need to investigate the potential value of combining novel molecularly-targeted drugs with chemo/radiotherapy. Conducting early phase clinical trials of novel agents in combination with chemo/radiotherapy represents a very significant regulatory, logistical, ethical and financial undertaking. It is essential, therefore, that any trial we undertake is underpinned by high quality data in a relevant preclinical model. There is a clinical and economic need for in vivo animal models that can provide a rationale that is translatable to the clinic. One approach involves “mouse avatar” models and co-clinical trials to inform the design of studies taking place in man.35
3.2 Cervical cancer xenograft models
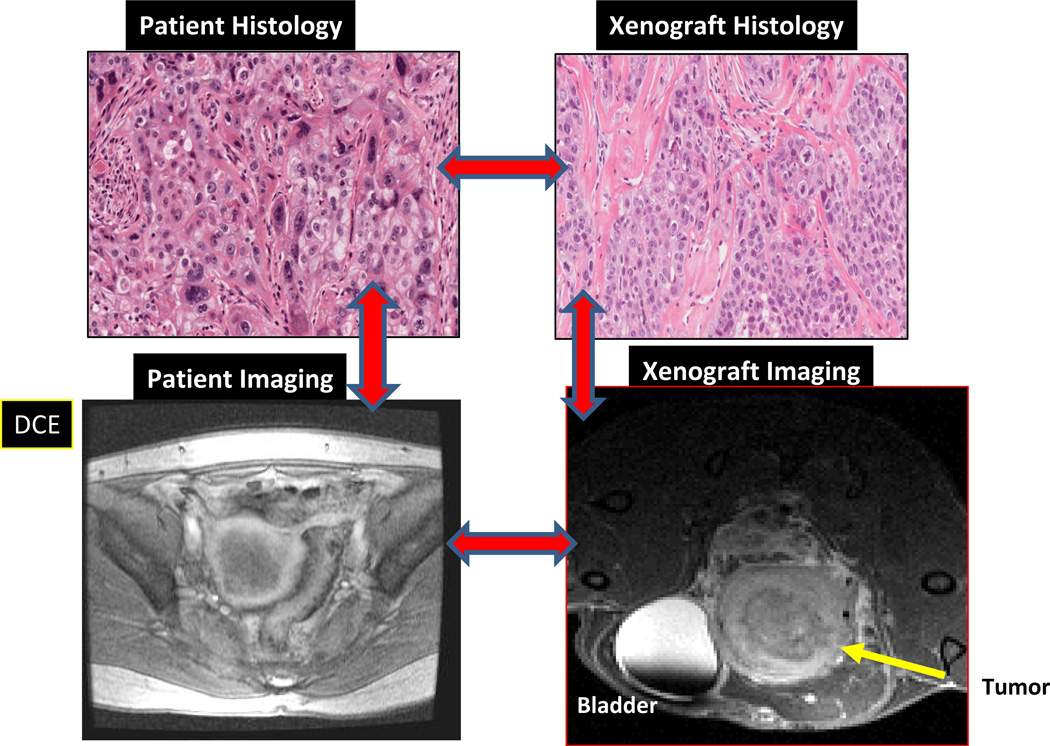
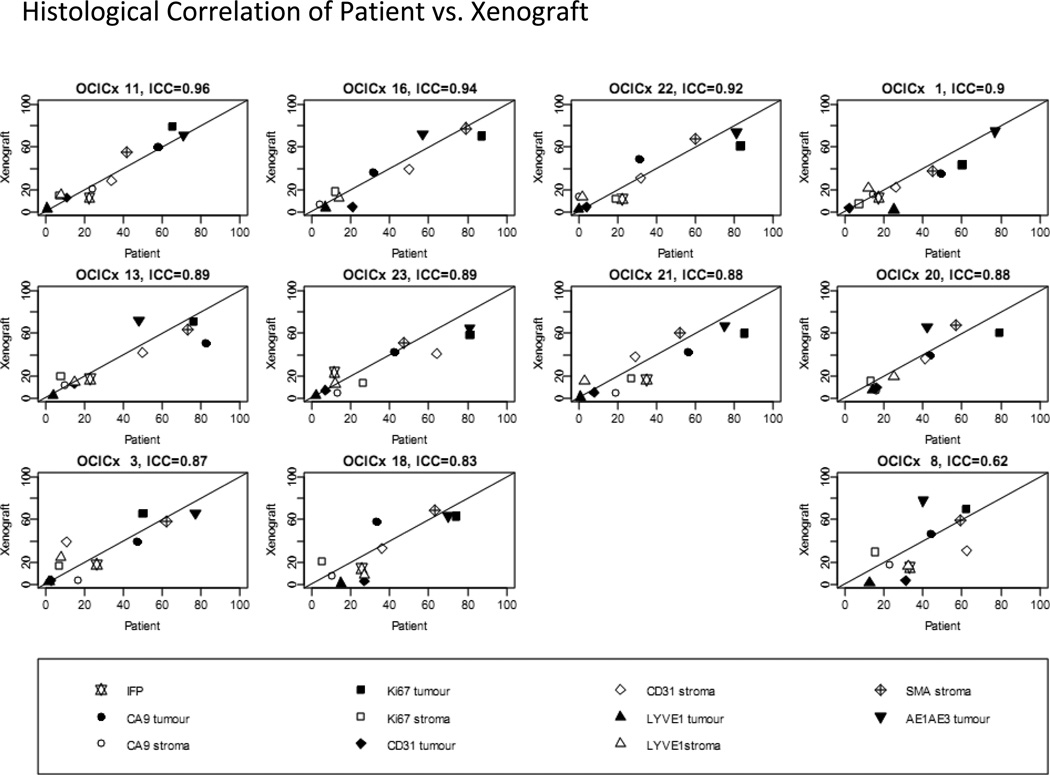
Most xenograft models of cervical cancer have been developed using commercially available cell lines which have limitations notably: genetic instability with multiple passaging;36 differences in gene expression patterns between a tumor and its corresponding cell line;37 cross contamination; and a lack of heterogeneity in cell lines that hinder our ability to study phenomena such as tumor initiating cells. Commercially available cell lines when grown in mice do not, therefore, adequately represent clinical characteristics particularly with regard to response to drugs and distant metastasis. The establishment of patient derived cervical cancer xenograft models utilizing samples taken from patients prior to treatment with chemoradiotherapy potentially addresses some of these issues. Most xenograft models are generated by subcutaneously implantation, as the accessibility of this site contributes to the relative ease of developing and testing novel agents. However, in these models the microenvironment of subcutaneous murine models may not reflect that of the original tumor.38 Recapitulation of the original tumor microenvironment has a greater likelihood of occurring in orthotopic models. The feasibility of growing cancers cells taken from patients and establishing serially transplantable, orthotopic (grown in the mouse cervix) xenografts has been demonstrated with an engraftment rate of 48% (Figure 4). These patient-derived cervical cancer xenograft models (OCICx) show a relatively stable retention of the original tumor characteristics including stromal content and tumor heterogeneity (Figure 5). To date engraftment has been successful and is independent of HPV status, patient clinico-pathological features and tumor histology.39 Successful xenograft propagation has been achieved for all histological subtypes of cervical cancer, although there are insufficient data to determine if there is a difference in the engraftment rates of individual histological or molecular subtypes. These primary xenograft models recapitulate the features of these tumors in the patient including development of lymph node and distant metastases.39,40 More importantly the tumor microenvironment appears to be preserved with strong correlation (at least up to 5 passages) of stromal content and architecture between patient tumor and xenograft models (Figure 5). This ability to recapitulate the microenvironment is reflected in comparisons of pathophysiological features measured in the patient’s in situ tumor and in the OCICX model. Direct measurements of interstitial fluid pressure (reflecting lymphatic drainage and vascular permeability) in the patient and mouse, together with measures of hypoxia and other microenvironment features show good correlation Figure 3.One important caveat, however, especially in a tumor associated with HPV infection and where data suggest the importance of the immune response pathways including the interferon-γ signaling pathway.41, 42 is that the OCICX models to date have been propagated in immunocompromised mouse models (NOD/SCID, NRG or Rag2/gamma T null mice) and therefore the mice lack an adaptive immune response. Exploration of transgenic models may help overcome this and are currently being actively explored but investigating immune response and the potential for therapeutic targeting remains a challenge.
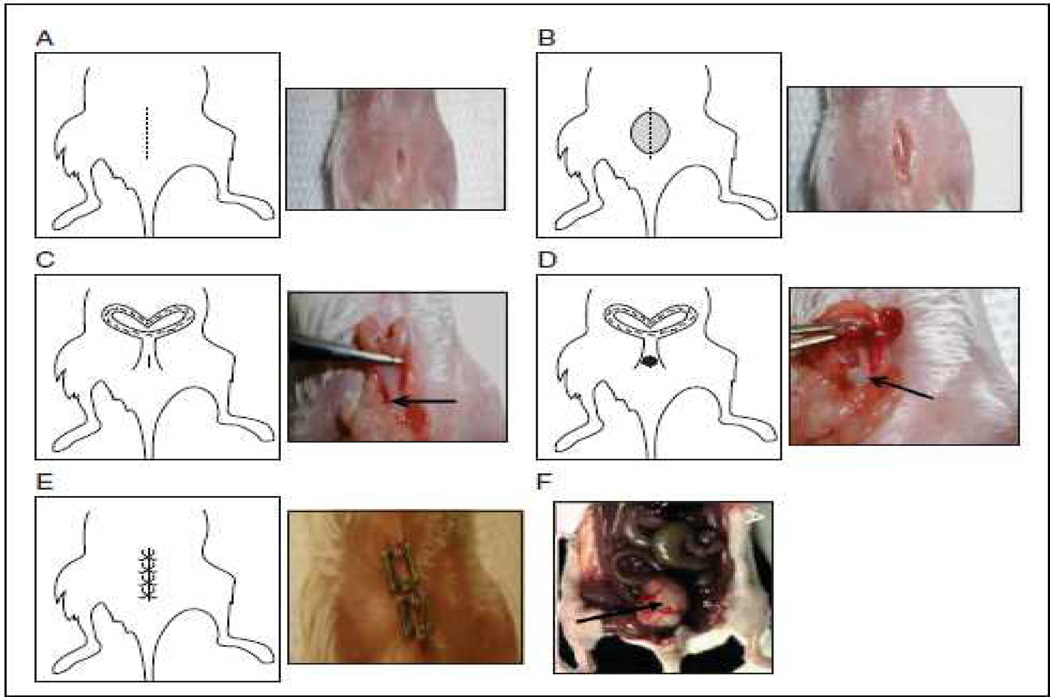
Figure 4. Surgical technique of orthotopic implantation onto the cervical site of SCID mice.
(A). A 1-cm incision in the skin of the lower abdomen. (B) Incision in the peritoneum. (C) Accessing the cervix (D) The suture with the tumor fragment threaded onto it is passed from the inside of the uterus to the outside and the at the fragment sutured to the cervix (arrow). (E) The uterus is then placed back into the peritoneal cavity. The peritoneum is closed. (F) Post-implant, approximately following 3 weeks, the growth of the primary cervix tumor is shown (indicated by arrow).
Figure 5. Comparison of histology and imaging of patient and tumor compared to imaging and histology in mouse primary xenograft model.
3.3 Biology of Cervical Cancer
OCICx models allow exploration of cervical cancer biology genomic and proteomic sequencing can elucidate tumoral heterogeneity. Mechanistic studies in primary xenografts allow further exploration of pathways of interest identified in patient samples,43–45 as data emerge about somatic mutations implicated in tumorigenesis, including in PIK3CA, PTEN, TP53, STK11 and KRAS. Whole exome sequencing is starting to suggest additional potential for targets following identification of significantly recurrent somatic mutations in the MAPK1 in squamous cell cervical cancers.41 In addition to evidence of ERBB2 activation by somatic mutation, amplification and HPV integration. Differences between adeno-and squamous carcinomas are emerging and investigating the effects of genomic changes in preclinical patient derived models is an attractive option.46
3.4 OCICX and preclinical studies
Availability of image-guided small animal irradiator technology means that the orthotopic, primary mouse xenograft models can be treated with fractionated radiation alone and in combination with weekly cisplatin chemotherapy in a manner which mimics clinical regimens.47 Tumors are grown to a size of 4–5 mm local irradiation using an 8 beam treatment plan can be used to deliver daily 2 Gy fractions (5 days/wk) (total 20 or 30 Gy) and cisplatin given weekly. Thus changes in biology associated with treatment can be investigated and potentially correlated with biopsies obtained from patients during treatment. In tandem, development of novel functional imaging protocols (particularly Magnetic Resonance Imaging), in the mouse models, allow for serial, non-invasive monitoring of dynamic changes in tumor metabolism, micro environmental characteristics and molecular background. Promising potential imaging or biomarker studies in the mouse can then be adapted for investigation in patients participating in clinical trials (Figure 6). Furthermore, treating tumors grown in the mouse cervix allows evaluation of infield toxicity (both early and late effects), again highly relevant for trial design.48, 49 Overcoming treatment resistance and repopulation in patients undergoing chemo-radiotherapy is critical if we are to improve long-term survival and “cure” for women diagnosed with cervical cancer. It has become clear that the current chemoradiotherapy regimens utilized in standard practice are delivered close to (or even at) the limits of normal tissue tolerance, such that further treatment intensification by increasing the cytotoxic drug dose or by adding different classes of cytotoxic agents is not a viable strategy.50 There is, therefore, an urgent need to investigate the potential value of combining novel molecularly-targeted drugs with chemo/radiotherapy. Gut clone assays are a well-established technique for investigating gastrointestinal acute toxicity; 49,48 and late effects can be evaluated using radiation induced fibrosis assays.51 These techniques can also be adapted to other relevant organs, such as the bladder. Currently, studies exploring potential novel combinations are underway.47
Figure 6. The relationship between passage 3 xenograft models and its matching biopsy.
Plots for IFP and tumor and stroma measures for hypoxia, blood and lymphatic vessels, and proliferation and smooth muscle actin. Lines plotted are (0,1) line of perfect concordance.
4. Conclusions
It is not feasible to develop “real time” mouse PDX for all individual patients participating in clinical trials, due to expense and the time taken to produce informative data (the time from engraftment to palpable tumor is approximately 3–4 months and follow up of treatment responses can approach a year). Furthermore, engraftment is not guaranteed and success rate is < 50% for certain gynecologic tumour subsets. However, it may be feasible in the recurrence setting, particularly in advanced EOC where there is a high –rate of recurrence and a substantial lead-time for drug sensitivity investigations. There is also the potential to run parallel “mouse trials” using mouse models with detailed molecular and functional annotation to inform dose escalation and treatment scheduling decisions in early phase frontline chemo/radiotherapy trials. Thus translational research coupled to pre-clinical models, utilizing commercially available novel agents, will hopefully lead to rapid optimization of the next generation of combined modality trials. Locally recurrent and distant metastases observed in the orthotopic, treated mouse model may also provide us with data on response rates and biological data, for both infield and out-of-field radiation efficacy and tumor characteristics, a crucial issue for some gynecologic cancer patients with advanced disease. Most importantly, the study of drug resistance, upon relapse of PDX tumor following first, second or third-line treatment, can address specific tumor/treatment contexts and may provide a wealth of material to underpin drug discovery. Innovative funding strategies to support large-scale in vivo PDX treatment programs which could uncover therapeutic requirements for drug efficacy and circumvention of drug resistance, could inform the existing, even more expensive, clinical trial arena, providing clinical trials for our EOC patients with a higher chance of long-term benefit.
KEY POINTS.
Many gynecologic cancer cell lines fail to adequately recapitulate molecular hallmarks of specific cancer subsets so new models are needed
Fresh gynecologic tumors without prior in vitro culture engraft in immunodeficient mice with a high success rate within a reasonable time frame
The resulting Patient Derived Xenografts (PDX) accurately recapitulate the patient’s tumour in histological, molecular and in vivo treatment response characteristics
Orthotopic analysis provides opportunities for understanding natural history
Matching detailed molecular and drug response annotation of individual PDX can guide “personalized” treatment with conventional and novel therapeutics
Acknowledgments
Supported in part by the Mayo Clinic Ovarian SPORE (P.H., CA136393), Ovarian Cancer Research Fund Program Project Development Grant (P.H.), and Minnesota Ovarian Cancer Alliance (P.H.); Fellowships and grants from the National Health and Medical Research Council (NHMRC Australia; Project Grant (CLS #1062702)); the Cancer Council Victoria (Sir Edward Dunlop Fellowship in Cancer Research, CLS); the Victorian Cancer Agency (Clinical Fellowship, CLS). This work was made possible through the Australian Cancer Research Foundation, the Victorian State Government Operational Infrastructure Support and Australian Government NHMRC IRIISS. The Australian Ovarian Cancer Study was supported by the U.S. Army Medical Research and Materiel Command under DAMD17-01-1-0729, The Cancer Council Tasmania and The Cancer Foundation of Western Australia and the National Health and Medical Research Council of Australia (NHMRC; ID400413). HM receives funding from Terry Fox Research Institute, Princess Margaret Foundation, COI Advisory for Roche and Astra Zeneca 3
Footnotes
Authors’ Disclosure of Potential Conflicts of Interests:
CLS receives in-kind collaborative laboratory support from Clovis Oncology.
PH is an unpaid consultant for Tesaro and Clovis Oncology and receives research funding from Tesaro, Clovis Oncology and Genentech.
REFERENCES
- 1.Adams RA. Heightened Immunity and Susceptibility toward Cheek Pouch Heterografts of a Mouse Leukemia in Syrian Hamsters. Cancer Res. 1963;23:1834–1840. [PubMed] [Google Scholar]
- 2.Carrel S, Sordat B, Merenda C. Establishment of a cell line (Co-115) from a human colon carcinoma transplanted into nude mice. Cancer Res. 1976;36:3978–3984. [PubMed] [Google Scholar]
- 3.Domcke S, Sinha R, Levine DA, Sander C, Schultz N. Evaluating cell lines as tumour models by comparison of genomic profiles. Nat Commun. 2013;4:2126. doi: 10.1038/ncomms3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scott CL, Becker MA, Haluska P, Samimi G. Patient-Derived Xenograft Models to Improve Targeted Therapy in Epithelial Ovarian Cancer Treatment. Front Oncol. 2013;3:295. doi: 10.3389/fonc.2013.00295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weroha SJ, Becker MA, Enderica-Gonzalez S, Harrington SC, Oberg AL, Maurer MJ, Perkins S, Al Hilli M, Butler K, McKinstry S, Fink SR, Jenkins RB, Hou X, Kalli KR, Goodman KM, Sarkaria JN, Karlan BY, Kumar A, Kaufmann SH, Hartmann LC, Haluska P. Tumorgrafts as in vivo surrogates for women with ovarian cancer. Clin Cancer Res. 2014 doi: 10.1158/1078-0432.CCR-13-2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hausser HJ, Brenner RE. Phenotypic instability of Saos-2 cells in long-term culture. Biochem Biophys Res Commun. 2005;333:216–222. doi: 10.1016/j.bbrc.2005.05.097. [DOI] [PubMed] [Google Scholar]
- 7.Wenger SL, Senft JR, Sargent LM, Bamezai R, Bairwa N, Grant SG. Comparison of established cell lines at different passages by karyotype and comparative genomic hybridization. Biosci Rep. 2004;24:631–639. doi: 10.1007/s10540-005-2797-5. [DOI] [PubMed] [Google Scholar]
- 8.Langdon SP, Hendriks HR, Braakhuis BJ, Pratesi G, Berger DP, Fodstad O, Fiebig HH, Boven E. Preclinical phase II studies in human tumor xenografts: a European multicenter follow-up study. Ann Oncol. 1994;5:415–422. doi: 10.1093/oxfordjournals.annonc.a058872. [DOI] [PubMed] [Google Scholar]
- 9.Massazza G, Tomasoni A, Lucchini V, Allavena P, Erba E, Colombo N, Mantovani A, D'Incalci M, Mangioni C, Giavazzi R. Intraperitoneal and subcutaneous xenografts of human ovarian carcinoma in nude mice and their potential in experimental therapy. Int J Cancer. 1989;44:494–500. doi: 10.1002/ijc.2910440320. [DOI] [PubMed] [Google Scholar]
- 10.Press JZ, Kenyon JA, Xue H, Miller MA, De Luca A, Miller DM, Huntsman DG, Gilks CB, McAlpine JN, Wang YZ. Xenografts of primary human gynecological tumors grown under the renal capsule of NOD/SCID mice show genetic stability during serial transplantation and respond to cytotoxic chemotherapy. Gynecol Oncol. 2008;110:256–264. doi: 10.1016/j.ygyno.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 11.Lee CH, Xue H, Sutcliffe M, Gout PW, Huntsman DG, Miller DM, Gilks CB, Wang YZ. Establishment of subrenal capsule xenografts of primary human ovarian tumors in SCID mice: potential models. Gynecol Oncol. 2005;96:48–55. doi: 10.1016/j.ygyno.2004.09.025. [DOI] [PubMed] [Google Scholar]
- 12.Garrett A, Quinn MA. Hormonal therapies and gynaecological cancers. Best Pract Res Clin Obstet Gynaecol. 2008;22:407–421. doi: 10.1016/j.bpobgyn.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 13.Schilder RJ, Sill MW, Chen X, Darcy KM, Decesare SL, Lewandowski G, Lee RB, Arciero CA, Wu H, Godwin AK. Phase II study of gefitinib in patients with relapsed or persistent ovarian or primary peritoneal carcinoma and evaluation of epidermal growth factor receptor mutations and immunohistochemical expression: a Gynecologic Oncology Group Study. Clin Cancer Res. 2005;11:5539–5548. doi: 10.1158/1078-0432.CCR-05-0462. [DOI] [PubMed] [Google Scholar]
- 14.Schilder RJ, Sill MW, Lee RB, Shaw TJ, Senterman MK, Klein-Szanto AJ, Miner Z, Vanderhyden BC. Phase II evaluation of imatinib mesylate in the treatment of recurrent or persistent epithelial ovarian or primary peritoneal carcinoma: a Gynecologic Oncology Group Study. J Clin Oncol. 2008;26:3418–3425. doi: 10.1200/JCO.2007.14.3420. [DOI] [PubMed] [Google Scholar]
- 15.Bookman MA, Darcy KM, Clarke-Pearson D, Boothby RA, Horowitz IR. Evaluation of monoclonal humanized anti-HER2 antibody, trastuzumab, in patients with recurrent or refractory ovarian or primary peritoneal carcinoma with overexpression of HER2: a phase II trial of the Gynecologic Oncology Group. J Clin Oncol. 2003;21:283–290. doi: 10.1200/JCO.2003.10.104. [DOI] [PubMed] [Google Scholar]
- 16.Verschraegen CF, Hu W, Du Y, Mendoza J, Early J, Deavers M, Freedman RS, Bast RC, Jr, Kudelka AP, Kavanagh JJ, Giovanella BC. Establishment and characterization of cancer cell cultures and xenografts derived from primary or metastatic Mullerian cancers. Clin Cancer Res. 2003;9:845–852. [PubMed] [Google Scholar]
- 17.Conover CA, Hartmann LC, Bradley S, Stalboerger P, Klee GG, Kalli KR, Jenkins RB. Biological characterization of human epithelial ovarian carcinoma cells in primary culture: the insulin-like growth factor system. Exp Cell Res. 1998;238:439–449. doi: 10.1006/excr.1997.3861. [DOI] [PubMed] [Google Scholar]
- 18.Stordal B, Timms K, Farrelly A, Gallagher D, Busschots S, Renaud M, Thery J, Williams D, Potter J, Tran T, Korpanty G, Cremona M, Carey M, Li J, Li Y, Aslan O, O'Leary JJ, Mills GB, Hennessy BT. BRCA1/2 mutation analysis in 41 ovarian cell lines reveals only one functionally deleterious BRCA1 mutation. Mol Oncol. 2013;7:567–579. doi: 10.1016/j.molonc.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vermeulen L, De Sousa EMF, van der Heijden M, Cameron K, de Jong JH, Borovski T, Tuynman JB, Todaro M, Merz C, Rodermond H, Sprick MR, Kemper K, Richel DJ, Stassi G, Medema JP. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat Cell Biol. 2010;12:468–476. doi: 10.1038/ncb2048. [DOI] [PubMed] [Google Scholar]
- 20.van Roozendaal CE, Gillis AJ, Klijn JG, van Ooijen B, Claassen CJ, Eggermont AM, Henzen-Logmans SC, Oosterhuis JW, Foekens JA, Looijenga LH. Loss of imprinting of IGF2 and not H19 in breast cancer, adjacent normal tissue and derived fibroblast cultures. FEBS Lett. 1998;437:107–111. doi: 10.1016/s0014-5793(98)01211-3. [DOI] [PubMed] [Google Scholar]
- 21.Rasmussen AA, Cullen KJ. Paracrine/autocrine regulation of breast cancer by the insulin-like growth factors. Breast Cancer Res Treat. 1998;47:219–233. doi: 10.1023/a:1005903000777. [DOI] [PubMed] [Google Scholar]
- 22.Guenot D, Guerin E, Aguillon-Romain S, Pencreach E, Schneider A, Neuville A, Chenard MP, Duluc I, Du Manoir S, Brigand C, Oudet P, Kedinger M, Gaub MP. Primary tumour genetic alterations and intra-tumoral heterogeneity are maintained in xenografts of human colon cancers showing chromosome instability. J Pathol. 2006;208:643–652. doi: 10.1002/path.1936. [DOI] [PubMed] [Google Scholar]
- 23.Tentler JJ, Tan AC, Weekes CD, Jimeno A, Leong S, Pitts TM, Arcaroli JJ, Messersmith WA, Eckhardt SG. Patient-derived tumour xenografts as models for oncology drug development. Nat Rev Clin Oncol. 2012;9:338–350. doi: 10.1038/nrclinonc.2012.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609–615. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kandoth C, Schultz N, Cherniack AD, Akbani R, Liu Y, Shen H, Robertson AG, Pashtan I, Shen R, Benz CC, Yau C, Laird PW, Ding L, Zhang W, Mills GB, Kucherlapati R, Mardis ER, Levine DA. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497:67–73. doi: 10.1038/nature12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elkas JC, Baldwin RL, Pegram M, Tseng Y, Slamon D, Karlan BY. A human ovarian carcinoma murine xenograft model useful for preclinical trials. Gynecol Oncol. 2002;87:200–206. doi: 10.1006/gyno.2002.6819. [DOI] [PubMed] [Google Scholar]
- 27.Topp M, Hartley L, Cook M, Heong V, Boehm E, McShane L, Pyman J, McNally O, Ananda S, Harrell M, Etemadmoghadam D, Galletta L, Alsop K, Mitchell G, Fox SB, Kerr JB, Hutt KJ, Kaufmann SH, Study AOC, Swisher EM, Bowtell DD, Wakefield M, Scott CL. Molecular correlates of platinum response in human high-grade serous ovarian cancer patient-derived xenografts. Mol Oncol. 2013 doi: 10.1016/j.molonc.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quintana E, Shackleton M, Sabel MS, Fullen DR, Johnson TM, Morrison SJ. Efficient tumour formation by single human melanoma cells. Nature. 2008;456:593–598. doi: 10.1038/nature07567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stewart JM, Shaw PA, Gedye C, Bernardini MQ, Neel BG, Ailles LE. Phenotypic heterogeneity and instability of human ovarian tumor-initiating cells. Proc Natl Acad Sci U S A. 2011;108:6468–6473. doi: 10.1073/pnas.1005529108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tewari KS, Sill MW, Long HJ, et al. Incorporation of bevacizumab in the treatment of recurrent and metastatic cervical cancer: a phrase III randomized trial of the Gynecologic Oncology Group. J Clin Oncol. 2013;31 doi: 10.3978/j.issn.2304-3865.2013.11.01. [DOI] [PubMed] [Google Scholar]
- 31.Morris M, Eifel PJ, Lu J, Grigsby PW, Levenback C, Stevens RE, Rotman M, Gershenson DM, Mutch DG. Pelvic radiation with concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancer. N Engl J Med. 1999;340:1137–1143. doi: 10.1056/NEJM199904153401501. [DOI] [PubMed] [Google Scholar]
- 32.Rose PG, Bundy BN, Watkins EB, Thigpen JT, Deppe G, Maiman MA, Clarke-Pearson DL, Insalaco S. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. N Engl J Med. 1999;340:1144–1153. doi: 10.1056/NEJM199904153401502. [DOI] [PubMed] [Google Scholar]
- 33.Keys HM, Bundy BN, Stehman FB, Okagaki T, Gallup DG, Burnett AF, Rotman MZ, Fowler WC., Jr Radiation therapy with and without extrafascial hysterectomy for bulky stage IB cervical carcinoma: a randomized trial of the Gynecologic Oncology Group. Gynecol Oncol. 2003;89:343–353. doi: 10.1016/s0090-8258(03)00173-2. [DOI] [PubMed] [Google Scholar]
- 34.Peters WA, 3rd, Liu PY, Barrett RJ, 2nd, Stock RJ, Monk BJ, Berek JS, Souhami L, Grigsby P, Gordon W, Jr, Alberts DS. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J Clin Oncol. 2000;18:1606–1613. doi: 10.1200/JCO.2000.18.8.1606. [DOI] [PubMed] [Google Scholar]
- 35.Malaney P, Nicosia SV, Dave V. One mouse, one patient paradigm: New avatars of personalized cancer therapy. Cancer Lett. 2013 doi: 10.1016/j.canlet.2013.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Staveren WC, Solis DW, Delys L, Duprez L, Andry G, Franc B, Thomas G, Libert F, Dumont JE, Detours V, Maenhaut C. Human thyroid tumor cell lines derived from different tumor types present a common dedifferentiated phenotype. Cancer Res. 2007;67:8113–8120. doi: 10.1158/0008-5472.CAN-06-4026. [DOI] [PubMed] [Google Scholar]
- 37.Ertel A, Verghese A, Byers SW, Ochs M, Tozeren A. Pathway-specific differences between tumor cell lines and normal and tumor tissue cells. Mol Cancer. 2006;5:55. doi: 10.1186/1476-4598-5-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.John T, Kohler D, Pintilie M, Yanagawa N, Pham NA, Li M, Panchal D, Hui F, Meng F, Shepherd FA, Tsao MS. The ability to form primary tumor xenografts is predictive of increased risk of disease recurrence in early-stage non-small cell lung cancer. Clin Cancer Res. 2011;17:134–141. doi: 10.1158/1078-0432.CCR-10-2224. [DOI] [PubMed] [Google Scholar]
- 39.Chaudary N, Pintilie M, Schwock J, Dhani N, Clarke B, Milosevic M, Fyles A, Hill RP. Characterization of the Tumor-Microenvironment in Patient-Derived Cervix Xenografts (OCICx) Cancers (Basel) 2012;4:821–845. doi: 10.3390/cancers4030821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chaudary N, Hedley D, Hill RP. Orthotopic xenograft model of cervical cancer for studying microenvironmental effects on metastasis formation and response to drug treatment. 2011 doi: 10.1002/0471141755.ph1419s53. Edited by. [DOI] [PubMed] [Google Scholar]
- 41.Ojesina AI, Lichtenstein L, Freeman SS, Pedamallu CS, Imaz-Rosshandler I, Pugh TJ, Cherniack AD, Ambrogio L, Cibulskis K, Bertelsen B, Romero-Cordoba S, Trevino V, Vazquez-Santillan K, Guadarrama AS, Wright AA, Rosenberg MW, Duke F, Kaplan B, Wang R, Nickerson E, Walline HM, Lawrence MS, Stewart C, Carter SL, McKenna A, Rodriguez-Sanchez IP, Espinosa-Castilla M, Woie K, Bjorge L, Wik E, Halle MK, Hoivik EA, Krakstad C, Gabino NB, Gomez-Macias GS, Valdez-Chapa LD, Garza-Rodriguez ML, Maytorena G, Vazquez J, Rodea C, Cravioto A, Cortes ML, Greulich H, Crum CP, Neuberg DS, Hidalgo-Miranda A, Escareno CR, Akslen LA, Carey TE, Vintermyr OK, Gabriel SB, Barrera-Saldana HA, Melendez-Zajgla J, Getz G, Salvesen HB, Meyerson M. Landscape of genomic alterations in cervical carcinomas. Nature. 2013 doi: 10.1038/nature12881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Meir H, Kenter GG, Burggraaf J, Kroep JR, Welters MJ, Melief CJ, van der Burg SH, van Poelgeest MI. The Need for Improvement of the Treatment of Advanced and Metastatic Cervical Cancer, the Rationale for Combined Chemo-Immunotherapy. Anticancer Agents Med Chem. 2013 doi: 10.2174/18715206113136660372. [DOI] [PubMed] [Google Scholar]
- 43.Chaudary N, Pintilie M, Hedley D, Fyles AW, Milosevic M, Clarke B, Hill RP, Mackay H. Hedgehog pathway signaling in cervical carcinoma and outcome after chemoradiation. Cancer. 2012;118:3105–3115. doi: 10.1002/cncr.26635. [DOI] [PubMed] [Google Scholar]
- 44.Hodgson L, Tavare J, Verkade P. Development of a quantitative Correlative Light Electron Microscopy technique to study GLUT4 trafficking. Protoplasma. 2014 doi: 10.1007/s00709-013-0597-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Akwari OE, Tucker A, Seigler HF, Itani KM. Hepatobiliary cystadenoma with mesenchymal stroma. Ann Surg. 1990;211:18–27. doi: 10.1097/00000658-199001000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wright AA, Howitt BE, Myers AP, Dahlberg SE, Palescandolo E, Van Hummelen P, MacConaill LE, Shoni M, Wagle N, Jones RT, Quick CM, Laury A, Katz IT, Hahn WC, Matulonis UA, Hirsch MS. Oncogenic mutations in cervical cancer: genomic differences between adenocarcinomas and squamous cell carcinomas of the cervix. Cancer. 2013;119:3776–3783. doi: 10.1002/cncr.28288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chaudary N, Pintille MSJ, et al. Hedgehog signaling in primary cervix xenograft (OCICX) models: a potential new therapeutic target. J Euro Soc Radiother Oncol. 2013;108:S78. [Google Scholar]
- 48.Withers HR, Mason K, Reid BO, Dubravsky N, Barkley HT, Jr, Brown BW, Smathers JB. Response of mouse intestine to neutrons and gamma rays in relation to dose fractionation and division cycle. Cancer. 1974;34:39–47. doi: 10.1002/1097-0142(197407)34:1<39::aid-cncr2820340107>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 49.Withers HR, Mason KA. The kinetics of recovery in irradiated colonic mucosa of the mouse. Cancer. 1974;34(suppl):896–903. doi: 10.1002/1097-0142(197409)34:3+<896::aid-cncr2820340717>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 50.Bentzen SM, Trotti A. Evaluation of early and late toxicities in chemoradiation trials. J Clin Oncol. 2007;25:4096–4103. doi: 10.1200/JCO.2007.13.3983. [DOI] [PubMed] [Google Scholar]
- 51.Haydont V, Bourgier C, Pocard M, Lusinchi A, Aigueperse J, Mathe D, Bourhis J, Vozenin-Brotons MC. Pravastatin Inhibits the Rho/CCN2/extracellular matrix cascade in human fibrosis explants and improves radiation-induced intestinal fibrosis in rats. Clin Cancer Res. 2007;13:5331–5340. doi: 10.1158/1078-0432.CCR-07-0625. [DOI] [PubMed] [Google Scholar]