Abstract
Background and Aims
Developing a conceptual and functional framework for simulating annual long-term carbohydrate storage and mobilization in trees has been a weak point for virtually all tree models. This paper provides a novel approach for solving this problem using empirical field data and details of structural components of simulated trees to estimate the total carbohydrate stored over a dormant season and available for mobilization during spring budbreak.
Methods
The seasonal patterns of mobilization and storage of non-structural carbohydrates in bark and wood of the scion and rootstock crowns of the trunks of peach (Prunus persica) trees were analysed subsequent to treatments designed to maximize differences in source–sink behaviour during the growing season. Mature peach trees received one of three treatments (defruited and no pruning, severe pruning to 1·0 m, and unthinned with no pruning) in late winter, just prior to budbreak. Selected trees of each treatment were harvested at four times (March, June, August and November) and slices of trunk and root crown tissue above and below the graft union were removed for carbohydrate analysis. Inner bark and xylem tissues from the first to fifth rings were separated and analysed for non-structural carbohydrates. Data from these experiments were then used to estimate the amount of non-structural carbohydrates available for mobilization and to parameterize a carbohydrate storage sub-model in the functional–structural L-PEACH model.
Key Results
The mass fraction of carbohydrates in all sample tissues decreased from March to June, but the decrease was greatest in the severely pruned and unthinned treatments. November carbohydrate mass fractions in all tissues recovered to values similar to those in the previous March, except in the older xylem rings of the severely pruned and unthinned treatment. Carbohydrate storage sink capacity in trunks was empirically estimated from the mean maximum measured trunk non-structural carbohydrate mass fractions. The carbohydrate storage source available for mobilization was estimated from these maximum mass fractions and the early summer minimum mass fractions remaining in these tissues in the severe treatments that maximized mobilization of stored carbohydrates. The L-PEACH sink–source carbohydrate distribution framework was then used along with simulated tree structure to successfully simulate annual carbohydrate storage sink and source behaviour over years.
Conclusions
The sink–source concept of carbohydrate distribution within a tree was extended to include winter carbohydrate storage and spring mobilization by considering the storage sink and source as a function of the collective capacity of active xylem and phloem tissue of the tree, and its annual behaviour was effectively simulated using the L-PEACH functional–structural plant model.
Keywords: Peach, Prunus persica, carbon budget, carbon reserves, carbon mobilization, stored carbohydrates, perennial plants, tree carbon budget modelling, xylem storage, functional–structural plant modelling, FSPM, L-PEACH
INTRODUCTION
Carbohydrate reserves, defined as carbohydrate resources accumulated in plant tissues that can be mobilized at a later date, are recognized to play an essential role in the survival and productivity of temperate deciduous fruit trees. They are essential to support early spring growth after winter dormancy when the metabolism of many parts of the plant increase and active growth begins but there are no leaves on the tree to supply photosynthates (Oliveira and Priestley, 1988; Kozlowski, 1992). Carbohydrate reserves have been studied in trees for more than a century (Hartig, 1858) and their nature, location and seasonal behaviour have been documented in many types of forest and fruit trees (Priestley, 1970; Oliveira and Priestley, 1988; Dickson, 1991; Kozlowski, 1992).
Starch is usually the most prevalent form of long-term carbohydrate reserve in temperate deciduous trees during the growing season until autumn, but during the transition from autumn to winter many species convert starch into soluble carbohydrates. Carbohydrate mass fractions tend to be higher in bark tissues but reserves stored in the woody parenchyma tissues of the root, trunk and stems are quantitatively most important (Kozlowski and Keller, 1966). In tissues of some species carbohydrate reserves can constitute 20–35 % of the total dry matter (Oliveira and Priestley, 1988).
The seasonal dynamics of carbohydrate reserves have been studied in numerous tree species, and almost all temperate deciduous tree species are characterized by having high mass fractions of reserves in late autumn and winter followed by depletion during the grand period of growth in spring and a gradual buildup of reserves during the summer and early autumn (Kozlowski et al., 1991). Deciduous fruit trees often have low rates of reserve accumulation during summer or early autumn periods that correspond to peak times of fruit growth (Ryugo and Davis, 1959; Priestley, 1970).
In spite of the essential nature of carbohydrate reserves for tree survival and productivity there is no clear understanding about how allocation of carbohydrates to storage reserves occurs in trees. The prevailing view has been that trees store carbohydrate reserves during times of excess photosynthate production (when current supply exceeds demands for growth and tissue metabolism) and deplete reserves when the potential rate of carbohydrate utilization exceeds the rate of current photosynthate production (Oliveira and Priestley, 1988; Dickson, 1991; Kozslowski et al., 1991). In 1994, Cannell and Dewar challenged this concept and argued that because storage reserves are so important for the survival of perennial plants, it may not be correct to treat storage sinks as passive reservoirs. They argued that reserves are not an ‘optional extra’. They cited cases when there appeared to be control mechanisms for the use of carbohydrate reserves and that storage sinks are refilled at the same time as the growth of other sinks for carbohydrates (Weinstein et al., 1991). Evaluation of the seasonal dynamics of reserve mobilization and accumulation that correspond to periods of shoot and fruit growth indicates that, although rates of reserve accumulation are generally lower when fruit growth rates are high, reserve accumulation still occurs during this period even though potential fruit growth rates are probably not saturated (Priestley, 1970; Ryugo et al., 1977). Similarly, although autumn appears to be the main period for accumulation of carbohydrate reserves in temperate deciduous trees, significant amounts of reserves are accumulated during active growth in the summer period (Barbaroux and Breda, 2002; Landhausser and Lieffers, 2003; Wong et al., 2003). Wargo (1979) reported that substantial storage of carbohydrates preceded radial growth of Acer saccharum roots and speculated that root reserve storage in maple may have a higher priority for transported carbohydrates than growth.
Silpi et al. (2007) attempted to test the Cannell and Dewar (1994) hypothesis directly by tapping rubber trees to deplete carbohydrate reserves during the growing period, and observed that the rate of carbohydrate reserve storage increased to more than compensate for the depletion of reserves caused by latex production. Thus, storage reserves appeared to be an active sink that could compete with growth for transported carbohydrates.
The lack of understanding and clear concepts regarding the dynamics of reserve storage and mobilization in perennial plants has been a major limitation in carbon-based models of tree growth (Lacointe, 2000; Le Roux et al., 2001) and is a substantial unresolved issue facing forest tree physiologists and ecologists (Epron et al., 2012; Sala et al., 2012). The view of reserve storage as a passive buffer means that carbohydrate partitioning into storage is modelled passively when all other sinks are satisfied. This was the approach that was used in an early carbon budget model of peach tree growth and productivity (Grossman and DeJong, 1994).
The L-PEACH functional–structural plant model (Allen et al., 2005; Lopez et al., 2008) simulates the development and growth of a plant's architecture, tracks all functional elements during growth and exchanges carbon and water (Da Silva et al., 2011) between the plant's elements while individual components are sensitive to local availability of carbon and water as well as environmental factors. This model has no set carbohydrate allocation patterns; instead carbohydrate distribution is governed by the relative carbon demands of each carbohydrate sink, the proximity of the sinks to carbohydrate sources and resistances along the transport pathways. Early versions of this model included the concept that carbohydrate storage is an active sink (and source during a re-mobilization period in the spring) (Allen et al., 2005; Lopez et al., 2008). However, the processes involved were never correctly implemented or validated because of lack of understanding and quantitative information about the dynamics of this long-term storage sink/source.
To treat the carbohydrate storage reserves as an active sink (and source in the spring) in a dynamic carbon distribution system requires that the potential carbohydrate demand of the storage sink (and the potential source when carbohydrates are mobilized) be defined and quantified. The most important carbohydrate storage organs in deciduous trees are the major roots, trunk and major branches (Kozlowski, 1992). While mass fractions of carbohydrates can be greater in phloem tissue than xylem, the relative mass of active xylem tissue is much greater than that of active phloem tissue; sapwood is thus the primary storage tissue of large trees (Kozlowski, 1992). Furthermore, virtually all of the xylem carbohydrates capable of being stored and subsequently mobilized are stored in radial and axial xylem parenchyma (Oliveira and Priestly, 1988; Kozlowski, 1992) and the distribution of xylem parenchyma in trees is characteristic of specific taxonomic groups (Zimmermann, 1971). This means that the upper limit of long-term carbohydrate storage in a deciduous tree is mainly determined by the mass fraction of xylem parenchyma characteristic of the sapwood of that species. Thus, the storage potential of perennial tissues of deciduous trees can be quantified by determining the mass fraction of stored carbohydrates in the sapwood of a tree under conditions in which the storage sink would be expected to be saturated and by quantifying the total amount of sapwood in the tree. By the same reasoning, the potential source of xylem carbohydrates that are available for mobilization would be represented by the difference between the maximum mass fraction of carbohydrates in the bark and sapwood and the minimum mass fraction at the end of spring, under conditions when it would be expected that all available reserves have been mobilized.
Research with several temperate deciduous species indicates that xylem tissue can store and mobilize reserve carbohydrates for many years after a secondary growth ring is formed. However, the extent to which carbohydrates are mobilized and restored in older tissue appears to decline with age and varies with species (Wargo, 1979; Barbaroux and Breda, 2002; Wong et al., 2003). Lacointe et al. (1993) reported that walnut trees mobilized few reserves that were formed more than 2 years previous to the mobilization period. Keller and Loescher (1989) also reported that seasonal storage and remobilization of non-structural carbohydrates were greatly reduced in wood more than 3 years old in mature sweet cherry trees. Definitive information about how much of the woody tissue of trees is active in storage and mobilization of carbohydrate reserves is essential for quantifying reserves available for mobilization and the capacity for reserve storage in peach trees.
The first objective of this research was to experimentally determine the mass fraction of non-structural carbohydrates present in the bark and wood of mature peach trees under conditions when it was likely that those mass fractions would represent the maximum and minimum in an annual cycle. Furthermore, the experiment was conducted in such a way as to determine how much the maximum and minimum mass fractions varied among woody tissues of different ages and to determine if storage tissue could be modelled as one unit or if sub-compartments of different aged tissue should be modelled individually. The second objective was to use the data from the field experiment to develop a better conceptual and quantitative understanding of how to handle carbohydrate storage and mobilization in carbon-driven dynamic simulation models of peach tree growth and development, and to fully implement a long-term carbohydrate storage sub-model in the functional–structural L-PEACH model.
To achieve the first objective and ensure that the results of the field study would reflect the capacity of peach trees to mobilize and replenish carbohydrate reserves in their trunks, we compared two treatments that were designed to maximize utilization of reserves (severe dormant pruning and unthinned) with a treatment we assumed would minimize the need for mobilization of reserves (defruited).
MATERIALS AND METHODS
Field study
The field study was performed on 16-year-old peach trees (Prunus persica, ‘O'Henry’ scion grafted on ‘Lovell’ rootstock) growing in a semi-commercial orchard at the University of California Wolfskill Experimental Orchard near Winters, CA, USA. The trees were planted in January 1990 in a Yolo clay loam soil and trained to have two major scaffold branches, as in the KAC-V system (DeJong et al., 1994). Prior to this experiment, which was initiated in 2005, pruning, irrigation, fertilization and pest control were conducted according to local commercial practices. The height of the trees at the beginning of the experiment was ∼4·0 m.
On 1 March [day of year (DoY) 61], 60 trees growing in four rows of the orchard were selected for the study based on their apparent health and uniformity. At that time six trees were cut down and the stumps and major roots were pulled out of the soil with a tractor. After removal from the soil, two radial slices (<1 cm thick) of the trunk (∼20 cm above the graft union) and root crown (∼10 cm below the graft union) were obtained using a chain saw. These samples were later used for carbohydrate analyses.
The remaining 54 trees were assigned to one of three treatments designed to have dramatic effects on source–sink relationships. Trees in one treatment received a severe pruning treatment in which the tops of the two main scaffolds were removed to a height of about 1 m above the ground. This treatment was designed to eliminate reproductive sink development and stimulate strong vegetative growth in the form of epicormic shoots (Pernice et al., 2006). The canopies of the trees in the other two treatments were left intact and did not receive any subsequent pruning. The second set of trees set a heavy crop and the fruit was not thinned in order to maximize the reproductive sink (unthinned treatment). All the flowers were removed from the third set of trees (defruited treatment) to eliminate the reproductive sinks and maximize the amount of carbohydrates available to be allocated to storage during the growing season.
On 6 June, 5 August and 5 November (DoY 158, 218 and 313, respectively), six trees from each of the three treatments were cut down and removed from the soil, and sample slices of the trunk and root crown (above and below the graft union, respectively) were obtained as described above for the initial six trees. Upon removal, the samples were placed in an ice-chest and subsequently stored at 0 °C until they were further dissected in the laboratory. In the laboratory, a minimum of 1·0 g of tissue was removed from the inner layer of the bark and each of the five most recent annual rings of xylem. However, only xylem tissue from the first, third and fifth rings (counting inwards from the outer ring) was analysed. Analysis was restricted to the five outer rings of xylem because, in the trees studied, it was apparent that heartwood began to form in rings older than 6 years.
The tissue samples were subsequently dried to a constant mass at 60 °C, weighed and ground to pass a 40-mesh (0·60 mm) screen. To decrease the number of samples required to be analysed, equal portions (by mass) of dried, ground tissue of each sample type from two trees from the same treatment and sampling date were combined and thoroughly mixed prior to tissue analysis. Thus, each tissue analysis was performed on a pooled tissue sample from two individual trees. The tissue samples were analysed for starch and soluble non-structural carbohydrates by standard methods (Smith, 1968) at the Division of Agriculture and Natural Resources Analytical Laboratory at the University of California, Davis, CA (ANR Analytical Lab, http://anlab.ucdavis.edu/). Starch was hydrolysed with amyloglucosidase, and glucose, sucrose, fructose and sorbitol were analysed by HPLC (Johansen et al., 1996) with a fast carbohydrate column (HPAP, Bio-Rad Laboratories, Hercules, CA). Mass fractions of total glucose after starch hydrolysis, sucrose, fructose and sorbitol were summed to give an estimate of total non-structural carbohydrates (TNC).
To estimate the significance of each of the four factors of this study, i.e. tissue age, date, treatment and location (scion or root), a four-way ANOVA was carried out. The statistical significances of the differences between the observed TNC values were then assessed using Tukey's honestly significant difference (HSD) test. These statistical analyses were performed using R software version 2·13·1.
Modelling
L-PEACH is a functional–structural model that simulates the development and growth of P. persica trees based on carbon and water exchanges (Allen et al., 2005; Lopez et al., 2008; Da Silva et al., 2011). The model is based on the premise that trees can be viewed as being composed of a collection of semi-autonomous organs interacting with each other and the environment and competing for resources based on their individual development and growth potentials and their location within the tree transport network. The transport network is supported by the tree topology, which is organized as Markovian sub-models. These sub-models control both the succession and branching patterns of growth units along axes in terms of shoot type (Guédon et al., 2001; Lopez et al., 2008), whereas shoot initiation is controlled by carbon and water availability and the environmental conditions. To distribute carbohydrate around the plant, L-PEACH contains multiple sub-models for calculating the potential source (photosynthesis and storage mobilization) and sink (growth, respiration and storage) behaviour of all the major organs of the tree in response to the environment and management practices over multiple years. All sub-models were estimated on previously collected data sets or adapted from the literature. Carbohydrate is then distributed as a function of the individual source and sink strength of all organs and the resistances along the pathways between them. Resistance varies depending on organ type (e.g. stem or petiole) and location (e.g. root or scion).
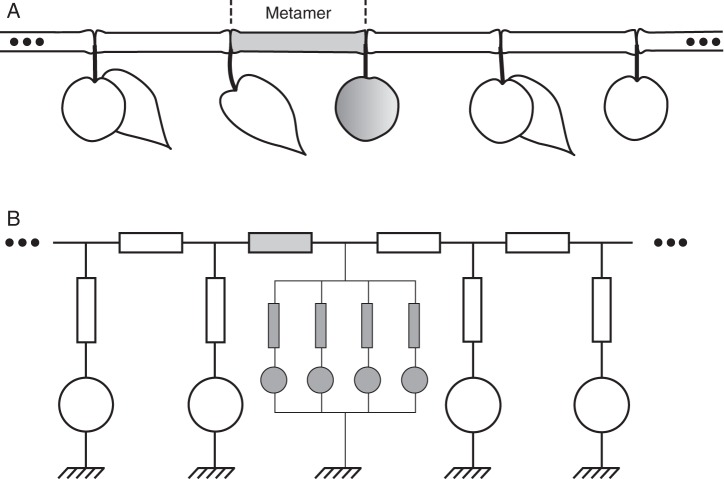
The underlying mechanism for modelling carbohydrate transport and partitioning uses an analogy with an electrical circuit to compute the flow between all components (Allen et al., 2005). A schematic representation of this modelling analogy is illustrated in Fig. 1. As stated above, the concept of storage being an active sink was included in previous versions of the model and the stored carbohydrate available for mobilization was modelled as a capacitor within the electrical sub-circuit representing the organ storage. This capacitor discharged when carbohydrate storage was mobilized, thus acting as an active source, and charged when storage was a sink. However, the switch between source and sink, i.e. the remobilization period, was user-defined, and a more quantitative approach to modelling the carbohydrate demand of the storage sink and the maximum carbohydrate available for mobilization needed to be developed.
Fig. 1.
The natural metamer structuration of plants (A) is used as a support to represent the tree as a network of electrical components in which each organ can be represented as an individual module within the branching network (B). Each individual module of the electrical network can in turn be decomposed into sub-circuits (shown in grey) representing physiological processes such as primary and secondary growth, respiration and storage. Rectangles denote resistances and circles denote sources of electromotive force (emf). Adapted from Prusinkiewicz et al. (2007).
In the electrical analogy, sources and sinks are defined by their electromotive force (emf), which can be viewed as their ‘strength’. The electromotive forces of the whole system are subsequently used to determine the voltage (or carbohydrate mass fraction) at each attachment point of sources and sinks. Simply put, for a pure sink emf = 0 while for a pure source emf = 1. In the case of a process that can act as both source and sink, a shift between these values is necessary to simulate the passage from sink to source and vice versa. However, such a drastic change is unlikely to be a natural process and, moreover, it requires additional information about when and why this switch occurs. To tackle this, we defined the storage emf as a function of the capacitor charge. It is 0 as long as TNC is below the minimum value and increases along a simple logistic curve to reach the value of 1 when TNC reaches its potential value. In this way, source or sink activity depends on the local conditions of carbohydrate allocation and, along with the pattern of TNC during the season, these activities are emergent properties of the model.
The carbohydrate demand of the storage sink and the maximum carbohydrate available for mobilization were defined and quantified from the experimental field data. Since storage in woody tissue represents the majority of carbohydrates stored in perennial tissues of deciduous trees, the mass fraction of stored carbohydrates in the active sapwood of a tree under conditions in which the storage sinks would be expected to be saturated was used as the potential sink storage (i.e. the mean maximum values measured in the non-fruited, non-pruned treatment in the field). By the same reasoning, the maximum amount of carbohydrate available for mobilization was assumed to be represented by the difference between the maximum mass fraction of carbohydrates in the sapwood and the minimum mass fraction under ‘healthy’ conditions, when it would be expected that all available reserves have been mobilized (i.e. the severe pruning or unthinned treatment in the field study without carbon starvation, water stress or any disease).
The L-PEACH model simulates the secondary growth of each metamer of shoot as a unit and does not explicitly simulate the internal structure of the shoot or root, i.e. bark and xylem rings. Therefore, an additional objective was to determine if the behaviour of these different tissues in terms of TNC should be explicitly taken into account in order to adequately model the dynamics of carbohydrate storage.
RESULTS
Field study
The source–sink manipulation treatments imposed at the outset of the experiment resulted in vastly different demands on the allocation of carbohydrates for supporting growth in the three different treatments. By 6 June and 5 August the mean fruit fresh weight of trees in the unthinned treatment was 23·8 ± 1·8 (s.e.) and 54·4 ± 5·2 kg/tree, respectively, while there was no crop in the other two treatments. On the other hand, the severely pruned treatment initially had very little leaf area development because all that remained after severe pruning was a trunk with the bases of two large scaffolds, but by 6 June the trees regenerated a substantial amount of photosynthetic canopy by producing a mean ± s.e. of 6·56 ± 0·43 kg/tree of epicormic shoots. Although leaf weight was not determined, previous research (Pernice et al., 2006) indicated that leaves account for ∼45 % of the weight of epicormic peach shoots at this time. Thus, the severely pruned trees had approximately 3 kg of leaves per tree by 6 June. While new vegetative shoot growth in trees of the defruited treatment was not quantitatively measured, visually it was clear that these trees did not produce as much new growth as the severely pruned treatment and vegetative growth in the heavily cropped treatment was also clearly less than that in either of the non-cropping treatments.
The statistical analysis showed that the main effects of all four factors were significant, as were also the two-way interactions involving date. The TNC mass fractions observed were significantly different for each wood age, location and date except for August and November, when they did not exhibit significant differences. Finally, the contrasting source–sink manipulations had clear effects, as the TNC mass fractions from the defruited treatment were significantly different from those in the two other treatments, i.e. severe pruning and unthinned (Supplementary Data Tables S1–5). To better analyse the seasonal patterns of TNC mass fractions, the results were separated by treatment and location, as displayed in Fig. 2. Two-way ANOVA and Tukey's HSD test were repeated on each of these subsets (Supplementary Data Tables 6–11).
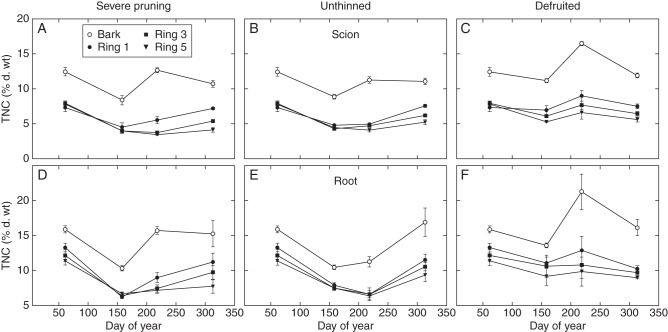
Fig. 2.
Seasonal patterns of mean (± s.e.) TNC ( % dry weight) mass fractions in the inner bark tissue and xylem tissue in the 1-, 3- and 5 yr-old xylem rings of the scion (A, B and C) and root (D, E and F) crown of peach trees subjected to three source–sink manipulation treatments: severe pruning (A, D), unthinned (B, E) and defruited (C, F).
Mean March TNC mass fractions in the scion wood were not significantly different (7·33–7·93 %) in rings 1, 3 and 5, while the mass fraction in the bark was significantly higher (12·43 %) (Table 1). Mean TNC mass fractions in all active root crown wood tissue in March (11·40–13·30 %) were significantly higher than in the trunk wood, and outer wood rings tended to have higher mass fractions than the inner rings (Table 1). Mean TNC mass fraction in the root crown bark tissue (15·90 %) was also significantly higher than that in the trunk bark (12·43 %).
Table 1.
Mean (±s.e.) non-structural carbohydrate mass fractions ( % dry weight) for inner bark and 1-, 3- and 5-yr xylem rings of root crown and trunk tissues of peach trees prior to bud-break (March) and at the sampling date when the minimum over all treatments was measured (June or August), and percentage decline in mass fraction between the two sampling dates
| March | Minimum | Decline ( %) | |
|---|---|---|---|
| Scion | |||
| Bark | 12·43 ± 0·62b | 8·37 ± 0·67ab | 32·66 |
| Year 1 | 7·33 ± 0·60c | 4·53 ± 0·61cd | 38·20 |
| Year 3 | 7·93 ± 0·33c | 3·97 ± 0·19cd | 49·94 |
| Year 5 | 7·80 ± 0·17c | 3·47 ± 0·14d | 55·51 |
| Root | |||
| Bark | 15·90 ± 0·51a | 10·33 ± 0·38a | 35·03 |
| Year 1 | 13·30 ± 0·62ab | 6·60 ± 0·92bc | 50·38 |
| Year 3 | 12·17 ± 0·85b | 6·63 ± 0·52bc | 45·52 |
| Year 5 | 11·40 ± 0·62b | 6·40 ± 0·46bc | 43·86 |
Values with different superscript letters differ significantly (P < 0·01).
Declines in mean TNC mass fractions in bark and wood tissues were apparent in both trunk and root crown by the June sampling time (Fig. 2). Declines in TNC mass fractions between the March and June sampling periods were greatest in trees in the unthinned and severely pruned treatments, while decreases were moderate or barely apparent in some defruited treatment samples.
At the August sampling date (DoY 218), bark TNC mass fractions exceeded what they had been in March in both the trunk and root crown samples of defruited treatment trees (Fig. 2C, F). During the same period, bark TNC mass fractions in the severely pruned treatment recovered to about the same level as in March (Fig. 2A, D), but the bark mass fractions of the unthinned treatment were the slowest to recover (Fig. 2B, E). In August, TNC mass fractions in the xylem tissues of the defruited trees had recovered to the March levels (Fig. 2C, F). In the woody tissues of the severely pruned trees, recovery of TNC mass fractions between June and August was apparent but values remained substantially less than the initial values in March (Fig. 2A, D). Mobilization of TNC out of the root xylem tissues apparently continued until the August sampling date in the unthinned treatment, but this was not as apparent in the trunk xylem tissue, and neither was TNC recovery (Fig. 1B, E).
By the November sampling (DoY 313), mean TNC mass fractions in almost all treatments and tissues had recovered to common levels with no significant difference from their respective levels in March (Fig. 2). The exceptions to this were some of the older xylem rings of the severe pruning and unthinned treatments, which remained significantly lower. The higher mass fraction in November than in March of some bark samples was not statistically significant.
To further elucidate potential differences in carbohydrate mobilization between tissues from different locations (ring age and trunk or root crown), the percentage declines in mean TNC mass fractions from the March samples to the minimum mass fractions registered for the same tissue type and age during the season were calculated (Table 1). These data represent estimates of the maximum TNC mobilization for the bark and different aged xylem rings in the trunk and the root crowns. The apparent maximum mobilization of TNC was greater in xylem tissue than in bark in both the root crown and trunk.
The TNC mass fraction in the March xylem samples seemed to decline with ring age in the root crown, and this was accompanied by a tendency to age-associated decreases in mean estimates of maximum mobilization of TNC in the same tissues (Table 1). However, this apparent decrease was not statistically significant. Similar tendencies in declines with age were not apparent in the trunk (scion) xylem tissue.
Modelling
In light of the observed patterns of carbohydrate storage and mobilization in the different tissues and in both scion and root, it appeared that a detailed model of the root and shoot structure was not necessary and could be replaced by a unique storage capacity representing the bark and the different rings. To this end, the carbohydrate storage capacity (CSC) of shoot and root was estimated from a weighted mean of the respective parts and potential (maximum) and minimum carbohydrate mass fractions measured in the field. For this weighted mean, the bark value accounted for one-sixth, whereas the three xylem ring values equally represented the remaining five-sixths.
| (1) |
These weighted means are presented in Table 2. The difference between these values indicated that an average of 44 % of the stored carbohydrate was available for mobilization under the conditions of the field experiment. We assumed that the potential CSC of the storage sink could be estimated from the biomass of the woody structures of the shoot and root and the potential TNC mass fraction of those structures, i.e.:
| (2) |
Table 2.
Mean (±s.e.) non-structural carbohydrate mass fractions (% dry weight) for root crown and trunk tissues of peach trees at their potential (March) and at the sampling date when the minimum was measured, and the percentage decline in the mass fraction between the two sampling dates
| Potential | Minimum | Decline ( %) | |
|---|---|---|---|
| Scion | 8·48 ± 1·91 | 4·72 ± 1·83 | 44·34 |
| Root | 12·89 ± 1·82 | 7·18 ± 1·68 | 44·30 |
Furthermore, we assumed that the amount of storage available for mobilization at any time could be deduced from the experimentally estimated percentage decline as the difference between the actual storage and the product of the CSC and the percentage decline.
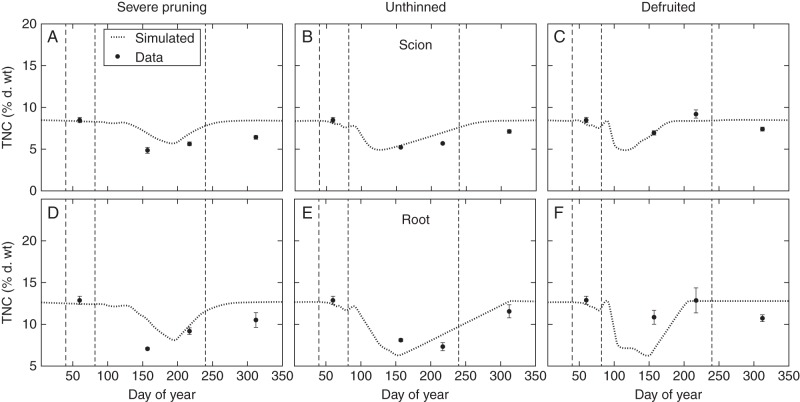
Simulations depicting carbohydrate storage and mobilization behaviour results were obtained on a virtual tree that was grown on the computer, where the potential and minimal TNC mass fractions were set according to the values shown in Table 2. After five normal years of simulated growth (i.e. grown and pruned to simulate the commercial practices of the orchard), at the beginning of the sixth year of simulated growth the tree was put in one of the treatment conditions of the field experiment: (1) severely pruned; (2) unthinned; or (3) defruited. As shown in Fig. 3, the simulated TNCs at the beginning of the season were the potential ones as defined in Table 2, showing that the model successfully simulated storage accumulation under the orchard conditions during the first 5 years. The simulated TNCs then decreased as storage mobilization took place and reached a minimum value slightly above the minimum indicated in Table 2. The mobilization period was followed by a period when carbohydrates accumulated again towards their initial ratio. The overall behaviour of mobilization and refill of storage carbohydrate generally matched the experimental pattern (whisker dots in Fig. 3). For the hard pruning experiment (Fig. 3A, D), the simulated carbohydrate mobilization seemed to be delayed and/or slower than the observed TNCs for both the scion and the root. The minimum was reached around DoY 200 and was above the observed minimum on DoY 150. In contrast, the simulated refill of storage happened faster and TNC attained their initial values between DoY 250 and 300, whereas the last observed carbohydrate mass fractions on DoY 313 were still substantially below the original values. In the unthinned experiment (Fig. 3B, E) the scion TNC reached a minimum value around DoY 125 before starting a refill slightly faster than the one suggested by the experimental data, and returned to its initial value between DoY 250 and 300, more quickly than the observed values. The mobilization period of root carbohydrate storage lasted longer than that for the scion but was faster than what the experimental data suggested. Root TNC reached its minimum by DoY 150, which was below the observed data at that time. For the defruited experiment (Fig. 3C, F) the scion TNC reached a minimum value around DoY 100 that was not visible in the experimental samples, this date being between two measurement dates. The refill speed consecutive to this minimum was on a par with the data except for the value above the target and the slight decrease at the end of the season. The mobilization in roots showed a similar but amplified pattern, with a large discrepancy at DOY 150, when the minimum was reached.
Fig. 3.
Seasonal patterns of weighted mean (± s.e.; for details, see Modelling section of the Results) TNC mass fractions ( % dry weight) in the scion (A, B, C) and root (D, E, F) crown of peach trees subjected to three source–sink manipulation treatments: severe pruning (A, D), unthinned (B, E) and defruited (C, F) and their simulated counterparts. Vertical dashed lines in each subplot indicate, from left to right, bud break, fruit growth start and harvest dates, as set in the L-PEACH model.
DISCUSSION
This study clearly indicates that in peach trees sapwood age up to 5 years had a relatively modest and statistically insignificant effect on the capacity of xylem tissue to either mobilize reserve carbohydrates in the spring or replenish reserves after spring mobilization. The minimal effect of wood age on the apparent annual carbohydrate storage/remobilization activity in this study was less than has been reported for wood of sweet cherry trees (Keller and Loescher, 1989) or walnut (Lacointe et al., 1995). The general rate of loss of this function with age appeared to be similar to that reported for wood of sessile oak (Quercus petraea) (Barbaroux and Breda, 2002). Interestingly, the percentage decrease in TNC mass fractions between the March sample and the minimum summer sample for a given age of wood (Table 1) tended to decline with age in the root crown samples but tended to increase with age in the trunk samples. This was partly because the apparent storage capacity of the root crown tissues (as reflected by the March TNC mass fractions) decreased with age while the apparent minimum TNC mass fractions remained similar, but the apparent storage capacity tended to remain constant in different-aged wood in the trunk while the apparent minimum TNC mass fraction declined.
The mean maximum TNC mass fractions (measured in March and November) were between 20 and 45 % higher in the root crown than in the scion trunk in spite of the fact that the distance between where these two samples were taken was not more than 30 cm. It is not possible to ascertain from this study if the differences between the root crown and trunk values indicate genotypic differences in the carbohydrate storage capacity of the two genotypes (rootstock versus scion) involved or root versus shoot storage characteristics. In previous research with mature peach trees involving two scion cultivars growing on six different rootstocks, analysis of plugs of wood tissue taken from above and below the graft union indicated consistently higher TNC mass fractions in the roots than the trunks (L. Solari, B. Basile and T. M. DeJong, unpubl. data). Thus, as suggested by Loescher et al. (1990), it appears likely that the higher mass fractions of carbohydrate storage in the root tissue compared with the trunk in this study are reflective of inherent differences between root and trunk tissue and not just a matter of genotype. The physiological basis for these differences is not readily apparent since no sign of scion/rootstock incompatibility was apparent in either the present study or the unpublished work mentioned above.
The range of TNC mass fractions in trunk wood for peach in this study was similar to values reported for almond (Esparza et al., 2001) but somewhat higher than reported for sweet cherry (Keller and Loescher, 1989). On the other hand, the root values were lower than reported for roots of the almond and sweet cherry in the same studies, but the root tissue analysed in the current study was from the root crown, whereas the previous studies used tissues from smaller roots.
The seasonal patterns of TNC mass fractions in woody tissues are consistent with the notion that carbohydrate storage should be viewed as an active process, as Cannell and Dewar (1994) asserted. The fact that the minimum TNC mass fractions in woody tissues of the defruited treatment remained higher than those in either the severely pruned or the unthinned treatment indicates that mobilization of carbohydrates in the spring may have been influenced by demand for carbohydrate reserves. Similarly, the fact that TNC mass fractions within the outer ring of xylem tissues returned to common levels similar to those at the beginning of the season, in spite of the fact that the time it took to return to the final common level varied strongly depending on the sink–source manipulations, is consistent with the notion of a sink saturation limit or a target mass fraction for each tissue age and location. Similar general seasonal patterns of starch or non-structural carbohydrates have been reported for many species, including peach (Stassen et al., 1982), pistachio (Spann et al., 2008) and walnut (Lacointe et al., 1993). The high TNC mass fractions in the August sample of bark tissues from the defruited treatment may indicate high availability of transport carbohydrates in the phloem tissues in late summer because of low sink activity in other parts of the plant. However, TNC mass fractions in both bark and wood were not significantly higher in November than in March, and thus TNC storage appeared to be saturated.
The strong effect of crop load on delaying the recovery of TNC storage, especially in the woody tissues, was expected and is consistent with the idea that fruits are very efficient sinks and also may have an advantage over the trunk because of their relative proximity to the photosynthetic sources (Cannell, 1985; Grossman and DeJong, 1994, 1995). The lag in the recovery of stored reserves in the severely pruned trees is consistent with the fact that there was probably a significant lag in photosynthetic production relative to the other treatments because of the initial loss of canopy scaffold branches with the heavy pruning. Also, the vigorous growth of epicormic shoots was very likely a strong competing sink for carbohydrates (Grossman and DeJong, 1998; Pernice et al., 2006). It is interesting to note that the patterns of carbohydrate mass fractions in the August samples are consistent with replenishment of carbohydrate storage proceeding from the bark inwards, as one might expect, since carbohydrate transport to the inner rings would presumably move from the phloem in the bark, inwards through the radial parenchyma in the xylem (Zimmermann, 1971).
Lacointe (2000) and Le Roux et al. (2001) asserted that the lack of understanding and clear concepts regarding the dynamics of reserve storage and mobilization in perennial plants is a major limitation in most current carbon-based models of tree growth. The carbon partitioning rationale in the PEACH (Grossman and DeJong, 1994) and L-PEACH (Allen et al., 2005; Lopez et al., 2008; Da Silva et al., 2011) models is based on the concept that each organ in the tree is governed by the genetic code of the plant and that the expression of that genetic code in the phenotype is conditioned by environmental conditions and availability of resources (DeJong, 1999). Based on the present research, it appears logical that modelling of the source–sink behaviour of carbohydrate storage in woody tissues could be conceptually approached in a manner similar to the growth of organs. The sapwood of the trunk and root crown of trees in this study ended the growing season with a fairly consistent and quantifiable, age-dependent, maximum mass fraction of carbohydrates after very different patterns of mobilization that depended on the treatments applied in the spring, supporting our approach.
The simulation results indicate that this approach did a reasonable job of recreating the general pattern of carbohydrate storage and mobilization observed in the experimental data. However, the slight offset in the patterns indicated by the experimental data and the simulated data suggests that spring carbohydrate mobilization occurred sooner than was simulated. This was apparent in the hard pruning simulation experiment because strong mobilization of carbohydrates in the model did not occur until new epicormic shoots were stimulated to grow on the stumps left after pruning and a substantial amount of shoot growth stimulated the mobilization of stored carbohydrates. On the other hand, for the same reason, the simulated shoot growth in the unthinned treatment on intact trees stimulated more carbohydrate mobilization than apparently occurred in the field experiment. However, it is possible that the storage mobilization suggested by the modelling actually occurred in the field, at least partially, since the timing of the simulated mobilization fell between two sampling dates. Finally, the large simulation–measurement discrepancy observed in the root of the defruited treatment at DOY 150 is likely a consequence of excessive initiation of shoots early in the season due to the extra carbohydrate available subsequent to the removal of fruit sinks. The growth of these shoots likely generated an additional carbohydrate demand that required extra storage mobilization to sustain their growth. It is clear that the model needs more development with regard to spring storage remobilization, since it is known that some spring carbohydrate mobilization is initiated prior to spring growth and probably starts as a signal-activated process, with stored starch being hydrolysed and sugars being unloaded into xylem vessels prior to shoot growth actually occurring (Sauter et al., 1973). Research is in progress to develop a winter chill-based phenology sub-model that can be incorporated into the model to provide such a signal.
The model simulated the replenishment of carbohydrates back into storage sink as a slower process than mobilization out of storage. It is interesting that refilling of the storage pool occurred more quickly in the simulations and field data of the defruited experiment than in the other two treatments. The speed of replenishment of storage sinks in the simulations was a function of the demand for carbohydrates by other sinks relative to the supply. This was presumably also what occurred in the unthinned and severely pruned field experiments, since unthinned peach trees can be severely carbon-limited during the entire period when the crop is on the tree (Grossman and DeJong, 1995) and severe pruning stimulates the production of epicormic shoots that continue to grow late in the season until environmental conditions become limiting (DeJong et al., 2012). The slower rate of storage refill that was apparent in field data compared with what was simulated might also be due to the differences between the biochemical processes necessary to transform sugar into starch and those required to hydrolyse starch in the spring. Further research is needed before these details can be explicitly modelled within the framework of L-PEACH.
This study attempted to experimentally quantify the overwintering carbohydrate storage sink and source of stored carbohydrates available for spring mobilization in peach trees by imposing contrasting field treatments and analysing non-structural structural carbohydrate mass fractions. Data from these experiments were then used to parameterize a storage sub-model within the L-PEACH framework that was able to reproduce the general pattern of storage mobilization and replenishment. The simulation of shoot storage behaviour was somewhat better than the behaviour of the root, which probably reflects the fact that the development of the L-PEACH model has concentrated on simulating shoot growth much more than root growth; this is a recognized limitation of the current model.
These additions to the L-PEACH model represent a conceptually simple approach to modelling carbohydrate storage and mobilization in trees but do require collection of substantial amounts of field data. The data set used in this study was rather limited and more accurate results could probably have been obtained if more frequent carbohydrate sampling had been done in the field experiment. The current improvements in the model can be used to indicate the specific times during the growing season when additional field sampling would be particularly valuable. Additionally, the discrepancies in model behaviour suggest that additional mechanisms are involved and detailed modelling of these processes may require specific parameterization for a given species. Indeed, the more detail that is incorporated into modelling specific processes, the closer the connection of the model with a specific genotype. Being able to determine what parameters/processes are genetically altered among genotypes and incorporating them into functional–structural models is a major challenge for the future.
The empirical and modelling results of this study are consistent with the concept of active carbohydrate reserve sinks and sources proposed by Cannell and Dewar (1994). We believe that the modelling approach we have used provides a clear path forward for understanding and modelling annual carbohydrate storage behaviour in trees.
SUPPLEMENTARY DATA
LITERATURE CITED
- Allen MT, Prusinkiewicz P, DeJong TM. Using L-systems for modeling source-sink interactions, architecture and physiology of growing trees: the L-PEACH model. New Phytologist. 2005;166:869–888. doi: 10.1111/j.1469-8137.2005.01348.x. [DOI] [PubMed] [Google Scholar]
- Barbaroux C, Breda N. Contrasting distribution and seasonal dynamics of carbohydrate reserves in stem wood of adult ring-porous sessile oak and diffuse-porous beech trees. Tree Physiology. 2002;22:1201–1210. doi: 10.1093/treephys/22.17.1201. [DOI] [PubMed] [Google Scholar]
- Cannell MGR. Dry matter partitioning in tree crops. In: Cannell MGR, Jackson JE, editors. Attributes of trees as crop plants. Huntingdon: Institute of Terrestrial Ecology; 1985. pp. 160–193. [Google Scholar]
- Cannell MGR, Dewar RC. Carbon allocation in trees: a review of concepts for modeling. In: Begon M, Fitter AH, editors. Advances in ecological research. Vol. 25. London: Academic Press; 1994. pp. 59–104. [Google Scholar]
- Da Silva D, Favreau R, Auzmendi I, DeJong TM. Linking water stress effects on carbon partitioning by introducing a xylem circuit into L-PEACH. Annals of Botany. 2011;108:1135–1145. doi: 10.1093/aob/mcr072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeJong TM. Developmental and environmental control of dry-matter partitioning in peach. HortScience. 1999;34:1037–1040. [Google Scholar]
- DeJong TM, Day KR, Doyle JF., Johnson RS. The Kearney Agricultural Center Perpendicular ‘V’ (KAC-V) orchard system for peaches and nectarine. HortTechnology. 1994;4:362–367. [Google Scholar]
- DeJong TM, Negron C, Favreau R, et al. Using concepts of shoot growth and architecture to understand and predict responses of peach trees to pruning. Acta Horticulturae. 2012;962:225–232. [Google Scholar]
- Dickson RE. Assimilate distribution and storage. In: Raghavendra AS, editor. Physiology of trees. New York: John Wiley & Sons; 1991. pp. 51–85. [Google Scholar]
- Epron D, Nouvellon Y, Ryan MG. Introduction to the invited issue on carbon allocation in forest trees. Tree Physiology. 2012;32:639–643. doi: 10.1093/treephys/tps055. [DOI] [PubMed] [Google Scholar]
- Esparza G, DeJong TM, Weinbaum SA. Effects of irrigation deprivation during the harvest period on nonstructural carbohydrate and nitrogen contents of dormant, mature almond trees. Tree Physiology. 2001;21:1081–1086. doi: 10.1093/treephys/21.14.1081. [DOI] [PubMed] [Google Scholar]
- Grossman YL, DeJong TM. PEACH: a simulation model of reproductive and vegetative growth in peach trees. Tree Physiology. 1994;14:329–345. doi: 10.1093/treephys/14.4.329. [DOI] [PubMed] [Google Scholar]
- Grossman YL, Dejong TM. Maximum fruit growth potential and seasonal patterns of resource dynamics during peach growth. Annals of Botany. 1995;75:553–560. [Google Scholar]
- Grossman YL, Dejong TM. Training and pruning system effects on vegetative growth potential, light interception, and cropping efficiency in peach trees. Journal of the American Society for Horticultural Science. 1998;123:1058–1064. [Google Scholar]
- Guédon Y, Barthélémy D, Caraglio Y, Costes E. Pattern analysis in branching and axillary flowering sequences. Journal of Theoretical Biology. 2001;212:481–520. doi: 10.1006/jtbi.2001.2392. [DOI] [PubMed] [Google Scholar]
- Hartig T. Ueber die Bewegung des Saftes in den Holzpflanzen. Botanische Zeitung. 1858;16:329–335. [Google Scholar]
- Johansen HN, Glitso V, Knudsen KEB. Influence of extraction solvent and temperature on the quantitative determination of oligosaccharides from plant materials by high-performance liquid chromatography. Journal of Agricultural and Food Chemistry. 1996;44:1470–1474. [Google Scholar]
- Keller JD, Loescher WH. Nonstructural carbohydrate partitioning in perennial parts of sweet cherry. Journal of the American Society for Horticultural Science. 1989;114:969. [Google Scholar]
- Kozlowski TT. Carbohydrate sources and sinks in woody plants. Botanical Review. 1992;58:107–222. [Google Scholar]
- Kozlowski TT, Keller T. Food relations of woody plants. Botanical Review. 1966;32:293–382. [Google Scholar]
- Kozlowski TT, Kramer PJ, Pallardy SG. The physiological ecology of woody plants. San Diego: Academic Press; 1991. [Google Scholar]
- Lacointe A. Carbon allocation among tree organs: a review of basic processes and representation in functional-structural plant models. Annals of Forest Science. 2000;57:521–533. [Google Scholar]
- Lacointe A, Kajji A, Daudet F-A, Archer P, Frossard J-S. Mobilization of carbon reserves in young walnut trees. Acta Botanica Gallica. 1993;140:435–441. [Google Scholar]
- Lacointe A, Kajji A, Daudet F-A, Archer P, Frossard J-S. Seasonal variation of photosynthetic carbon flow into young walnuts and its partitioning among the plant organs and functions. Journal of Plant Physiology. 1995;146:222–230. [Google Scholar]
- Landhausser SM, Lieffers VJ. Seasonal changes in carbohydrate reserves in mature northern Populus tremuloides clones. Trees. 2003;17:471–476. [Google Scholar]
- Le Roux X, Lacointe A, Escobar-Gutierrez A, Le Dizes S. Carbon-based models of individual tree growth: a critical appraisal. Annals of Forest Science. 2001;58:469–506. [Google Scholar]
- Loescher WH, McCamant T, Keller JD. Carbohydrate reserves, translocation and storage in woody plant roots. HortScience. 1990;25:274–281. [Google Scholar]
- Lopez G, Favreau RR, Smith C, Costes E, Prusinkiewicz P, Dejong TM. Integrating simulation of architectural development and source-sink behaviour of peach trees by incorporating Markov chains and physiological organ function sub-models into L-PEACH. Functional Plant Biology. 2008;35:761–771. doi: 10.1071/FP08039. [DOI] [PubMed] [Google Scholar]
- Oliveira CM, Priestley CA. Carbohydrate reserves in deciduous fruit trees. Horticultural Reviews. 1988;10:403–430. [Google Scholar]
- Pernice F, Solari L, Dejong TM. Comparison of growth potentials of epicormic shoots of nectarine trees grown on size-controlling and vigorous rootstocks. Journal of Horticultural Science and Biotechnology. 2006;81:211–218. [Google Scholar]
- Priestley CA. Carbohydrate storage and utilization. In: Luckwill LC, Cutting CV, editors. Physiology of tree crops. London: Academic Press; 1970. pp. 113–127. [Google Scholar]
- Prusinkiewicz P, Allen M, Escobar-Gutiérrez A, DeJong TM. Numerical methods for transport-resistance source-sink allocation models. In: Vos J, de Visser LFM, Struick PC, Evers JB, editors. Functional-structural plant modeling in crop production. 2007. pp. 123–137. Dordrecht: Springer. [Google Scholar]
- Ryugo K, Davis LD. The effect of the time of ripening on the starch content of bearing peach branches; Proceedings of the American Society for Horticultural Science; 1959. pp. 130–133. [Google Scholar]
- Ryugo K, Nii N, Iwata M, Carlson RM. Effect of fruiting on carbohydrate and mineral composition of stems and leaves of French prunes. Journal of the American Society for Horticultural Science. 1977;102:813–816. [Google Scholar]
- Sala A, Woodruff DR, Meinzer FC. Carbon dynamics in trees: feast or famine? Tree Physiology. 2012;32:764–775. doi: 10.1093/treephys/tpr143. [DOI] [PubMed] [Google Scholar]
- Sauter J, Iten W, Zimmermann MH. Studies on the release of sugar into the vessels of sugar maple (Acer saccharum) Canadian Journal of Botany. 1973;51:1–8. [Google Scholar]
- Silpi U, Lacointe A, Kasempsap P, et al. Carbohydrate reserves as a competing sink: evidence from tapping rubber trees. Tree Physiology. 2007;27:881–889. doi: 10.1093/treephys/27.6.881. [DOI] [PubMed] [Google Scholar]
- Smith D. Removing and analyzing total nonstructural carbohydrates from plant tissue. 1968. Wisconsin Agricultural Experiment Station Research Report 41.
- Spann TM, Beede RH, Dejong TM. Seasonal carbohydrate storage and mobilization in bearing and non-bearing pistachio (Pistacia vera) trees. Tree Physiology. 2008;28:207–213. doi: 10.1093/treephys/28.2.207. [DOI] [PubMed] [Google Scholar]
- Stassen PJC, Bergh O, Bester CW J, Du Preez MM. Reserves in full bearing beach trees: carbohydrate reserves and their implications to orchard practices. Deciduous Fruit Grower. 1982;32:424–430. [Google Scholar]
- Wargo PM. Starch storage and radial growth in woody roots of sugar maple. Canadian Journal of Forest Research. 1979;9:49–56. [Google Scholar]
- Weinstein DA, Beloin RM, Yanai RD. Modelling changes in red spruce carbon balance and allocation in response to interacting ozone and nutrient stresses. Tree Physiology. 1991;9:127–146. doi: 10.1093/treephys/9.1-2.127. [DOI] [PubMed] [Google Scholar]
- Wong BL, Baggett KL, Rye AH. Seasonal patterns of reserve and soluble carbohydrates in mature sugar maple (Acer saccharum) Canadian Journal of Botany. 2003;81:780–788. [Google Scholar]
- Zimmermann MH. Storage, mobilization and circulation of assimilates. In: Zimmermann MH, Brown CL, editors. Trees: structure and function. New York: Springer; 1971. pp. 307–322. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.