Abstract
Case-control analyses of resistant versus susceptible isolates have implicated fluoroquinolone exposure as a strong risk factor for fluoroquinolone-resistant isolates of Enterobacteriaceae. We suspect that such methodology may overestimate this association. A total of 84 cases with fluoroquinolone-resistant isolates and 578 cases with fluoroquinolone-susceptible isolates of Escherichia coli or Klebsiella pneumoniae were compared with 608 hospitalized controls in parallel multivariable analyses. For comparison of previous estimates, the results of 10 published case-control studies of risk for fluoroquinolone resistance in isolates of Enterobacteriaceae were pooled by using a random-effects model. Exposure to fluoroquinolones was significantly positively associated with fluoroquinolone resistance (odds ratio [OR], 3.17) and negatively associated with fluoroquinolone susceptibility (OR, 0.18). Multivariable analyses yielded similar estimates (ORs, 2.04 and 0.10, respectively). As data on antibiotic exposure were limited to inpatient prescriptions, misclassification of fluoroquinolone exposure in persons who received fluoroquinolones as outpatients may have led to an underestimation of the true effect size. Pooling the results of previously published studies in which a direct comparison of fluoroquinolone-resistant and fluoroquinolone-susceptible cases was used resulted in a markedly higher effect estimate (OR, 18.7). Had we directly compared resistant and susceptible cases, our univariate OR for the association between fluoroquinolone use and the isolation of resistant Enterobacteriaceae would have been 19.3, and the multivariate OR would have been 16.5. Fluoroquinolone use is significantly associated with the isolation of fluoroquinolone-resistant Enterobacteriaceae; however, previous studies likely exaggerated the magnitude of this association.
Since their introduction in the 1980s, fluoroquinolones have been used extensively due to their broad antimicrobial spectrum, availability in both oral and parenteral formulations, and low toxicity profile. Fluoroquinolones have become the first-line treatment for urinary tract infections in many areas where resistance to trimethoprim-sulfamethoxazole is prevalent (37), and with the introduction of newer spectra of quinolones, their use for respiratory infections has increased dramatically (36).
Large surveillance studies of clinical isolates in North America and Europe have detected fluoroquinolone resistance in a wide range (1 to 20%) of Escherichia coli and Klebsiella pneumoniae isolates, depending on the organism and agent being tested (7, 17, 24, 32-34). Of most concern is that resistance may be increasing; Livermore et al. identified an increasing prevalence of fluoroquinolone resistance among blood culture isolates of E. coli (from 0.8 to 3.7%) and K. pneumoniae (from 3.5 to 7.1%) between 1990 and 1999 (23). Another large survey recently confirmed an increase in ciprofloxacin resistance from 1994 to 2000 among common gram-negative organisms, including E. coli and K. pneumoniae (27).
A variety of mechanisms may lead to quinolone resistance; these include target modification of the DNA gyrase and topoisomerases and decreased drug concentrations within bacteria mediated by efflux pumps and a loss of porins. Organisms such as E. coli are believed to require several mutational events to develop resistance to fluoroquinolones (16). Although it is possible to select fluoroquinolone-resistant isolates of Enterobacteriaceae by performing serial passages in the presence of subinhibitory concentrations of various fluoroquinolones (9), it is considered relatively difficult (35). In contrast to these laboratory observations, it is believed that resistance to fluoroquinolones emerges often in clinical isolates of Enterobacteriaceae.
Numerous case-control studies have examined risk factors for the isolation of fluoroquinolone-resistant Enterobacteriaceae from clinical specimens. All have identified previous fluoroquinolone exposure—either as prophylaxis (4, 28) or as treatment (5, 6, 8, 10, 21, 25, 29, 30)—as a significant risk factor. Notably, all of these prior studies compared cases with fluoroquinolone-resistant organisms to controls with susceptible organisms. As was shown in previous studies (3, 13-15), this methodology distorts and likely overestimates the association of prior antimicrobial use, since patients infected with susceptible organisms are unlikely to have previously received an agent that would be active against these organisms.
In order to provide a less biased estimate of the association between fluoroquinolone use and resistance for E. coli and K. pneumoniae, we performed a case-case-control study as suggested by Kaye et al. (18). In this study, we compared cases with fluoroquinolone-resistant E. coli or K. pneumoniae isolates to hospitalized controls without positive clinical cultures. We conducted a parallel analysis in which cases with fluoroquinolone-susceptible isolates and hospitalized controls were compared. In order to demonstrate how our study results deviate from those of studies with the “conventional” methodology, we also conducted a meta-analysis of case-control studies that examined risks for fluoroquinolone resistance by using a comparison of resistant cases and susceptible controls.
MATERIALS AND METHODS
Study design.
We performed a retrospective case-case-control study at the Beth Israel Deaconess Medical Center, a 508-bed tertiary-care hospital in Boston, Mass. Patients were eligible for inclusion in the study if they were admitted to a medical or surgical service between October 1999 and August 2001. Pediatric, obstetrics and gynecology, and psychiatry patients were not included in the study. The study was reviewed and approved by the Committee on Clinical Investigations of the Beth Israel Deaconess Medical Center.
Data were abstracted from the hospital computerized clinical, administrative, pharmacy, and laboratory data repositories by using data management software (Microsoft Access, Redmond, Wash.). We identified the two groups of cases by using the microbiology database. Case patients with fluoroquinolone-resistant E. coli or K. pneumoniae isolated from clinical specimens after at least 48 h of hospitalization were assigned to the resistant case group. Case patients with fluoroquinolone-susceptible E. coli or K. pneumoniae isolates obtained after at least 48 h of hospitalization were assigned to the susceptible case group. Each patient was included as a subject only once, at the time of the initial positive culture. Isolates were identified to the species level. MICs were determined by using the Vitek 2 system (bioMeriéux, Inc., Durham, N.C.). Isolates were considered resistant to fluoroquinolones when the MIC of levofloxacin was ≥4 μg/ml or when the MIC of ciprofloxacin was ≥2 μg/ml.
Control subjects were randomly selected from patients who were admitted for at least 48 h during the study period and who had no positive cultures for the study organisms. The control group was used as the comparison group for both case groups.
Patient data.
For all patients, information on demographics, comorbidities, and antibiotic exposures was obtained from hospital relational databases. Risk factors assessed included age (<65 years or ≥65 years), gender, elective admission (versus urgent or emergent), transfer from another site, surgical procedure or intensive care unit stay during admission, and days at risk (days of hospitalization prior to isolation of organisms for cases or days of hospitalization for controls). Comorbidities were acquired from International Classification of Diseases (ninth revision) diagnostic codes. Antibiotic use during the hospitalization but prior to the isolation of a positive culture was documented and categorized by agent. There were no missing data for the outcome variable or other variables. All risk factors were analyzed as binary variables, except for days at risk, which was a continuous variable.
Statistical analysis.
We performed parallel case-control analyses of resistant cases versus controls and susceptible cases versus controls to evaluate the relationship between each variable and the likelihood of isolating an organism. For the univariate analysis, discrete variables were assessed by using the chi-square test. P values, odds ratios (ORs), and 95% confidence intervals were obtained. The continuous variable days at risk was assessed by using the Wilcoxon rank-sum test.
In an additional step prior to the construction of multivariable models, time-adjusted univariate analysis was performed. The effect of each variable in combination with the variable days at risk was studied for association with the outcome by using logistic regression. The purpose of this step was to control for the influence of duration of hospitalization prior to the isolation of an organism for cases or length of stay for controls. We also examined the association between fluoroquinolone use and the isolation of either E. coli or K. pneumoniae separately in a stratified analysis. We did not pursue a multivariable analysis stratified in this manner due to an insufficient number of cases.
Variables with a P value of <0.05 in a time-adjusted univariate analysis were included in parallel multivariable analyses by using logistic regression. The variable days at risk was forced into both models. Each model was further refined by removing all nonsignificant variables that were not confounders. A number of variables did change the β estimate by >10% and were considered confounders and included in the final model. No colinear variables were identified (as determined by a change in the standard error of >10%). For ease of comparison between the parallel case-control studies, two final models were constructed by using identical variables. For a variable to be included, it had to be significantly associated with either outcome in a time-adjusted univariate analysis. This property did not have a significant impact on the variables of interest, nor did it introduce collinearity or confounding. A bootstrap analysis of 1,000 repetitions was performed on the final models to test for overfitting. Finally, to illustrate the marked divergence in effect estimates obtained by standard case-control analysis compared to those obtained by our case-case-control analysis, we performed standard univariate and multivariable analyses with our own data. The statistical software used for these analyses was SAS version 8 (SAS Institute, Cary, N.C.).
Comparison to previous studies.
Case-control studies from English language articles published between 1966 and September 2002 were identified with a Medline search by using the following key words: drug resistance, quinolones, risk factors or case-control studies, and Enterobacteriaceae or Escherichia coli or Klebsiella pneumoniae. Studies were included if they were case-control studies comparing cases with fluoroquinolone-resistant E. coli or K. pneumoniae clinical isolates to controls with fluoroquinolone-susceptible isolates. In these studies, fluoroquinolone exposure could be in the context of treatment or prophylaxis. Studies were evaluated for suitability by two of us (M.K.B. and Y.C.) based on the above-mentioned predefined criteria.
The risk of isolating fluoroquinolone-resistant E. coli or K. pneumoniae following fluoroquinolone exposure was abstracted from each study. Crude ORs and 95% confidence intervals were calculated by using the primary data reported for the study. We used the DerSimonian-Laird random-effects model to obtain pooled estimates of the ORs and 95% confidence intervals. We tested for heterogeneity in the results of different studies by using the Q statistic and considered heterogeneity to be significant when the P value was <0.10. The statistical software used for these analyses was STATA version 7.0 (Stata Corporation, College Station, Tex.).
RESULTS
Patient characteristics:.
In our center, the prevalence of fluoroquinolone resistance rose 14% over the short time period of this study; in the year from August 1999 to July 2000, 7% of E. coli and 14% of K. pneumoniae isolates were resistant to levofloxacin. In the following year, from August 2000 to July 2001, 8% of E. coli and 16% of K. pneumoniae isolates were resistant to levofloxacin.
We identified 84 subjects with fluoroquinolone-resistant Enterobacteriaceae isolated from clinical specimens. A total of 33 of the organisms were E. coli (39%), and 51 were K. pneumoniae (61%). The median age of the population was 72 years, the median days at risk was 8.5 days (mean, 13.5), and 45% were male. There were 578 subjects with fluoroquinolone-susceptible Enterobacteriaceae; 381 of the organisms were E. coli (66%), and 197 were K. pneumoniae (34%). The median age of the population was 70 years, the median days at risk was 4.0 days (mean, 6.3), and 32% were male. The majority of the resistant isolates were from urine cultures (45.1%), while respiratory isolates represented 34.2% of samples and blood cultures represented 6.1% of samples. The majority of susceptible isolates were also from urine cultures (71.1%), with respiratory isolates contributing 14.9% and blood isolates contributing 4.4%. These two groups were compared to 608 control subjects with no target Enterobacteriaceae isolated during their admissions. The median age of controls was 64 years, the median length of stay was 4.0 days (mean, 5.5), and 49% were male. Table 1 shows the distribution of demographics, comorbidities, and previous antibiotic use for each of these populations.
TABLE 1.
Time-adjusted univariate risk factors for isolation of fluoroquinolone-resistant and fluoroquinolone-susceptible E. coli and K. pneumoniaea
| Variable | No. (%) of:
|
Univariate result for the following comparison:
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Cases
|
Controls (n = 608) | Resistant vs control
|
Susceptible vs control
|
||||||
| Resistant (n = 84) | Susceptible (n = 578) | OR | 95% Confidence interval | P | OR | 95% Confidence interval | P | ||
| Demographics | |||||||||
| Age of ≥65 yr | 49 (58.33) | 360 (62.28) | 300 (49.34) | 1.50 | 0.91-2.47 | 0.11 | 1.69 | 1.34-2.13 | <0.0001 |
| Male | 38 (45.24) | 185 (32.01) | 299 (49.18) | 0.81 | 0.49-1.33 | 0.41 | 0.48 | 0.38-0.61 | <0.0001 |
| Elective admission | 7 (8.33) | 100 (17.30) | 117 (19.24) | 0.51 | 0.23-1.16 | 0.11 | 0.90 | 0.67-1.20 | 0.47 |
| Transfer | 18 (21.43) | 140 (24.22) | 80 (13.16) | 1.71 | 0.91-3.22 | 0.09 | 2.08 | 1.53-2.81 | <0.0001 |
| Surgery | 34 (40.48) | 227 (39.27) | 176 (28.95) | 1.23 | 0.74-2.07 | 0.43 | 1.52 | 1.19-1.95 | 0.0008 |
| Intensive care unit stay | 50 (59.52) | 246 (42.56) | 108 (17.76) | 4.26 | 2.51-7.20 | <0.0001 | 3.41 | 2.59-4.48 | <0.0001 |
| Comorbidities | |||||||||
| Cardiovascular disease | 68 (80.95) | 425 (73.53) | 386 (63.49) | 2.34 | 1.28-4.31 | 0.006 | 1.59 | 1.24-2.04 | 0.0003 |
| Respiratory disease | 47 (55.95) | 172 (29.76) | 136 (22.37) | 2.98 | 1.79-4.95 | <0.0001 | 1.41 | 1.09-1.84 | 0.0103 |
| Renal disease | 24 (28.57) | 79 (13.67) | 54 (8.88) | 2.63 | 1.42-4.86 | 0.002 | 1.55 | 1.07-2.24 | 0.021 |
| Hepatic disease | 14 (16.67) | 59 (10.21) | 46 (7.57) | 2.24 | 1.10-4.57 | 0.027 | 1.37 | 0.92-2.07 | 0.13 |
| Transplant | 2 (2.38) | 5 (0.87) | 11 (1.81) | 1.64 | 0.35-7.65 | 0.53 | 0.48 | 0.17-1.39 | 0.18 |
| Malignancy | 7 (8.33) | 105 (18.17) | 93 (15.30) | 0.44 | 0.19-1.06 | 0.067 | 1.22 | 0.90-1.65 | 0.21 |
| Diabetes mellitus | 25 (29.76) | 131 (22.66) | 157 (25.82) | 1.12 | 0.65-1.94 | 0.68 | 0.84 | 0.65-1.10 | 0.21 |
| Antibiotic exposure | |||||||||
| Fluoroquinolones | 53 (63.10) | 47 (8.13) | 158 (25.99) | 3.17 | 1.89-5.31 | <0.0001 | 0.18 | 0.13-0.27 | <0.0001 |
| Levofloxacin | 36 (42.86) | 25 (4.33) | 101 (16.61) | 2.62 | 1.55-4.42 | 0.0003 | 0.16 | 0.10-0.26 | <0.0001 |
| Ciprofloxacin | 25 (29.76) | 29 (5.02) | 65 (10.69) | 2.15 | 1.16-3.97 | 0.015 | 0.38 | 0.24-0.61 | <0.0001 |
| Vancomycin | 47 (55.95) | 101 (17.47) | 63 (10.36) | 6.63 | 3.73-11.76 | <0.0001 | 1.68 | 1.17-2.42 | 0.005 |
| Penicillins | 21 (25.00) | 65 (11.25) | 65 (10.69) | 2.08 | 1.12-3.84 | 0.020 | 0.97 | 0.67-1.41 | 0.87 |
| β-Lactamase inhibitorsb | 3 (3.57) | 12 (2.08) | 17 (2.80) | 0.95 | 0.26-3.46 | 0.93 | 0.63 | 0.29-1.35 | 0.23 |
| Narrow-spectrum and extended- spectrum cephalosporins | 13 (15.48) | 84 (14.53) | 143 (23.52) | 0.46 | 0.23-0.93 | 0.030 | 0.52 | 0.38-0.70 | <0.0001 |
| Broad-spectrum cephalosporins | 27 (32.14) | 36 (6.23) | 58 (9.54) | 2.14 | 1.13-4.02 | 0.019 | 0.51 | 0.32-0.81 | 0.004 |
| Imipenem | 2 (2.38) | 3 (0.52) | 3 (0.49) | 0.40 | 0.04-4.53 | 0.46 | 0.73 | 0.14-3.83 | 0.71 |
| Clindamycin | 7 (8.33) | 37 (4.67) | 31 (5.10) | 1.10 | 0.41-2.99 | 0.85 | 0.88 | 0.52-1.49 | 0.63 |
| Aminoglycosides | 19 (22.62) | 31 (5.36) | 27 (4.44) | 3.92 | 1.87-8.23 | 0.0003 | 1.12 | 0.66-1.92 | 0.67 |
| Metronidazole | 39 (46.43) | 78 (13.49) | 83 (13.65) | 3.74 | 2.20-6.34 | <0.0001 | 0.87 | 0.62-1.23 | 0.44 |
Time-adjusted univariate results were obtained by examining the effect of each variable in combination with days at risk on the association with outcome of the isolation of a resistant or susceptible organism.
Beta-lactam-beta-lactamase inhibitor combinations.
Univariate results. (i) Comparison of fluoroquinolone-resistant cases with controls.
Time-adjusted univariate results for the outcome of isolation of fluoroquinolone-resistant organisms are presented in Table 1. Cases were more likely to have been in the intensive care unit. Of the comorbidities evaluated, cardiovascular disease, respiratory disease, renal disease, and hepatic disease were all more common in cases. Prior use of a number of antimicrobial agents was associated with the isolation of fluoroquinolone-resistant organisms; these agents included fluoroquinolones, levofloxacin, ciprofloxacin, vancomycin, penicillins, broad-spectrum cephalosporins, aminoglycosides, and metronidazole. Prior use of narrow-spectrum and extended-spectrum cephalosporins was the only variable that was protective against the isolation of fluoroquinolone-resistant organisms. Fluoroquinolone use was a risk factor for the isolation of resistant E. coli (OR, 4.03; P = 0.0002) and K. pneumoniae (OR, 2.59; P = 0.006) when the organisms were analyzed separately.
(ii) Comparison of fluoroquinolone-susceptible cases with controls.
Time-adjusted univariate results for the isolation of fluoroquinolone-susceptible E. coli or K. pneumoniae are presented in Table 1. Cases were more likely to be older than 65, have been transferred from another institution, have had surgery, have had an intensive care unit stay, and have cardiovascular disease, respiratory disease, and renal disease. Male gender was protective against the isolation of susceptible organisms. Of the antimicrobial agents studied, only prior vancomycin use was associated with the isolation of fluoroquinolone-susceptible organisms. A number of antimicrobial agents were protective against the isolation of fluoroquinolone-susceptible organisms; these agents included fluoroquinolones, levofloxacin, ciprofloxacin, and narrow-spectrum, extended-spectrum, and broad-spectrum cephalosporins. Fluoroquinolone use was protective against the isolation of susceptible E. coli (OR, 0.10; P < 0.0001) and K. pneumoniae (OR, 0.36; P < 0.0001) when the organisms were analyzed separately.
Multivariable results. (i) Comparison of fluoroquinolone-resistant cases with controls.
Table 2 presents the results of multivariable logistic regression for the outcome of isolation of fluoroquinolone-resistant Enterobacteriaceae. These results are adjusted for days at risk, as well as for other factors found to be significant by univariate analysis at a P value of <0.05. Variables that were found to be significant included an intensive care unit stay, cardiovascular disease, prior fluoroquinolone use, vancomycin use, and aminoglycoside use. Bootstrapping confirmed the stability of this model. A separate model was run to examine the significance of prior use of the individual fluoroquinolone antibiotics. Ciprofloxacin was identified as a risk factor (OR, 2.5; P = 0.01), but levofloxacin was not (OR, 1.6; P = 0.16). Intensive care unit stay, cardiovascular disease, vancomycin use, and aminoglycoside use remained significant risk factors with this substitution.
TABLE 2.
Multivariable model of risk factors for isolation of fluoroquinolone-resistant and fluoroquinolone-susceptible Enterobacteriaceaea
| Variable | Fluoroquinolone-resistant isolates
|
Fluoroquinolone-susceptible isolates
|
||||
|---|---|---|---|---|---|---|
| OR | 95% Confidence interval | P | OR | 95% Confidence interval | P | |
| Days at risk | 1.02 | 0.98-1.06 | 0.43 | 1.04 | 1.01-1.07 | 0.024 |
| Age of ≥65 yr | 1.22 | 0.67-2.21 | 0.52 | 1.61 | 1.20-2.15 | 0.002 |
| Male | 0.71 | 0.40-1.24 | 0.23 | 0.40 | 0.30-0.53 | <0.0001 |
| Transfer | 0.99 | 0.46-2.11 | 0.98 | 1.47 | 1.03-2.09 | 0.034 |
| Surgery | 1.26 | 0.66-2.40 | 0.48 | 1.74 | 1.26-2.42 | 0.001 |
| Intensive care unit stay | 2.65 | 1.38-5.10 | 0.003 | 3.33 | 2.36-4.71 | <0.0001 |
| Cardiovascular disease | 2.17 | 1.02-4.62 | 0.044 | 0.96 | 0.70-1.32 | 0.80 |
| Respiratory disease | 1.71 | 0.94-3.14 | 0.081 | 1.47 | 1.07-2.01 | 0.017 |
| Fluoroquinolones | 2.04 | 1.09-3.81 | 0.026 | 0.10 | 0.06-0.17 | <0.0001 |
| Vancomycin | 3.17 | 1.62-6.19 | 0.001 | 1.46 | 0.90-2.37 | 0.121 |
| Narrow-spectrum and extended-spectrum cephalosporins | 0.54 | 0.24-1.19 | 0.13 | 0.33 | 0.23-0.49 | <0.0001 |
| Broad-spectrum cephalosporins | 0.98 | 0.44-2.17 | 0.96 | 0.42 | 0.24-0.76 | 0.004 |
| Aminoglycosides | 3.73 | 1.51-9.23 | 0.004 | 0.89 | 0.43-1.83 | 0.76 |
| Metronidazole | 1.33 | 0.68-2.61 | 0.41 | 2.15 | 1.31-3.51 | 0.002 |
Also controlled for renal disease, hepatic disease, and penicillin use, which were not significant in either model. The final models were constructed by using identical variables for ease of comparison between parallel case-control studies. For a variable to be included, it had to be significantly associated with either outcome (fluoroquinolone-resistant or fluoroquinolone-susceptible organism) in a time-adjusted univariate analysis. Simplified models were also examined; in these, only variables significantly related to a single outcome in a time-adjusted univariate analysis were included. In the simplified model of risk factors for fluoroquinolone-resistant organisms, the risk associated with fluoroquinolone use had an OR of 2.42 and a confidence interval of 1.35 to 3.45. In the simplified model of risk factors for fluoroquinolone-susceptible organisms, the risk associated with fluoroquinolone use had an OR of 0.10 and a confidence interval of 0.06 to 0.17.
(ii) Comparison of fluoroquinolone-susceptible cases with controls.
Table 2 presents the results of multivariable logistic regression for isolation of fluoroquinolone-susceptible Enterobacteriaceae. Significant risk factors included days at risk, age greater than 65 years, transfer from an outside institution, surgery, intensive care unit stay, and respiratory disease. Male gender was protective against the isolation of susceptible organisms. Of the antimicrobial agents studied, only metronidazole use proved to be a risk factor for the isolation of susceptible organisms. Antimicrobial agents that were protective against the isolation of susceptible organisms included fluoroquinolones and narrow-spectrum, extended-spectrum, and broad-spectrum cephalosporins. Bootstrapping confirmed the stability of this model. When levofloxacin use and ciprofloxacin use were substituted for fluoroquinolone use, both were found to be significant protective factors (ORs, 0.08 and 0.27, respectively; P values for both, <0.0001).
When we performed a standard case-control analysis of our data (i.e., comparing patients with resistant isolates to patients with susceptible isolates), our univariate OR for the association between fluoroquinolone use and the outcome of isolation of resistant Enterobacteriaceae was 19.3 (P < 0.0001), and the multivariable OR was 16.5 (P < 0.0001). These values are about six to eight times higher than those obtained by case-case-control analysis—3.17 and 2.04, respectively.
Meta-analysis.
Ten case-control studies comparing cases with resistant organisms to controls with susceptible organisms met the criteria for inclusion (4-6, 8, 10, 21, 25, 28-30). A case-control study by Muder et al. was not included because fewer than 10% of the gram-negative organisms isolated were either E. coli or K. pneumoniae (26). Another study was not included because it was published after our analysis was complete (20).
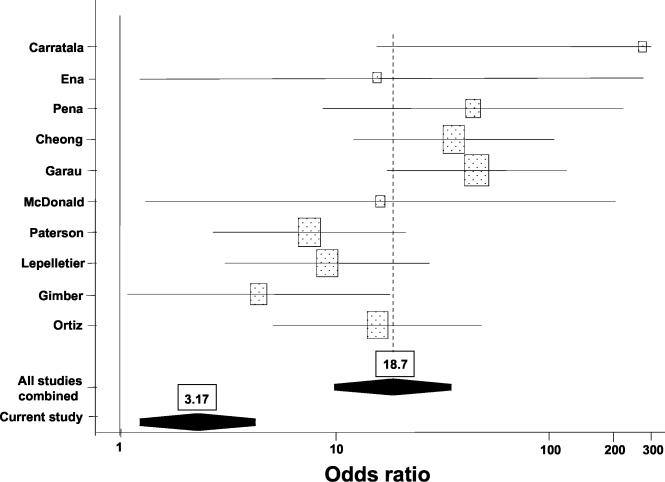
The included studies and their associated ORs for the effect of previous fluoroquinolone exposure upon the outcome of isolation of a fluoroquinolone-resistant organism are presented in Table 3. For most of the studies, only univariate results were available. Fluoroquinolone use was a risk factor for the isolation of resistant E. coli or K. pneumoniae in all of these studies; the ORs ranged from 4.4 to 273. Pooling the univariate ORs from these studies led to an OR of 18.7 (95% confidence interval, 10.0 to 34.9) for fluoroquinolone use. The results of these studies were heterogeneous (Q = 17.8 [df, 9]; P = 0.038). Figure 1 shows plots of the univariate ORs from the various studies as well as the pooled OR.
TABLE 3.
Case-control studies of fluoroquinolone-resistant and fluoroquinolone-susceptible Enterobacteriaceae
| Reference | Yr | OR | 95% Confidence interval |
|---|---|---|---|
| 4 | 1995 | 273 | 15.7-4751 |
| 6a | 1995 | 15.7 | 2.2-680.5 |
| 30b | 1995 | 44.2 | 8.8-221.9 |
| 10 | 1998 | 4.4 | 1.1-19 |
| 8c | 1999 | 46 | 17-117 |
| 21 | 1999 | 9.2 | 3-27 |
| 28 | 1999 | 15.8 | 5.3-49.9 |
| 29 | 2000 | 7.6 | 2.7-21.5 |
| 5 | 2001 | 35.9 | 12.2-105 |
| 25 | 2001 | 16.3 | 1.3-203 |
Due to the presence of a zero cell in the primary data, recalculation of the OR required the addition of 1 to each cell. The choice to add 1 rather than 0.5 was believed to be more conservative, as the effect on the OR was minimized. The adjusted OR and confidence interval were 19.09 and 2.2 to 166, respectively.
Adjusted OR and confidence interval, 31.3 and 3.3 to 291, respectively.
Adjusted OR, 14.
FIG. 1.
Effect of fluoroquinolone use on the isolation of fluoroquinolone-resistant Enterobacteriaceae. Results from standard case-control studies are shown. Stippled boxes, ORs for the individual studies; black regions, pooled ORs. From top to bottom, the names at the left correspond to references 4, 6, 30, 5, 8, 25, 29, 21, 10, and 28.
DISCUSSION
Fluoroquinolone use and resistance to fluoroquinolones are increasing. In our institution, in the year from August 2002 to July 2003, resistance to levofloxacin was present in 12% of E. coli isolates and 25% of Klebsiella spp., representing a greater than 70% increase in resistance since the beginning of the study period and continuing the concerning trend noted during that period. At the same time, dollars expended to purchase fluoroquinolones in our center increased by 27% from fiscal year 2001 to fiscal year 2003. The main finding of our study using the case-case-control methodology was that fluoroquinolone use was indeed a risk factor for the isolation of fluoroquinolone-resistant E. coli or K. pneumoniae from clinical specimens. However, this association appears to have been overestimated in previous studies, as evidenced by the comparison of the results of the current study with a meta-analysis of previous case-control studies. The meta-analysis revealed that the standard case-control approach yields higher effect estimates of the association between fluoroquinolone use and resistance. We have demonstrated that these estimates may be biased, as fluoroquinolones were actually found to be highly protective against the isolation of fluoroquinolone-susceptible organisms in our case-case-control analysis. As a consequence, using subjects with susceptible isolates as the control group inflates the risk of prior fluoroquinolone use upon the isolation of fluoroquinolone-resistant organisms. This finding was emphasized by performing a standard case-control analysis with our data; this analysis led to higher ORs that were on the same order of magnitude as the ORs from the meta-analysis of case-control studies.
A variety of antimicrobial agents appeared to be associated with the subsequent isolation of both susceptible and resistant Enterobacteriaceae. Many of the antimicrobial agents with spectra of activity against gram-negative organisms were identified on univariate analysis but were eliminated on multivariable analysis. Fluoroquinolone, aminoglycoside, and vancomycin use remained a significant risk factor on multivariable analysis, and bootstrapping validated these results.
Both aminoglycosides and vancomycin may have been identified as risk factors because they are frequently used as empirical broad-spectrum coverage for the most ill patients. These antibiotics may simply be serving as markers for these very ill patients, who are often prescribed multiple antibiotics and are vulnerable to infection with resistant organisms. Another possible explanation for this finding is the principle of coselection; i.e., a bacterium that is resistant to multiple drugs (e.g., aminoglycosides and fluoroquinolones) may be selected for by any one of those drugs. Alternatively, certain bacterial resistance mechanisms may confer resistance to more than one agent—efflux pumps exemplify this principle. In a survey of fluoroquinolone resistance among clinical isolates, Sahm et al. found that fluoroquinolone resistance never occurred independently of resistance to at least one other agent (33). Acquisition of resistance may occur in a stepwise manner and be dependent upon exposure to several agents (12). Since hospitalized patients often receive more than one antibiotic, it is difficult to determine which agent is responsible for the development of resistance and cross-resistance.
The role of vancomycin as a putative risk factor for the isolation of fluoroquinolone-resistant E. coli or K. pneumoniae defies these explanations, since vancomycin is not active against gram-negative bacteria and the mechanisms of resistance appear to be completely independent. Other case-control studies of gram-negative organisms (14, 31) have identified vancomycin as a risk factor. A possible explanation could be the effect of vancomycin upon normal gram-positive flora, which clears the way for colonization with nosocomial enteric gram-negative species. This observation deserves further study.
Fluoroquinolone use was protective against the isolation of susceptible organisms. This was true for both ciprofloxacin and levofloxacin. In addition to this finding, the use of all spectra of cephalosporins was protective against the isolation of susceptible Enterobacteriaceae. This finding is likely due to their known activity against these organisms. Only one antibiotic agent, metronidazole, was identified as a risk factor for the isolation of fluoroquinolone-susceptible Enterobacteriaceae. This agent has been implicated as an important risk factor for other resistant organisms, including vancomycin-resistant enterococci (11). Its effects are believed to occur through its activity against anaerobic gut flora; through the reduction of colonization resistance, an ecological niche is provided for resistant enteric organisms.
The chief limitation of our study is the lack of data on outpatient use of antibiotics. As the fluoroquinolone class is so commonly used in the ambulatory setting, a number of patients may have received outpatient therapy and been misclassified as not having exposure to these agents, a situation which could have led to an underestimation of the magnitude of association between fluoroquinolone exposure and the subsequent isolation of resistant bacteria. There is no question that knowing the impact of outpatient antibiotic use upon the development of bacterial resistance would be invaluable, if only such information could be reliably ascertained. The efforts of Lautenbach et al. in this regard serve as a model for future investigation; these authors performed extensive chart review in order to more accurately characterize antibiotic exposure in the 30 days prior to infection of patients at outside hospitals and long-term care facilities and in the outpatient setting (20). The size of the study population in the current study made such chart review not feasible.
As a case-control study, this work was not designed to prove causality, which would require a clinical trial. Additionally, the patient populations in our study differed substantially from one another. We used multivariable analysis to control for this difference as a source of confounding but cannot rule out the possibility of unmeasured confounders influencing our results. Other investigators using case-case-control methodology have used as a control group a random sample of all patients without resistant organisms isolated from cultures; this strategy leads to the inclusion of a small number of cases with susceptible organisms in the control group (14). While we acknowledge that this situation is more accurate from an epidemiologic perspective, it would have eliminated the possibility of using the same comparison group for both case groups. In our study, patients with susceptible target isolates comprised less than 1% of the total number of patients admitted. We believe that the removal of such patients from our control group would be an acceptably small source of bias.
Although all of our microbiology information was from clinical specimens, we did not try to distinguish between true infection and colonization with these organisms. Presumably, cultures would be obtained only if there were clinical suspicion of infection. However, for epidemiologic purposes, this situation should not be of major importance, as both groups contribute to the burden of resistance, as described by the colonization pressure (1). Additionally, both colonized and infected patients can transmit resistant organisms, and those colonized may become infected with the same organism later in the study period. Indeed, the lack of surveillance could have resulted in the misclassification of individuals in the control group as not infected with either resistant or susceptible organisms. The lack of surveillance cultures may also contribute to detection bias, which is a concern given the very real differences between characteristics of the case group population and those of the control group population. The patient characteristics that prompted clinicians to treat with antimicrobial agents likely also prompted the acquisition of clinical cultures and could have contributed to the observed association between treatment with antimicrobial agents and subsequent infections. One method to control for this situation is to use propensity scoring—e.g., for the propensity to be treated with fluoroquinolones (2). Propensity scoring was not performed in this study because of our focus on the case-case-control methodology.
We did not perform clonal typing of these organisms; thus, we cannot conclude definitely whether any outbreak or clonal spread influenced our results. If clonal dissemination of resistant strains were responsible for some portion of the resistant organisms present, then we might expect to find a weaker association between antibiotic exposure and resistance. Review of infection control records failed to demonstrate any outbreak of fluoroquinolone-resistant Enterobacteriaceae during the study period. Previous investigators suggested that horizontal transmission contributes minimally to the emergence of resistance in nosocomial gram-negative organisms (19, 22).
As we have noted, fluoroquinolones are prescribed frequently in our institution—more than a quarter of the subjects in our control group were prescribed a fluoroquinolone antimicrobial agent. Disturbingly, this practice appears to be increasing at our center and across the United States (27). Such a high prevalence of fluoroquinolone use may have influenced the magnitude of the association that we found between fluoroquinolone use and resistance. While our extensive fluoroquinolone use may make our results less generalizable to centers with more restrained use, we nonetheless believe that our practices are reflective of those of many other centers.
We conclude that fluoroquinolone exposure was associated with the subsequent isolation of fluoroquinolone-resistant Enterobacteriaceae. However, the magnitude of the association was smaller than has been described by previous case-control studies. We acknowledge that a single study based on 84 resistant isolates cannot invalidate the findings of multiple previous studies, but we do suggest that others attempt to confirm our findings at other centers in order to more accurately estimate the association between fluoroquinolone exposure and subsequent resistance. We acknowledge that the lack of data on outpatient fluoroquinolone exposure may have contributed to the finding of a weaker association between exposure and resistance, and we suggest that future studies attempt to capture data on outpatient antibiotic use. It must be stated that it is not the intent of these authors to exonerate fluoroquinolones as agents that select for antimicrobial drug resistance or to encourage their use. Prudent and responsible use of all antimicrobial drugs remains crucial in preventing the selection of resistant bacteria.
Acknowledgments
We thank Bayer Pharmaceuticals for providing an unrestricted educational grant that contributed to this work.
REFERENCES
- 1.Bonten, M. J., S. Slaughter, A. W. Ambergen, M. K. Hayden, J. van Voorhis, C. Nathan, and R. A. Weinstein. 1998. The role of “colonization pressure” in the spread of vancomycin-resistant enterococci: an important infection control variable. Arch. Intern. Med. 158:1127-1132. [DOI] [PubMed] [Google Scholar]
- 2.Carmeli, Y., J. Castro, G. M. Eliopoulos, and M. H. Samore. 2001. Clinical isolation and resistance patterns of and superinfection with 10 nosocomial pathogens after treatment with ceftriaxone versus ampicillin-sulbactam. Antimicrob. Agents Chemother. 45:275-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carmeli, Y., M. H. Samore, and C. Huskins. 1999. The association between antecedent vancomycin treatment and hospital-acquired vancomycin-resistant enterococci: a meta-analysis. Arch. Intern. Med. 159:2461-2468. [DOI] [PubMed] [Google Scholar]
- 4.Carratala, J., A. Fernandez-Sevilla, F. Tubau, M. Callis, and F. Gudiol. 1995. Emergence of quinolone-resistant Escherichia coli bacteremia in neutropenic patients with cancer who have received prophylactic norfloxacin. Clin. Infect. Dis. 20:557-560. [DOI] [PubMed] [Google Scholar]
- 5.Cheong, H. J., C. W. Yoo, J. W. Sohn, W. J. Kim, M. J. Kim, and S. C. Park. 2001. Bacteremia due to quinolone-resistant Escherichia coli in a teaching hospital in South Korea. Clin. Infect. Dis. 33:48-53. [DOI] [PubMed] [Google Scholar]
- 6.Ena, J., C. Amador, C. Martinez, and V. Ortiz de la Tabla. 1995. Risk factors for acquisition of urinary tract infections caused by ciprofloxacin resistant Escherichia coli. J. Urol. 153:117-120. [DOI] [PubMed] [Google Scholar]
- 7.Fluit, A. C., M. E. Jones, F. J. Schmitz, J. Acar, R. Gupta, and J. Verhoef. 2000. Antimicrobial susceptibility and frequency of occurrence of clinical blood isolates in Europe from the SENTRY Antimicrobial Surveillance Program, 1997 and 1998. Clin. Infect. Dis. 30:454-460. [DOI] [PubMed] [Google Scholar]
- 8.Garau, J., M. Xercavins, M. Rodriguez-Carballeira, J. R. Gomez-Vera, I. Coll, D. Vidal, T. Llovet, and A. Ruiz-Bremon. 1999. Emergence and dissemination of quinolone-resistant Escherichia coli in the community. Antimicrob. Agents Chemother. 43:2736-2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilbert, D. N., S. J. Kohlhepp, K. A. Slama, G. Grunkemeier, G. Lewis, R. J. Dworkin, S. E. Slaughter, and J. E. Leggett. 2001. Phenotypic resistance of Staphylococcus aureus, selected Enterobacteriaceae, and Pseudomonas aeruginosa after single and multiple in vitro exposures to ciprofloxacin, levofloxacin, and trovafloxacin. Antimicrob. Agents Chemother. 45:883-892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gimber, E. A., M. D. Shields, H. N. Canawati, F. L. Sapico, A. Krishnaswamy, R. El-Farra, K. N. Maeder, and J. Z. Montgomerie. 1998. Bacteriuria with Escherichia coli resistant to ciprofloxacin in patients with spinal-cord injury. Infect. Control Hosp. Epidemiol. 19:85-86. [DOI] [PubMed] [Google Scholar]
- 11.Harbarth, S., S. Cosgrove, and Y. Carmeli. 2002. Effects of antibiotics on nosocomial epidemiology of vancomycin-resistant enterococci. Antimicrob. Agents Chemother. 46:1619-1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harris, A., C. Torres-Viera, L. Venkataraman, P. DeGirolami, M. Samore, and Y. Carmeli. 1999. Epidemiology and clinical outcomes of patients with multiresistant Pseudomonas aeruginosa. Clin. Infect. Dis. 28:1128-1133. [DOI] [PubMed] [Google Scholar]
- 13.Harris, A. D., T. B. Karchmer, Y. Carmeli, and M. H. Samore. 2001. Methodological principles of case-control studies that analyzed risk factors for antibiotic resistance: a systematic review. Clin. Infect. Dis. 32:1055-1061. [DOI] [PubMed] [Google Scholar]
- 14.Harris, A. D., E. Perencevich, M. C. Roghmann, G. Morris, K. S. Kaye, and J. A. Johnson. 2002. Risk factors for piperacillin-tazobactam-resistant Pseudomonas aeruginosa among hospitalized patients. Antimicrob. Agents Chemother. 46:854-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harris, A. D., M. H. Samore, and Y. Carmeli. 2000. Control group selection is an important but neglected issue in studies of antibiotic resistance. Ann. Intern. Med. 133:159. [DOI] [PubMed] [Google Scholar]
- 16.Hooper, D. C. 2001. Emerging mechanisms of fluoroquinolone resistance. Emerg. Infect. Dis. 7:337-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones, R. N., and M. A. Pfaller. 2002. Ciprofloxacin as broad-spectrum empiric therapy—are fluoroquinolones still viable monotherapeutic agents compared with beta-lactams: data from the MYSTIC Program (US)? Diagn. Microbiol. Infect. Dis. 42:213-215. [DOI] [PubMed] [Google Scholar]
- 18.Kaye, K. S., A. D. Harris, H. Gold, and Y. Carmeli. 2000. Risk factors for recovery of ampicillin-sulbactam-resistant Escherichia coli in hospitalized patients. Antimicrob. Agents Chemother. 44:1004-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kern, W. V., E. Andriof, M. Oethinger, P. Kern, J. Hacker, and R. Marre. 1994. Emergence of fluoroquinolone-resistant Escherichia coli at a cancer center. Antimicrob. Agents Chemother. 38:681-687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lautenbach, E., N. O. Fishman, W. B. Bilker, A. Castiglioni, J. P. Metlay, P. H. Edelstein, and B. L. Strom. 2002. Risk factors for fluoroquinolone resistance in nosocomial Escherichia coli and Klebsiella pneumoniae infections. Arch. Intern. Med. 162:2469-2477. [DOI] [PubMed] [Google Scholar]
- 21.Lepelletier, D., N. Caroff, A. Reynaud, and H. Richet. 1999. Escherichia coli: epidemiology and analysis of risk factors for infections caused by resistant strains. Clin. Infect. Dis. 29:548-552. [DOI] [PubMed] [Google Scholar]
- 22.Lipsitch, M., and M. H. Samore. 2002. Antimicrobial use and antimicrobial resistance: a population perspective. Emerg. Infect. Dis. 8:347-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Livermore, D. M., D. James, M. Reacher, C. Graham, T. Nichols, P. Stephens, A. P. Johnson, and R. C. George. 2002. Trends in fluoroquinolone (ciprofloxacin) resistance in enterobacteriaceae from bacteremias, England and Wales, 1990-1999. Emerg. Infect. Dis. 8:473-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mathai, D., R. N. Jones, and M. A. Pfaller. 2001. Epidemiology and frequency of resistance among pathogens causing urinary tract infections in 1,510 hospitalized patients: a report from the SENTRY Antimicrobial Surveillance Program (North America). Diagn. Microbiol. Infect. Dis. 40:129-136. [DOI] [PubMed] [Google Scholar]
- 25.McDonald, L. C., F. J. Chen, H. J. Lo, H. C. Yin, P. L. Lu, C. H. Huang, P. Chen, T. L. Lauderdale, and M. Ho. 2001. Emergence of reduced susceptibility and resistance to fluoroquinolones in Escherichia coli in Taiwan and contributions of distinct selective pressures. Antimicrob. Agents Chemother. 45:3084-3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muder, R. R., C. Brennen, A. M. Goetz, M. M. Wagener, and J. D. Rihs. 1991. Association with prior fluoroquinolone therapy of widespread ciprofloxacin resistance among gram-negative isolates in a Veterans Affairs medical center. Antimicrob. Agents Chemother. 35:256-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neuhauser, M. M., R. A. Weinstein, R. Rydman, L. H. Danziger, G. Karam, and J. P. Quinn. 2003. Antibiotic resistance among gram-negative bacilli in US intensive care units: implications for fluoroquinolone use. JAMA 289:885-888. [DOI] [PubMed] [Google Scholar]
- 28.Ortiz, J., M. C. Vila, G. Soriano, J. Minana, J. Gana, B. Mirelis, M. T. Novella, S. Coll, M. Sabat, M. Andreu, G. Prats, R. Sola, and C. Guarner. 1999. Infections caused by Escherichia coli resistant to norfloxacin in hospitalized cirrhotic patients. Hepatology 29:1064-1069. [DOI] [PubMed] [Google Scholar]
- 29.Paterson, D. L., L. Mulazimoglu, J. M. Casellas, W. C. Ko, H. Goossens, A. Von Gottberg, S. Mohapatra, G. M. Trenholme, K. P. Klugman, J. G. McCormack, and V. L. Yu. 2000. Epidemiology of ciprofloxacin resistance and its relationship to extended-spectrum beta-lactamase production in Klebsiella pneumoniae isolates causing bacteremia. Clin. Infect. Dis. 30:473-478. [DOI] [PubMed] [Google Scholar]
- 30.Pena, C., J. M. Albareda, R. Pallares, M. Pujol, F. Tubau, and J. Ariza. 1995. Relationship between quinolone use and emergence of ciprofloxacin-resistant Escherichia coli in bloodstream infections. Antimicrob. Agents Chemother. 39:520-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Richard, P., M. H. Delangle, D. Merrien, S. Barille, A. Reynaud, C. Minozzi, and H. Richet. 1994. Fluoroquinolone use and fluoroquinolone resistance: is there an association? Clin. Infect. Dis. 19:54-59. [DOI] [PubMed] [Google Scholar]
- 32.Robert, J., E. Cambau, K. Grenet, D. Trystram, Y. Pean, M. H. Fievet, and V. Jarlier. 2001. Trends in quinolone susceptibility of Enterobacteriaceae among inpatients of a large university hospital: 1992-98. Clin. Microbiol. Infect. 7:553-561. [DOI] [PubMed] [Google Scholar]
- 33.Sahm, D. F., I. A. Critchley, L. J. Kelly, J. A. Karlowsky, D. C. Mayfield, C. Thornsberry, Y. R. Mauriz, and J. Kahn. 2001. Evaluation of current activities of fluoroquinolones against gram-negative bacilli using centralized in vitro testing and electronic surveillance. Antimicrob. Agents Chemother. 45:267-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmitz, F. J., J. Verhoef, A. Fluit, et al. 1999. Geographical distribution of quinolone resistance among Staphylococcus aureus, Escherichia coli and Klebsiella spp. isolates from 20 European university hospitals. J. Antimicrob. Chemother. 43:431-434. [DOI] [PubMed] [Google Scholar]
- 35.Smith, J. T. 1986. The mode of action of 4-quinolones and possible mechanisms of resistance. J. Antimicrob. Chemother. 18(Suppl. D):21-29. [DOI] [PubMed] [Google Scholar]
- 36.Talan, D. A. 2001. Clinical perspectives on new antimicrobials: focus on fluoroquinolones. Clin. Infect. Dis. 32(Suppl. 1):S64-S71. [DOI] [PubMed] [Google Scholar]
- 37.Warren, J. W., E. Abrutyn, J. R. Hebel, J. R. Johnson, A. J. Schaeffer, W. E. Stamm, et al. 1999. Guidelines for antimicrobial treatment of uncomplicated acute bacterial cystitis and acute pyelonephritis in women. Clin. Infect. Dis. 29:745-758. [DOI] [PubMed] [Google Scholar]