Abstract
Autophagy, a catabolic process by which a cell “eats” itself, turning over its own cellular constituents, plays a key role in cellular homeostasis. In an effort to maintain normal cellular function, autophagy is often up-regulated in response to environmental stresses and excessive organelle damage to facilitate aggregated protein removal. In the eye, virtually all cell types from those comprising the cornea in the front of the eye to the retinal pigment epithelium (RPE) providing a protective barrier for the retina at the back of the eye, rely on one or more aspects of autophagy to maintain structure and/or normal physiological function. In the lens autophagy plays a critical role in lens fiber cell maturation and the formation of the organelle free zone. Numerous studies delineating the role of Atg5, Vsp34 as well as FYCO1 in maintenance of lens transparency are discussed. Corneal endothelial dystrophies are also characterized as having elevated levels of autophagic proteins. Therefore, novel modulators of autophagy such as lithium and melatonin are proposed as new therapeutic strategies for this group of dystrophies. In addition, we summarize how corneal Herpes Simplex Virus (HSV-1) infection subverts the cornea’s response to infection by inhibiting the normal autophagic response. Using glaucoma models we analyze the relative contribution of autophagy to cell death and cell survival. The cytoprotective role of autophagy is further discussed in an analysis of photoreceptor cell heath and function. We focus our analysis on the current understanding of autophagy in photoreceptor and RPE health, specifically on the diverse role of autophagy in rods and cones as well as its protective role in light induced degeneration. Lastly, in the RPE we highlight hybrid phagocytosis-autophagy pathways. This comprehensive review allows us to speculate on how alterations in various stages of autophagy contribute to glaucoma and retinal degenerations.
Keywords: autophagy, lens, cornea, photoreceptors, retinal pigment epithelium, glaucoma and retinal degeneration
1. Introduction
Autophagy is the major eukaryotic degradative process for cytosolic organelles, long–lived proteins as well as misfolded protein aggregates, playing an important role in development, adaptation, starvation, tumor suppression, and aging as well as innate and adaptive immunity. Although mostly referred to strictly as a degradative process, autophagy is also often described as a renewal process; a pathway by which metabolites generated through autophagic degradation are reused either as sources of energy or building blocks for synthesis of new macromolecules.
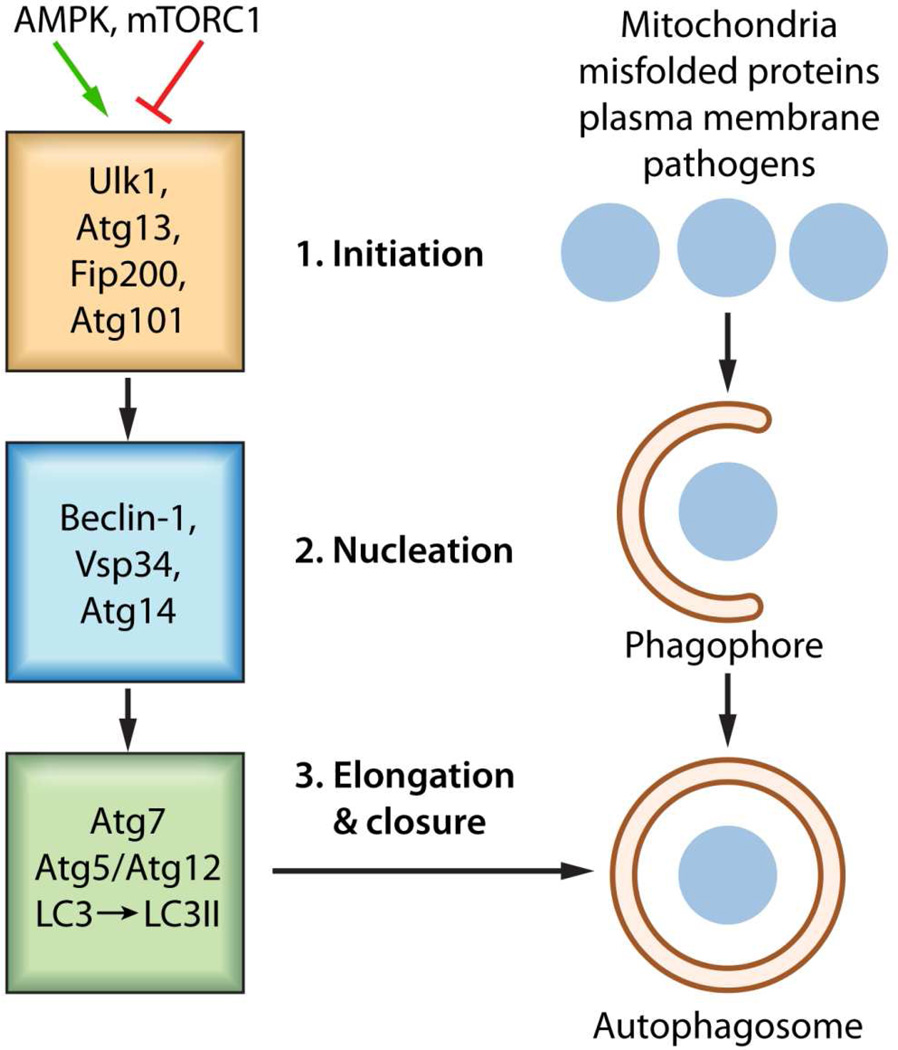
Macro-autophagy hereafter referred to as autophagy is the primary mechanism by which cells ‘self-eat’ delivering sequestered cytosolic cargo to lysosomes for proteolytic degradation by lysosomal proteases (Xie & Klionsky, 2007). Sequestration vesicles, known as autophagosomes, form through a highly regulated pathway requiring over 20 autophagy related proteins (Atg) (Thumm et al, 1994; Tsukada & Ohsumi, 1993). Canonical autophagosome (AP) formation is evolutionarily conserved, requiring initiation (1), nucleation (2), elongation and closure (3), as illustrated in Figure 1, with the key proteins essential at each stage indicated (Mizushima et al, 2011). In addition, there is a recycling stage as well as the final degradative stage. The initiation stage requires a UNC51-like kinase (ULK1) containing complex consisting of the kinase and its substrates, Atg13 and FIP200. ULK1 activity is stimulated by AMP-activated protein kinase (AMPK, in response to increased AMP/ATP ratios) and inhibited by mammalian target of rapamycin complex 1 (mTORC1). Critical in the nucleation stage is a protein complex consisting of BCL-2 interacting myosin/moesin-like coiled-coiled protein (Beclin1), the phosphatidylinositol 3-kinase called Vsp34, and Atg14. During this stage, the Beclin1-Vsp34-Atg14 complex mediated production of PtdIns3P recruits WIPI1 and Atg2 (Walker et al, 2008) in the formation of a nascent autophagosome. Subsequently, autophagosome elongation and closure requires an Atg7 dependent Atg12-Atg5 conjugation system that is responsible for the lipidation, by phosphatidyethanolamine (PE), of microtubule-associated protein 1 light chain 3 (LC3). Lipids are supplied to the expanding autophagosomal membrane via an Atg9 dependent pathway. Closure of the autophagosome ensures complete inclusion of the autophagosome cargo. Ultimately, degradation of this cargo relies upon the autophagosome’s association and fusion with lysosomes in a structure referred to as an autolysosomes. A component of this process, the FYVE coiled-coil domain containing 1 (FYCO1) protein functions as a Rab7 effector to mediate the microtubule dependent transport of autophagic vacuoles (Pankiv et al, 2010). Details of the individual stages can be found in several excellent reviews (Boya et al, 2013; Codogno et al, 2011; Mehrpour et al, 2010).
Figure 1. Basic autophagic processes.
The stages of autophagosome formation are illustrated. Autophagosome formation, an evolutionarily conserved process utilizes an Initiation stage (1), a Nucleation stage (2) as well as an Elongation and Closure stage (3), ultimately the degradation of autophagosomal content proceeds through fusion of autophagosome with lysosome in an autolysosome in a degradation stage. 1. The mTOR complex (shown as mTORC1) represses autophagy when amino acids energy and growth factors are abundant through the inhibition of ULK1 activity. Upon energy deprivation the Initiation Stage commences through the stimulation of Ulk1 kinase activity and phosphorylation of its substrates Atg13 and FIP100. Rapamycin removes the mTOR mediated inhibition of autophagy 2. During the nucleation stage, the generation of PtdIns3P by the Vsp34 allows for the recruitment of additional factors. The functional relationship between the individual components of the initiation stage and nucleation stage is unclear. 3. The expansion and subsequent closure of the autophagosome depends on the Atg5/Atg12 conjugate system which facilitates the lipidation of LC3 to LC3II by phosphatidylethanolamine (PE).
In the eye, virtually all cell types cells from those comprising the cornea in the front of the eye to the retinal pigment epithelium (RPE) providing a protective barrier for the retina at the back of the eye, rely on one or more aspects of autophagy to maintain structure and/or normal physiological function. The essentiality of autophagic processes for healthy vision is best illustrated by a comparison of autophagy related proteins expressed in various cells of the eye. Gene mutations in some of these contribute directly to ocular disease, while in other cases cellular homeostasis relies on regulated interplay between basal and stress induced autophagic pathways. In this review we focus our attention on the current understanding of the role of autophagy in the health and pathology of the lens, cornea, photoreceptor and RPE, and emphasize the proposed role of this degradative pathway in glaucoma and retinal degenerative disease.
2. Autophagy in the Lens
The lens is comprised of a layer of simple cuboidal epithelium at the anterior pole that continues to differentiate at the equatorial region to form fiber cells. Upon maturation, these differentiating fiber cells lose their organelles to produce the organelle free zone (OFZ) thus contributing to lens transparency. Congenital cataracts may result from the improper clearance of proteins and organelles producing lens opacity. Consistent with a role for autophagy in lens organelle degradation, mutations in the FYCO1 gene were identified as a cause of autosomal recessive congenital cataracts (Chen et al, 2011). FYCO1 is a PI(3)P, Rab7 and LC3 binding protein that mediates microtubule plus end-directed vesicle transport of autophagosomes, a process essential in autolysosome formation (Pankiv et al, 2010). FYCO1 was one of 40+ autophagy genes identified and characterized by Brennan et al (2012), in a comprehensive analysis of autophagy gene and protein expression. Autophagy genes associated with all stages of autophagy, including, initiation, autophagosome elongation and closure as well as autolysosomal degradation were found in lens epithelium and fibers. LC3 and FYCO1 were expressed throughout the newborn mouse lens, in both the lens epithelium and fiber cells with highest levels detected in nuclear fibers suggesting that autophagy plays a role in lens fiber cell differentiation (Brennan et al, 2012). These studies also document an elevation of LC3II in the human lens fiber cells measured as an increased number of LC3 positive autophagosomes, under stress conditions. These authors did not directly measure autophagic flux. Consistent with the hypothesis that dysfunctional FYCO1 may contribute to altered autophagy and lens opacity are studies by Chen et al (2011) who document the partial co-localization of FYCO1 with LC3 enriched vesicles, albeit they present no confirmatory immuno-EM analysis.
Costello et al (2013), suggest that autophagy is a key process involved in lens fiber cell maturation and formation of the OFZ. They demonstrate, by EM and confocal microscopy, the presence of autophagic vesicles in embryonic and adult lens (Costello et al, 2013). In these cells, serum starvation results in increased colocalization of mitochondria and autophagy markers, suggesting that the lens cells can respond to environmental stress through the induction of autophagy and mitophagy (the selective removal of mitochondria by autophagy). Collectively, these studies are consistent with the hypothesis that FYCO1 is required for organelle degradation and lens epithelial cell differentiation. FYCO1 is expressed in both the embryonic and adult lens where it localizes to autophagosomes and lysosomes. The loss of FYCO1 may inhibit transport of APs to lysosomes resulting in an accumulation of LC3II positive vesicles and ultimately lead to a loss of lens transparency. Furthermore, they support the idea that loss of lens resistance to stress may result in cataract formation.
In studies focused on events upstream of LC3 lipidation, specifically the role of the Atg5/Atg12 complex in lens organelle degradation, Matsui, et al (2006), observe autophagic vacuoles in lens epithelial cells, however they demonstrate that organelle degradation in lens cells occurs normally in the absence of Atg5, in the Atg5−/− mouse (Matsui et al, 2006). While these studies are important in understanding the role of Atg5 in lens development, they provide little insight into the role of autophagy in long-term lens homeostasis given that the Atg5−/− mice die only ten hours after birth. This lethality problem was overcome in studies using lens specific ablation of Atg5 in Atg5flox/flox; MRL10-Cre mice, Morishita and coworkers (2013), show that Atg5 is required for the suppression of age-related cataracts but has no effect on programmed organelle degradation. Thus the authors posit that Atg5 is correlated with age related cataract likely through compromised intracellular quality control due to the accumulation of p62, oxidized proteins and insoluable crystalins in an autophagy independent manner (Morishita et al, 2013). In the context of these studies it is important to note several recent studies that suggest autophagosome elongation may occur in an Atg5-independent pathway utilizing instead the monomeric rabGTPase, Rab9, for elongation and closure. The resulting noncannonical double membrane autophagosome can serve to degrade and recycle cargo as need during lens development and cell homeostasis (Ao et al, 2014; Codogno et al, 2011).
Vsp34, a class III phosphatidylinositol 3-kinase, is involved in an Atg5-independent autophagy pathway that depends on upstream autophagic events requiring Ulk1 (see Figure 1) (Nishida et al, 2009); Vsp34 also complexes with beclin1. To determine if lens organelle degradation utilized a non-cannonical pathway requiring Vsp34 and independent of Atg5, a lens-specific Vps34 deletion was analyzed. The loss of Vsp34 did not affect lens organelle degradation suggesting cataract formation is not due to disrupted autophagy initiation (Morishita et al, 2013). Loss of Vsp34, however did lead to congenital cataracts and defective lens development.
Connexins are the building blocks of gap junctions; their short half-life suggests that their degradation and renewal is critical in maintaining cell-cell contacts. CX50 is a connexin expressed specifically in the lens, and the CX50 P88S mutant has been shown to be associated with inherited congenital cataracts. Lichtenstein et al (2011) verify that CX50 colocalizes with LC3 and demonstrate that starvation leads to decreased wild-type connexin levels. Inhibition of autophagy by ATG5 knockdown decreases this effect (Lichtenstein et al, 2011). The mutant connexin, CX50 P88S, also accumulates and colocalizes with autophagy markers ultimately being degraded after starvation. These studies suggest that autophagy can regulate both wild-type connexin levels and mutant, CX50 P88S accumulation. Mediation of autophagy could therefore be an appropriate strategy to ameliorate disease pathogenesis. However, as Lichtenstein, et al, (Lichtenstein et al, 2011) suggest autophagic degradation is not the complete story; although lysosomes are involved in starvation-induced autophagy the starvation-induced degradation of WT connexins is only partially dependent on lysosome activity as treatment with chloroquine to alkalinize lysosomes and inactivate degradative lysosomal enzymes does not completely prevent the starvation-induced decrease in connexin levels.
The autophagy related studies reviewed here underscore the need to understand how autophagic degradation and likely even more importantly metabolic and macromolecular constituent renewal contribute to lens development and later to lens homeostasis and transparency. This is especially important given the post mitotic nature of the lens fiber cells which must adapt to a lifetime of stressors. Multiple converging and redundant degradative pathways have been suggested as necessary for long term control of organelle degradation and lens transparency (Wride, 2011). As such autophagy is one of many lysosome mediated clearance mechanisms and emphasis on the interplay between several converging degradative mechanisms using in vivo models when possible will provide valuable insight into how specific disruptions in autophagy contributes to disease. It is important to note that while several studies demonstrate increases in LC3II by Western blot few look at autophagy induction or autophagic flux using conventional autophagy assays employing bafilomycin A1, DQ-BSA or chloroquine for example (Ha et al, 2010; Klionsky et al, 2008a; Klionsky et al, 2008b; Vazquez & Colombo, 2009).
3. Autophagy in the Cornea
Corneal endothelial dystrophies are characterized by the progressive degeneration of corneal endothelium, due in part to genetic predisposition (Schmedt et al, 2012). Granular corneal dystrophy type 2 (GCD2) is caused by a point mutation in the Transforming Growth Factor Beta 1 (TGFβ1) gene resulting in age-dependent accumulation of aggregated proteins in the corneal epithelia and stroma, which interferes with corneal transparency (Choi et al, 2011). The TGFβ1 protein is a critical component of the extracellular matrix (ECM), mediating cell adhesion and migration through integrins. Analysis by Choi et al (2012) reveals that the aggregate prone mutant TGFβ1 protein extensively colocalizes with LC3 enriched vesicles and requires autophagy for its clearance (Choi et al, 2012). Although LC3II was increased in GCD2 corneal fibroblasts, the LC3II levels were unaltered upon treatment with the vacuolar H+-ATPase inhibitor, bafilomycin A1, known to block fusion of autophagosomes with lysosomes. Treatment with the proteasome inhibitor MG132 coupled with ubiquitin immunoblot analysis suggests that TGFβ1 clearance does not involve the ubiquitin/proteasome-dependent pathway allowing the authors to infer that autophagy is the main intracellular degradation system. Consistent with this interpretation, enhancing autophagy with rapamycin to inhibit the negative regulator of autophagy, mTOR, led to decreased levels of mutant TGFβ1 suggesting defective autophagy may contribute to the pathogenesis of GCD2 (Choi et al, 2012).
In addition to rapamycin, lithium also enhances autophagy and is proposed in the treatment of neurodegenerative diseases in which the toxic protein is an autophagy substrate. Lithium induces autophagy in an mTOR independent pathway that likely inhibits inositol monophosphatase thus affecting the autophagy pathway at a point downstream of decreased myo-inositol-1,4,5-triphosphate (IP3) levels (Sarkar et al, 2005). In the presence of lithium there is reduced expression of TGFβ1 protein with a concomitant increase in the ratio of LC3II:I in corneal fibroblasts (Choi et al, 2011). Based on these observations Choi, et al, 2011, suggest that lithium mediated modulation of autophagy could be used in the treatment of GCD2. Data on the autophagic flux in these cells would strengthen the rationale for lithium as a potential therapeutic approach. Modulation of autophagy as a GCD2 therapeutic is further supported in studies by Choi et al (2013) in which they document a melatonin mediated induction of autophagy in an mTOR dependent manner, resulting in increased beclin-1 levels. Melatonin activates autophagy in both wild type and GCD2 fibroblasts, consequently eliminating mutant TGFβ1 in GCD corneal fibroblasts with a combination of rapamycin and melatonin having an additive effect on mutant TGFβ1 protein clearance (Choi et al, 2013b).
Fuchs endothelial corneal dystrophy (FECD), characterized by a decrease in corneal endothelial cell (CEC) density, ECM deposits, and thickening of the Descemet basement membrane of the CEC, has also been linked to alterations in autophagic processes. The primary component of the basement membrane is collagen type VIII, thus it is not surprising that the Col8a2 Q455K knockin mouse exhibits a FECD-like endothelial morphology and is used as a model of early onset disease (Matthaei et al, 2013). Morphological and molecular analysis of the Col8a2 Q455K mouse reveals an increase in damage-regulated autophagy modulator Dram1, a p53 target gene encoding a lysosomal protein that induces macroautophagy (Meng et al, 2013). These studies also document an increase in LC3 with a decrease in Atg12-Atg5 in mutant endothelium compared to age matched controls. Transmission electron microscopy (TEM) identifies large vacuoles containing partially degraded organelles, suggesting altered degradation. The relevance of these findings to human disease is reinforced by the observation that human FECD patients exhibit a 10.4 fold increase in DRAM1 gene expression, although protein levels have not been measured. Although compelling, these human studies are largely correlative and would benefit from an assessment of defects in AP formation at the ultrastructural level in human FECD donor cells, or further analysis of autophagy flux (fusion of the AP with the lysosome, and subsequent protein degradation) in the Col8a2 Q455K knock-in mouse.
Autophagy is also critical for the degradation of invading microorganisms in a process known as xenography (Deretic, 2005; Deretic & Levine, 2009), albeit numerous microorganisms subvert this normal response to persist and survive (Krummenacher et al, 2011). The human cornea is a primary target for herpes simplex infection (HSV-1), which enters endothelial cells through endocytosis (Shah et al, 2010). Entry of herpes simplex into target cells is complex and proceeds by a sequential succession of interactions between 5 viral envelope glycoproteins (designated gB, gC, gD, gH and gL) and receptors on the cell membrane. After attachment to the cell (gC), there is an interaction with a specific entry receptor (gD), followed by internalization of the virion, and membrane fusion (gB, and gH/gL). The viral genome is then delivered to the cell nucleus to initiate viral replication (Eisenberg et al, 2012; Krummenacher et al, 2013). The corneal pathogen HSV-1 actively inhibits autophagy through interactions with the autophagy promoting protein beclin-1. In the host cell, beclin-1 through its association with Vsp34 is necessary in the formation of autophagosomes (Figure 1). Using a murine model of corneal HSV-1 infection, Leib, et al., 2009, demonstrate that the HSV gene product, ICP34.5, antagonizes the normal autophagic response through its interaction with beclin-1 via a beclin binding domain (BBD). These investigators generated two recombinant HSV-1 viruses, recombinant virus Δ68H in which the beclin1 binding domain was deleted and its marker counterpart 68HR. Both Δ68H and Δ68HR replicated at similar rates early in infection however, Δ68H was cleared more rapidly after corneal infection, leading to less ocular disease. The authors propose that enhanced clearance is due to autophagy mediated degradation. Using the same recombinant HSV-1 viruses, Zhang et al. (2013), sought to determine how loss of the beclin binding domain affects viral ocular infection by following the spread of Δ68H and Δ68HR from the eye to the brain after anterior chamber inoculation. Mice injected with Δ68H showed enhanced conversion of LC3 to LCII in both the eye and brain 4–6 days post infection. This elevation in LC3II was interpreted by the authors as enhanced autophagy, though again autophagic flux was not measured. Δ68H infection also led to NLRP3 inflammasome activation, measured as cleavage of caspase 1, leading to an enhanced innate immune response. Both Δ68H and Δ68HR appeared to replicate equivalently in the injected eyes, however, less severe ipsilateral retinal infection was observed with Δ68H than in eyes injected with Δ68HR. Collectively, these studies suggest that autophagy’s role in controlling HSV-1 infection is via a more rapid induction of the innate immune response. Whether inflammasome activation occurs through an autophagy dependent mechanism remains to be determined.
4. Glaucoma and Autophagy
Glaucomas, are a group of eye diseases characterized by the progressive degeneration of the optic nerve and, are the second leading cause of permanent blindness. Primary Open Angle Glaucoma (POAG), the most prevalent form of the disease, is a late onset disorder often associated with an increase in intraocular pressure (IOP), that likely results from failure of the trabecular meshwork (TM) to maintain aqueous humor outflow at an appropriate rate (Athanasiou et al, 2013). Decreased aqueous humor outflow results in increased IOP which can eventually result in damage to the optic nerve head and retinal ganglion cells (RGCs).
The TM lies between the cornea and sclera and consists of collagen and elastin beams surrounded by endothial-like cells. TM cells are post-mitotic, differentiated cells with a low proliferative capacity (Liton et al, 2009). The post-mitotic nature of these cells highlights the critical need to efficiently remove damaged organelles and misfolded proteins to avoid toxicity and degeneration. Cells in the TM are exposed to a considerable level of reactive oxygen species (ROS) resulting in the accumulation of non-degradable material in lysosomes and a decrease in lysosome activity (Liton et al, 2009). Lysosomal alkalinization is accompanied by a decrease in autophagic flux in stressed TM cells (Porter et al, 2013), ultimately, leading to a decline in TM cell function and the progression of POAG.
While autophagy is often associated with cell death, Rodriguez-Muela et al. (2012) suggest that autophagy can promote the survival of RGCs after optic nerve axotomy (Rodriguez-Muela et al, 2012). Optic nerve axotomy is an acute model for RGC death in glaucoma, and axotomy was found to increase autophagic processes at both the mRNA and protein levels, with detection of an increase in GFP-LC3 puncta and double-membrane bound autophagosomes by EM. Axotomy in RGC-specific Atg5 knockout mice results in increased cell death versus the wild-type control, while rapamycin treatment decrease the RGC loss, both suggesting autophagy promotes cell survival in the axotomy model. This result is not unexpected given that axotomy of peripheral neurons and motor neurons can lead to a robust regenerative response without any neuronal death (Rubinsztein et al, 2005).
Mutations in the optineurin (OPTN) gene are associated with normal-tension glaucoma (Rezaie et al, 2002), a disorder where RGCs are lost in a pattern analogous to that in POAG but with the IOP within normal range. E50K is the most common disease causing mutation of optineurin (Chalasani et al, 2007), and overexpression of the optineurin E50K mutation in mice led to a loss of RGCs and other retinal cell types with no change in IOP (Chi et al, 2010). Optineurin was identified as an autophagy receptor involved in the elimination of cytosolic bacteria (Wild et al, 2011). Wild and coworkers identified a LC3-interacting motif (LIR) in optineurin, documenting its localization to LC3-positive structures upon induction of autophagy. Semi-quantitative immunohistochemistry suggested an increase in LC3 levels in the RGCs of mice overexpressing OPTNE50K (Shen et al, 2011). While this study also demonstrated rapamycin was protective in RGC5 cells overexpressing E50K, the unresolved nature of the RGC5 cells, combined with the loss of several retinal cell types in the mouse over expressing E50K, makes the relevance to glaucoma difficult to interpret. Whether autophagy protects RGCs by specific degradation and elimination of accumulated mutant proteins such as OPTN and its binding partners is an important hypothesis, however. In this regard, Tezel et al. (2012) find POAG patient serum samples contain optineurin and huntingtin thought to be due to failure of autophagic proteolytic fragmentation (Tezel et al, 2012a).
In contrast to the protective contribution of autophagy, Park et al. (2012) suggest that activation of autophagy RGCs after chronic elevation of IOP plays a role in cell death (Park et al, 2012). Ultrastructural analysis by EM shows autophagosomes accumulation in dendrites and cytoplasm of RGCs in a chronic hypertensive glaucoma model with a concomitant upregulation of LC3II and Beclin-1. Inhibition of autophagy by 3-methyladenine (3-MA) decreases apoptosis. The authors suggest that autophagy may be compartmentally regulated since it is initially activated in dendrites of the RGC and then in the cytoplasm. They propose that IOP first activates dendritic autophagy in response to stress in the early stages, and later stages leads to a cytoplasmic autophagy resulting in death. However it is important to note that not all TUNEL positive RGCs are autophagic suggesting an alternative mechanism of cell death after IOP elevation. Clearly, the response of autophagic pathways need to be taken into account when assessing the effectiveness of other neuroprotective approaches.
Although RGCs are thought to be the final target in glaucoma, other cell types including glial cells also play a role. Using an IOP rat model Tezel et al. (2012) document astrocyte mediated inflammatory processes in glaucoma (Tezel et al, 2012b). In a global proteomic profile, the authors demonstrate upregulation of mTOR, Atg3, and Atg7 proteins, and a phosphorylation-mediated activation of mTOR, in astrocytes and not RGCs of ocular hypertensive rats. IRGM (immunity-related autophagy) protein expression is also increased. This suggests properly functioning autophagy helps to prevent glial cell death, and aberrant autophagy may contribute to immunogenic neurodegenerative disease. Whether autophagy has parallel roles in astrocytes and RGCs at different time points after IOP elevation is an important issue for future studies.
5. RPE and Photoreceptors
Retinal pigment epithelia serve numerous diverse functions in the maintenance of retinal homeostasis; they transports nutrients and ions between the retina and the choriocapillaris, serve as a protective barrier and prevent photo-oxidative product toxicity through the daily phagocytosis and digestion of photoreceptor outer segment (POS) (Kevany & Palczewski, 2010). This renewal of POSs is critical for the maintenance of photoreceptor structural integrity and function (La Vail, 1976). Retinal degenerative diseases due to both genetic and cumulative environmental factors lead to a pleiotropic series of phenotypes often linked by the accumulation of partially digested phagocytic debris in the form of lipofuscin. The age-related increase in lipofuscin accumulation that occurs in a wide variety of highly metabolically active, post mitotic cells throughout the body is proposed to be the result of autophagy-dependent removal of damaged organelles and their subsequent degradation in autolysosomes. Autophagy is proposed to protect the retina from light-induced degeneration (Chen et al, 2013) with a non-canonical form of autophagy likely involved in maintaining the visual cycle (Kim et al, 2013). Autophagic processes play several different, but mutually complimentary roles in photoreceptor and RPE cells. In photoreceptors, autophagy is involved in maintaining inner segment turnover, photoreceptor survival, and autophagy-dependent apoptosis. Within RPE cells, autophagy is involved in aspects of development, cell survival in response to stress, melanin degradation, as well as the degradation of toxic cellular components/damaged organelles.
5.1 Autophagy in the RPE
Numerous studies in a variety of cell types suggest that autophagy is linked to aging through its role in the degradation and recycling of protein aggregates and damaged organelles (Choi et al, 2013a; Couve et al, 2013; Pyo et al, 2013; Zhu et al, 2013). Like most other cells, the RPE maintains basal autophagy for cellular homeostasis, with autophagic variations common in aging and diseased cells (Kaarniranta et al, 2013; Krohne et al, 2010; Mitter et al, 2012). RPE cells are constantly exposed to environmental stresses including high levels of light as well as internal stresses due in part to their post-mitotic nature. Defects in the ability to protect cells from such damage results in compromised homeostasis, loss of function and eventually cell death. Age-related macular degeneration (AMD) is the leading cause of irreversible blindness in the developed world. AMD is a progressive eye disease that results in loss of central vision and is characterized by abnormalities in RPE cells such as a build-up of debris known as lipofuscin in lysosomes, and thickening of the Bruch’s membrane (BM), caused by drusen deposits. Abnormal autophagy has been cited as an important feature in the pathogenesis of AMD and other blinding retinal diseases due to defective lysosomal-autophagic degradation in the RPE.
The RPE is a post-mitotic phagocyte, therefore optimal degradation of both ingested and intracellular components is essential for cell function. Early studies by Burke and Skumatz (1998), identified auto-fluorescent debris in long-term post confluent cultures of human donor RPE cells (Burke & Skumatz, 1998). Morphometric and spectral studies suggested that the accumulated debris likely derives from two sources, heterophagy of photoreceptor outer segments (POS) as well as defects in constitutive macroautophagy. It is important to note that understanding the relative contribution of each pathway is critical given that numerous studies have shown that RPE cells accumulate autofluorescent granules with many of the features of lipofuscin in the absence of a photoreceptor substrate (Boulton et al, 1989; Burke & Skumatz, 1998; Krohne et al, 2010; Kurz et al, 2009). Thus basal autophagy likely also contributes to lipofuscin–like debris.
Bergmann et al (2004) observed reduced autophagic sequestration of endogenous proteins in cultured RPE cells. The decreased protein degradation was followed using autoradiography in the presence of autophagic inhibitors, further confirming an essential role for this degradative process in RPE health (Bergmann et al, 2004). The aging RPE is also associated with decreased ATP levels, suggested by some to contribute to reduced autophagy and POS uptake (Schutt et al, 2013; Schutt et al, 2012). Drusen in AMD donor eyes contains autophagic proteins and markers of exosomes, which are also found in the Bruch’s membrane (BM) of aged mice (Wang et al, 2009a; Wang et al, 2009b). To understand the molecular relationship between autophagy and exosomes, RPE cultures were treated with rotenone (an inhibitor of mitochondrial complex 1) which did not alter their phagocytic activity but instead resulted in elevated LC3II/LC3 with a concomitant increase in Atg5-Atg12 conjugates. Concurrently, Wang and coworkers observed a decrease in lysosome function and an increase in exocytic activity as well as chemoattractant release. The exosomes released by the stressed RPE were coated with C3 complement suggesting that they are a potential target for complement factor H (CFH), mutations of which have been linked to AMD (Loyet et al, 2012). The authors speculate that in fact autophagy is increased in the aged RPE and exosome release by the aged RPE contributes to drusen formation with the aged RPE/choroid serving to recruit complement factor H. This apparent contradiction reinforces the need to analyze autophagic protein degradation, and not simply autophagy protein levels, to determine if in fact autophagy is enhanced or inhibited in the aged or diseased retina.
Lipid peroxidation products have long been known to affect lipofuscinogenesis (Kopitz et al, 2004). The addition of HNE or MDA-modified POS to RPE cells in vitro not only results in the accumulation of auto-fluorescent lipofuscin–like granules (Krohne et al, 2010) but also contributes to decreased autophagic activity, as measured by turnover of metabolically labeled endogenous proteins in ARPE19 cells. While these studies suggest that lipid peroxidation may contribute to defects in autophagy resulting in accumulation of toxic lipid debris, it would be beneficial to confirm this result in primary RPE cells. Collectively, these studies reinforce the emerging notion that autophagic dysfunction contributes to impaired RPE cell function.
In an in vivo postnatal mitochondrial oxidative phosphorylation ablation study, in which RPE cells lost mitochondrial oxidative phosphorylation capacity, RPE cells where shown to dedifferentiate. In these studies activation of the AKT/mTOR pathway was shown to drive this dedifferentiation phenotype (Zhao et al, 2011a). A similar dedifferentiation profile was observed upon chemical oxidative damage with the enhancement of autophagy by rapamycin serving to inhibit dedifferentiation thereby aiding in the preservation of photoreceptors. These results reveal an in vivo response of the mature RPE to diverse stressors that utilizes autophagy to prolong RPE cell survival. The authors conclude that mTOR pathway inhibition may be a potential therapeutic strategy for retinal degenerative diseases involving RPE stress. Consistent with this notion, Zhao (2011) document age-related retinopathy in the NRF-2 deficient mouse; NRF-2 is a transcription factor with a role in retinal antioxidant and detox response. They suggest dysregulated autophagy as a likely mechanistic link between oxidative injury and inflammation proposing that the Nrf2−/− mouse is a useful model for AMD mechanistic research (Zhao et al, 2011b).
Photo-stress in the form of UVB irradiation also leads to increased LC3II levels in a dose-dependent manner suggesting an induced autophagic response (Li et al, 2013). Interestingly these studies also suggest that epigallocatechin-gallate (EGCG), a polyphenol found in green tea reduces LC3II and autophagosome number thereby repressing autophagy. Rapamycin inhibits this effect suggesting that an Ulk1 dependent, mTOR mediated pathway is involved. The authors suggest EGCG could be used as a potential therapeutic agent for the treatment of eye diseases associated with abnormal autophagy. It would perhaps be of interest to confirm this result in primary RPE cells and not simply ARPE-19 cells, an RPE cell model known to potentially contain differentiation defects (Ahmado et al, 2011)
Within the RPE, melanosomes provide protection against photo-oxidative stress (Peters & Schraermeyer, 2001; Pilat et al, 2013; Sarangarajan & Apte, 2005; Sarna et al, 2003; Schraermeyer & Heimann, 1999; Sundelin et al, 2001), with the accumulation of lipofuscin in melano-lysosomes over time. Using the rhesus monkeys, a species shown to have numerous AMD-like phenotypes, Gouras, et al (2011), show that melanosomes decrease with age and smaller melanosomes become more common on the basolateral side of the RPE. Among these small melanosomes they identify an organelle in various stages of losing melanin and in some cases nearly devoid of melanin. They suggest this unique type of organelle, designated the ‘type 1 lysosome’, is an autophagic melano-lysosome due to the self-degradation of melanin. In contrast the ‘type 2 lysosome’ is rarely found without melanin (Gouras et al, 2011). The authors report that the levels of each type of lysosome differentially change with age, with the type 1 lysosome decreasing and type 2 lysosomes building up with age, suggesting the lysosomal and autophagic pathways are involved in melanin decline and the pathogenesis of AMD. These seminal studies are largely based on EM and organelle quantitation and would be strengthened with reliable biochemical analysis.
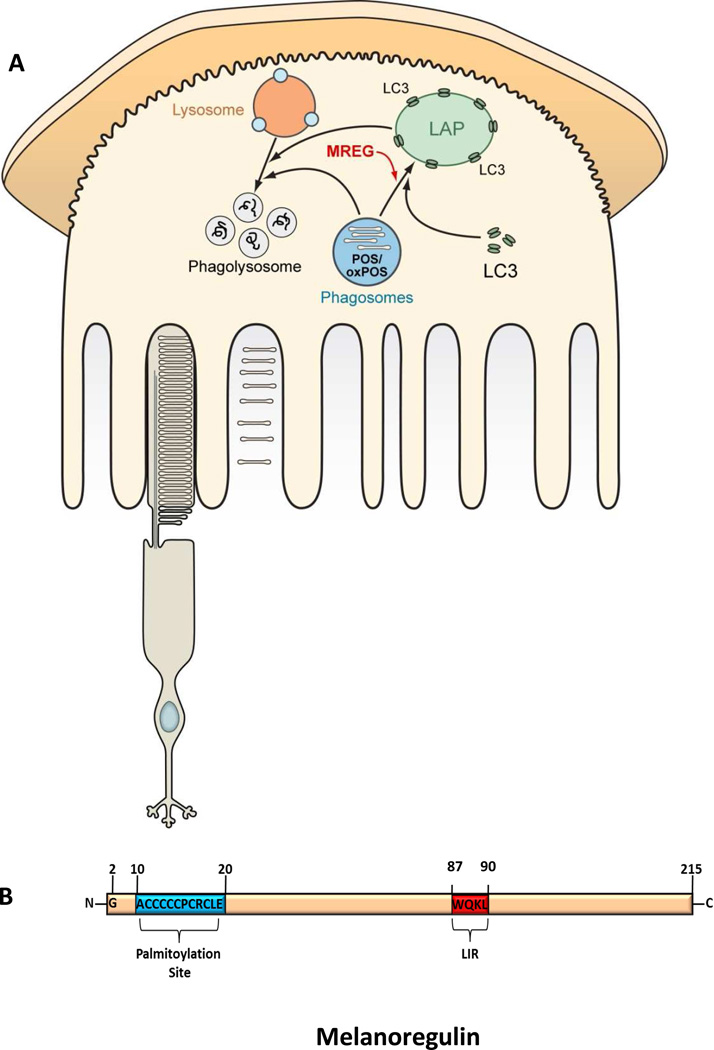
Phagocytic cells have also evolved a novel hybrid degradative pathway utilizing aspect of two highly conserved digestion processes, phagocytosis and autophagy. The capacity of phagocytic cells to breakdown ingested material is predicted to be increased through this hybrid process known as microtubule-associated protein 1 light chain 3 (LC3) associated phagocytosis (LAP) in which LC3 is recruited to phagosomes (Florey et al, 2011; Florey & Overholtzer, 2012; Henault et al, 2012; Martinez et al, 2011; Sanjuan et al, 2007; Sanjuan et al, 2009). LC3 recruitment to maturing phagosomes occurs in an Atg5 dependent, Ulk1 independent manner. Kim et al, 2013 (Kim et al, 2013) looked at the role of autophagy in POS degradation suggesting that the circadian burst of RPE phagocytosis coincides with LC3 lipidation. They report that Atg5 and Beclin1 are involved in association of RFP-LC3 with POS but not the autophagy initiation complex containing Ulk1. We propose that the extent of LC3 association with phagosomes is regulated by the intracellular sorting protein melanoregulin (MREG) (Damek-Poprawa et al, 2009) as illustrated in Figure 2A, through an LC3 interacting region LIR (Popovica et al, 2012) (see Figure 2B). We have previously shown that loss of MREG leads to the accumulation of phagosomes and an abundance of the toxic photoproduct, A2E in older mice (Damek-Poprawa et al, 2009). Given that the majority of phagosomes do not associate with endogenous LC3, LAP may in a manner analogous to macrophages, be required to clear debris during stress conditions or as the RPE ages (Sanjuan et al, 2007; Sanjuan et al, 2009). Because LC3 association with phagosomes is independent of autophagosome formation, additional studies are required to understand the relative contribution of LAP to RPE mediated phagocytic processing of ingested OS under normal and disease states, as well as the balance between autophagic clearance of cytoplasmic material and phagocyte maturation. Both autophagy and phagocytosis are lysosome-mediated waste clearance pathways, in which acidification of the endo-lysosomal system is modulated by the V-ATPase proton pump (Oczypok et al, 2013; Valapala et al, 2014). Recent studies by Valapala, et al, 2104, show that CRYBA1/βA3/A1 crystalin is not only an RPE lysosome associated protein but that it also modulates V-ATPase and ultimately the terminal degradation phase of autophagy (Valapala et al, 2014). Thus not only are autophagy and phagocytosis linked at the level of LC3 but at the degradation phase through sorting of V-ATPase.
Figure 2. LC3 associated phagocytosis.
A. Schematic representation of the proposed role of LC3 associated phagocytosis and MREG in the degradation of ingested POS as well as oxidized POS. B. Schematic of the intracellular sorting protein, melanoregulin (MREG), the C-terminal palmitoylation site is indicated in blue (Wu et al, 2012) and the LC3 interacting region (LIR), residues 87 to 90 in red (Popovica et al, 2012).
5.2 Autophagy in Photoreceptors
The presence of misfolded proteins has been linked to numerous diseases, including cystic fibrosis, as well as neurodegenerative diseases such as Huntingtons and Parkinson’s Disease (Bross et al, 1999; Gilbert et al, 1998; Goldberg, 2003; Gregersen, 2006). The accumulation of misfolded or aggregated proteins is also associated with retinopathies including, Retinitis Pigmentosa, Best vitelliform macular dystrophy, and Stargardt’s disease (Allikmets et al, 1997; Burgess et al, 2008; Kaushal, 2006).
Early studies by Reme and coworkers suggested that rhodopsin-containing discs are removed by an autophagy-like process in response to an increase in light intensity (Reme et al, 1984; Reme et al, 1999). This maintains steady state levels of wild-type rhodopsin in the photoreceptor (PR) and may therefore be important in preventing retinal pathologies. Consistent with the work of Reme and coworkers, Iwasaki and Inomata (1988) report that lipofuscin granules are found in the myoids of inner segments with vacuoles related to autophagy found in the myoids of rods and cones (Iwasaki & Inomata, 1988). They speculate that lipofuscin granules are an accumulation of residual bodies of autophagy in photoreceptor inner segments.
In a detailed molecular analyses of the role of autophagic processes in photoreceptor degeneration, rapamycin was shown to decreases the level of mutant P23H opsin, associated with Retinitis Pigmentosa (RP), with the autophagic proteins, Atg7, LC3 and LAMP-1 shown to colocalize with P23H opsin (Kaushal, 2006). Based on these findings Kaushal et al (2006) suggest that rapamycin (as an autophagy enhancer) may be useful in clearing misfolded proteins associated with retinal degeneration. Consistent with a protective role for autophagy against PR cell death Besirli et al (2011) suggest that autophagy activation in the injured PR, inhibits Fas-mediated apoptosis (Besirli et al, 2011). Here, Retina-RPE separation results in an increase in Atg5 and LC3 as well as the lysosomal enzymes, Cathepsin-B and Cathepsin-D. Inhibition of autophagy through treatment with 3-methyladenine (3-MA) or through ATG5 knockdown accelerated caspase 8 activation and photoreceptor TUNEL suggesting autophagy plays a protective role against photoreceptor demise in response to injury.
In contrast, Kunchithapautham and Rohrer (2007) demonstrate that apoptotic and autophagic genes may be co-expressed in PRs undergoing degeneration (Kunchithapautham & Rohrer, 2007). They suggest that autophagy participates in photoreceptor cell death possibly by initiating apoptosis. These studies open the door to the identification of upstream regulators of autophagy as potential therapeutic targets in PR degeneration. Consistent with this, Lohr et al (2006) report caspase and non-caspase dependent mechanisms contribute to PR cell death with apoptosis, autophagy and complement mediated lysis all playing a role (Lohr et al, 2006). Giansanti et al (2013) reconcile these seemingly inconsistent viewpoints and show that autophagy may play an initial survival role, but eventually becomes impaired (as indicated by p62 accumulation) resulting in cell death (Giansanti et al, 2013). The work of Cho and coworkers supports this idea; Cho et al (2012) find that treatment of RPE-derived, ARPE19 cells and photoreceptor-derived, 661W cells, with tamoxifen results in an increase in LC3II and autophagic flux within 1h. Death occurs by 18h suggesting that autophagy plays an initial protective role in these cells, however the autophagic process becomes impaired over time leading to eventual cell demise (Cho et al, 2012).
Autophagy may also play diverse roles in rod and cone photoreceptors with the addition of rapamycin having differing effects on rods and cones in an albino mouse light damage model (Kunchithapautham et al, 2011). Rapamycin treatment improves rod photoreceptor survival and function, reduces apoptosis and normalizes cytokine levels that were elevated by light. However, Kunchithapautham and coworkers report that autophagic vacuole (AV) formation is seen exclusively in cones with concomitant reduction in cone photoreceptor function. This suggests the rapamycin acts differentially in stressed PRs. The authors speculate that AV-dependent autophagy may require photon capture in cone PRs, which is consistent with the work of Reme et al (1999) who demonstrate that AVs are generated in response to intense light and may be involved in shortening POSs to reduce photon capture. Kunchithapautham et al (2011) also suggest that autophagy may play a role in apoptosis either by delaying it, or by initiating it, consistent with previous studies.
In a recent series of studies, Chen et al, 2013 sought to determine if autophagy serves a protective role in light induced degeneration in rod cells (Chen et al, 2013). Using the Abca4−/− Rdh8−/− mouse, which exhibits delayed clearance of all trans retinal they demonstrate increased expression of LC3II as well as the mitophagy specific regulator Park2 in response to light. They go on to directly address the role of autophagy, specifically at the induction stage in light induced cell death using a beclin 1+/− mouse (the beclin-1−/− is embryonic lethal) as well as a rod specific Atg7 knockout mouse. Both Atg7 and beclin1 are necessary in the induction phase of autophagosome formation. Rod cell morphology in the beclin 1+/− mouse was normal under room light however under intense light these mice developed severe retinal degeneration; whereas the rod specific decrease in Atg7 resulted in retinal degeneration even under normal light. These studies led these authors to suggest that under relatively mild stress autophagy is activated to cope with this cellular stress whereas impaired autophagy is associated with increased stress ultimately contributing to cell death. Cleary the molecular mechanisms contributing to impaired autophagy and cell death need to be elucidated (Marino et al, 2014). The studies by Chen, et al, (2013) are even more provocative when coupled with the work of Kim, et al, (2013), who show that a non cannonical form of autophagy is utilized by RPE cells in POS phagocytosis to maintain the visual cycle (Kim et al, 2013). These two studies underscore the need to address the role of autophagy in vivo when possible given the complexity of photoreceptor-RPE interactions necessary for POS renewal and visual pigment recycling.
6. Summary
Although often labeled as a degradative process, autophagy can be considered a recycling pathway; it is in this context that we best understand its cytoprotective role and contribution to cell homoeostasis. Metabolites generated through autophagic processes are used for energy or reused as building blocks in the synthesis of new macromolecules. In an analysis of autophagic pathways in the maintenance of cell health some common themes emerge including the role of autophagy in post-mitotic cells the interplay between autophagy and apoptosis two process that both focus on “self-consumption” and in the case of photoreceptors the recycling of visual pigments.
The post-mitotic nature of the lens fiber cells, RGCs, photoreceptor cells, and the RPE cells puts them at an elevated risk for demise through a defective autophagic pathway. As with other post mitotic cells residual material resulting from impaired clearance slowly accumulates within and around these cells throughout life. Lens fibers cells maintain transparency by preventing the build-up of cellular waste and misfolded proteins through autophagy. The RPE maintains normal morphology and function through regulated basal autophagy as well as newly described role of autophagy in phagocytosis (Kim et al, 2013). Autophagy is a key pathway by which these cells sequester toxic waste and target it for degradation by lysosomes. Defects in any stage of autophagosome formation (reviewed in Figure 1), autophagic vacuole trafficking or association and fusion with lysosomes will disrupt this “cleaning” function thereby contributing to the buildup of toxic debris. Such debris is often in the form of Pick or Lewey bodies. It is likely that mutations in autophagy genes could be a contributing factor to susceptibility to cataracts or AMD.
Autophagy is thought to play a dual role in cell survival and cell death (Chaabane et al, 2013; Eskelinen, 2005; Levine & Yuan, 2005; Subramani & Malhotra, 2013; Yu et al, 2004). Photoreceptor death is proposed to occur mainly through apoptotic mechanisms. Consistent with this, models of retinal degenerations show the caspase family of cysteine proteases are elevated (Murakami et al, 2013; Zacks et al, 2003; Zacks et al, 2004). However, recent evidence suggests non-apoptotic forms of cell death such as autophagy and necrosis may play a role (for review see (Murakami et al, 2013)). In both photoreceptors and RGCs, it appears that the interplay between autophagy and apoptosis is critical for cell health. The interrelationship between these two processes both considered by some as mechanisms of programmed cell death is complex (Marino et al, 2014). One commonality between RGCs and photoreceptor cells is that autophagy and apoptosis often occur in the same cell, with autophagy preceding apoptosis. This sequence of events is likely due to the fact that stress will stimulate the autophagic response up until a point when that level of stress, whether due to time or dose, becomes lethal. These cells appear to utilize autophagy as a cytoprotective mechanism unless or until a critical stress threshold or duration is exceeded at which point apoptotic mechanism are activated. Once activated apoptosis inactivates autophagy in part due to the inactivation of autophagy proteins by caspase cleavage. Thus it is critical both in advancing our understanding of glaucoma as well as AMD that we define those factors that regulate that critical threshold; we determine how genetic predisposition alters the precarious balance between autophagy and apoptosis and how metabolism regulates this interrelationship.
Finally, from a technical standpoint, numerous autophagy studies would benefit from a more unified approach designed to accurately assess autophagic flux. For example, it is more appropriate to measure GFP-LC3 cleavage in the presence or absence of chloroquine or bafilomycin A1 together with other autophagy markers, such as p62 and endogenous LC3-II because the amount of free GFP does not correlate with autophagic flux if the lysosomal activity and pH are changed (Ni et al, 2011) as is the case for example in the Abcr4−/− mouse model of Stargardts disease (Liu et al, 2008). Furthermore, immunblot quantitation of LC3 and LC3II are not directly indicative of autophagic flux (Wang et al, 2009a). Ideally the use of in vivo models is preferred with the analysis of endogenous levels of autophagy proteins.
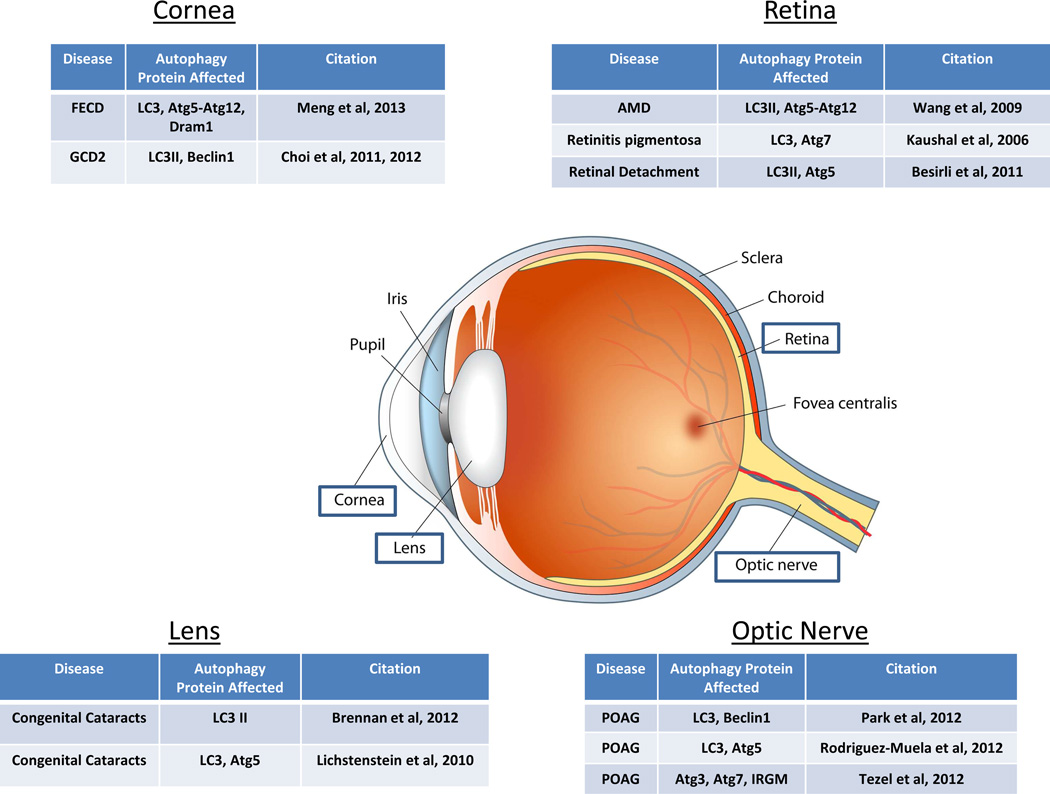
Figure 3. Alterations in autophagy in various regions of the eye.
Schematic of the anatomy of the eye with Tables displaying autophagy proteins affected in various regions, some leading to ocular disease.
Highlights.
Overview of autophagic processes in the eye.
Emphasis on current studies of this degradative pathway in maintaining normal structure and function of the lens, cornea, photoreceptors and retinal pigment epithelium.
Role of autophagy in retinal degeneration and glaucoma.
Acknowledgments
EY-010420 (KBB), EY-015537 and EY-013434 (CHM), and P30EY001583 (KBB & CHM)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmado A, Carr AJ, Vugler AA, Semo M, Gias C, Lawrence JM, Chen LL, Chen FK, Turowski P, da Cruz L, Coffey PJ. Induction of differentiation by pyruvate and DMEM in the human retinal pigment epithelium cell line ARPE-19. Invest Ophthalmol Vis Sci. 2011;52:7148–7159. doi: 10.1167/iovs.10-6374. [DOI] [PubMed] [Google Scholar]
- Allikmets R, Singh N, Sun H, Shroyer NF, Hutchinson A, Chidambaram A, Gerrard B, Baird L, Stauffer D, Peiffer A, Rattner A, Smallwood P, Li Y, Anderson KL, Lewis RA, Nathans J, Leppert M, Dean M, Lupski JR. A photoreceptor cell-specific ATP-binding transporter gene (ABCR) is mutated in recessive Stargardt macular dystrophy. Nature genetics. 1997;15:236–246. doi: 10.1038/ng0397-236. [DOI] [PubMed] [Google Scholar]
- Ao X, Zou L, Wu Y. Regulation of autophagy by the Rab GTPase network. Cell death and differentiation. 2014;21:348–358. doi: 10.1038/cdd.2013.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athanasiou D, Aguila M, Bevilacqua D, Novoselov SS, Parfitt DA, Cheetham ME. The cell stress machinery and retinal degeneration. FEBS letters. 2013;587:2008–2017. doi: 10.1016/j.febslet.2013.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann M, Schutt F, Holz FG, Kopitz J. Inhibition of the ATP-driven proton pump in RPE lysosomes by the major lipofuscin fluorophore A2-E may contribute to the pathogenesis of age-related macular degeneration. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2004;18:562–564. doi: 10.1096/fj.03-0289fje. [DOI] [PubMed] [Google Scholar]
- Besirli CG, Chinskey ND, Zheng QD, Zacks DN. Autophagy activation in the injured photoreceptor inhibits fas-mediated apoptosis. Investigative ophthalmology & visual science. 2011;52:4193–4199. doi: 10.1167/iovs.10-7090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulton M, McKechnie NM, Breda J, Bayly M, Marshall J. The formation of autofluorescent granules in cultured human RPE. Investigative ophthalmology & visual science. 1989;30:82–89. [PubMed] [Google Scholar]
- Boya P, Reggiori F, Codogno P. Emerging regulation and functions of autophagy. Nat Cell Biol. 2013;7:713–720. doi: 10.1038/ncb2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan LA, Kantorow WL, Chauss D, McGreal R, He S, Mattucci L, Wei J, Riazuddin SA, Cvekl A, Hejtmancik JF, Kantorow M. Spatial expression patterns of autophagy genes in the eye lens and induction of autophagy in lens cells. Molecular vision. 2012;18:1773–1786. [PMC free article] [PubMed] [Google Scholar]
- Bross P, Corydon TJ, Andresen BS, Jorgensen MM, Bolund L, Gregersen N. Protein misfolding and degradation in genetic diseases. Human mutation. 1999;14:186–198. doi: 10.1002/(SICI)1098-1004(1999)14:3<186::AID-HUMU2>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Burgess R, Millar ID, Leroy BP, Urquhart JE, Fearon IM, De Baere E, Brown PD, Robson AG, Wright GA, Kestelyn P, Holder GE, Webster AR, Manson FD, Black GC. Biallelic mutation of BEST1 causes a distinct retinopathy in humans. American journal of human genetics. 2008;82:19–31. doi: 10.1016/j.ajhg.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke JM, Skumatz CM. Autofluorescent inclusions in long-term postconfluent cultures of retinal pigment epithelium. Investigative ophthalmology & visual science. 1998;39:1478–1486. [PubMed] [Google Scholar]
- Chaabane W, User SD, El-Gazzah M, Jaksik R, Sajjadi E, Rzeszowska-Wolny J, Los MJ. Autophagy, apoptosis, mitoptosis and necrosis: interdependence between those pathways and effects on cancer. Archivum immunologiae et therapiae experimentalis. 2013;61:43–58. doi: 10.1007/s00005-012-0205-y. [DOI] [PubMed] [Google Scholar]
- Chalasani ML, Radha V, Gupta V, Agarwal N, Balasubramanian D, Swarup G. A glaucoma-associated mutant of optineurin selectively induces death of retinal ganglion cells which is inhibited by antioxidants. Investigative ophthalmology & visual science. 2007;48:1607–1614. doi: 10.1167/iovs.06-0834. [DOI] [PubMed] [Google Scholar]
- Chen J, Ma Z, Jiao X, Fariss R, Kantorow WL, Kantorow M, Pras E, Frydman M, Pras E, Riazuddin S, Riazuddin SA, Hejtmancik JF. Mutations in FYCO1 cause autosomal-recessive congenital cataracts. American journal of human genetics. 2011;88:827–838. doi: 10.1016/j.ajhg.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Sawada O, Kohno H, Le YZ, Subauste C, Maeda T, Maeda A. Autophagy protects the retina from light-induced degeneration. J Biol Chem. 2013;288:7506–7518. doi: 10.1074/jbc.M112.439935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi ZL, Akahori M, Obazawa M, Minami M, Noda T, Nakaya N, Tomarev S, Kawase K, Yamamoto T, Noda S, Sasaoka M, Shimazaki A, Takada Y, Iwata T. Overexpression of optineurin E50K disrupts Rab8 interaction and leads to a progressive retinal degeneration in mice. Human molecular genetics. 2010;19:2606–2615. doi: 10.1093/hmg/ddq146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho KS, Yoon YH, Choi JA, Lee SJ, Koh JY. Induction of autophagy and cell death by tamoxifen in cultured retinal pigment epithelial and photoreceptor cells. Investigative ophthalmology & visual science. 2012;53:5344–5353. doi: 10.1167/iovs.12-9827. [DOI] [PubMed] [Google Scholar]
- Choi AM, Ryter SW, Levine B. Autophagy in human health and disease. The New England journal of medicine. 2013a;368:1845–1846. doi: 10.1056/NEJMc1303158. [DOI] [PubMed] [Google Scholar]
- Choi SI, Kim BY, Dadakhujaev S, Jester JV, Ryu H, Kim TI, Kim EK. Inhibition of TGFBIp expression by lithium: implications for TGFBI-linked corneal dystrophy therapy. Investigative ophthalmology & visual science. 2011;52:3293–3300. doi: 10.1167/iovs.10-6405. [DOI] [PubMed] [Google Scholar]
- Choi SI, Kim BY, Dadakhujaev S, Oh JY, Kim TI, Kim JY, Kim EK. Impaired autophagy and delayed autophagic clearance of transforming growth factor beta-induced protein (TGFBI) in granular corneal dystrophy type 2. Autophagy. 2012;8:1782–1797. doi: 10.4161/auto.22067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SI, Kim KS, Oh JY, Jin JY, Lee GH, Kim EK. Melatonin induces autophagy via an mTOR-dependent pathway and enhances clearance of mutant-TGFBIp. Journal of pineal research. 2013b;54:361–372. doi: 10.1111/jpi.12039. [DOI] [PubMed] [Google Scholar]
- Codogno P, Mehrpour M, Proikas-Cezanne T. Canonical and noncanonical autophagy: variations on a common theme of self-eating? Nat Rev Mol Cell. 2011;13:7–12. doi: 10.1038/nrm3249. [DOI] [PubMed] [Google Scholar]
- Costello MJ, Brennan LA, Basu S, Chauss D, Mohamed A, Gilliland KO, Johnsen S, Menko AS, Kantorow M. Autophagy and mitophagy participate in ocular lens organelle degradation. Experimental eye research. 2013;116:141–150. doi: 10.1016/j.exer.2013.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couve E, Osorio R, Schmachtenberg O. The amazing odontoblast: activity, autophagy, and aging. Journal of dental research. 2013;92:765–772. doi: 10.1177/0022034513495874. [DOI] [PubMed] [Google Scholar]
- Damek-Poprawa M, Diemer T, Lopes VS, Lillo C, Harper DC, Marks MS, Wu Y, Sparrow JR, Rachel RA, Williams DS, Boesze-Battaglia K. Melanoregulin (MREG) modulates lysosome function in pigment epithelial cells. J Biol Chem. 2009;284:10877–10889. doi: 10.1074/jbc.M808857200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deretic V. Autophagy in innate and adaptive immunity. Trends Immunol. 2005;26:523–528. doi: 10.1016/j.it.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Deretic V, Levine B. Autophagy, immunity, and microbial adaptations. Cell Host Microbe. 2009;5:527–549. doi: 10.1016/j.chom.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg RJ, Atanasiu D, Cairns TM, Gallagher JR, Krummenacher C, Cohen GH. Herpes virus fusion and entry: a story with many characters. Viruses. 2012;4:800–832. doi: 10.3390/v4050800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskelinen EL. Doctor Jekyll and Mister Hyde: autophagy can promote both cell survival and cell death. Cell death and differentiation. 2005;12(Suppl 2):1468–1472. doi: 10.1038/sj.cdd.4401721. [DOI] [PubMed] [Google Scholar]
- Florey O, Kim SE, Sandoval CP, Haynes CM, Overholtzer M. Autophagy machinery mediates macroendocytic processing and entotic cell death by targeting single membranes. Nat Cell Biol. 2011;13:1335–1343. doi: 10.1038/ncb2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florey O, Overholtzer M. Autophagy proteins in macroendocytic engulfment. Trends in cell biology. 2012;22:374–380. doi: 10.1016/j.tcb.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giansanti V, Rodriguez GE, Savoldelli M, Gioia R, Forlino A, Mazzini G, Pennati M, Zaffaroni N, Scovassi AI, Torriglia A. Characterization of stress response in human retinal epithelial cells. Journal of cellular and molecular medicine. 2013;17:103–115. doi: 10.1111/j.1582-4934.2012.01652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert A, Jadot M, Leontieva E, Wattiaux-De Coninck S, Wattiaux R. Delta F508 CFTR localizes in the endoplasmic reticulum-Golgi intermediate compartment in cystic fibrosis cells. Experimental cell research. 1998;242:144–152. doi: 10.1006/excr.1998.4101. [DOI] [PubMed] [Google Scholar]
- Goldberg AL. Protein degradation and protection against misfolded or damaged proteins. Nature. 2003;426:895–899. doi: 10.1038/nature02263. [DOI] [PubMed] [Google Scholar]
- Gouras P, Brown K, Ivert L, Neuringer M. A novel melano-lysosome in the retinal epithelium of rhesus monkeys. Experimental eye research. 2011;93:937–946. doi: 10.1016/j.exer.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregersen N. Protein misfolding disorders: pathogenesis and intervention. Journal of inherited metabolic disease. 2006;29:456–470. doi: 10.1007/s10545-006-0301-4. [DOI] [PubMed] [Google Scholar]
- Ha SD, Ham B, Mogridge J, Saftig P, Lin S, Kim SO. Cathepsin B-mediated autophagy flux facilitates the anthrax toxin receptor 2-mediated delivery of anthrax lethal factor into the cytoplasm. The Journal of biological chemistry. 2010;285:2120–2129. doi: 10.1074/jbc.M109.065813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henault J, Martinez J, Riggs JM, Tian J, Mehta P, Clarke L, Sasai M, Latz E, Brinkmann MM, Iwasaki A, Coyle AJ, Kolbeck R, Green DR, Sanjuan MA. Noncanonical autophagy is required for type I interferon secretion in response to DNA-immune complexes. Immunity. 2012;37:986–997. doi: 10.1016/j.immuni.2012.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki M, Inomata H. Lipofuscin granules in human photoreceptor cells. Investigative ophthalmology & visual science. 1988;29:671–679. [PubMed] [Google Scholar]
- Kaarniranta K, Sinha D, Blasiak J, Kauppinen A, Vereb Z, Salminen A, Boulton ME, Petrovski G. Autophagy and heterophagy dysregulation leads to retinal pigment epithelium dysfunction and development of age-related macular degeneration. Autophagy. 2013;9:973–984. doi: 10.4161/auto.24546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushal S. Effect of rapamycin on the fate of P23H opsin associated with retinitis pigmentosa (an American Ophthalmological Society thesis) Transactions of the American Ophthalmological Society. 2006;104:517–529. [PMC free article] [PubMed] [Google Scholar]
- Kevany BM, Palczewski K. Phagocytosis of Retinal Rod and Cone Photoreceptors. Physiology. 2010;25:8–15. doi: 10.1152/physiol.00038.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JY, Zhao H, Martinez J, Doggett TA, Kolesnikov AV, Tang PH, Ablonczy Z, Chan CC, Zhou Z, Green DR, Ferguson TA. Noncanonical autophagy promotes the visual cycle. Cell. 2013;154:365–376. doi: 10.1016/j.cell.2013.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klionsky DJ, Abeliovich H, Agostinis P, Agrawal DK, Aliev G, Askew DS, Baba M, Baehrecke EH, Bahr BA, Ballabio A, Bamber BA, Bassham DC, Bergamini E, Bi X, Biard-Piechaczyk M, Blum JS, Bredesen DE, Brodsky JL, Brumell JH, Brunk UT, Bursch W, Camougrand N, Cebollero E, Cecconi F, Chen Y, Chin LS, Choi A, Chu CT, Chung J, Clarke PG, Clark RS, Clarke SG, Clave C, Cleveland JL, Codogno P, Colombo MI, Coto-Montes A, Cregg JM, Cuervo AM, Debnath J, Demarchi F, Dennis PB, Dennis PA, Deretic V, Devenish RJ, Di Sano F, Dice JF, Difiglia M, Dinesh-Kumar S, Distelhorst CW, Djavaheri-Mergny M, Dorsey FC, Droge W, Dron M, Dunn WA, Jr., Duszenko M, Eissa NT, Elazar Z, Esclatine A, Eskelinen EL, Fesus L, Finley KD, Fuentes JM, Fueyo J, Fujisaki K, Galliot B, Gao FB, Gewirtz DA, Gibson SB, Gohla A, Goldberg AL, Gonzalez R, Gonzalez-Estevez C, Gorski S, Gottlieb RA, Haussinger D, He YW, Heidenreich K, Hill JA, Hoyer-Hansen M, Hu X, Huang WP, Iwasaki A, Jaattela M, Jackson WT, Jiang X, Jin S, Johansen T, Jung JU, Kadowaki M, Kang C, Kelekar A, Kessel DH, Kiel JA, Kim HP, Kimchi A, Kinsella TJ, Kiselyov K, Kitamoto K, Knecht E, Komatsu M, Kominami E, Kondo S, Kovacs AL, Kroemer G, Kuan CY, Kumar R, Kundu M, Landry J, Laporte M, Le W, Lei HY, Lenardo MJ, Levine B, Lieberman A, Lim KL, Lin FC, Liou W, Liu LF, Lopez-Berestein G, Lopez-Otin C, Lu B, Macleod KF, Malorni W, Martinet W, Matsuoka K, Mautner J, Meijer AJ, Melendez A, Michels P, Miotto G, Mistiaen WP, Mizushima N, Mograbi B, Monastyrska I, Moore MN, Moreira PI, Moriyasu Y, Motyl T, Munz C, Murphy LO, Naqvi NI, Neufeld TP, Nishino I, Nixon RA, Noda T, Nurnberg B, Ogawa M, Oleinick NL, Olsen LJ, Ozpolat B, Paglin S, Palmer GE, Papassideri I, Parkes M, Perlmutter DH, Perry G, Piacentini M, Pinkas-Kramarski R, Prescott M, Proikas-Cezanne T, Raben N, Rami A, Reggiori F, Rohrer B, Rubinsztein DC, Ryan KM, Sadoshima J, Sakagami H, Sakai Y, Sandri M, Sasakawa C, Sass M, Schneider C, Seglen PO, Seleverstov O, Settleman J, Shacka JJ, Shapiro IM, Sibirny A, Silva-Zacarin EC, Simon HU, Simone C, Simonsen A, Smith MA, Spanel-Borowski K, Srinivas V, Steeves M, Stenmark H, Stromhaug PE, Subauste CS, Sugimoto S, Sulzer D, Suzuki T, Swanson MS, Tabas I, Takeshita F, Talbot NJ, Talloczy Z, Tanaka K, Tanida I, Taylor GS, Taylor JP, Terman A, Tettamanti G, Thompson CB, Thumm M, Tolkovsky AM, Tooze SA, Truant R, Tumanovska LV, Uchiyama Y, Ueno T, Uzcategui NL, van der Klei I, Vaquero EC, Vellai T, Vogel MW, Wang HG, Webster P, Wiley JW, Xi Z, Xiao G, Yahalom J, Yang JM, Yap G, Yin XM, Yoshimori T, Yu L, Yue Z, Yuzaki M, Zabirnyk O, Zheng X, Zhu X, Deter RL. Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy. 2008a;4:151–175. doi: 10.4161/auto.5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klionsky DJ, Elazar Z, Seglen PO, Rubinsztein DC. Does bafilomycin A1 block the fusion of autophagosomes with lysosomes? Autophagy. 2008b;4:849–850. doi: 10.4161/auto.6845. [DOI] [PubMed] [Google Scholar]
- Kopitz J, Holz FG, Kaemmerer E, Schutt F. Lipids and lipid peroxidation products in the pathogenesis of age-related macular degeneration. Biochimie. 2004;86:825–831. doi: 10.1016/j.biochi.2004.09.029. [DOI] [PubMed] [Google Scholar]
- Krohne TU, Stratmann NK, Kopitz J, Holz FG. Effects of lipid peroxidation products on lipofuscinogenesis and autophagy in human retinal pigment epithelial cells. Experimental eye research. 2010;90:465–471. doi: 10.1016/j.exer.2009.12.011. [DOI] [PubMed] [Google Scholar]
- Krummenacher C, Brown A, Edrington TV, Shenker BJ, Boesze-Battaglia K. Mechanisms by Which Pathogens Hijack and Utilize Membrane Domains To Mediate Cytotoxicity. In: Y PL, editor. The Structure of Biological Membranes. Boca Raton, FL: CRC Press; 2011. [Google Scholar]
- Krummenacher C, Carfi A, Eisenberg RJ, Cohen GH. Entry of herpesviruses into cells: the enigma variations. Advances in experimental medicine and biology. 2013;790:178–195. doi: 10.1007/978-1-4614-7651-1_10. [DOI] [PubMed] [Google Scholar]
- Kunchithapautham K, Coughlin B, Lemasters JJ, Rohrer B. Differential effects of rapamycin on rods and cones during light-induced stress in albino mice. Investigative ophthalmology & visual science. 2011;52:2967–2975. doi: 10.1167/iovs.10-6278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunchithapautham K, Rohrer B. Apoptosis and autophagy in photoreceptors exposed to oxidative stress. Autophagy. 2007;3:433–441. doi: 10.4161/auto.4294. [DOI] [PubMed] [Google Scholar]
- Kurz T, Karlsson M, Brunk UT, Nilsson SE, Frennesson C. ARPE-19 retinal pigment epithelial cells are highly resistant to oxidative stress and exercise strict control over their lysosomal redox-active iron. Autophagy. 2009;5:494–501. doi: 10.4161/auto.5.4.7961. [DOI] [PubMed] [Google Scholar]
- La Vail MM. Rod outer segment disk shedding in rat retina: relationship to cyclic lighting. Science. 1976;194:1071–1074. doi: 10.1126/science.982063. [DOI] [PubMed] [Google Scholar]
- Levine B, Yuan J. Autophagy in cell death: an innocent convict? The Journal of clinical investigation. 2005;115:2679–2688. doi: 10.1172/JCI26390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CP, Yao J, Tao ZF, Li XM, Jiang Q, Yan B. Epigallocatechin-gallate (EGCG) regulates autophagy in human retinal pigment epithelial cells: a potential role for reducing UVB light-induced retinal damage. Biochemical and biophysical research communications. 2013;438:739–745. doi: 10.1016/j.bbrc.2013.07.097. [DOI] [PubMed] [Google Scholar]
- Lichtenstein A, Minogue PJ, Beyer EC, Berthoud VM. Autophagy: a pathway that contributes to connexin degradation. Journal of cell science. 2011;124:910–920. doi: 10.1242/jcs.073072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liton PB, Lin Y, Gonzalez P, Epstein DL. Potential role of lysosomal dysfunction in the pathogenesis of primary open angle glaucoma. Autophagy. 2009;5:122–124. doi: 10.4161/auto.5.1.7304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Lu W, Reigada DNJ, Laties AM, Mitchell CH. Restoration of lysosomal pH in RPE cells from cultured human and ABCA4(-/-) mice: pharmacologic approaches and functional recovery. Invest Ophthalmol Vis Sci. 2008;49:772–780. doi: 10.1167/iovs.07-0675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohr HR, Kuntchithapautham K, Sharma AK, Rohrer B. Multiple, parallel cellular suicide mechanisms participate in photoreceptor cell death. Experimental eye research. 2006;83:380–389. doi: 10.1016/j.exer.2006.01.014. [DOI] [PubMed] [Google Scholar]
- Loyet KM, Deforge LE, Katschke KJ, Jr., Diehl L, Graham RR, Pao L, Sturgeon L, Lewin-Koh SC, Hollyfield JG, van Lookeren Campagne M. Activation of the alternative complement pathway in vitreous is controlled by genetics in age-related macular degeneration. Investigative ophthalmology & visual science. 2012;53:6628–6637. doi: 10.1167/iovs.12-9587. [DOI] [PubMed] [Google Scholar]
- Marino G, Niso-Santano M, Baehrecke EH, Kroemer G. Self-consumption: the interplay of autophagy and apoptosis. Nature reviews Molecular cell biology. 2014;15:81–94. doi: 10.1038/nrm3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez J, Almendinger J, Oberst A, Ness R, Dillon CP, Fitzgerald P, Hengartner MO, Green DR. Microtubule-associated protein 1 light chain 3 alpha (LC3)-associated phagocytosis is required for the efficient clearance of dead cells. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:17396–17401. doi: 10.1073/pnas.1113421108. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Matsui M, Yamamoto A, Kuma A, Ohsumi Y, Mizushima N. Organelle degradation during the lens and erythroid differentiation is independent of autophagy. Biochemical and biophysical research communications. 2006;339:485–489. doi: 10.1016/j.bbrc.2005.11.044. [DOI] [PubMed] [Google Scholar]
- Matthaei M, Hu J, Meng H, Lackner EM, Eberhart CG, Qian J, Hao H, Jun AS. Endothelial cell whole genome expression analysis in a mouse model of early-onset Fuchs' endothelial corneal dystrophy. Investigative ophthalmology & visual science. 2013;54:1931–1940. doi: 10.1167/iovs.12-10898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehrpour M, Esclatine A, Beau I, Codogno P. Overview of macroautophagy regulation in mammalian cells. Cell Res. 2010;20:748–762. doi: 10.1038/cr.2010.82. [DOI] [PubMed] [Google Scholar]
- Meng H, Matthaei M, Ramanan N, Grebe R, Chakravarti S, Speck CL, Kimos M, Vij N, Eberhart CG, Jun AS. L450W and Q455K Col8a2 knock-in mouse models of Fuchs endothelial corneal dystrophy show distinct phenotypes and evidence for altered autophagy. Investigative ophthalmology & visual science. 2013;54:1887–1897. doi: 10.1167/iovs.12-11021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitter SK, Rao HV, Qi X, Cai J, Sugrue A, Dunn WA, Jr., Grant MB, Boulton ME. Autophagy in the retina: a potential role in age-related macular degeneration. Advances in experimental medicine and biology. 2012;723:83–90. doi: 10.1007/978-1-4614-0631-0_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima N, Yoshimori T, Ohsumi Y. The role of Atg proteins in autophagosome formation. Cell Dev Biol. 2011;27:107–132. doi: 10.1146/annurev-cellbio-092910-154005. [DOI] [PubMed] [Google Scholar]
- Morishita H, Eguchi S, Kimura H, Sasaki J, Sakamaki Y, Robinson ML, Sasaki T, Mizushima N. Deletion of autophagy-related 5 (Atg5) and Pik3c3 genes in the lens causes cataract independent of programmed organelle degradation. J Biol Chem. 2013;288:11436–11447. doi: 10.1074/jbc.M112.437103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami Y, Notomi S, Hisatomi T, Nakazawa T, Ishibashi T, Miller JW, Vavvas DG. Photoreceptor cell death and rescue in retinal detachment and degenerations. Progress in retinal and eye research. 2013;37:114–140. doi: 10.1016/j.preteyeres.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni HM, Bockus A, Wozniak AL, Jones K, Weinman S, Yin XM, Ding WX. Dissecting the dynamic turnover of GFP-LC3 in the autolysosome. Autophagy. 2011;7:188–204. doi: 10.4161/auto.7.2.14181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida Y, Arakawa S, Fujitani K, Yamaguchi H, Mizuta T, Kanaseki T, Komatsu M, Otsu K, Tsujimoto Y, Shimizu S. Discovery of Atg5/Atg7-independent alternative macroautophagy. Nature. 2009;461:654–658. doi: 10.1038/nature08455. [DOI] [PubMed] [Google Scholar]
- Oczypok EA, Oury TD, Chu CT. It's a cell-eat-cell world: autophagy and phagocytosis. The American journal of pathology. 2013;182:612–622. doi: 10.1016/j.ajpath.2012.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankiv S, Alemu EA, Brech A, Bruun J-A, Lamark T, Øvervatn A, Bjørkøy G, Johansen T. FYCO1 is a Rab7 effector that binds to LC3 and PI3P to mediate microtubule plus end-directed vesicle transport. The Journal of cell biology. 2010;188:253–269. doi: 10.1083/jcb.200907015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HY, Kim JH, Park CK. Activation of autophagy induces retinal ganglion cell death in a chronic hypertensive glaucoma model. Cell death & disease. 2012;3:e290. doi: 10.1038/cddis.2012.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters S, Schraermeyer U. [Characteristics and functions of melanin in retinal pigment epithelium] Der Ophthalmologe : Zeitschrift der Deutschen Ophthalmologischen Gesellschaft. 2001;98:1181–1185. doi: 10.1007/s003470170011. [DOI] [PubMed] [Google Scholar]
- Pilat A, Herrnreiter AM, Skumatz CM, Sarna T, Burke JM. Oxidative stress increases HO-1 expression in ARPE-19 cells, but melanosomes suppress the increase when light is the stressor. Investigative ophthalmology & visual science. 2013;54:47–56. doi: 10.1167/iovs.12-11153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovica D, Akutsua M, Novakb I, Harperc JW, Behrendsa C, Dikic I. Rab GTPase-Activating Proteins in Autophagy: Regulation of Endocytic and Autophagy Pathways by Direct Binding to Human ATG8 Modifiers. Mol Cell biol. 2012;32 doi: 10.1128/MCB.06717-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter K, Nallathambi J, Lin Y, Liton PB. Lysosomal basification and decreased autophagic flux in oxidatively stressed trabecular meshwork cells: implications for glaucoma pathogenesis. Autophagy. 2013;9:581–594. doi: 10.4161/auto.23568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyo JO, Yoo SM, Jung YK. The Interplay between Autophagy and Aging. Diabetes & metabolism journal. 2013;37:333–339. doi: 10.4093/dmj.2013.37.5.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reme C, Drinker CK, Aeberhard B. Modification of autophagic degradation by medium- and illumination conditions in frog visual cells in vitro. Documenta ophthalmologica Advances in ophthalmology. 1984;56:377–383. doi: 10.1007/BF00155682. [DOI] [PubMed] [Google Scholar]
- Reme CE, Wolfrum U, Imsand C, Hafezi F, Williams TP. Photoreceptor autophagy: effects of light history on number and opsin content of degradative vacuoles. Investigative ophthalmology & visual science. 1999;40:2398–2404. [PubMed] [Google Scholar]
- Rezaie T, Child A, Hitchings R, Brice G, Miller L, Coca-Prados M, Heon E, Krupin T, Ritch R, Kreutzer D, Crick RP, Sarfarazi M. Adult-onset primary open-angle glaucoma caused by mutations in optineurin. Science. 2002;295:1077–1079. doi: 10.1126/science.1066901. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Muela N, Germain F, Marino G, Fitze PS, Boya P. Autophagy promotes survival of retinal ganglion cells after optic nerve axotomy in mice. Cell death and differentiation. 2012;19:162–169. doi: 10.1038/cdd.2011.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinsztein DC, DiFiglia, Heintz N, Nixon RA, Qin ZH, Ravikumar B, Stefanis L, Tolkovsky A. Autophagy and Its Possible Roles in Nervous System Diseases, Damage and Repair. Autophagy. 2005;1:11–22. doi: 10.4161/auto.1.1.1513. [DOI] [PubMed] [Google Scholar]
- Sanjuan MA, Dillon CP, Tait SW, Moshiach S, Dorsey F, Connell S, Komatsu M, Tanaka K, Cleveland JL, Withoff S, Green DR. Toll-like receptor signalling in macrophages links the autophagy pathway to phagocytosis. Nature. 2007;450:1253–1257. doi: 10.1038/nature06421. [DOI] [PubMed] [Google Scholar]
- Sanjuan MA, Milasta S, Green DR. Toll-like receptor signaling in the lysosomal pathways. Immunol Rev. 2009;227:203–220. doi: 10.1111/j.1600-065X.2008.00732.x. [DOI] [PubMed] [Google Scholar]
- Sarangarajan R, Apte SP. Melanization and phagocytosis: implications for age related macular degeneration. Molecular vision. 2005;11:482–490. [PubMed] [Google Scholar]
- Sarkar S, Floto RA, Berger Z, Imarisio S, Cordenier A, Pasco M, Cook LJ, Rubinsztein DC. Lithium induces autophagy by inhibiting inositol monophosphatase. The Journal of cell biology. 2005;170:1101–1111. doi: 10.1083/jcb.200504035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarna T, Burke JM, Korytowski W, Rozanowska M, Skumatz CM, Zareba A, Zareba M. Loss of melanin from human RPE with aging: possible role of melanin photooxidation. Experimental eye research. 2003;76:89–98. doi: 10.1016/s0014-4835(02)00247-6. [DOI] [PubMed] [Google Scholar]
- Schmedt T, Silva MM, Ziaei A, Jurkunas U. Molecular bases of corneal endothelial dystrophies. Experimental eye research. 2012;95:24–34. doi: 10.1016/j.exer.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schraermeyer U, Heimann K. Current understanding on the role of retinal pigment epithelium and its pigmentation. Pigment cell research / sponsored by the European Society for Pigment Cell Research and the International Pigment Cell Society. 1999;12:219–236. doi: 10.1111/j.1600-0749.1999.tb00755.x. [DOI] [PubMed] [Google Scholar]
- Schutt F, Aretz S, Auffahrt GU, Kopitz J. [Role of energy metabolism in retinal pigment epithelium] Der Ophthalmologe : Zeitschrift der Deutschen Ophthalmologischen Gesellschaft. 2013;110:346–352. doi: 10.1007/s00347-012-2752-3. [DOI] [PubMed] [Google Scholar]
- Schutt F, Aretz S, Auffarth GU, Kopitz J. Moderately reduced ATP levels promote oxidative stress and debilitate autophagic and phagocytic capacities in human RPE cells. Investigative ophthalmology & visual science. 2012;53:5354–5361. doi: 10.1167/iovs.12-9845. [DOI] [PubMed] [Google Scholar]
- Shah A, Farooq AV, Tiwari V, Kim MJ, Shukla D. HSV-1 infection of human corneal epithelial cells: receptor-mediated entry and trends of re-infection. Molecular vision. 2010;16:2476–2486. [PMC free article] [PubMed] [Google Scholar]
- Shen X, Ying H, Qiu Y, Park JS, Shyam R, Chi ZL, Iwata T, Yue BY. Processing of optineurin in neuronal cells. J Biol Chem. 2011;286:3618–3629. doi: 10.1074/jbc.M110.175810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramani S, Malhotra V. Non-autophagic roles of autophagy-related proteins. EMBO reports. 2013;14:143–151. doi: 10.1038/embor.2012.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundelin SP, Nilsson SE, Brunk UT. Lipofuscin-formation in cultured retinal pigment epithelial cells is related to their melanin content. Free radical biology & medicine. 2001;30:74–81. doi: 10.1016/s0891-5849(00)00444-5. [DOI] [PubMed] [Google Scholar]
- Tezel G, Thornton IL, Tong MG, Luo C, Yang X, Cai J, Powell DW, Soltau JB, Liebmann JM, Ritch R. Immunoproteomic analysis of potential serum biomarker candidates in human glaucoma. Investigative ophthalmology & visual science. 2012a;53:8222–8231. doi: 10.1167/iovs.12-10076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tezel G, Yang X, Luo C, Cai J, Powell DW. An astrocyte-specific proteomic approach to inflammatory responses in experimental rat glaucoma. Investigative ophthalmology & visual science. 2012b;53:4220–4233. doi: 10.1167/iovs.11-9101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thumm M, Egner R, Koch B, Schlumpberger M, Straub M, Veenhuis M, Wolf DH. Isolation of autophagocytosis mutants of Saccharomyces cerevisiae. FEBS Lett. 1994;349:275–280. doi: 10.1016/0014-5793(94)00672-5. [DOI] [PubMed] [Google Scholar]
- Tsukada M, Ohsumi Y. Isolation and characterization of autophagy-defective mutants of Saccharomyces cerevisiae. FEBS Lett. 1993;333:169–174. doi: 10.1016/0014-5793(93)80398-e. [DOI] [PubMed] [Google Scholar]
- Valapala M, Wilson C, Hose S, Bhutto IA, Grebe R, Dong A, Greenbaum S, Gu L, Sengupta S, Cano M, Hackett S, Xu G, Lutty GA, Dong L, Sergeev Y, Handa JT, Campochiaro P, Wawrousek E, Zigler JS, Sinha D. Lysosomal-mediated waste clearance in retinal pigment epithelial cells is regulated by CRYBA1/betaA3/A1-crystallin via V-ATPase-MTORC1 signaling. Autophagy. 2014;10 doi: 10.4161/auto.27292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez CL, Colombo MI. Assays to assess autophagy induction and fusion of autophagic vacuoles with a degradative compartment, using monodansylcadaverine (MDC) and DQ-BSA. Methods in enzymology. 2009;452:85–95. doi: 10.1016/S0076-6879(08)03606-9. [DOI] [PubMed] [Google Scholar]
- Walker S, Chandra P, Manifava M, Axe E, Ktistakis NT. Making autophagosomes: localized synthesis of phosphatidylinositol 3-phosphate holds the clue. Autophagy. 2008;4:1093–1096. doi: 10.4161/auto.7141. [DOI] [PubMed] [Google Scholar]
- Wang AL, Boulton ME, Dunn WA, Jr., Rao HV, Cai J, Lukas TJ, Neufeld AH. Using LC3 to monitor autophagy flux in the retinal pigment epithelium. Autophagy. 2009a;5:1190–1193. doi: 10.4161/auto.5.8.10087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang AL, Lukas TJ, Yuan M, Du N, Tso MO, Neufeld AH. Autophagy, exosomes and drusen formation in age-related macular degeneration. Autophagy. 2009b;5:563–564. doi: 10.4161/auto.5.4.8163. [DOI] [PubMed] [Google Scholar]
- Wild P, Farhan H, McEwan DG, Wagner S, Rogov VV, Brady NR, Richter B, Korac J, Waidmann O, Choudhary C, Dotsch V, Bumann D, Dikic I. Phosphorylation of the autophagy receptor optineurin restricts Salmonella growth. Science. 2011;333:228–233. doi: 10.1126/science.1205405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wride MA. Lens fibre cell differentiation and organelle loss:many paths lead to clarity. Phil Trans R Soc B. 2011;366:1219–1233. doi: 10.1098/rstb.2010.0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu XS, Martina JA, Hammer JA. Melanoregulin is stably targeted to the melanosome membrane by palmitoylation. Biochemical and biophysical research communications. 2012;426:209–214. doi: 10.1016/j.bbrc.2012.08.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, Klionsky DJ. Autophagosome formation: core machinery and adaptations. Nat Cell Biol. 2007;9:1102–1109. doi: 10.1038/ncb1007-1102. [DOI] [PubMed] [Google Scholar]
- Yu L, Lenardo MJ, Baehrecke EH. Autophagy and caspases: a new cell death program. Cell cycle. 2004;3:1124–1126. [PubMed] [Google Scholar]
- Zacks DN, Hanninen V, Pantcheva M, Ezra E, Grosskreutz C, Miller JW. Caspase activation in an experimental model of retinal detachment. Investigative ophthalmology & visual science. 2003;44:1262–1267. doi: 10.1167/iovs.02-0492. [DOI] [PubMed] [Google Scholar]
- Zacks DN, Zheng QD, Han Y, Bakhru R, Miller JW. FAS-mediated apoptosis and its relation to intrinsic pathway activation in an experimental model of retinal detachment. Investigative ophthalmology & visual science. 2004;45:4563–4569. doi: 10.1167/iovs.04-0598. [DOI] [PubMed] [Google Scholar]
- Zhao C, Yasumura D, Li X, Matthes M, Lloyd M, Nielsen G, Ahern K, Snyder M, Bok D, Dunaief JL, LaVail MM, Vollrath D. mTOR-mediated dedifferentiation of the retinal pigment epithelium initiates photoreceptor degeneration in mice. The Journal of clinical investigation. 2011a;121:369–383. doi: 10.1172/JCI44303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Chen Y, Wang J, Sternberg P, Freeman ML, Grossniklaus HE, Cai J. Age-related retinopathy in NRF2-deficient mice. PloS one. 2011b;6:e19456. doi: 10.1371/journal.pone.0019456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu XC, Yu JT, Jiang T, Tan L. Autophagy modulation for Alzheimer's disease therapy. Molecular neurobiology. 2013;48:702–714. doi: 10.1007/s12035-013-8457-z. [DOI] [PubMed] [Google Scholar]