Abstract
The purpose of this study was to quantitatively compare, in a rabbit keratitis model, the levels of effectiveness of moxifloxacin, levofloxacin, and ciprofloxacin for the treatment of Staphylococcus aureus isolates of diverse antibiotic susceptibilities. Rabbit eyes were intrastromally injected with approximately 100 CFU of methicillin-sensitive or methicillin-resistant S. aureus (MSSA or MRSA, respectively) organisms that were either sensitive or resistant to ofloxacin. One drop of moxifloxacin (0.5%), levofloxacin (0.5%), or ciprofloxacin (0.3%) was topically applied hourly from 4 to 9 (early) or 10 to 15 (late) h postinfection. At 1 h after cessation of therapy, the corneas were harvested, and the number of CFU per cornea was determined. For the ofloxacin-sensitive strains, early treatment of MSSA or MRSA with moxifloxacin, levofloxacin, or ciprofloxacin produced approximately a 5-log decrease in CFU per cornea relative to that in untreated eyes (P ≤ 0.0001). For late therapy of ofloxacin-sensitive strains, moxifloxacin, levofloxacin, and ciprofloxacin produced approximately 5-, 4-, and 2- to 3-log reductions in CFU per cornea, respectively (P ≤ 0.0001). Early treatment of the ofloxacin-resistant strains with either moxifloxacin or levofloxacin produced a ≥4-log or ≥3-log decrease, respectively, in the MSSA or MRSA strains (P ≤ 0.0001), whereas ciprofloxacin treatment produced a 1-log decrease in CFU per cornea relative to that in untreated eyes (P = 0.1540). For late treatment of ofloxacin-resistant strains, levofloxacin and ciprofloxacin failed to significantly reduce the number of CFU per cornea (P ≥ 0.3627), whereas moxifloxacin produced a significant reduction in CFU per cornea of approximately 1 log (P ≤ 0.0194). Therefore, for three of the four treatments tested, moxifloxacin demonstrated greater effectiveness than either levofloxacin or ciprofloxacin.
Staphylococcus aureus is a major cause of bacterial keratitis (2, 15, 21). S. aureus ocular infections can cause severe inflammation, pain, corneal perforation, scarring, and loss of visual acuity (6, 15). S. aureus has a long history of evolving to more resistant states, and this trend is expected to continue (13, 22). Therefore, new antibiotics and new antibiotic formulations are needed to manage future cases of S. aureus-induced keratitis.
Throughout the 1990s, S. aureus acquired more resistance to the commonly prescribed antibiotics (15; K. R. Wilhelmus and R. L. Penland, Investig. Opthalmol. Vis. Sci. 37:S877, 1996). Of particular concern is that S. aureus strains resistant to multiple antibiotics, including methicillin-resistant S. aureus (MRSA), have become more prevalent in the hospital setting, dictating the empirical use of vancomycin for nosocomial Staphylococcus infections (13, 22). Additionally, MRSA strains have been isolated in increasing numbers among nonhospitalized individuals who have no recent history of antibiotic usage or a hospital stay (18). Of potential importance to treating bacterial keratitis is the finding that MRSA strains are typically resistant to cephalosporins and aminoglycosides and are more frequently resistant to the extended-spectrum and broad-spectrum fluoroquinolones in use today (Wilhelmus and Penland, Investig. Opthalmol. Vis. Sci. 37:S877, 1996). The distribution of such MRSA strains among the outpatient population implies that drug-resistant forms of S. aureus will become more common causes of ocular infections. Moreover, there is a long-term concern that rare strains expressing intermediate resistance to vancomycin may become more prevalent (13). These changes in S. aureus itself and in the distribution of MRSA into the outpatient population imply that the future treatment of keratitis may be considerably more challenging.
Moxifloxacin and gatifloxacin are “fourth generation” fluoroquinolone antibiotics that target bacterial DNA gyrase (topoisomerase II) and topoisomerase IV (1, 4, 11, 14, 23, 26). These fourth generation fluoroquinolones have in vitro activity similar to that of ciprofloxacin and ofloxacin (extended-spectrum fluoroquinolones) against gram-negative bacteria but enhanced activity against gram-positive bacteria, including S. aureus (3, 8, 9). Levofloxacin (a broad-spectrum fluoroquinolone) is the optically active l isomer of the racemate ofloxacin. Levofloxacin has activity against gram-negative bacteria similar to that of ciprofloxacin or ofloxacin and has a greater potency in vitro against gram-positive bacteria (8, 10, 24).
This study compares ciprofloxacin, levofloxacin, and moxifloxacin for their in vivo effectiveness in treating experimental S. aureus keratitis. The four S. aureus strains used in this study were selected for their differences in susceptibility to antibiotics, including strains with established resistance to ofloxacin. Treatment times were chosen to compare the effectiveness of fluoroquinolones during both a period of active bacterial replication (early therapy) and a subsequent phase with reduced bacterial replication (late therapy) in the cornea (5). The results demonstrate the potency of these antibiotics and reveal the enhanced value of moxifloxacin as a potential new ocular therapy.
MATERIALS AND METHODS
Rabbits.
New Zealand White rabbits (2.0 to 3.0 kg) were treated and maintained in accordance with the tenets of the ARVO Resolution on the Use of Animals in Ophthalmic and Vision Research. Rabbits were anesthetized by subcutaneous injection of a 1:5 mixture of xylazine (100 mg/ml; Rompum; Miles Laboratories, Shawnee, Kans.) and ketamine hydrochloride (100 mg/ml; Ketaset; Bristol Laboratories, Syracuse, N.Y.). Proparacaine hydrochloride (0.5% Alcaine; Alcon Laboratories, Fort Worth, Tex.) was topically applied to each eye before intrastromal injection.
Bacteria and corneal inoculation.
S. aureus strains 8325-4 (methicillin and ofloxacin sensitive), MRSA 301 (methicillin resistant and ofloxacin sensitive), 60171 (methicillin sensitive and ofloxacin resistant), and 30155 (methicillin and ofloxacin resistant) were incubated overnight in tryptic soy broth (TSB; Difco, Detroit, Mich.) at 37°C. This culture was diluted (1:100) in fresh TSB, and a log-phase culture was grown to an optical density of 0.325 at 650 nm and then diluted in TSB to approximately 10,000 CFU per ml. Each cornea was intrastromally injected with a 10-μl volume containing approximately 100 CFU as previously described (5). MRSA 301 is a clinical isolate that has previously been used in antibiotic studies (5, 7). Strains 60171 and 30155 are clinical isolates that were provided by David Stroman (Alcon Laboratories).
Treatment schedule.
Rabbits were topically treated every hour from 4 to 9 (early) or 10 to 15 (late) h postinfection (p.i.) with a single topical drop of ciprofloxacin (0.3% ophthalmic solution; Ciloxan; Alcon Laboratories), moxifloxacin (0.5% ophthalmic solution; Alcon Laboratories), or levofloxacin (0.5% ophthalmic solution; Quixin; Santen Oy, Tampere, Finland). At 4 h p.i., when early therapy started, the eyes contained approximately 4.7 log CFU per cornea, and at 10 h p.i., when late therapy started, the eyes contained approximately 6.6 log CFU per cornea. Rabbits were sacrificed 1 h after the final treatment by an intravenous injection of sodium pentobarbital solution (100 mg/ml; The Butler Co., Columbus, Ohio).
Antibiotic susceptibility.
The MICs of ciprofloxacin, moxifloxacin, and levofloxacin were determined by the tube broth dilution method with Mueller-Hinton broth (Difco) supplemented with 5% sodium chloride as previously described (7).
Bacterial quantification.
Corneas were aseptically removed, dissected, and homogenized in sterile phosphate-buffered saline with a tissue homogenizer (Tekmar, Cincinnati, Ohio). Aliquots of corneal homogenates were serially diluted in buffered saline, plated in triplicate on tryptic soy agar (Difco), and incubated for 24 h at 37°C. The number of viable S. aureus organisms per cornea was expressed as base 10 logarithms.
Statistical analysis.
Data were analyzed by using the Statistical Analysis System program for personal computers. For CFU determinations, analysis of variance and Student's t tests between least-squared means from each group showing statistical variances were performed. P values of <0.05 were considered significant.
RESULTS
In vitro susceptibility of S. aureus strains to fluoroquinolones. The MICs for the four strains of S. aureus analyzed are presented in Table 1.
TABLE 1.
Determination of the MICs of ciprofloxacin, moxifloxacin, and levofloxacin for S. aureus strainsa
| Strain | Drug sensitivity
|
MIC (μg/ml)
|
|||
|---|---|---|---|---|---|
| Methicillin | Ofloxacin | Ciprofloxacin | Levofloxacin | Moxifloxacin | |
| 8325-4 | Sensitive | Sensitive | 0.12 | 0.07 | 0.06 |
| MRSA 301 | Resistant | Sensitive | 0.25 | 0.24 | 0.07 |
| 60171 | Sensitive | Resistant | 29.17 | 9.38 | 4.26 |
| 30155 | Resistant | Resistant | 16.88 | 7.81 | 2.20 |
MICs of ciprofloxacin, moxifloxacin, and levofloxacin for the S. aureus strains tested were determined using the tube broth dilution method with Mueller-Hinton broth supplemented with 5% sodium chloride. Approximately 105 CFU of S. aureus per ml was added to doubling dilutions of antibiotics and incubated at 35°C for 24 h. The MIC was designated as the lowest concentration that inhibited growth of Staphylococcus as determined by the absence of turbidity. The values presented are the means of three or more separate determinations.
Treatment of ofloxacin-sensitive S. aureus keratitis.
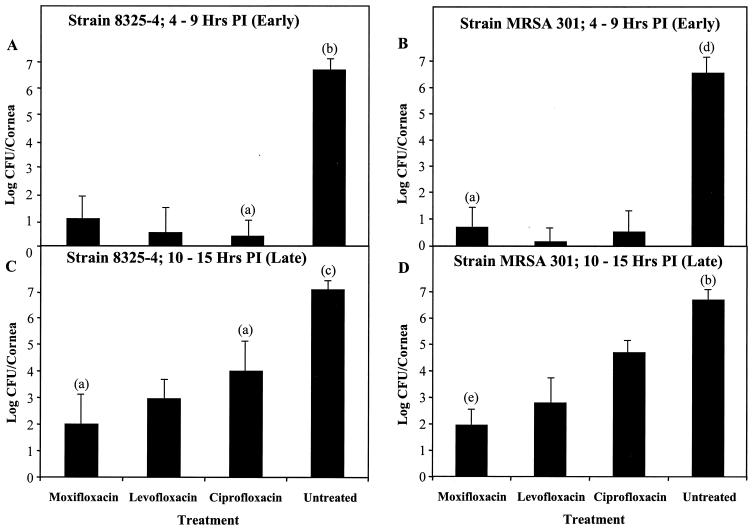
Early treatment of rabbit eyes infected with ofloxacin-sensitive S. aureus strain 8325-4, a methicillin-sensitive S. aureus strain (MSSA), or MRSA 301 from 4 to 9 h p.i. with moxifloxacin, levofloxacin, or ciprofloxacin reduced the number of S. aureus organisms by approximately 5 log CFU/cornea compared to that of the untreated control group (P ≤ 0.0001) (Fig. 1A and B). There were no significant differences in numbers of CFU per cornea among the antibiotic-treated groups (P ≥ 0.0709).
FIG. 1.
Fluoroquinolone treatment of rabbit eyes infected with ofloxacin-sensitive S. aureus strains. Rabbit corneas were infected with ofloxacin-sensitive S. aureus and treated with moxifloxacin (0.5%), levofloxacin (0.5%), or ciprofloxacin (0.3%). Rabbit eyes were treated every hour from 4 to 9 (A and B) or from 10 to 15 (C and D) h p.i. Following sacrifice of the rabbits, the numbers of viable S. aureus organisms per cornea was quantified and expressed as base 10 logarithms ± standard deviations (indicated by error bars). The number of eyes per group (n) was 6 unless specified by one of the following labels above the error bars: (a), n = 12; (b), n = 15; (c), n = 22; (d), n = 10; (e), n = 18.
Late treatment of eyes infected with the ofloxacin-sensitive strains with any of the three antibiotics from 10 to 15 h p.i. produced a significant decrease in the number of CFU per cornea relative to that of the untreated control (P ≤ 0.0001). Moxifloxacin produced approximately a 5-log reduction in CFU per cornea, while levofloxacin produced approximately 4-log reductions in CFU per cornea relative to that of the control (P ≤ 0.0001) (Fig. 1C and D). Ciprofloxacin was less effective than either of the other drugs, producing 2- to 3-log reductions in CFU per cornea (P ≤ 0.0051). Levofloxacin and ciprofloxacin were not as effective during late therapy as they were during early therapy, but moxifloxacin demonstrated its effectiveness in both the early and late treatments.
Treatment of ofloxacin-resistant S. aureus keratitis.
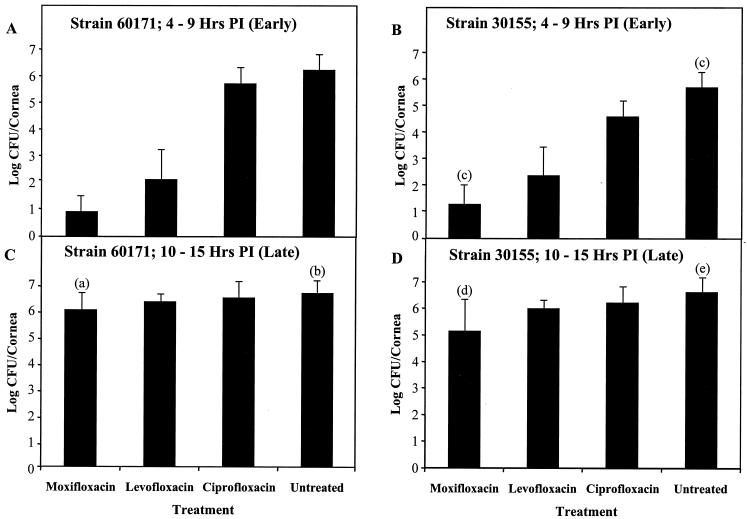
For early treatment (4 to 9 h p.i.) of rabbit eyes infected with S. aureus strains resistant to ofloxacin, 60171 (an MSSA strain) and 30155 (an MRSA strain), moxifloxacin produced approximately 4- to 5-log reductions in the number of CFU per cornea relative to that of the untreated eyes (P ≤ 0.0001) (Fig. 2A and B). Early treatment with levofloxacin produced 3- to 4-log reductions (P ≤ 0.0001), and treatment with ciprofloxacin produced 0.5- to 1-log reductions in CFU per cornea compared to that of the untreated group (P = 0.1540). Moxifloxacin had significantly greater effectiveness than levofloxacin or ciprofloxacin for these ofloxacin-resistant strains during the early therapy (P ≤ 0.0132).
FIG. 2.
Fluoroquinolone treatment of rabbit eyes infected with ofloxacin-resistant S. aureus strains. Rabbit corneas were infected with ofloxacin-resistant S. aureus and treated with moxifloxacin (0.5%), levofloxacin (0.5%), or ciprofloxacin (0.3%). Rabbit eyes were treated every hour from 4 to 9 (A and B) or from 10 to 15 (C and D) h p.i. Following sacrifice of the rabbits, the numbers of viable S. aureus organisms per cornea were quantified and expressed as base 10 logarithms ± standard deviations (indicated by error bars). The number of eyes per group (n) was 6 unless specified by one of the following labels above the error bar: (a), n = 17; (b), n = 13; (c), n = 12; (d), n = 26; (e), n = 20.
Late therapy (10 to 15 h p.i.) of the eyes infected with the ofloxacin-resistant strains (60171 and 30155) with either levofloxacin or ciprofloxacin did not produce significant reductions in the number of CFU per cornea relative to that of the untreated control (P ≥ 0.3627) (Fig. 2C and D). Late therapy of these two ofloxacin-resistant strains with moxifloxacin significantly reduced the number of CFU per cornea by approximately 1 log compared to that of the untreated group (P values of 0.0194 and ≤0.0001 for strains 60171 and 30155, respectively). Moxifloxacin was significantly more effective than levofloxacin or ciprofloxacin in treating eyes infected with strain 30155 (P ≤ 0.0103).
DISCUSSION
The present study demonstrates the effectiveness of each of the spectrum types of fluoroquinolones tested for treating experimental keratitis caused by S. aureus isolates of diverse antibiotic susceptibilities. Typically, early therapy was significantly more effective than late therapy; however, moxifloxacin retained greater activity in late therapy, especially against the fluoroquinolone-sensitive S. aureus strains. Moxifloxacin therapy had significantly greater activity against the ofloxacin-resistant strains than levofloxacin or ciprofloxacin therapy. These results concur with previous in vitro studies that reported moxifloxacin to be more potent against S. aureus than either levofloxacin or ciprofloxacin (16, 17, 25, 27). These results demonstrate that there was a correlation between the MIC and the in vivo effectiveness of these three fluoroquinolone formulations when the number of bacteria in the cornea was reduced by 2 to 5 logs per cornea relative to that of the control.
A major objective of this study was to compare the levels of effectiveness of different types of fluoroquinolones for treating strains of S. aureus that were either susceptible or resistant to ofloxacin. The antibiotic solutions tested were the commercially available formulations that differ in concentration from 0.3 to 0.5%. The strains susceptible to ofloxacin in vitro were readily killed in the early stage of infection by ciprofloxacin, levofloxacin, or moxifloxacin. However, the effectiveness of treatment for these ofloxacin-susceptible strains was reduced when the antibiotics were applied late in infection.
There was a major difference in the levels of antibiotic effectiveness in early phase versus late-phase therapy of strains resistant to ofloxacin. Ciprofloxacin and levofloxacin produced 1- and 3-log reductions in CFU per cornea, respectively, when they were applied early in infection, but they essentially lost all effectiveness during late-phase therapy of the ofloxacin-resistant strains. Moxifloxacin produced approximately a 5-log reduction in CFU per cornea during early therapy and was the only antibiotic to significantly reduce the number of CFU of ofloxacin-resistant strains per cornea during the late phase of infection. Although moxifloxacin produced a significant reduction in CFU per cornea, the difficulty in treating ofloxacin-resistant S. aureus late in infection is apparent.
The marked reduction in bacterial killing after rapid bacterial growth relates to an important point in considering treatment for established infections. Research in the rabbit model of keratitis has demonstrated that tissue damage and inflammation are produced by the action of specific toxins secreted by S. aureus. These toxins are produced primarily after the bacteria have completed rapid growth (19, 20). The implication from this rabbit model is that a patient with significant clinical signs of keratitis has a large population of toxin-producing, slowly replicating bacteria. The present study demonstrates that once considerable inflammation and tissue damage are occurring in the rabbit, effective killing of bacteria is compromised by the reduced bacterial replication. This reduced rate of bacterial killing applies to all fluoroquinolones but to a lesser extent to moxifloxacin, a fourth generation fluoroquinolone. This concept is consistent with the benefits of prompt antibiotic treatment and the need for repeated application of antibiotics in high concentrations once the infection is well established.
The present findings show that moxifloxacin, a fourth generation fluoroquinolone, can manifest activity against S. aureus that is superior to that of ciprofloxacin and levofloxacin. One potential aspect of this superior action is that moxifloxacin therapy may provide less opportunity for emergence of fluoroquinolone-resistant populations of Staphylococcus. Lister (16) showed that although both levofloxacin and moxifloxacin are bactericidal, some S. aureus strains developed resistance to levofloxacin during administration. Such resistance did not occur during therapy with moxifloxacin. This finding by Lister may have important ramifications, because Wright et al. (27) have demonstrated in vitro that an S. aureus subpopulation exposed to a sublethal dose of a fluoroquinolone may express resistance to the same and other fluoroquinolones. There has been an increase in resistance to fluoroquinolones among isolates of S. aureus in recent years (12), and the advent of the fourth generation fluoroquinolones may provide a means to slow this growing and dangerous process of increasing resistance.
Acknowledgments
This work was supported by a donation from Alcon Research, Inc., to the LSU Health Sciences Center Foundation and by NEI grant EY10974 and NEI core grant EY02377.
REFERENCES
- 1.Adams, D. E., E. M. Shekhtman, E. L. Zechiedrich, M. B. Schmidt, and N. R. Cozzarelli. 1992. The role of topoisomerase IV in partitioning bacterial replicons and the structure of catenated intermediates in DNA replication. Cell 71:277-288. [DOI] [PubMed] [Google Scholar]
- 2.Alexandrakis, G., E. C. Alfonso, and D. Miller. 2000. Shifting trends in bacterial keratitis in south Florida and emerging resistance to fluoroquinolones. Ophthalmology 107:1497-1502. [DOI] [PubMed] [Google Scholar]
- 3.Biedenbach, D. J., and R. N. Jones. 1996. The comparative antimicrobial activity of levofloxacin tested against 350 clinical isolates of streptococci. Diagn. Microbiol. Infect. Dis. 25:47-51. [DOI] [PubMed] [Google Scholar]
- 4.Blanche, F., B. Cameron, F. X. Bernard, L. Maton, B. Manse, L. Ferrero, N. Ratet, C. Lecoq, A. Goniot, D. Bisch, and J. Crouzet. 1996. Differential behaviors of Staphylococcus aureus and Escherichia coli type II DNA topoisomerases. Antimicrob. Agents Chemother. 40:2714-2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Callegan, M. C., J. A. Hobden, J. M. Hill, M. S. Insler, and R. J. O'Callaghan. 1992. Topical antibiotic therapy for the treatment of experimental Staphylococcus aureus keratitis. Investig. Ophthalmol. Vis. Sci. 33:3017-3023. [PubMed] [Google Scholar]
- 6.Chusid, M. J., and S. D. Davis. 1979. Experimental bacterial keratitis in neutropenic guinea pigs: polymorphonuclear leukocytes in corneal host defense. Infect. Immun. 24:948-952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dajcs, J. J., E. B. Hume, J. M. Moreau, A. R. Caballero, B. M. Cannon, and R. J. O'Callaghan. 2000. Lysostaphin treatment of methicillin-resistant Staphylococcus aureus keratitis in the rabbit. Investig. Ophthalmol. Vis. Sci. 41:1432-1437. [PubMed] [Google Scholar]
- 8.Dalhoff, A., U. Petersen, and R. Endermann. 1996. In vitro activity of BAY 12-8039, a new 8-methoxyquinolone. Chemotherapy 42:410-425. [DOI] [PubMed] [Google Scholar]
- 9.Davis, R., and H. M. Bryson. 1994. Levofloxacin. A review of its antibacterial activity, pharmacokinetics and therapeutic efficacy. Drugs 47:677-700. [DOI] [PubMed] [Google Scholar]
- 10.Dholakia, N., K. V. Rolston., D. H. Ho, B. LeBlanc, and G. P. Bodey. 1994. Susceptibilities of bacterial isolates from patients with cancer to levofloxacin and other quinolones. Antimicrob. Agents Chemother. 38:848-852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fasching, C. E., F. C. Tenover, T. G. Slama, L. M. Fisher, S. Sreedharan, M. Oram, K. Willard, L. M. Sinn, D. N. Gerding, and L. R. Peterson. 1991. gyrA mutations in ciprofloxacin-resistant, methicillin-resistant Staphylococcus aureus from Indiana, Minnesota, and Tennessee. J. Infect. Dis. 164:976-979. [DOI] [PubMed] [Google Scholar]
- 12.Goldstein, M. H., R. P. Kowalski, Y. J. Gordon. 1999. Emerging fluoroquinolone resistance in bacterial keratitis: a 5-year review. Ophthalmology 106:1313-1318. [PubMed] [Google Scholar]
- 13.Hiramatsu, K., N. Aritaka, H. Hanaki, S. Kawasaki, Y. Hosoda, S. Hori, Y. Fukuchi, and I. Kobayashi. 1997. Dissemination in Japanese hospitals of strains of Staphylococcus aureus heterogeneously resistant to vancomycin. Lancet 350:1670-1673. [DOI] [PubMed] [Google Scholar]
- 14.Kato, J., H. Suzuki, and H. Ikeda. 1992. Purification and characterization of DNA topoisomerase IV in Escherichia coli. J. Biol. Chem. 267:25676-25684. [PubMed] [Google Scholar]
- 15.Liesegang, T. J. 1998. Bacterial and fungal keratitis, p. 159-219. In H. E. Kaufman (ed.), The cornea, 2nd ed. Butterworth-Heinemann, Boston, Mass.
- 16.Lister, P. D. 2001. Pharmacodynamics of moxifloxacin and levofloxacin against Staphylococcus aureus and Staphylococcus epidermidis in an in vitro pharmacodynamic model. Clin. Infect. Dis. 32:S33-S38. [DOI] [PubMed] [Google Scholar]
- 17.Mather, R., L. M. Karenchak, E. G. Romanowski, and R. P. Kowalski. 2002. Fourth generation fluoroquinolones: new weapons in the arsenal of ophthalmic antibiotics. Am. J. Ophthalmol. 133:463-466. [DOI] [PubMed] [Google Scholar]
- 18.O'Brien, F. G., J. W. Pearman, M. Gracey, T. V. Riley, and W. B. Grubb. 1999. Community strain of methicillin-resistant Staphylococcus aureus involved in a hospital outbreak. J. Clin. Microbiol. 37:2858-2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O'Callaghan, R. J., M. C. Callegan, J. M. Moreau, L. C. Green, T. J. Foster, O. M. Hartford, L. S. Engel, and J. M. Hill. 1997. Specific roles of alpha-toxin and beta-toxin during Staphylococcus aureus corneal infection. Infect. Immun. 65:1571-1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O'Reilly, M., B. Kreiswirth, and T. J. Foster. 1990. Cryptic alpha-toxin gene in toxic shock syndrome and septicemia strains of Staphylococcus aureus. Mol. Microbiol. 11:1947-1955. [DOI] [PubMed] [Google Scholar]
- 21.Ormerod, L. D., E. Hertzmark, D. S. Gomez, R. G. Stabiner, D. J. Schanzlin, and R. E. Smith. 1987. Epidemiology of microbial keratitis in southern California. A multivariate analysis. Ophthalmology 94:1322-1333. [DOI] [PubMed] [Google Scholar]
- 22.Peterson, D. L. 1999. Vancomycin-resistant Staphylococcus aureus. Infect. Med. 16:235-238. [Google Scholar]
- 23.Shen, L. L. 1994. Molecular mechanisms of DNA gyrase inhibition by quinolone antibacterials. Adv. Pharmacol. 29A:285-304. [DOI] [PubMed] [Google Scholar]
- 24.Souli, M., C. B. Wennersten, and G. M. Eliopoulos. 1998. In vitro activity of BAY 12-8039, a new fluoroquinolone, against species representative of respiratory tract pathogens. Int. J. Antimicrob. Agents 10:23-30. [DOI] [PubMed] [Google Scholar]
- 25.Takei, M., H. Fukuda, R. Kishii, and M. Hosaka. 2001. Target preference of 15 quinolones against Staphylococcus aureus, based on antibacterial activities and target inhibition. Antimicrob. Agents Chemother. 45:3544-3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang, J. C. 1991. DNA topoisomerases: why so many? J. Biol. Chem. 266:6659-6662. [PubMed] [Google Scholar]
- 27.Wright, D. H., B. W. Gunderson, L. B. Hovde, G. H. Ross, K. H. Ibrahim, and J. C. Rotschafer. 2002. Comparative pharmacodynamics of three newer fluoroquinolones versus six strains of staphylococci in an in vitro model under aerobic and anaerobic conditions. Antimicrob. Agents Chemother. 46:1561-1563. [DOI] [PMC free article] [PubMed] [Google Scholar]