Abstract
Topical microbicides designed to prevent acquisition of sexually transmitted infections are urgently needed. Nonoxynol-9, the only commercially available spermicide, damages epithelium and may enhance human immunodeficiency virus transmission. The observation that herpes simplex virus (HSV) and human immunodeficiency virus bind heparan sulfate provided the rationale for the development of sulfated or sulfonated polymers as topical agents. Although several of the polymers have advanced to clinical trials, the spectrum and mechanism of anti-HSV activity and the effects on soluble mediators of inflammation have not been evaluated. The present studies address these gaps. The results indicate that PRO 2000, polystyrene sulfonate, cellulose sulfate, and polymethylenehydroquinone sulfonate inhibit HSV infection 10,000-fold and are active against clinical isolates, including an acyclovir-resistant variant. The compounds formed stable complexes with glycoprotein B and inhibit viral binding, entry, and cell-to-cell spread. The effects may be long lasting due to the high affinity and stability of the sulfated compound-virus complex, as evidenced by surface plasmon resonance studies. The candidate microbicides retained their antiviral activities in the presence of cervical secretions and over a broad pH range. There was little reduction in cell viability following repeated exposure of human endocervical cells to these compounds, although a reduction in secretory leukocyte protease inhibitor levels was observed. These studies support further development and rigorous evaluation of these candidate microbicides.
Genital herpes is one of the most prevalent sexually transmitted infections (STIs) worldwide. Studies in developing countries show that the rates of herpes simplex virus (HSV) type 2 (HSV-2) seroprevalence among young adults range from 60 to 80% (23). What makes HSV so difficult to control is that most transmission occurs during periods of unrecognized or asymptomatic shedding (20). Epidemiological studies have shown that genital herpes enhances the transmission of human immunodeficiency virus (HIV) (6). HSV may facilitate HIV acquisition by disrupting epithelial cells, which release proinflammatory cytokines, and may activate target cells for HIV (27). The increasing prevalence of genital herpes, the frequency of clinical recurrences and asymptomatic shedding, and the association of HSV with HIV transmission highlight the urgent need for new strategies to control HSV.
One approach is the development of topical microbicides, self-administered agents designed for vaginal or rectal application to prevent STIs. Microbicides in development include (i) detergents or surfactants such as C31G that disrupt the viral envelope (17); (ii) acid-buffering agents that maintain the natural vaginal acidity (1, 38, 42); and (iii) sulfated or sulfonated polysaccharides (SPs), including cellulose sulfate (CS), polystyrene sulfonate (PSS), polymethylenehydroquinone sulfonate (PMHS), and PRO 2000, that may prevent viral entry (12, 26). Additional compounds in development include fusion inhibitors, natural antimicrobial peptides, and a mandelic acid condensation polymer designated SAMMA, which has no surfactant properties and which does not contain sulfur (13, 35).
Goals for microbicide development are that they be safe, affordable, stable, and active against multiple STIs. A major limitation of detergents or surfactants is their cytotoxicity; nonoxynol-9 (N-9), sodium dodecyl sulfate, and C31G are toxic to primary vaginal cells (14, 21). The in vitro findings for N-9 have been validated in clinical trials, as a result of which a recently completed study revealed a higher risk of HIV infection with frequent N-9 use relative to that with the use of a placebo (37).
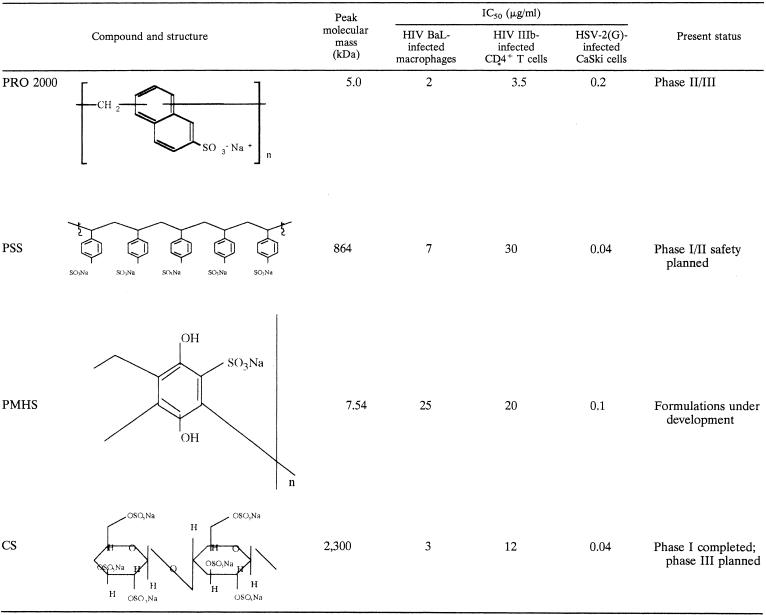
In contrast, SPs and SAMMA exhibit little or no cytotoxicity in vitro and are active against HIV, HSV, Neisseria gonorrhoeae, Chlamydia trachomatis, and human papillomavirus (Table 1) (2, 3, 12). PRO 2000 and PSS prevent genital herpes in murine models (4, 12). Several phase I trials demonstrate that PRO 2000 is safe and well tolerated following vaginal or penile application (22, 33). For most clinical trials, safety is monitored by colposcopic examination for cervical lesions (11). However, little is known about the more subtle effects of the compounds on the mucosal environment, including induction of inflammation and interference with host defense mechanisms. In addition, microbicides may alter the production of soluble factors that directly or indirectly protect against STIs, such as secretory leukocyte protease inhibitor (SLPI) (24).
TABLE 1.
Summary of structures, antiviral activities, and present statuses of SPs
The studies described here were undertaken to expand our understanding of the in vitro safety and anti-HSV activities of several SPs. Specifically, we explored (i) the spectrum of antiviral activities with clinical isolates; (ii) the effects of repeated application of SPs on human cervical epithelial cell viability, cytokines, and SLPI; (iii) the stabilities of the compounds in the presence of cervical fluid and over a broad pH range; and (iv) the mechanism of anti-HSV activity.
MATERIALS AND METHODS
Cells.
CaSki cells (a human cervical cell line) were obtained from the American Type Culture Collection (Manassas, Va.) and maintained as described previously (15). Immortalized human epithelial cells (End1/E6E7 cells; a generous gift from R. N. Fichorova and D. J. Anderson, Harvard Medical School, Boston, Mass.) derived from a healthy human endocervix were maintained in keratinocyte serum-free medium (GIBCO BRL Life Technologies, Gaithersburg, Md.), as described previously (7, 8).
Viral strains and glycoproteins.
The HSV-2 strains used were laboratory wild-type isolates 333 and G. Clinical isolates MMA and WTWIA (gifts from P. Spear, Northwestern University) were obtained from patients attending an STI clinic and have been partially sequenced (34). DT-1 and DT-2 are acyclovir-sensitive and -resistant strains, respectively, isolated from a neonate (25). HSV-2 glycoprotein B (gB-2) was generated by using the Bac-to-Bac system (Gibco) and was purified by heparin-affinity chromatography (13).
Reagents.
N-9 and chondroitin sulfate C were purchased from Sigma, St. Louis, Mo. Topical Prevention for Conception and Disease (Chicago, Ill.) provided CS, PSS, PMHS, and SAMMA. PRO 2000, a naphthalene sulfonate polymer, was from Indevus Pharmaceuticals Inc., Lexington, Mass. (29).
Infectivity assays.
Plaque assays were conducted to evaluate the effects of the candidate microbicides on HSV infection (14). End1/E6E7 cells were plated in 96-well dishes, exposed to serial dilutions (0.01 to 100 μg/ml) of the candidate microbicides for 1 h at 37°C, and then challenged with serial 10-fold dilutions of HSV (0.01 to 1,000 PFU/cell) in triplicate. The viral titer in the presence of each concentration of drug was determined by counting the plaques at 48 h postinfection after immunostaining by the black plaque assay (15). Only wells in which the number of plaques ranged from 25 to 250 were used to calculate the viral titer. Alternatively, duplicate wells of CaSki cells were infected with clinical isolates of HSV-2 in the absence or presence of different concentrations of each candidate microbicide. The plaques were counted at 48 h postinfection, and the concentrations of the drugs that inhibited 50 and 90% of the viral infection (IC50 and IC90, respectively) were determined from dose-response curves generated from three independent experiments.
Synchronized infection assays.
Time course assays were conducted to determine which steps in viral infection were inhibited by the SPs (12). CaSki cells were precooled to 4°C, and each well was inoculated with ∼200 to 1,000 PFU of virus for 3 h. Unbound virus was removed by washing the wells, and the cells were then shifted to 37°C to allow penetration for 15 min. The cell monolayers were exposed to citrate buffer (pH 3.0) for 1 min to inactivate any nonpenetrated virus. The cells were washed and overlaid with medium. The drugs were added either during the 4°C period, at the time that the cells were transferred to 37°C, or immediately after citrate treatment for 1 h followed by a wash to determine if the SPs act at the level of binding, penetration, or postentry, respectively.
Preincubation of virus or cells with microbicides.
To examine whether the drugs interact primarily with HSV or epithelial cells, or both, the SPs were preincubated with ∼104 PFU of HSV-2(G) per ml for 1 h at 37°C, and the mixture was diluted 50-fold to yield ∼200 PFU/well on control plates and inoculated onto monolayers of CaSki cells in duplicate on six-well dishes (13). For comparison, diluted virus (∼200 PFU) was preincubated for 1 h with various concentrations of drug and the mixture was plated (without dilution) onto the cells. Alternatively, the drugs (or phosphate-buffered saline [PBS] as a control) were preincubated with the cells for 1 h at 37°C and then either washed extensively or not washed prior to inoculation with HSV-2(G).
Binding studies.
Binding studies were conducted to determine if the SPs inhibit binding of HSV-2 or gB-2 to cells (13). CaSki cells were exposed to purified HSV-2(G) for 5 h at 4°C or to recombinant gB-2 at 37°C for 1 h in the absence or presence of various concentrations of the SPs. Unbound virus or gB-2 and compound were removed by washing, and the cell-bound virus or glycoprotein was analyzed by preparing Western blots of cell lysates and probing the blots with anti-gD or anti-gB monoclonal antibodies (MAbs), respectively (MAb 1103 for virus and MAb 1123 for gB-2; Goodwin Institute, Plantation, Fla.).
SPR assays.
Surface plasmon resonance (SPR) analyses were performed on a BIAcore 3000 system with CM5 sensor chips (Biacore Inc., Piscataway, N.J.). gB-2 was diluted to a final concentration of 40 μg/ml in 10 mM sodium acetate buffer at pH 4.5 and immobilized on the chip surface by the amine coupling method. The carboxyl groups on the sensor surfaces were activated with a mixture of 0.2 M N-ethyl-N′-(3-diethylamino-propyl) carbodiimide (EDC) and 0.05 M N-hydroxysuccinimide (NHS), and the protein was coupled under the continuous flow of HBS-EP buffer (10 mM HEPES with 0.15 M NaCl, 3.4 mM EDTA, and 0.005% surfactant P20 [pH 7.4]). A flow cell containing an unmodified dextran surface treated with EDC and NHS and not exposed to the coupling protein served as the control flow-cell surface for each chip. Residual reactive groups were blocked with 1.0 M ethanolamine hydrochloride at pH 8.5 in control and test flow-cell surfaces. Immobilization levels of 200 resonance units were achieved for gB-2. For kinetic binding determinations, increasing concentrations of compounds were diluted in HBS-EP buffer, injected over the test and control flow-cell surfaces at 30 μl/min for specified times, and then dissociated from the protein over time. The time parameters were as follows: (i) PRO 2000, injection time, 150 s; dissociation time, 1,000 s; (ii) PMHS, injection time, 300 s; dissociation time, 1,800 s; (iii) SAMMA, injection time, 60 s; dissociation time, 300 s; and (iv) PSS, injection time, 90 s; dissociation time, 500 s. The chip surfaces were regenerated with 100-s injections of 0.05% sodium dodecyl sulfate. Data transformation and overlay plots were prepared with BIA evaluation (version 3.1) software. Binding signals were corrected for nonspecific binding by subtracting the signal for the control flow cell. The affinities of the binding interactions between gB-2 and the compounds were calculated by using the 1:1 Langmuir binding model in the BIA evalution software.
Infectious center assays.
Infectious center assays were performed as described previously (28, 31). CaSki cells (donor cells) were exposed to virus at 37°C to allow entry (multiplicity of infection [MOI], 5 PFU/cell). Cells were washed with citrate buffer to inactivate residual extracellular virus 1 to 2 h after infection. Then, 4 to 5 h after infection, the infected cells were detached with trypsin-EDTA and counted, and ∼500 to 1,000 infected cells were plated onto duplicate monolayers of uninfected cells in medium containing pooled human immunoglobulin G (IgG; Sigma) in the presence of the SPs or control buffer. The pooled human immunoglobulin neutralizes infection by virus released into the medium. Plaques were quantified after 48 h by a black plaque immunoassay.
Cytotoxicity and detection of soluble immunobiological mediators.
Cell viability following exposure of End1/E6E7 cells to microbicides (dose range, 0.1 to 1 mg/ml) was determined using a cell proliferation assay (CellTiter96; Promega). End1/E6E7 cells were grown in 96-well plates to ∼80% confluence and were exposed to compounds diluted in keratinocyte serum-free medium for 24 h (acute toxicity study) or for 2 h daily for 6 consecutive days (chronic toxicity study). Controls included cells exposed to medium containing no compound. For the chronic toxicity study, the cells were exposed to microbicides for 2 h, washed thrice, and overlaid with fresh medium daily. Cell viability was assayed either following the 24-h exposure (acute) or 24 h after the 6th consecutive day of exposure (D7). In parallel, cell culture supernatants were collected on D0, D3 (24 h after the second application of drug), and D7 (24 h after the sixth application of drug) and assayed for cytokines (IL-1α and IL-1β), the chemokine IL-8, and SLPI with ELISA kits (R & D Systems, Cambridge, Mass.). To determine whether residual drug in the culture supernatants interfered with the ELISAs, culture supernatants obtained from D7 samples were spiked with the recombinant standards, and the results were compared with those obtained with standards diluted in the kit diluent.
Stability of microbicides in cervical fluid or over the pH range 4.0 to 8.0.
Following institutional review board (Mount Sinai School of Medicine) approval, cervicovaginal lavage (CVL) fluid samples were obtained from volunteers by washing with 10 ml of sterile normal saline directed at the cervical os and posterior vaginal wall. The CVL fluid sample was immediately transported on ice to the laboratory and centrifuged at 2,000 to 2,500 rpm (Beckman Coulter Allegra GR) for 10 to 15 min at 4°C. A cocktail of antibiotics was added, and the supernatants from the CVL fluid samples were aliquoted and stored at −80°C. The CVL fluid samples had a pH of 5.0 and a protein concentration of ∼0.2 mg/ml. SPs were diluted in CVL or control buffer (sterile saline [pH 5.0] with 0.2 mg of bovine serum albumin [BSA] per ml) for 1 h at 37°C. After incubation, the mixtures were further diluted 1:10 in PBS-BSA so that the final pH was 7.0, mixed with ∼200 to 500 PFU of HSV-2(G) (200 μl of mixture and 100 μl of virus), and plated onto 12-well dishes of CaSki cells in triplicate. Plaques were counted after 48 h. For the pH experiments, the SPs were mixed with Tris-buffered saline (TBS; pH range, 4.0 to 8.0). After 1 h, the mixtures were diluted 1:10 in TBS (pH 7.0) such that the final concentration of the SPs was 0.01 to 100 μg/ml and were then mixed with virus for plaque assays.
Statistical analysis.
The effects of the microbicides on cytokines were compared by a Mann-Whitney test and were done with the StatView program.
RESULTS
Antiviral activity.
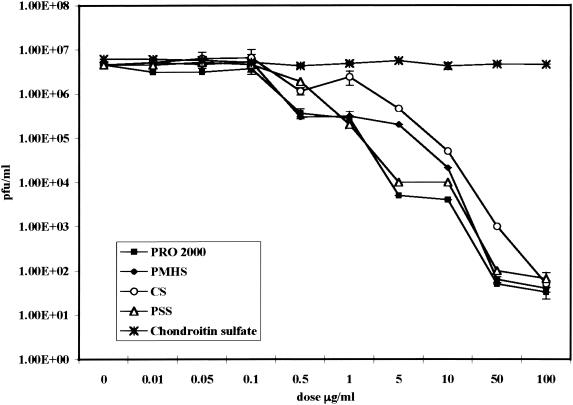
Previous studies (19) have shown that at concentrations between 0.04 and 0.2 μg/ml the four SPs inhibit at least 50% of HSV-2(G) infection when CaSki cells were challenged with ∼0.01 PFU/cell (Table 1). We extended these observations and quantified the anti-HSV activities following exposure of End1/E6E7 cells to the virus at 0.01 to 1,000 PFU/cell by determining the viral titer in the presence of each concentration of drug, as indicated in Materials and Methods. Chondroitin sulfate C, a sulfated glycosaminoglycan, which does not inhibit HSV infection, was included as a control (41). The results are summarized in Fig. 1 and show that, at a concentration of 100 μg/ml, the SPs produce a 5-log reduction in HSV-2 infection, whereas chondroitin sulfate had no antiviral activity. The SPs also inhibited infection by clinical isolates of HSV-2 (MMA, WTWIA, DT-1) as well as an acyclovir-resistant strain (DT-2). The IC50 and IC90 of each drug are shown in Table 2.
FIG. 1.
Viral titers in the presence of candidate SPs. End1E6/E7 cells were pretreated with the indicated dose of compound for 1 h and were then challenged with serial 10-fold dilutions of HSV-2(G) (MOI, 0.01 to 1,000 PFU/cell). Plaques were counted 48 h postinfection. The results are the means ± standard deviations of two independent experiments conducted in duplicate.
TABLE 2.
Effects of candidate SPs on infection with clinical isolatesa
| Clinical isolate | IC50 (μg/ml)/IC90 (μg/ml)
|
|||
|---|---|---|---|---|
| PRO 2000 | PSS | CS | PMHS | |
| WTWIA | 0.1/1.0 | 0.07/0.7 | 0.6/8.0 | 0.4/1.0 |
| MMA | 0.1/1.0 | 0.07/0.7 | 0.6/8.0 | 0.4/1.0 |
| DT-1 | 0.1/1.0 | 0.07/0.7 | 0.6/8.0 | 0.4/1.0 |
| DT-2 | 0.1/1.0 | 0.07/0.7 | 0.6/8.0 | 0.4/1.0 |
CaSki cells were infected with clinical isolates (MMA, WTWIA, DT-1) or an acyclovir-resistant clinical isolate (DT-2) of HSV-2 in the absence or presence of different concentrations of each candidate drug. The IC50s and IC90s were determined from dose-response curves generated from three independent experiments performed in duplicate.
Mechanism of anti-HSV activity.
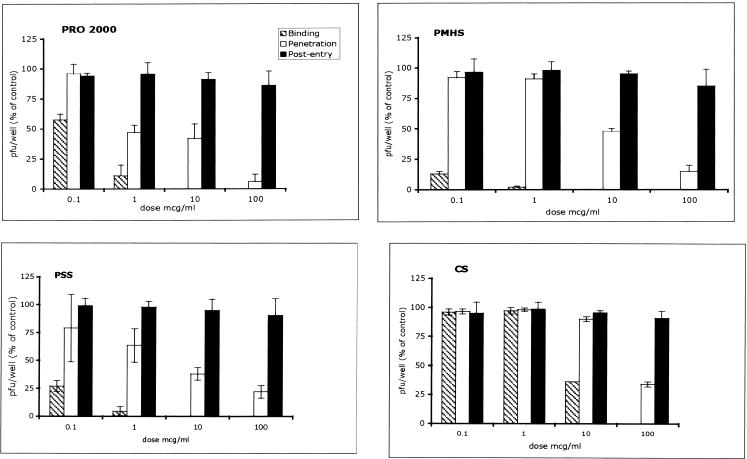
Several approaches were adopted to examine which steps in HSV-2 infection that SPs inhibit. Studies with synchronized infections were conducted; and the compounds were added during binding (4°C), at the time of the shift of the temperature to 37°C (penetration), or immediately after citrate treatment. The results are shown in Fig. 2. At concentrations <1 μg/ml, PRO 2000, PMHS, and PSS inhibited HSV infection if each of the drugs was added at the time of binding. Tenfold higher concentrations of CS were required to inhibit viral infection. Notably, the SPs retained variable amounts of activity when they were added at the time that the cells were transferred to 37°C (penetration) but lost activity if they were added postentry. The anti-HSV activity persisted if the HSV particles were pretreated with drugs for 1 h, followed by dilution of the mixture 50-fold. The antiviral activity was reduced, but not abolished, if the cells were exposed to the drug for 1 h and then washed prior to infection (Table 3). These results suggest that the primary mechanism of anti-HSV activity is inhibition of viral binding by targeting the viral envelope glycoproteins.
FIG. 2.
Mechanism of anti-HSV activity. Synchronized infections were conducted as described in Materials and Methods. Each panel shows a dose-response to the indicated compound when the compound was added during the 4°C binding period (binding; hatched bars), at the time of temperature shift (penetration; open bars), or immediately postentry (black bars). The results are presented as the PFU formed in the presence of drug as a percentage of the PFU formed in the presence of medium and are the means ± standard deviations of three independent experiments performed in duplicate.
TABLE 3.
Infection following preincubation of cells or virus for 1 h with SPs or control medium
| SP | Antiviral activity (% control activity) at the following times with the indicated dose (μg/ml)a:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Pretreatment of cells with no washing
|
Pretreatment of cells with washing
|
Pretreatment of virus, no dilution
|
Pretreatment of virus, 50× dilutionb
|
|||||
| 1 | 10 | 1 | 10 | 1 | 10 | 0.02 | 0.2 | |
| PRO 2000 | 0 | 0 | 46 + 2 | 22 ± 4 | 0 | 0 | 0 | 0 |
| PMHS | 3 ± 3 | 0 | 37 + 0 | 20 ± 4 | 0 | 0 | 1 ± 0.7 | 0 |
| PSS | 2 ± 3 | 0 | 69 ± 3 | 55 ± 2 | 7 ± 3 | 0 | 0 | 0 |
| CS | 7 ± 8 | 0 | 62 + 22 | 53 ± 1 | 15 ± 0 | 0 | 27 ± 4 | 16 ± 1.4 |
The results are presented as the means ± standard deviations of at least two independent experiments conducted in duplicate.
The doses are the final concentrations of the SP after dilution.
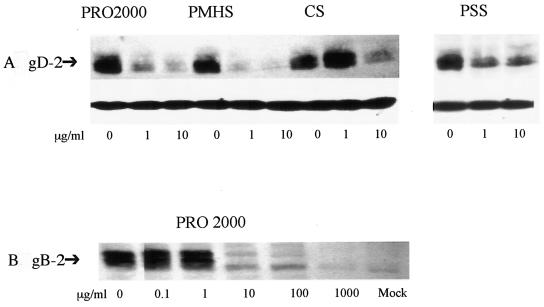
To test this hypothesis further, the effects of SP on HSV-2(G) or gB-2 binding to cells were examined. gB-2 plays the key role in mediating binding of HSV-2 to cells and is also required for viral penetration and cell-to-cell spread (5). At concentrations between 1 and 10 μg/ml, the SPs substantially reduced the levels of binding of HSV-2(G) to cells (Fig. 3A). In addition, the four SPs also reduced the levels of binding of gB-2 to cells; a representative blot with PRO 2000 is shown in Fig. 3B. Similar results were obtained with PSS, PMHS, and CS. The abilities of the SPs to target gB-2 were further explored by SPR analyses. gB-2 was immobilized on the surface of the chip, and the affinities of the interactions between gB-2 and the test compounds were calculated. SAMMA, a chemically distinct microbicide that prevents viral binding, was included for comparison (13). The results are shown in Table 4. The compounds formed stable complexes with gB-2; PSS bound to gB-2 with a higher affinity than any of the other compounds. SAMMA had a substantially lower affinity of binding. We were unable to obtain conclusive results for CS, possibly caused by steric interference due to its higher molecular mass.
FIG. 3.
Effects of SP on binding of HSV-2(G) or recombinant gB-2 to cells. CaSki cells were exposed to purified HSV-2(G) (0.1 PFU/cell in the presence or absence of the indicated concentrations of SP) (A). Bound virus was detected by analyzing Western blots of cell lysates for gD; the blots were also probed with an MAb to β-actin to control for cell loading. Alternatively, cells were exposed to recombinant gB-2 (10−5 μg/cell) in the absence or presence of increasing concentrations of PRO 2000, and bound gB-2 was detected by analyzing Western blots of cell lysates (B). Blots are representative of those found in three independent experiments.
TABLE 4.
Kinetic analysis of gB-2 binding to candidate topical microbicidesa
| Microbicide | Ka (M−1 s−1) | Kd (s−1) | KD (kd/Ka) (M) |
|---|---|---|---|
| PRO 2000 | 1,340 | 8.71 × 10−4 | 6.5 × 10−7 |
| PSS | 159,000 | 1.5 × 10−3 | 9.41 × 10−9 |
| PMHS | 26,900 | 5.3 × 10−4 | 1.97 × 10−8 |
| SAMMA | 183 | 4.61 × 10−3 | 2.52 × 10−5 |
The doses tested for each compound were (i) 3.125, 6.25, 12.5, 25, 50, 75, 100, and 200 μg/ml for PRO 2000; (ii) 62.5, 125, and 250 μg/ml for PSS; (iii) 0.825, 1.65, 3.3, 6.6, 13.2, 26.4, 52.8, and 105.6 μg/ml for PMHS; and (iv) 1, 50, 100, 200, 500, and 1,000 μg/ml for SAMMA. Binding signals were corrected for nonspecific binding by subtracting the signal of the control flow cell. The rate of association (Ka), rate of dissociation (Kd), and affinity constant (KD) were calculated by using the 1:1 Langmuir binding model in the BIA evaluation software.
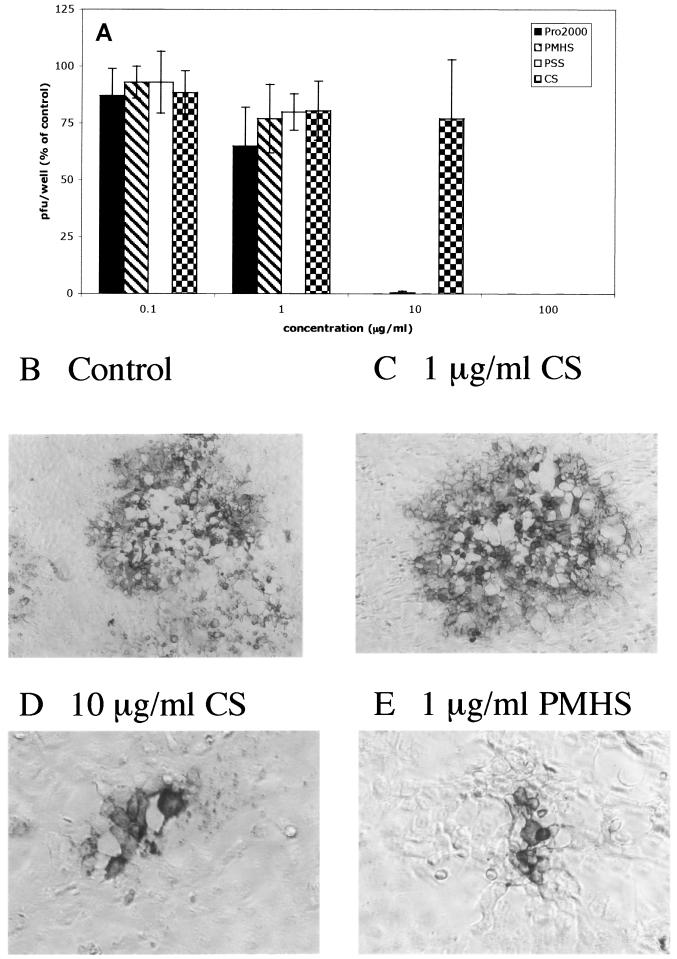
To explore the possibility that SP might also prevent the spread of virus from infected cells to neighboring uninfected cells, infectious center assays were conducted. Uninfected cells were cocultured with ∼500 to 1,000 infected cells in the presence of pooled immunoglobulin. Under these conditions, HSV presumably spreads via intercellular junctions created by local fusion events between the plasma membranes of infected and uninfected cells, but the virus released is neutralized by IgG (28, 31). The SPs prevented cell-to-cell spread, as evidenced by complete inhibition of plaque formation by PRO 2000, PMHS, and PSS, each at a concentration of 10 μg/ml. Tenfold higher concentrations of CS were required to achieve the same degree of inhibition (Fig. 4A). Moreover, the plaques that formed in the presence of lower concentrations of the SPs were substantially smaller than control plaques. Representative results obtained with CS and PMHS are shown (Fig. 4B to E). Similar results were observed with PRO 2000 and PSS. These findings suggest that the SPs prevent cell-to-cell spread of virus and may be effective at inhibiting transmission of cell-associated HSV.
FIG. 4.
SPs prevent cell-to-cell spread. From 500 to 1,000 infected cells (harvested at 4 to 5 h postinfection) were plated onto duplicate monolayers of uninfected cells in medium containing pooled human IgG (Sigma) in the presence of an SP or control buffer, and the number of plaques formed were counted following immunostaining 48 h later. The results are presented as the PFU formed in the presence of each drug as a percentage of the PFU formed in the presence of control medium, and each value is the mean ± standard deviation obtained from at least two independent experiments conducted in duplicate (A). Representative plaques viewed under ×100 (B and C) or ×200 (D and E) magnification are shown.
Cytotoxicity.
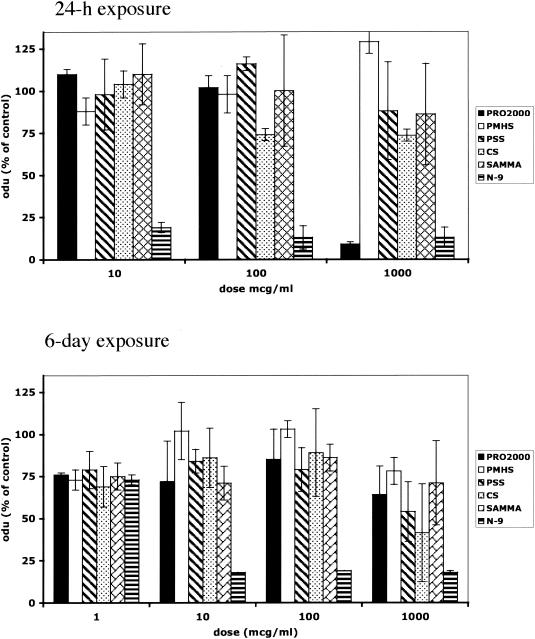
Cell viability and the effects on cytokines and other soluble immune mediators following acute or chronic exposure of End1/E6E7 cells to SP, N-9, or SAMMA were examined. At concentrations as high as 1 mg/ml, PMHS, PSS, CS, and SAMMA had little or no effect on End1/E6E7 cell viability following a 24-h exposure (Fig. 5A). However, at 1 mg/ml, PRO 2000 did markedly reduce cell viability. Importantly, little cytotoxicity was observed in the setting of repeated exposure (2 h daily) to any of the candidate microbicides (Fig. 5B). In contrast, N-9 was highly toxic following either acute or repeated exposure at all concentrations tested.
FIG. 5.
Cell viability was quantified by an MTS assay following acute exposure (24 h of exposure) or repeated exposure (exposure for 2 h/day for 6 days) of End1/E6E7 cells to the indicated concentrations of SPs, SAMMA, or N-9. Results are presented as optical densitometry units (odu) obtained from cells treated with the indicated concentration of drug as a percentage of the optical densitometry units obtained from cells incubated in medium, and each value is the mean ± standard deviation obtained from at least two independent experiments conducted in triplicate. The concentrations of N-9 used in these experiments were 0.001, 0.01, and 0.1% for acute toxicity and 0.0001, 0.001, 0.01, and 0.1% for chronic toxicity.
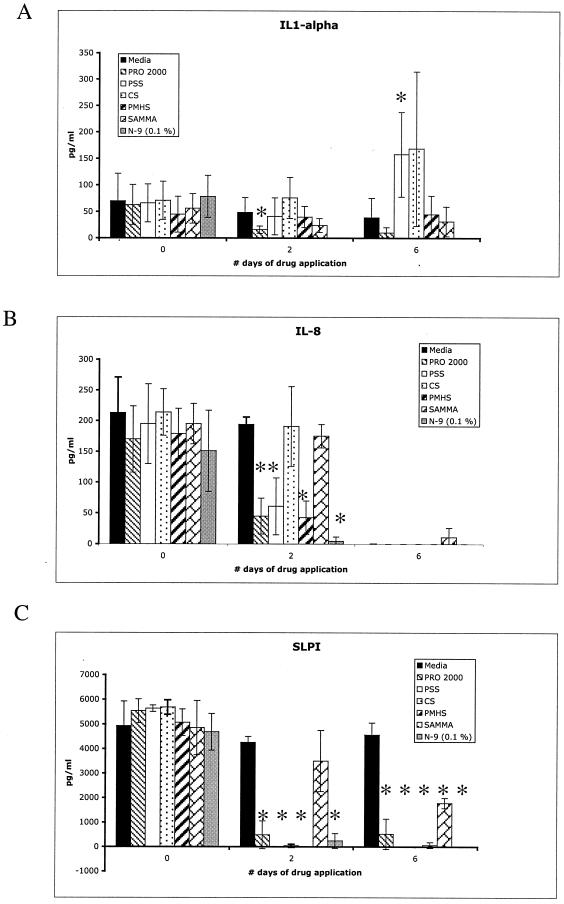
To explore the possible impact of repeated applications of compounds on soluble mediators of inflammation, cell culture supernatants were collected at the baseline (D0) and 24 h following the second or sixth daily application of 100 μg of SP or SAMMA per ml or 0.01% N-9 and evaluated for IL-1α, IL-1β, IL-8, and SLPI levels by ELISA. These soluble mediators were selected because they are expressed by End1/E6E7 cells and may be important in host responses to STIs (7). Dilution of the protein standards for each of the ELISAs in culture supernatants obtained from cells treated with drugs did not interfere with protein recovery (data not shown). There was a significant decrease in IL-1α levels following 2 days of exposure but not 6 days of exposure to PRO 2000 (P = 0.04). Exposure to N-9 significantly reduced IL-1α levels at both time points (P = 0.02) (Fig. 6A). In contrast, PSS increased IL-1α levels following six daily applications in vitro (P = 0.02). IL-8 levels were significantly reduced relative to those for cells in medium only following 2 days of exposure to PRO 2000, PMHS, PSS, and N-9 but not following 2 days of exposure to SAMMA or CS (Fig. 6B). Following 6 days in culture, IL-8 was not detected in any of the culture supernatants, even in the absence of the microbicides. The IL-1β levels were very low (<10 pg/ml) in all samples (data not shown). The biological significance of the modest increase in IL-1α levels following exposure to PSS and the reduction in IL-8 levels following 2 days of exposure to several of the SPs is unclear. Notably, the concentration of SLPI, which may be important in protecting the host from HIV, was substantially reduced following acute or chronic exposure to the SPs at all time points (P = 0.02) (Fig. 6C). In contrast, SAMMA had no significant effect following 2 days of exposure, but it did modestly reduce SLPI levels after the 6th day of exposure (P = 0.02). Exposure to N-9 led to marked reductions in the levels of all of the soluble mediators, presumably reflecting cell death.
FIG. 6.
Levels of IL-1-α (A), IL-8 (B), and SLPI (C) in culture supernatants of End1/E6E7 cells at the baseline (D0) and 24 h after 2 or 6 days of repeated exposure to 100 μg of SP or SAMMA per ml and 0.1% N-9. Each value is the mean ± standard deviation obtained from at least two independent experiments conducted in triplicate. The asterisks indicate significant differences (P < 0.05) between the measured values obtained in the presence of the indicated drug compared with the values obtained with medium only (Mann-Whitney test).
Stabilities of the SPs over a broad pH range and in the presence of cervical secretions.
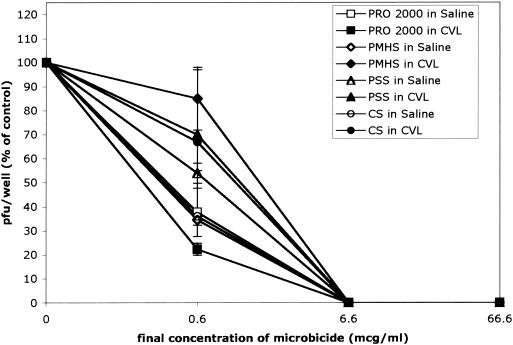
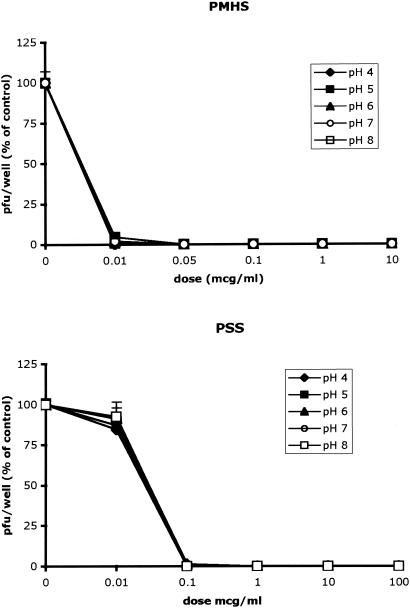
To test whether the SPs retained anti-HSV activities in the presence of cervical fluid and over the pH range 4.0 to 8.0, the microbicides were diluted in CVL fluid obtained from three subjects, control buffer, or 0.1 M Tris buffers (pH 4.0 to 8.0) for 1 h; and plaque assays were conducted as described in Materials and Methods. The SPs retained their anti-HSV activities in the presence of cervical secretions and completely inhibited viral infection at concentrations <10 μg/ml (Fig. 7). Moreover, the SPs retained their antiviral activities following preincubation over the full pH range; representative results for PMHS and PSS are shown in Fig. 8, and similar results were obtained with CS and PRO 2000.
FIG. 7.
The SP compounds (concentrations, 0 to 1 mg/ml) were diluted in CVL fluid (pH ∼5.0) or saline (pH ∼5.0) containing 0.2 mg of BSA per ml for 1 h at 37°C and neutralized in PBS (pH 7.4) before they were mixed with virus and inoculated onto CaSki cells. The results are presented as the PFU formed in the presence of drug as a percentage of the PFU formed in the absence of drug. The results are the means of three independent experiments, each conducted in duplicate with CVL fluid obtained from a different subject; error bars indicate standard deviations.
FIG. 8.
The microbicides (concentrations, 0 to 1 mg/ml) were diluted in Tris at pH 4.0, 5.0, 6.0, 7.0, or 8.0 for 1 h at 37°C and neutralized in Tris (pH 7.0) before they were mixed with virus and inoculated onto CaSki cells. The results are presented as the PFU formed in the presence of drug as a percentage of the PFU formed in the absence of drug. The results are the means of three independent experiments each conducted in duplicate; error bars indicate standard deviations.
DISCUSSION
With a growing epidemic of genital herpes and with more than 40 million people worldwide living with HIV infection, there is an urgent need to identify safe and effective topical microbicides (18). These studies extend our understanding of one major class of microbicides, SPs, by focusing on their activities against HSV. Using immortalized human endocervical cells, we found that the SPs reduce the levels of HSV infection ∼100,000-fold, are active even when the cells are exposed to virus at a very high MOI, and retain their activities against clinical isolates. Additionally, the infectious center studies indicate that SPs also prevent infection by cell-associated virus and cell-to-cell spread. This may be due to the ability of the drugs to bind to the envelope glycoproteins required for cell-to-cell spread. The inhibitory effect on cell-to-cell spread may be important, because the source of sexually transmitted virus may be intracellular.
One of the targets of SP is gB-2. gB-2 plays the predominant role in mediating HSV-2 binding to cells, but it is also required (along with gD and the heterodimeric complex of gH and gL) for penetration and cell-to-cell spread (5, 32). We used optical biosensor technology to quantify the affinity (KD) and the stability (Kd) of the interactions between gB-2 and microbicides. The values obtained show that the complexes of gB-2 and SPs are highly stable (Kd range, 1.5 × 10−3 to 9 × 10−4 s−1). In an earlier report, biosensor technology was used to investigate the interaction of a truncated form of gB-2 with heparin (39). The gB-2 ectodomain bound to biosensor surfaces coated with heparin with a KD of 7.7 × 10−7 M and a Kd of 4 × 10−4 s−1. The experimental conditions in our studies differed, in that the biosensor surfaces were coated with gB-2. The results obtained, however, suggest that the complexes of microbicides and gB-2 are at least as stable as gB-2 and heparin complexes and have affinities comparable to those observed for gB-2 and heparin. Moreover, the affinities and stabilities are at least as great as those observed for gC-1 and gC-2 with heparan sulfate and greater than those observed for gD and one of its coreceptors, HveA (30, 40). The stability of the SP-glycoprotein complexes may be critical for their activities as microbicides, since a highly stable complex may function to irreversibly block the binding of HSV to cells. This notion is supported by the observation that anti-HSV activity is retained if virus is first pretreated with the drug, followed by dilution to subinhibitory concentrations (Table 3). It should be noted that SPs must also interact with gC-2, gC-1, and gB-1. This follows from the observations that SPs inhibit the binding of HSV-1 to cells, mediated primarily by gC-1 (16), as well as the binding of variants of both serotypes with gC or gB deletions (data not shown). The affinities of the SPs for these other glycoproteins and the abilities of these compounds to bind to other envelope glycoproteins were not evaluated.
Factors in the genital tract and changes in pH may influence the activities of microbicides. Vaginal secretions from healthy women of reproductive age are characteristically acidic (pH 4 to 5) but are neutralized by alkaline semen (pH 7 to 8). In addition to pH, antimicrobial polypeptides and other components of vaginal fluid could interfere with microbicide activity. These studies demonstrate that SPs retain their activities against HSV over a broad pH range (4.0 to 8.0) and in the presence of cervical fluid. However, these studies should be replicated once an optimal formulation has been identified, as the excipients in the formulation may modify activity or stability.
A fundamental principle in microbicide development is ensuring that candidate compounds are nontoxic to the genital mucosa. The importance of more fully investigating the toxicity profile of any potential agent has been highlighted by a recently completed phase III clinical trial which showed that N-9 may actually increase the rate of HIV transmission (37). The relevant assays for monitoring toxicity in vitro or in vivo have not been established. Inflammatory responses to topical agents may increase the rates of transmission or acquisition of agents of STIs by several mechanisms. An inflammatory response may recruit target cells into the area; may activate macrophages or quiescent T cells, rendering them more susceptible to HIV or HSV; and may induce HIV replication in the reservoir of latently infected T cells (9). A recent study showed that N-9 caused IL-1α and IL-1β release and a decrease in SLPI levels by cervical epithelial cells in vitro (8). These in vitro findings may help explain the observed increase in the rate of HIV acquisition among sex workers who frequently applied N-9 (37).
We examined the in vitro effects of the SPs and SAMMA on IL-1α, IL-1β, IL-8, and SLPI production following repeated exposure of immortalized human endocervical cells to these compounds. The most striking finding was that SLPI levels were substantially reduced following exposure to the SPs but not following exposure to SAMMA. SLPI is present in saliva and cervical and bronchial secretions and has been shown to inhibit HIV infection in vitro (24). Its effects on HSV are unknown. The biological significance of these findings is unclear, particularly in view of the proteinase-inhibitory activity of at least PSS (2) and PMHS (R. A. Anderson, unpublished data). It remains to be determined whether the reductions in SLPI levels observed in these studies would increase susceptibility to HIV. This possibility needs to be verified in vitro, in animal models, and in pilot clinical studies.
These studies did not uncover substantial differences in the mechanisms of anti-HSV activity between the four SPs and SAMMA, despite the structural dissimilarities between SAMMA and the SPs. Higher concentrations of CS were needed to achieve the same degree of antiviral activity. However, the peak molecular mass of CS is greater than those of the other SPs, which may contribute to the differences noted when the drugs are compared on a weight basis. Notably, differences were observed with respect to cytotoxicity and effects on SLPI levels.
These studies support the concept that SPs should provide protection against HSV by preventing viral binding, entry, and cell-to-cell spread. The effects may be long lasting due to the high affinity and stability of the SP-virus complex. Importantly, these compounds retain their antiviral activities in the presence of cervical secretions and over a broad pH range. Prior to the initiation of large-scale clinical trials, more rigorous evaluation of the safety of this class of compounds and all topical formulations should include a thorough investigation of the changes in inflammatory cells and cytokines and the effects on host defenses following repeated applications.
Acknowledgments
This work was supported by Public Health Service grants AI37940, HD43733, and HD41763; subproject CIG-99-39, provided by the CICCR Program of the Contraceptive Research and Development (CONRAD) Program, Eastern Virginia Medical School; and contract CSA-98-229 from CONRAD.
We thank R. Fichorova and D. Anderson for the generous gift of the immortalized human endocervical cells and Carolyn Bertozzi (Department of Chemistry, University of California, Berkeley) for use of the BIAcore 3000 instrument.
The views expressed by the authors do not necessarily reflect the views of the funding agencies.
REFERENCES
- 1.Amaral, E., A. Faundes, L. Zaneveld, D. Waller, and S. Garg. 1999. Study of the vaginal tolerance to Acidform, an acid-buffering, bioadhesive gel. Contraception 60:361-366. [DOI] [PubMed] [Google Scholar]
- 2.Anderson, R. A., K. Feathergill, X. Diao, M. Cooper, R. Kirkpatrick, P. Spear, D. P. Waller, C. Chany, G. F. Doncel, B. Herold, and L. J. Zaneveld. 2000. Evaluation of poly(styrene-4-sulfonate) as a preventive agent for conception and sexually transmitted diseases. J. Androl. 21:862-875. [PubMed] [Google Scholar]
- 3.Anderson, R. A., K. A. Feathergill, X. H. Diao, M. D. Cooper, R. Kirkpatrick, B. C. Herold, G. F. Doncel, C. J. Chany, D. P. Waller, W. F. Rencher, and L. J. Zaneveld. 2002. Preclinical evaluation of sodium cellulose sulfate (Ushercell) as a contraceptive antimicrobial agent. J. Androl. 23:426-438. [PubMed] [Google Scholar]
- 4.Bourne, N., D. I. Bernstein, J. Ireland, A. J. Sonderfan, A. T. Profy, and L. R. Stanberry. 1999. The topical microbicide PRO 2000 protects against genital herpes infection in a mouse model. J. Infect. Dis. 180:203-205. [DOI] [PubMed] [Google Scholar]
- 5.Cheshenko, N., and B. C. Herold. 2002. Glycoprotein B plays a predominant role in mediating herpes simplex virus type 2 attachment and is required for entry and cell-to-cell spread. J. Gen. Virol. 83:2247-2255. [DOI] [PubMed] [Google Scholar]
- 6.Cohen, M. S. 1998. Sexually transmitted diseases enhance HIV transmission: no longer a hypothesis. Lancet 351:5-7. [DOI] [PubMed] [Google Scholar]
- 7.Fichorova, R. N., and D. J. Anderson. 1999. Differential expression of immunobiological mediators by immortalized human cervical and vaginal epithelial cells. Biol. Reprod. 60:508-514. [DOI] [PubMed] [Google Scholar]
- 8.Fichorova, R. N., L. D. Tucker, and D. J. Anderson. 2001. The molecular basis of nonoxynol-9-induced vaginal inflammation and its possible relevance to human immunodeficiency virus type 1 transmission. J. Infect. Dis. 184:418-428. [DOI] [PubMed] [Google Scholar]
- 9.Garcia-Blanco, M. A., and B. R. Cullen. 1991. Molecular basis of latency in pathogenic human viruses. Science 254:815-820. [DOI] [PubMed] [Google Scholar]
- 10.Garg, S., R. A. Anderson, C. J. Chany II, D. P. Waller, X. H. Diao, K. Vermani, and L. J. Zaneveld. 2001. Properties of a new acid-buffering bioadhesive vaginal formulation (ACIDFORM). Contraception 64:67-75. [DOI] [PubMed] [Google Scholar]
- 11.Harrison, P. F., Z. Rosenberg, and J. Bowcut. 2003. Topical microbicides for disease prevention: status and challenges. Clin. Infect. Dis. 36:1290-1294. [DOI] [PubMed] [Google Scholar]
- 12.Herold, B. C., N. Bourne, D. Marcellino, R. Kirkpatrick, D. M. Strauss, L. J. Zaneveld, D. P. Waller, R. A. Anderson, C. J. Chany, B. J. Barham, L. R. Stanberry, and M. D. Cooper. 2000. Poly(sodium 4-styrene sulfonate): an effective candidate topical antimicrobial for the prevention of sexually transmitted diseases. J. Infect. Dis. 181:770-773. [DOI] [PubMed] [Google Scholar]
- 13.Herold, B. C., I. Scordi-Bello, N. Cheshenko, D. Marcellino, M. Dzuzelewski, F. Francois, R. Morin, V. M. Casullo, R. A. Anderson, C. Chany II, D. P. Waller, L. J. Zaneveld, and M. E. Klotman. 2002. Mandelic acid condensation polymer: novel candidate microbicide for prevention of human immunodeficiency virus and herpes simplex virus entry. J. Virol. 76:11236-11244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herold, B. C., R. Kirkpatrick, D. Marcellino, A. Travelstead, V. Pilipenko, H. Krasa, J. Bremer, L. J. Dong, and M. D. Cooper. 1999. Bile salts: natural detergents for the prevention of sexually transmitted diseases. Antimicrob. Agents Chemother. 43:745-751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herold, B. C., A. Siston, J. Bremer, R. Kirkpatrick, G. Wilbanks, P. Fugedi, C. Peto, and M. Cooper. 1997. Sulfated carbohydrate compounds prevent microbial adherence by sexually transmitted disease pathogens. Antimicrob. Agents Chemother. 41:2776-2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herold, B. C., D. WuDunn, N. Soltys, and P. G. Spear. 1991. Glycoprotein C of herpes simplex virus type 1 plays a principal role in the adsorption of virus to cells and in infectivity. J. Virol. 65:1090-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Howett, M. K., E. B. Neely, N. D. Christensen, B. Wigdahl, F. C. Krebs, D. Malamud, S. D. Patrick, M. D. Pickel, P. A. Welsh, C. A. Reed, M. G. Ward, L. R. Budgeon, and J. W. Kreider. 1999. A broad-spectrum microbicide with virucidal activity against sexually transmitted viruses. Antimicrob. Agents Chemother. 43:314-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.The Joint United Nations Program on HIV/AIDS. 2002. Report on the global HIV/AIDS epidemic. December 2002 update. The Joint United Nations Program on HIV/AIDS, Geneva, Switzerland.
- 19.Keller, M. J., M. E. Klotman, and B. C. Herold. 2003. Development of topical microbicides for prevention of human immunodeficiency virus and herpes simplex virus. Am. J. Reprod. Immunol. 49:279-284. [DOI] [PubMed] [Google Scholar]
- 20.Koelle, D. M., and A. Wald. 2000. Herpes simplex virus: the importance of asymptomatic shedding. J. Antimicrob. Chemother 45(Suppl. T3):1-8. [DOI] [PubMed] [Google Scholar]
- 21.Krebs, F. C., S. R. Miller, B. J. Catalone, P. A. Welsh, D. Malamud, M. K. Howett, and B. Wigdahl. 2000. Sodium dodecyl sulfate and C31G as microbicidal alternatives to nonoxynol 9: comparative sensitivity of primary human vaginal keratinocytes. Antimicrob. Agents Chemother. 44:1954-1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mayer, K. H., S. A. Karim, C. Kelly, L. Maslankowski, H. Rees, A. T. Profy, J. Day, J. Welch, and Z. Rosenberg. 2003. Safety and tolerability of vaginal PRO 2000 gel in sexually active HIV-uninfected and abstinent HIV-infected women. AIDS 17:321-329. [DOI] [PubMed] [Google Scholar]
- 23.Mbopi-Keou, F. X., G. Gresenguet, P. Mayaud, H. A. Weiss, R. Gopal, M. Matta, J. L. Paul, D. W. Brown, R. J. Hayes, D. C. Mabey, and L. Belec. 2000. Interactions between herpes simplex virus type 2 and human immunodeficiency virus type 1 infection in African women: opportunities for intervention. J. Infect. Dis. 182:1090-1096. [DOI] [PubMed] [Google Scholar]
- 24.McNeely, T. B., D. C. Shugars, M. Rosendahl, C. Tucker, S. P. Eisenberg, and S. M. Wahl. 1997. Inhibition of human immunodeficiency virus type 1 infectivity by secretory leukocyte protease inhibitor occurs prior to viral reverse transcription. Blood 90:1141-1149. [PubMed] [Google Scholar]
- 25.Oram, R. J., D. Marcellino, D. Strauss, E. Gustafson, C. L. Talarico, A. K. Root, P. L. Sharma, K. Thompson, J. D. Fingeroth, C. Crumpacker, and B. C. Herold. 2000. Characterization of an acyclovir-resistant herpes simplex virus type 2 strain isolated from a premature neonate. J. Infect. Dis. 181:1458-1461. [DOI] [PubMed] [Google Scholar]
- 26.Pearce-Pratt, R., and D. M. Phillips. 1996. Sulfated polysaccharides inhibit lymphocyte-to-epithelial transmission of human immunodeficiency virus-1. Biol. Reprod. 54:173-182. [DOI] [PubMed] [Google Scholar]
- 27.Poli, G., and A. S. Fauci. 1995. A role of cytokine in the pathogenesis of human immunodeficiency virus infection, p. 421-449. In B. Aggarwahl and R. Puri (ed.), Human cytokines: their role in disease and therapy. Blackwell Science, Cambridge, Mass.
- 28.Roller, R. J., and B. C. Herold. 1997. Characterization of a BHK(TK−) cell clone resistant to postattachment entry by herpes simplex virus types 1 and 2. J. Virol. 71:5805-5813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rusconi, S., M. Moonis, D. P. Merrill, P. V. Pallai, E. A. Neidhardt, S. K. Singh, K. J. Willis, M. S. Osburne, A. T. Profy, J. C. Jenson, and M. S. Hirsch. 1996. Naphthalene sulfonate polymers with CD4-blocking and anti-human immunodeficiency virus type 1 activities. Antimicrob. Agents Chemother. 40:234-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rux, A. H., H. Lou, J. D. Lambris, H. M. Friedman, R. J. Eisenberg, and G. H. Cohen. 2002. Kinetic analysis of glycoprotein C of herpes simplex virus types 1 and 2 binding to heparin, heparan sulfate, and complement component C3b. Virology 294:324-332. [DOI] [PubMed] [Google Scholar]
- 31.Sinha, S., N. Cheshenko, R. I. Lehrer, and B. C. Herold. 2003. NP-1, a rabbit alpha-defensin, prevents the entry and intercellular spread of herpes simplex virus type 2. Antimicrob. Agents Chemother. 47:494-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spear, P. G., and R. Longnecker. 2003. Herpesvirus entry: an update. J. Virol. 77:10179-10185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tabet, S. R., M. M. Callahan, C. K. Mauck, F. Gai, A. S. Coletti, A. T. Profy, T. R. Moench, L. E. Soto-Torres, I. A. Poindexter, R. G. Frezieres, T. L. Walsh, C. W. Kelly, B. A. Richardson, L. Van Damme, and C. L. Celum. 2003. Safety and acceptability of penile application of 2 candidate topical microbicides: BufferGel and PRO 2000 Gel: 3 randomized trials in healthy low-risk men and HIV-positive men. J. Acquir. Immune Defic. Syndr. 33:476-483. [DOI] [PubMed] [Google Scholar]
- 34.Terhune, S. S., K. T. Coleman, R. Sekulovich, R. L. Burke, and P. G. Spear. 1998. Limited variability of glycoprotein gene sequences and neutralizing targets in herpes simplex virus type 2 isolates and stability on passage in cell culture. J. Infect. Dis. 178:8-15. [DOI] [PubMed] [Google Scholar]
- 35.Turpin, J. A. 2002. Considerations and development of topical microbicides to inhibit the sexual transmission of HIV. Expert Opin. Investig. Drugs 11:1077-1097. [DOI] [PubMed] [Google Scholar]
- 36.Van Damme, L., V. Chandeying, G. Ramjee, H. Rees, P. Sirivongrangson, M. Laga, J. Perriens, et al. 2000. Safety of multiple daily applications of COL-1492, a nonoxynol-9 vaginal gel, among female sex workers. AIDS 14:85-88. [DOI] [PubMed] [Google Scholar]
- 37.Van Damme, L., G. Ramjee, M. Alary, B. Vuylsteke, V. Chandeying, H. Rees, P. Sirivongrangson, L. Mukenge-Tshibaka, V. Ettiegne-Traore, C. Uaheowitchai, S. S. Karim, B. Masse, J. Perriens, and M. Laga. 2002. Effectiveness of COL-1492, a nonoxynol-9 vaginal gel, on HIV-1 transmission in female sex workers: a randomised controlled trial. Lancet 360:971-977. [DOI] [PubMed] [Google Scholar]
- 38.van De Wijgert, J., A. Fullem, C. Kelly, S. Mehendale, S. Rugpao, N. Kumwenda, Z. Chirenje, S. Joshi, T. Taha, N. Padian, R. Bollinger, and K. Nelson. 2001. Phase 1 trial of the topical microbicide BufferGel: safety results from four international sites. J. Acquir. Immune Defic. Syndr. 26:21-27. [DOI] [PubMed] [Google Scholar]
- 39.Williams, R. K., and S. E. Straus. 1997. Specificity and affinity of binding of herpes simplex virus type 2 glycoprotein B to glycosaminoglycans. J. Virol. 71:1375-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Willis, S. H., A. H. Rux, C. Peng, J. C. Whitbeck, A. V. Nicola, H. Lou, W. Hou, L. Salvador, R. J. Eisenberg, and G. H. Cohen. 1998. Examination of the kinetics of herpes simplex virus glycoprotein D binding to the herpesvirus entry mediator, using surface plasmon resonance. J. Virol. 72:5937-5947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.WuDunn, D., and P. G. Spear. 1989. Initial interaction of herpes simplex virus with cells is binding to heparan sulfate. J. Virol. 63:52-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zeitlin, L., T. E. Hoen, S. L. Achilles, T. A. Hegarty, A. E. Jerse, J. W. Kreider, S. S. Olmsted, K. J. Whaley, R. A. Cone, and T. R. Moench. 2001. Tests of Buffergel for contraception and prevention of sexually transmitted diseases in animal models. Sex. Transm. Dis. 28:417-423. [DOI] [PubMed] [Google Scholar]