Abstract
A novel aminoglycoside resistance gene, aac(6′)-Iad, encoding aminoglycoside 6′-N-acetyltransferase, was identified in Acinetobacter genospecies 3 strain A-51. The gene encoded a 144-amino-acid protein, which shared modest identity (up to 36.7%) with some of the aminoglycoside 6′-N-acetyltransferases. The results of high-pressure liquid chromatography assays confirmed that the protein is a functional aminoglycoside 6′-N-acetyltransferase. The enzyme conferred resistance to amikacin, tobramycin, sisomicin, and isepamicin but not to gentamicin. The prevalence of this gene among Acinetobacter clinical isolates in Japan was then investigated. Of 264 Acinetobacter sp. strains isolated from geographically diverse areas in Japan in 2002, 16 were not susceptible to amikacin, and aac(6′)-Iad was detected in 7. Five of the producers of aminoglycoside 6′-N-acetyltransferase type Iad were identified as Acinetobacter baumannii, and two were identified as Acinetobacter genospecies 3. These results suggest that aac(6′)-Iad plays a substantial role in amikacin resistance among Acinetobacter spp. in Japan.
Acinetobacter spp., especially Acinetobacter baumannii, are emerging pathogens responsible for causing a variety of nosocomial infections, including pneumonia, urinary tract infections, and septicemia (1). Outbreaks have been increasingly reported in the past 2 decades, particularly from intensive care units, where patients undergo invasive procedures and receive broad-spectrum antimicrobial agents, resulting in higher mortality rates (5, 27). Furthermore, because Acinetobacter spp. have an ability to readily accept foreign DNA, including genetic determinants for antimicrobial resistance, so as to adapt to and survive in environments that are hazardous to bacterial growth (6, 17), they have a propensity for developing resistance to multiple classes of useful antimicrobial agents, including broad-spectrum cephalosporins, fluoroquinolones, and aminoglycosides (1).
Aminoglycosides are widely used to treat infections caused by gram-negative bacilli, including Acinetobacter spp. (1). However, resistance rates to classic aminoglycosides such as gentamicin and kanamycin are now high among Acinetobacter spp. in many geographic regions (15). The mechanisms of Acinetobacter sp. resistance to newer semisynthetic aminoglycosides such as amikacin, tobramycin, sisomicin, and isepamicin are diverse and commonly involve production of aminoglycoside-modifying enzymes such as aminoglycoside acetyltransferases (AAC), aminoglycoside nucleotidyltransferases (ANT, or AAD), and/or aminoglycoside phosphotransferases (APH). Production of AAC(3)-I, APH(3′)-VI, and ANT(3")-I was reported to be predominant by worldwide surveys on Acinetobacter spp., but there were considerable regional differences in their genotypes (14, 15, 21). In Japan, although the prevalence of amikacin resistance was estimated to be high, especially among non-carbapenem-susceptible Acinetobacter strains (25), the overall prevalence of aminoglycoside resistance and the mechanisms of resistance among Acinetobacter spp. have not been elucidated to date.
MATERIALS AND METHODS
Bacterial strains, plasmids, and media.
In March 2002, 264 nonrepetitive strains identified as belonging to Acinetobacter spp. were collected from 88 hospitals located in geographically diverse areas in Japan. Among these, 16 strains (6.1%) that were not susceptible to amikacin (MICs, >16 μg/ml) by preliminary susceptibility testing were selected for further study. Species identification was carried out with API 20NE (bioMérieux Japan, Ltd., Tokyo, Japan) complemented by a carbon source utilization test and growth at 41 and 44°C (2). Escherichia coli XL1-Blue was used as the host for cloning experiments with vector pBCSK+ (Stratagene, La Jolla, Calif.). E. coli BL21(DE3)pLysS was used with vector pET29a(+) (Novagen, Madison, Wis.) for expression of aac(6′)-Iad. The strains were grown in Luria-Bertani (LB) broth or medium (Becton Dickinson Diagnostic Systems, Sparks, Md.) supplemented with appropriate antimicrobial agents, unless described otherwise.
Antimicrobial agents and susceptibility testing.
Antimicrobial agents were obtained from the following sources: amikacin, Bristol Pharmaceuticals K. K., Tokyo, Japan; arbekacin, kanamycin, ribostamycin, and streptomycin, Meiji Seika Kaisha Ltd., Tokyo, Japan; chloramphenicol, Sankyo Co., Ltd., Tokyo, Japan; gentamicin and sisomicin, Schering-Plough K. K., Osaka, Japan; isepamicin, Asahi Kasei Corporation, Tokyo, Japan; neomycin, Nippon Kayaku Co., Ltd., Tokyo, Japan; rifampin, Daiichi Pharmaceutical Co., Ltd., Tokyo, Japan; tobramycin, Shionogi Pharmaceutical Co., Osaka, Japan.
MICs were determined by the agar dilution method with Mueller-Hinton agar (Becton Dickinson Diagnostic Systems) according to the protocol recommended by the National Committee for Clinical Laboratory Standards (16).
Transfer of aminoglycoside resistance genes.
Conjugation experiments were conducted by using rifampin-resistant E. coli CSH2 and Acinetobacter calcoaceticus DU1, a rifampin-resistant derivative of A. calcoaceticus ATCC 33305, as the recipients by the broth mating method (7). Transconjugants were selected on LB agar supplemented with rifampin (50 μg/ml) and kanamycin (10 μg/ml).
Cloning and sequencing of the aminoglycoside resistance gene.
The genomic DNA of Acinetobacter genospecies 3 strain A-51 was partially digested with Sau3AI, and the resultant fragments were ligated to the BamHI-cleaved cloning site of plasmid vector pBCSK+ (Stratagene). Electrocompetent E. coli XL1-Blue was transformed with these recombinant plasmids carrying total-DNA restriction fragments of various sizes prepared from the aminoglycoside-resistant strain. Transformants were selected by their resistance to chloramphenicol (30 μg/ml) and kanamycin (25 μg/ml). The enzymes used for gene manipulation were purchased from New England Biolabs, Inc. (Beverly, Mass.), or TAKARA Bio, Inc. (Ohtsu, Japan). The DNA sequences were determined on both strands by using BigDye Terminator Cycle Sequencing Ready Reaction kits and an ABI 3100 DNA sequence analyzer (Applied Biosystems, Foster City, Calif.). Alignments of nucleotide and amino acid sequences were performed with the GENETYX-MAC computer program (version 10.1.1; Software Development Co., Ltd., Tokyo, Japan).
Purification of the acetyltransferase.
For use in N-terminal sequencing and high-pressure liquid chromatography (HPLC) assays, AAC(6′)-Iad was purified by using a histidine tag purification system. The entire coding region of aac(6′)-Iad and its upstream sequence were amplified by PCR with primers AAC-F (5′-GCT CTA GAA GAC TGA CTT CGC ATT G-3′) and AAC-R (5′-CCC AAG CTT GAG CTG CTT TGT AAA AC-3′). The product was double digested with XbaI and HindIII and then ligated with pET29a(+) (Novagen) digested with the same enzymes. Electrocompetent E. coli XL1-Blue was transformed with the recombinant plasmids, and transformants were selected on LB agar containing kanamycin (25 μg/ml). Several of the colonies obtained were found to harbor plasmids with inserts encoding AAC(6′)-Iad tagged with six histidine residues at the C-terminal end. E. coli BL21(DE3)pLysS (Novagen) was transformed with one such plasmid, pA51H7. The transformants were cultured in 1 liter of LB broth supplemented with kanamycin (25 μg/ml) to an A620 of approximately 0.7. The pellet was washed once with 50 mM phosphate buffer (pH 7.0) and suspended in 20 mM phosphate buffer (pH 7.4) containing 10 mM of imidazole. The suspension was passed twice through a French pressure cell (Ohtake Works Co., Ltd., Tokyo, Japan) at 120 MPa and then centrifuged at 30,000 × g for 30 min. Histidine-tagged AAC(6′)-Iad contained in the supernatant was purified by using HiTrap Chelating HP, included in the HisTrap kit (Amersham Biosciences, K. K., Tokyo, Japan), according to the manufacturer's instructions. It was eluted at an imidazole concentration of 300 mM and was estimated to be more than 95% pure by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Finally, the enzyme was dialyzed twice at 4°C against 500 volumes of 50 mM phosphate buffer (pH 7.4) and was stored in aliquots at −80°C until use. N-terminal sequencing of the purified enzyme was performed by Shimadzu Corporation (Kyoto, Japan).
Preparation of crude extracts.
As positive controls for acetylation reactions and HPLC assays, the following strains were used: AAC(2′)-producing Streptomyces lividans TK21/pANT12-1′, AAC(3)-producing S. lividans TK21/pANT3-1, and AAC(6′)-producing S. lividans TK21/pANTS-2 (8). They were cultured in 100 ml of TS medium containing 10 μg of ribostamycin/ml and 10 μg of thiostrepton/ml (Sigma-Aldrich Japan K. K., Tokyo, Japan) for 48 h. The cells were then harvested, washed once with 50 mM phosphate buffer (pH 7.0), and suspended in the same buffer. The suspension was passed twice through a French pressure cell (Ohtake Works) at 120 MPa and then centrifuged at 30,000 × g for 30 min. The supernatant was used as the crude enzyme.
Acetylation.
Reaction mixtures for acetylation contained 25 μmol of Tris-hydrochloride buffer (pH 7.6), 7.5 μmol of MgCl2, 200 nmol of acetyl coenzyme A (acetyl-CoA), and 50 μmol of either tobramycin or neomycin in a final volume of 500 μl. Acetylation was initiated by adding 50 μl of the enzyme and was carried out at 37°C for 30 min. ortho-Phthalaldehyde derivatization was then performed by adding equal volumes of 2-propanol and the derivatization reagent to the reaction mixture and heating at 60°C for 10 min. The derivatization reagent consisted of 80 mM o-phthalaldehyde, 1 M boric acid, and 250 mM thioglycolic acid with the pH adjusted to 10.4 with 40% potassium hydroxide.
HPLC assay.
HPLC was performed to identify the site of acetylation of substrate aminoglycosides according to the methods described by Lovering et al. (12). The system consisted of a Separations module 2690 (Waters Corporation, Milford, Mass.), a Dual λ absorbance detector set at 330 nM (Waters), and a Chemcobond 5-ODS-H column (4.6 by 100 mm; Chemco Scientific Co., Ltd., Osaka, Japan). The mobile phase consisted of methanol-water-acetic acid (61.25:33.75:5) plus 5 g of 1-heptanesulfonic acid sodium salt per liter at a flow rate of 2 ml/min.
PCR amplification.
PCR analysis was performed for the 16 non-amikacin-susceptible Acinetobacter strains with primers ABA-F (5′-TTT GGC TAT GAT CCT ATG-3′) and ABA-R (5′-CAT GTC GAA CAA GTA CGC-3′) to amplify an internal fragment of the aac(6′)-Iad gene. The conditions used have been described previously (7). When amplicons were obtained, they were directly sequenced with the same primers.
Nucleotide sequence accession number.
The nucleotide sequence of aac(6′)-Iad will appear in GenBank under accession no. AB119105.
RESULTS
Prevalence and resistance profile of Acinetobacter strains with aac(6′)-Iad.
Of the 16 non-amikacin-susceptible Acinetobacter strains included in this study, 7 were PCR positive for aac(6′)-Iad. Five were phenotypically identified as A. baumannii, whereas the remaining two were identified as Acinetobacter genospecies 3. When the amplicons were sequenced, all were identical to aac(6′)-Iad. The MICs of aminoglycosides for Acinetobacter strains possessing aac(6′)-Iad are shown in Table 1. All the strains studied were resistant to kanamycin, amikacin, tobramycin, sisomicin, isepamicin, and streptomycin. In addition, strain A-51 was resistant to all of the aminoglycosides tested, including arbekacin, gentamicin, and neomycin.
TABLE 1.
Susceptibilities of Acinetobacter spp. and E. coli strains with aac(6′)-Iad to various aminoglycosides
| Strain | Hospital | Specimen | MIC (μg/ml) of the following aminoglycosidea:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| KAN | TOB | AMK | ABK | GEN | SISO | ISP | NEO | STR | |||
| Acinetobacter genomic species 3, strain A-51 | A | Sputum | >1,024 | >1,024 | 1,024 | 1,024 | >1,024 | >1,024 | >1,024 | 64 | >1,024 |
| A. baumannii A-67 | B | Urine | >1,024 | 64 | 128 | 32 | 8 | 1,024 | 256 | 8 | 256 |
| A. baumannii A-74 | B | Pus | >1,024 | 512 | 128 | 32 | 8 | 512 | 256 | 8 | 256 |
| A. baumannii A-87 | C | Sputum | 512 | 128 | 32 | 16 | 4 | 256 | 256 | 4 | 256 |
| A. baumannii A-88 | C | Sputum | 256 | 64 | 128 | 32 | 4 | 128 | 128 | 8 | 256 |
| Acinetobacter genomic species 3, strain A-178 | D | Sputum | 128 | 16 | 32 | 8 | 1 | 64 | 64 | 1 | 64 |
| A. baumannii A-260 | E | Sputum | 512 | 256 | 128 | 16 | 4 | 256 | 128 | 8 | 128 |
| E. coli XL1-Blue(pA51S3) | 256 | 64 | 128 | 16 | 1 | 64 | 64 | 4 | 4 | ||
| E. coli XL1-Blue(pA51SG5) | 512 | 32 | 1 | 0.13 | 32 | 32 | 0.13 | 0.25 | 2 | ||
| E. coli XL1-Blue(pBCSK+) | 0.5 | 0.25 | 0.5 | 0.13 | 0.13 | 0.13 | 0.25 | 0.25 | 1 | ||
KAN, kanamycin; TOB, tobramycin; AMK, amikacin; ABK, arbekacin; GEN, gentamicin; SISO, sisomicin; ISP, isepamicin; NEO, neomycin; STR, streptomycin.
Molecular characterization of aminoglycoside resistance genes.
Several transformants were obtained by selection with kanamycin and chloramphenicol. When these colonies were inoculated onto plates containing either amikacin (5 μg/ml) or gentamicin (5 μg/ml), they grew only on one or the other plate. The colonies on the plates containing amikacin or gentamicin were found to harbor recombinant plasmids of various sizes with inserts originating from the genomic DNA of strain A-51. Among these, the smallest plasmids (pA51S3 from an amikacin-resistant colony and pA51SG5 from a gentamicin-resistant colony) were selected out for further study. The MICs of aminoglycosides for E. coli XL1-Blue(pA51S3) and XL1-Blue(pA51SG5) are listed in Table 1. pA51S3 conferred resistance to kanamycin, amikacin, tobramycin, sisomicin, and isepamicin, while pA51SG5 conferred resistance to kanamycin, gentamicin, tobramycin, and sisomicin. Neither plasmid conferred resistance to streptomycin or neomycin.
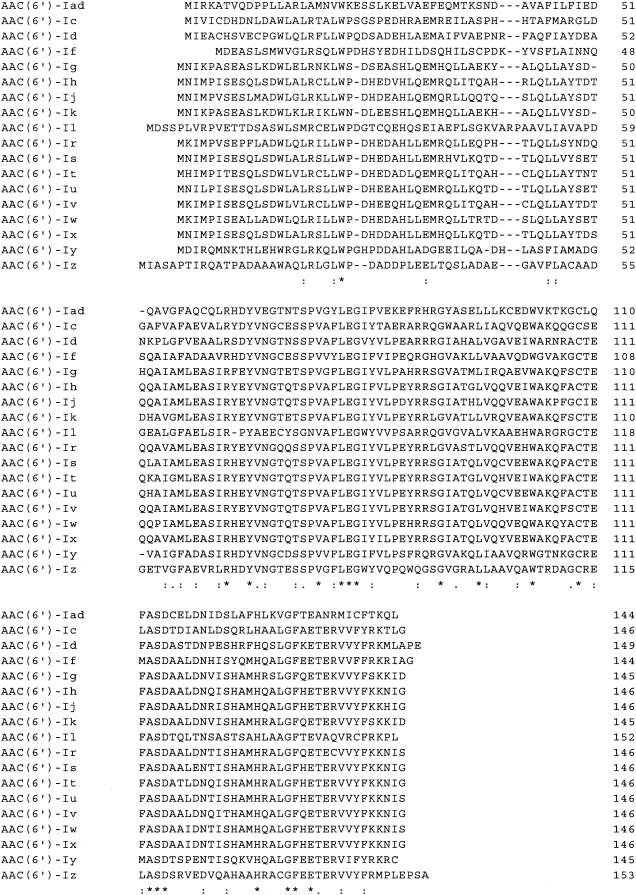
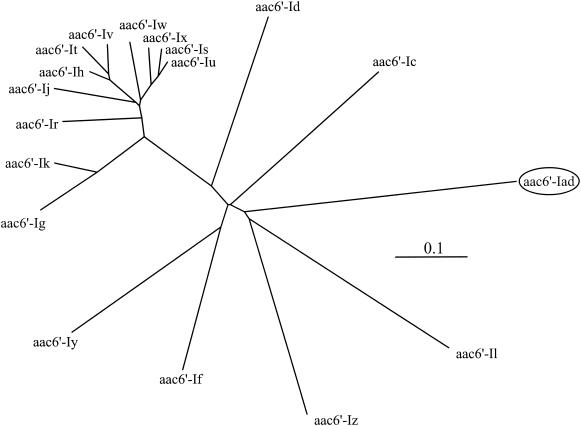
pA51S3 contained a 1.0-kb insert with one open reading frame, though several possible start codons were recognized. Therefore, N-terminal sequencing of the purified protein was carried out. Consequently, it was confirmed that the open reading frame encodes 144 amino acids and has a G+C content of 36.1%. The deduced amino acid sequence displayed the highest identity with that of AAC(6′)-Iy (36.7%) (13). It also showed moderate identities with the sequences of other aminoglycoside acetyltransferases [35.2% with AAC(6′)-If, 34.6% with AAC(6′)-Ic, 33.3% with AAC(6′)-Iz, and 29.7% with AAC(6′)-Il] (3, 11, 23, 26). The motifs that are conserved among the aminoglycoside 6′-N-acetyltransferases (24) were also found in the newly identified enzyme. This novel aminoglycoside acetyltransferase gene was thus designated aac(6′)-Iad. The deduced amino acid sequence of AAC(6′)-Iad is shown in Fig. 1, along with those of known aminoglycoside acetyltransferases. The dendrogram of phylogenetic relationships among aminoglycoside acetyltransferases is shown in Fig. 2. The 1.1-kb insert of pA51SG5 contained an aminoglycoside (2′) adenylyltransferase gene, ant(2")-Ia (4).
FIG. 1.
Alignment of the deduced amino acid sequences of AAC(6′)-Iad and other aminoglycoside acetyltransferases, including AAC(6′)-Ic (GenBank accession no. M94066), AAC(6′)-Id (X12618), AAC(6′)-If (X55353), AAC(6′)-Ig (L09246), AAC(6′)-Ih (L29044), AAC(6′)-Ij (L29045), AAC(6′)-Ik (L29510), AAC(6′)-Il (Z54241, U13880), AAC(6′)-Ir (AF031326), AAC(6′)-Is (AF031327), AAC(6′)-It (AF031328), AAC(6′)-Iu (AF031329), AAC(6′)-Iv (AF031330), AAC(6′)-Iw (AF031331), AAC(6′)-Ix (AF031332), AAC(6′)-Iy (AF144880), and AAC(6′)-Iz (AF140221). Asterisks indicate identical amino acids. Conservative amino acid substitutions are indicated by dots.
FIG. 2.
Dendrogram for aminoglycoside 6′-N-acetyltransferases belonging to the subfamily represented by AAC(6′)-Ic. The dendrogram was calculated by the ClustalW computer program, available on the National Institute of Genetics website (http://www.ddbj.nig.ac.jp/E-mail/clustalw-e.html), and illustrated with the TreeViewPPC computer program (version 1.6.5 for Macintosh). Branch lengths correspond to the numbers of amino acid exchanges.
Identification of site of modification.
The results of HPLC assays are shown in Table 2. The retention times of o-phthalaldehyde derivatives of tobramycin and neomycin after the acetylation reaction with AAC(6′)-Iad coincided only with those of positive controls for AAC(6′), confirming that AAC(6′)-Iad is a functional acetyltransferase and modifies position 6′ of aminoglycosides.
TABLE 2.
Retention times of aminoglycoside modification products after acetylation reactions
| Aminoglycoside acetyltransferase | Retention time (min) of aminoglycoside modification product
|
|||
|---|---|---|---|---|
| Tobramycin
|
Neomycin
|
|||
| With acetyl-CoA | Without acetyl-CoA | With acetyl-CoA | Without acetyl-CoA | |
| AAC(6′)-Iad | 3.3 | 17.0 | 4.9 | 11.8 |
| Positive controls | ||||
| AAC(6′) | 3.3 | 16.9 | 4.9 | 11.8 |
| AAC(2′) | 11.3 | 16.9 | 10.5 | 11.9 |
| AAC(3) | 4.4 | 16.9 | 6.7 | 11.9 |
Transfer of aminoglycoside resistance.
The amikacin resistance determinant of A. baumannii A-67 and A-74 could be transferred to the recipient A. calcoaceticus DU1 by conjugation at a frequency of approximately 5 × 10−4 to 1 × 10−3 and was confirmed by PCR to be aac(6′)-Iad. It was not transferred to E. coli CSH2. For the rest of the strains, amikacin resistance was not transferable to A. calcoaceticus DU1 or E. coli CSH2. The DNA probes for detection of aac(6′)-Iad hybridized with the large plasmids (>50 kb) harbored by all seven strains (data not shown).
DISCUSSION
A variety of aminoglycoside 6′-N-acetyltransferase genes from Acinetobacter species have been described to date (Fig. 2). aac(6′)-Ib and aac(6′)-Ih have been identified previously as the most prevalent plasmid-mediated aac(6′)-I genes among A. baumannii strains (18), while other genes have been associated with specific species. aac(6′)-Ig is specific to Acinetobacter haemolyticus (10), whereas aac(6′)-Ij and aac(6′)-Ik are specific to Acinetobacter genospecies 13 and 6, respectively (9, 19). aac(6′)-Ir, aac(6′)-Is, aac(6′)-It, aac(6′)-Iu, aac(6′)-Iv, aac(6′)-Iw, and aac(6′)-Ix have also been described for various Acinetobacter species (20). However, aac(6′)-Iad demonstrated considerable phylogenetic distance from these aminoglycoside-modifying enzymes (as shown in Fig. 2), suggesting the emergence of a novel subgroup of aminoglycoside 6′-N-acetyltransferases.
In the present study, we report identification of a novel aminoglycoside 6′-N-acetyltransferase gene, aac(6′)-Iad, in seven clinical isolates belonging to A. baumannii and Acinetobacter genospecies 3. The spectrum of resistance conferred by the gene product included kanamycin, tobramycin, amikacin, isepamicin, and sisomicin, a pattern typical of AAC(6′)-I (22). Preliminary sequencing results suggest that aac(6′)-Iad is located on a transposon (data not shown); in view of this possibility, along with the fact that the gene is transferable by conjugation in some of the producers of the enzyme, it is likely that aac(6′)-Iad is carried by a plasmid.
Three subgroups have been identified among aminoglycoside 6′-N-acetyltransferases (22). AAC(6′)-Iad is closest to the largest subfamily, which contains the proteins mentioned above as identified in Acinetobacter species, but the amino acid sequence identity between AAC(6′)-Iad and these proteins is limited (≤36.7%) (Fig. 1). Considering the low G+C content (36.1%) of aac(6′)-Iad for Acinetobacter species, we may speculate that the gene was acquired from some environmental species with an intrinsically low G+C content.
PFGE of the seven strains that produce AAC(6′)-Iad showed five distinct digestion patterns, except for those isolated from the same hospital (data not shown). Taken together, it is likely that aac(6′)-Iad was disseminated among Acinetobacter spp. via plasmid- and transposon-mediated lateral transfer, which is now responsible for reduced susceptibility to amikacin among Acinetobacter spp. in nearly half of the cases (7 out of 16 non-amikacin-susceptible strains) in Japan.
When the susceptibilities of the AAC(6′)-Iad producers to other classes of antimicrobial agents were tested, we found that none were susceptible to ceftazidime, moxalactam, or aztreonam, and two were resistant to ciprofloxacin as well. Only imipenem and meropenem were uniformly effective in vitro among the agents tested. The emergence and spread of plasmid-mediated aac(6′)-Iad genes could contribute to further acquisition of a multidrug-resistant phenotype among Acinetobacter spp. in Japan, thus limiting the treatment options in clinical settings in the near future.
Acknowledgments
We thank all the participating medical institutions for providing bacterial strains. We also thank Kunimoto Hotta for the gift of AAC-producing strains and Kumiko Kai for technical assistance.
This work was supported by grants H12-Shinkou-19, H12-Shinkou-20, H15-Shinkou-9, and H15-Shinkou-10 from the Ministry of Health, Labor, and Welfare of Japan.
REFERENCES
- 1.Bergogne-Berezin, E., and K. J. Towner. 1996. Acinetobacter spp. as nosocomial pathogens: microbiological, clinical, and epidemiological features. Clin. Microbiol. Rev. 9:148-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bouvet, P. J. M., and P. A. D. Grimont. 1986. Taxonomy of the genus Acinetobacter with the recognition of Acinetobacter baumannii sp. nov., Acinetobacter haemolyticus sp. nov., Acinetobacter johnsonii sp. nov., and Acinetobacter junii sp. nov., and emended descriptions of Acinetobacter calcoaceticus and Acinetobacter lwoffii. Int. J. Syst. Bacteriol. 36:228-240. [Google Scholar]
- 3.Bunny, K. L., R. M. Hall, and H. W. Stokes. 1995. New mobile gene cassettes containing an aminoglycoside resistance gene, aacA7, and a chloramphenicol resistance gene, catB3, in an integron in pBWH301. Antimicrob. Agents Chemother. 39:686-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cameron, F. H., D. J. Groot Obbink, V. P. Ackerman, and R. M. Hall. 1986. Nucleotide sequence of the AAD(2") aminoglycoside adenylyltransferase determinant aadB. Evolutionary relationship of this region with those surrounding aadA in R538-1 and dhfrII in R388. Nucleic Acids Res. 14:8625-8635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cisneros, J. M., and J. Rodriguez-Bano. 2002. Nosocomial bacteremia due to Acinetobacter baumannii: epidemiology, clinical features and treatment. Clin. Microbiol. Infect. 8:687-693. [DOI] [PubMed] [Google Scholar]
- 6.de Vries, J., and W. Wackernagel. 2002. Integration of foreign DNA during natural transformation of Acinetobacter sp. by homology-facilitated illegitimate recombination. Proc. Natl. Acad. Sci. USA 99:2094-2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doi, Y., K. Yokoyama, K. Yamane, J. Wachino, N. Shibata, T. Yagi, K. Shibayama, H. Kato, and Y. Arakawa. 2004. Identification of a plasmid-mediated 16S rRNA methylase in Serratia marcescens conferring high-level resistance to various aminoglycosides. Antimicrob. Agents Chemother. 48:491-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hotta, K., A. Sunada, Y. Ikeda, and S. Kondo. 2000. Double stage activity in aminoglycoside antibiotics. J. Antibiot. (Tokyo) 53:1168-1174. [DOI] [PubMed] [Google Scholar]
- 9.Lambert, T., G. Gerbaud, and P. Courvalin. 1994. Characterization of the chromosomal aac(6′)-Ij gene of Acinetobacter sp. 13 and the aac(6′)-Ih plasmid gene of Acinetobacter baumannii. Antimicrob. Agents Chemother. 38:1883-1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lambert, T., G. Gerbaud, M. Galimand, and P. Courvalin. 1993. Characterization of Acinetobacter haemolyticus aac(6′)-Ig gene encoding an aminoglycoside 6′-N-acetyltransferase which modifies amikacin. Antimicrob. Agents Chemother. 37:2093-2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lambert, T., M. C. Ploy, F. Denis, and P. Courvalin. 1999. Characterization of the chromosomal aac(6′)-Iz gene of Stenotrophomonas maltophilia. Antimicrob. Agents Chemother. 43:2366-2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lovering, A. M., L. O. White, and D. S. Reeves. 1984. Identification of aminoglycoside-acetylating enzymes by high-pressure liquid chromatographic determination of their reaction products. Antimicrob. Agents Chemother. 26:10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Magnet, S., P. Courvalin, and T. Lambert. 1999. Activation of the cryptic aac(6′)-Iy aminoglycoside resistance gene of Salmonella by a chromosomal deletion generating a transcriptional fusion. J. Bacteriol. 181:6650-6655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller, G. H., F. J. Sabatelli, R. S. Hare, Y. Glupczynski, P. Mackey, D. Shlaes, K. Shimizu, K. J. Shaw, et al. 1997. The most frequent aminoglycoside resistance mechanisms—changes with time and geographic area: a reflection of aminoglycoside usage patterns? Clin. Infect. Dis. 24(Suppl. 1):S46-S62. [DOI] [PubMed] [Google Scholar]
- 15.Miller, G. H., F. J. Sabatelli, L. Naples, R. S. Hare, K. J. Shaw, et al. 1995. The most frequently occurring aminoglycoside resistance mechanisms—combined results of surveys in eight regions of the world. J. Chemother. 7(Suppl. 2):17-30. [PubMed] [Google Scholar]
- 16.National Committee for Clinical Laboratory Standards. 2003. Performance standards for antimicrobial susceptibility testing; 13th informational supplement. M100-S13. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 17.Nielsen, K. M., M. D. van Weerelt, T. N. Berg, A. M. Bones, A. N. Hagler, and J. D. van Elsas. 1997. Natural transformation and availability of transforming DNA to Acinetobacter calcoaceticus in soil microcosms. Appl. Environ. Microbiol. 63:1945-1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ploy, M. C., H. Giamarellou, P. Bourlioux, P. Courvalin, and T. Lambert. 1994. Detection of aac(6′)-I genes in amikacin-resistant Acinetobacter spp. by PCR. Antimicrob. Agents Chemother. 38:2925-2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rudant, E., P. Bourlioux, P. Courvalin, and T. Lambert. 1994. Characterization of the aac(6′)-Ik gene of Acinetobacter sp. 6. FEMS Microbiol. Lett. 124:49-54. [DOI] [PubMed] [Google Scholar]
- 20.Rudant, E., P. Bouvet, P. Courvalin, and T. Lambert. 1999. Phylogenetic analysis of proteolytic Acinetobacter strains based on the sequence of genes encoding aminoglycoside 6′-N-acetyltransferases. Syst. Appl. Microbiol. 22:59-67. [DOI] [PubMed] [Google Scholar]
- 21.Seward, R. J., T. Lambert, and K. J. Towner. 1998. Molecular epidemiology of aminoglycoside resistance in Acinetobacter spp. J. Med. Microbiol. 47:455-462. [DOI] [PubMed] [Google Scholar]
- 22.Shaw, K. J., P. N. Rather, R. S. Hare, and G. H. Miller. 1993. Molecular genetics of aminoglycoside resistance genes and familial relationships of the aminoglycoside-modifying enzymes. Microbiol. Rev. 57:138-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shaw, K. J., P. N. Rather, F. J. Sabatelli, P. Mann, H. Munayyer, R. Mierzwa, G. L. Petrikkos, R. S. Hare, G. H. Miller, P. M. Bennett, and P. Downey. 1992. Characterization of the chromosomal aac(6′)-Ic gene from Serratia marcescens. Antimicrob. Agents Chemother. 36:1447-1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shmara, A., N. Weinsetel, K. J. Dery, R. Chavideh, and M. E. Tolmasky. 2001. Systematic analysis of a conserved region of the aminoglycoside 6′-N-acetyltransferase type Ib. Antimicrob. Agents Chemother. 45:3287-3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sugino, Y., Y. Iinuma, T. Nada, Y. Tawada, H. Amano, T. Nakamura, Y. Hasegawa, K. Shimokata, N. Shibata, and Y. Arakawa. 2001. Antimicrobial activities and mechanisms of carbapenem resistance in clinical isolates of carbapenem-resistant Pseudomonas aeruginosa and Acinetobacter spp. Kansenshogaku Zasshi 75:662-670. (In Japanese.) [DOI] [PubMed] [Google Scholar]
- 26.Teran, F. J., J. E. Suarez, and M. C. Mendoza. 1991. Cloning, sequencing, and use as a molecular probe of a gene encoding an aminoglycoside 6′-N-acetyltransferase of broad substrate profile. Antimicrob. Agents Chemother. 35:714-719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Theaker, C., B. Azadian, and N. Soni. 2003. The impact of Acinetobacter baumannii in the intensive care unit. Anaesthesia 58:271-274. [DOI] [PubMed] [Google Scholar]