Abstract
DNA topoisomerase II-α (Topo II-α) is essential for numerous cell processes, including DNA replication, transcription, recombination, and chromosome separation and condensation. Altered Topo II-α expression may lead to carcinogenesis and cancer progression. The aim of the present study was to investigate the association between Topo II-α expression levels and clinicopathological data from laryngeal cancer patients. Immunohistochemistry was used to analyze Topo II-α expression in laryngeal squamous cell carcinoma and distant healthy tissues obtained from 70 patients. In addition, fluorescence in situ hybridization was used to detect Topo II-α amplification and chromosome 17 ploidy using a laryngeal cancer tissue microarray. The expression of Topo II-α protein was detected in 71.43% (50/70) of laryngeal carcinoma tissues, in contrast to 9% of healthy tissues (2/22). Furthermore, the expression of Topo II-α protein was found to be associated with tumor de-differentiation and advanced tumor T stage. However, the expression of Topo II-α protein was not identified to be associated with Topo II-α amplification in laryngeal carcinoma, although was found to positively correlate with chromosome 17 aneuploidy (P<0.05). A higher aneuploidy rate contributed to increased expression levels of Topo II-α protein. Aberrant Topo II-α expression and chromosome 17 aneuploidy contributed to the development and progression of laryngeal cancer, indicating that targeting Topo II-α may provide a treatment strategy for patients with laryngeal cancer.
Keywords: DNA topoisomerase II-α, immunohistochemistry, chromosome 17 aneuploidy, laryngeal squamous cell carcinoma, fluorescence in situ hybridization
Introduction
Laryngeal cancer is a common type of head and neck malignancy. In the United States, there were an estimated 12,260 novel cases of laryngeal cancer in 2013 (1). Tobacco smoking and alcohol consumption are the most significant risk factors for laryngeal cancer, thus, smoking cessation and decreased alcohol intake may reduce the incidence of this cancer (2). The prognosis of patients with laryngeal cancer is closely associated with the tumor size, location, histological grade, patient age and the presence of lymph node or distant metastasis (3). For example, the five-year survival rate of early laryngeal cancer may be as high as 80–90%, whereas the five-year survival rate of advanced laryngeal cancer declines to ~60% (4). Thus, early diagnosis is considered to be critical for improving the survival of patients. Novel approaches to identify biomarkers may facilitate clinicians with the early identification of laryngeal cancer and studies on the biological behavior of this cancer may provide valuable information for clinical treatment.
The DNA topoisomerase II-α (Topo II-α) gene, localized at chromosome 17q21–22, encodes a 170-kD protein that regulates the dynamic changes in the spatial structure of nucleic acids (5). Topo II-α is a key enzyme, which maintains the physiological functions of nucleic acids (6) and is important in numerous cellular processes, including DNA replication, recombination, chromosome separation, and condensation and gene transcription (7). At present, the majority of the anticancer agents that have been developed interfere with DNA replication, recombination and gene expression in tumor cells. Thus, Topo II-α is the common target of antitumor drugs, including anthracycline, actinomycin and podophyllotoxin. These agents promote enzyme-mediated DNA cleavage by stabilizing the dissociation-prone complex between Topo II-α and DNA, leading to the accumulation of DNA double-strand breaks and subsequently resulting in tumor cell apoptosis (8). Thus far, Topo II-α amplification and protein expression have been extensively investigated in a variety of cancers, including breast cancer, testicular teratoma, bladder transitional cell carcinoma, meningioma, glioma, liver cancer and endometrial cancer (9–12). Topo II-α expression was found to be associated with tumor invasion and recurrence, as well as with the prognosis of different cancers (13,14). Previous studies have shown that levels of Topo II-α are associated with the responsiveness of tumors to chemotherapy and radiotherapy (15). Thus, in the current study, the expression of Topo II-α protein was analyzed in laryngeal cancer and adjacent tissues using immunohistochemistry, and its association with clinicopathological data was determined. Topo II-α amplification was also examined in addition to chromosome 17 aneuploidy using fluorescence in situ hybridization with the aim of identifying the mechanisms by which Topo II-α protein expression is regulated in laryngeal cancer.
Patients and methods
Patients
A total of 77 patients with pathologically confirmed laryngeal squamous cell carcinoma were enrolled in the present study and the patients underwent surgical tumor resection at Shanxi Cancer Hospital (Taiyuan, China) between January 2005 and December 2007. No patients received radiotherapy or chemotherapy prior to surgery. This study included 71 males and six females with a mean age of 59.3 years (range, 41–79 years). Regarding tumor localization, 40 (51.95%) patients were diagnosed with supraglottic cancer, 30 (3.90%) with glottic cancer, six (7.79%) with subglottic cancer and one (1.30%) patient was diagnosed with an unknown cancer location due to incomplete data. Histologically, five (6.49%) tumors were well-differentiated, 64 (83.12%) were moderately differentiated and eight (10.39%) were poorly differentiated squamous cell carcinomas. Regarding the clinical T stage, four patients (5.2%) were identified with the T1 stage of disease, 26 patients (33.77%) with the T2 stage, 31 patients (40.26%) with the T3 stage, 11 patients (14.29) with the T4 stage and five patients (6.49%) with an unknown T stage due to incomplete information. A total of 15 patients (19.48%) exhibited lymph node metastasis, however, no patients were identified to exhibit distant metastases. A total of 22 pairs of laryngeal cancer and distant healthy tissues were available for the study, whereas in the remaining 55 cases only laryngeal cancer tissues were available. The present study was approved by the Ethics Committee of Shanxi Cancer Hospital and informed consent was obtained from all patients prior to enrollment.
Immunohistochemistry
A mouse monoclonal anti-human Topo II-α antibody and PV-9000 general-purpose two-step immunohistochemical detection kit were purchased from Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd. (Beijing, China). A 3,3′-diaminobenzidine kit was purchased from Fuzhou Maixin Biotechnology Development Co., Ltd. (Fuzhou, China). For immunohistochemistry,77 laryngeal squamous cell carcinoma tissue sections and 22 distant healthy tissue specimens were deparaffinized, rehydrated and subjected to antigen retrieval using a citrate buffer (Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd.) at high pressure. All procedures were performed according to the manufacturer’s instructions. Phosphate-buffered saline (Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd.) served as the blank control to replace the primary antibody. The presence of Topo II-α protein in the nucleus of positive tumor cells was observed as uniform brown and yellow particles. Three high-power fields were randomly selected from each tissue section and the mean percentage of positive cells was defined as the percentage of positive cells in each tissue section. Percentage (%) of staining of Topo II-α protein was graded as follows (16): (−), <10% positive cells; (+), 10–25% positive cells; (++), 26–75% positive cells; and (+++), 76–100% positive cells. The grades between (+) and (+++) were considered to represent positive Topo II-α expression, while the grades between (−) and (+) were considered to represent low Topo II-α expression and grades between (++) and (+++) as high Topo II-α expression.
Fluorescence in situ hybridization
Paraffin blocks from 55 laryngeal squamous cell carcinoma tissues and 22 distant healthy tissue specimens were retrieved from the Pathology Department of Shanxi Cancer Hospital and were used to prepare two 5×6 tissue microarrays (2.0 mm) for fluorescence in situ hybridization.
The tissue microarray sections were heated at 65°C for 120 min and deparaffinized with xylene, rehydrated in a series of ethanol, and washed in distilled water for 1 min. For fluorescence in situ hybridization, the sections were placed in a water bath at 90°C for 30 min and rinsed twice for 5 min in 2X saline-sodium citrate (SSC) solution at room temperature. Next, the sections were denatured and hybridized with a fluorescent-labeled Topo II-α cRNA probe (Beijing Golden Bodhisattva Jia Medical Technology Co., Ltd., Beijing, China) at 37°C overnight in a hybridization oven (ThermoBrite 5500-24; Abbott Laboratories, Abbott Park, IL, USA) according to the manufacturer’s instructions. The sections were submerged in 2X SSC until the coverslip detached naturally. Next, the sections were counterstained with 4′,6-diamidino-2-phenylindole for 10–20 min and were visualized using a laser scanning confocal microscope (Leica SP-5; Leica, Mannheim, Germany). The fluorescent signal of the tissue specimens was reviewed and quantified to determine the DNA content using the copy number of Topo II-α. Digoxigenin-labeled human papillomaviruses 16/18-positive tissue sections served as the positive control and glycerol replaced the probes and served as a negative control. In healthy cells, the single interphase nucleus contained two red and two green signals. In tumor cells, particularly those with abnormal Topo II-α amplification, the single interphase nucleus contained more than two red signals. The ratio was calculated as follows: Ratio = total number of red signals in 30 nuclei/total number of green signals in 30 nuclei. A ratio of <1.8 indicated no Topo II-α gene amplification and a ratio of >2.2 indicated the presence of Topo II-α gene amplification according to previous Her2-neu studies (17). When the ratio was between 1.8 and 2.2, the number of cells counted was increased to 100 to determine the final result. To determine chromosome 17 aneuploidy, the mean number of chromosome 17 in each cell was determined, whereby values between1.76 and 2.25 indicated diploidy, whereas a value ≥2.26 indicated polyploidy (18).
Statistical analysis
SPSS version 15.0 statistical software (SPSS, Inc., Chicago, IL, USA) was used for all statistical analyses. Comparison between the groups was performed using χ2 test or Fisher’s exact test. The association between Topo II-α gene expression and other factors was analyzed using Spearman’s test. P<0.05 was considered to indicate a statistically significant difference.
Results
Expression of Topo II-α protein in laryngeal carcinoma tissue specimens
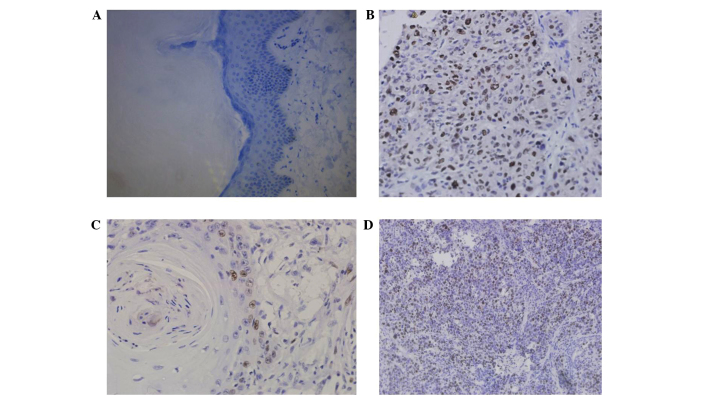
During immunohistochemical staining, certain tissue sections samples detached from the glass slides, and thus, only 70 samples were available for data analysis. Topo II-α was observed to be predominantly expressed in the nucleus of the cells, appearing as yellow, or brown and yellow in color (Fig. 1). Topo II-α expression was positive in 71.43% (50/70) laryngeal cancer tissue specimens with a low expression rate of 52.11% (37/70) and high expression rate of 47.14% (33/70; Table I). By contrast, the Topo II-α protein was only expressed in 9% (2/22) of the distant healthy laryngeal tissues (P<0.05).
Figure 1.
Immunohistochemical detection of Topo II-α protein expression in laryngeal cancer tissue specimens compared with distant healthy tissues. (A) Healthy tissue without Topo II-α expression. (B) Laryngeal cancer tissue with Topo II-α expression (tumor cell nuclei appeared brown and yellow in color). (C) Well-differentiated laryngeal cancer. (D) Poorly differentiated laryngeal cancer. (Magnification: A, ×400; B, ×400; C, ×400; and D, ×200).
Table I.
Topo II-α expression in 70 laryngeal squamous cell carcinoma tissues.
| Topo II-α expression, n | |||||
|---|---|---|---|---|---|
|
|
|||||
| Tissue | Patients, n | (−) | (+) | (++) | (+++) |
| Cancer | 70 | 20 | 17 | 29 | 4 |
| Healthy | 22 | 20 | 2 | 0 | 0 |
The association between Topo II-α expression and patient’s clinicopathological data was investigated. It was found that when compared with moderately and poorly differentiated tumors, well-differentiated tumors expressed the Topo II-α protein at a significantly lower level (P<0.05; Table II). Furthermore, when compared with the stage T3 + T4 group, the T1 + T2 tumors also expressed low levels of Topo II-α protein (P<0.05). In tumors without lymph node metastasis, the expression of Topo II-α protein was low, whereas in tumors with lymph node metastasis, the expression of Topo II-α protein was higher (50% [7/14]), however no significant differences were identified (P>0.05; Table II). In addition, 54.05% (20/37) of supraglottic cancer exhibited low Topo II-α expression, whereas 45.95% (17/37) of tumors exhibited high expression. Similarly, 53.85% (14/26) of glottic cancer exhibited low expression levels of Topo II-α protein, whereas 46.15% (12/26) of glottic cancers exhibited high levels of Topo II-α protein expression. By contrast, low and high levels of Topo II-α protein were observed in equal numbers of subglottic cancer tissue samples (50% [3/6]), however, no significant difference was identified between the expression of Topo II-α protein and the different tumor localizations (P>0.05; Table II).
Table II.
Association of Topo II-α expression with clinicopathological parameters of laryngeal squamous cell carcinoma patients.
| Topo II-α expression, n
|
||||
|---|---|---|---|---|
| Clinicopathological parameter | Patients, n | (−/+) | (++/+++) | P-value |
| Gender | ||||
| Male | 69 | 36 | 33 | >0.05 |
| Female | 1 | 1 | 0 | |
| Age, years | ||||
| <60 | 36 | 20 | 16 | >0.05 |
| ≥60 | 34 | 17 | 17 | |
| Clinical type | ||||
| Supraglottic | 37 | 20 | 17 | >0.05 |
| Glottic | 26 | 14 | 12 | |
| Subglottic | 6 | 3 | 3 | |
| Pathological grade | ||||
| Well-differentiated | 5 | 4 | 1 | <0.05 |
| Moderately/poorly differentiated | 65 | 33 | 32 | |
| T stage | ||||
| T1 + T2 | 26 | 17 | 9 | <0.05 |
| T3 + T4 | 40 | 20 | 20 | |
| N stage | ||||
| N0 | 56 | 30 | 26 | >0.05 |
| N1–3 | 14 | 7 | 7 | |
Chromosome 17 ploidy and its association with the expression of Topo II-α protein in laryngeal carcinoma tissue specimens
The overall aneuploidy rate of chromosome 17 was 45.83% (22/48) in the laryngeal carcinoma tissue specimens. Chromosome 17 aneuploidy was also found in 6/27 (22.22%) of cases with (−/+) expression of Topo II-α protein when compared with 16/21 (76.19%) in cases with (++/+++) expression of Topo II-α protein (P<0.05). These results revealed that a higher rate of chromosome 17 aneuploidy is associated with a higher expression of Topo II-α protein (Table III). Chromosome 17 aneuploidy was also found to be associated with the clinicopathological data (Table IV; Fig. 2).
Table III.
Association between Topo II-α protein expression and chromosome 17 ploidy in 48 laryngeal cancer patients.
| Group | Patients, n | Diploidy, n (%) | Polyploidy, n (%) |
|---|---|---|---|
| IHC (−)/FISH (−) | 14 | 12 (85.71) | 2 (14.29) |
| IHC (+)/FISH (−) | 11 | 7 (63.64) | 4 (36.36) |
| IHC (++)/FISH (−) | 12 | 4 (33.33) | 8 (66.67) |
| IHC (+++)/FISH (−) | 4 | 1 (25.00) | 3 (75.00) |
| IHC (−)/FISH (+) | 1 | 1 (100.00) | 0 |
| IHC (+)/FISH (+) | 1 | 1 (100.00) | 0 |
| IHC (++)/FISH (+) | 5 | 0 | 5 (100.00) |
IHC, immunohistochemistry; FISH, fluorescence in situ hybridization.
Table IV.
Association between chromosome 17 aneuploidy and clinicopathological data from laryngeal cancer patients.
| Chromosome 17 aneuploidy | ||||
|---|---|---|---|---|
|
|
||||
| Clinicopathological parameter | Patients, n | Diploidy | Aneuploidy | P-value |
| Age, years | ||||
| <60 | 22 | 15 | 7 | >0.05 |
| ≥60 | 26 | 11 | 15 | |
| Gender | ||||
| Male | 47 | 26 | 21 | >0.05 |
| Female | 1 | 0 | 1 | |
| Clinical type | ||||
| Supraglottic | 25 | 16 | 9 | >0.05 |
| Glottic | 18 | 8 | 10 | |
| Subglottic | 5 | 2 | 3 | |
| Pathological grade | ||||
| Well-differentiated | 3 | 2 | 1 | >0.05 |
| Moderately/poorly differentiated | 45 | 24 | 21 | |
| T stage | ||||
| T1 + T2 | 15 | 12 | 3 | <0.05 |
| T3 + T4 | 31 | 13 | 18 | |
| N stage | ||||
| N0 | 36 | 20 | 16 | >0.05 |
| N1–3 | 12 | 6 | 6 | |
Figure 2.
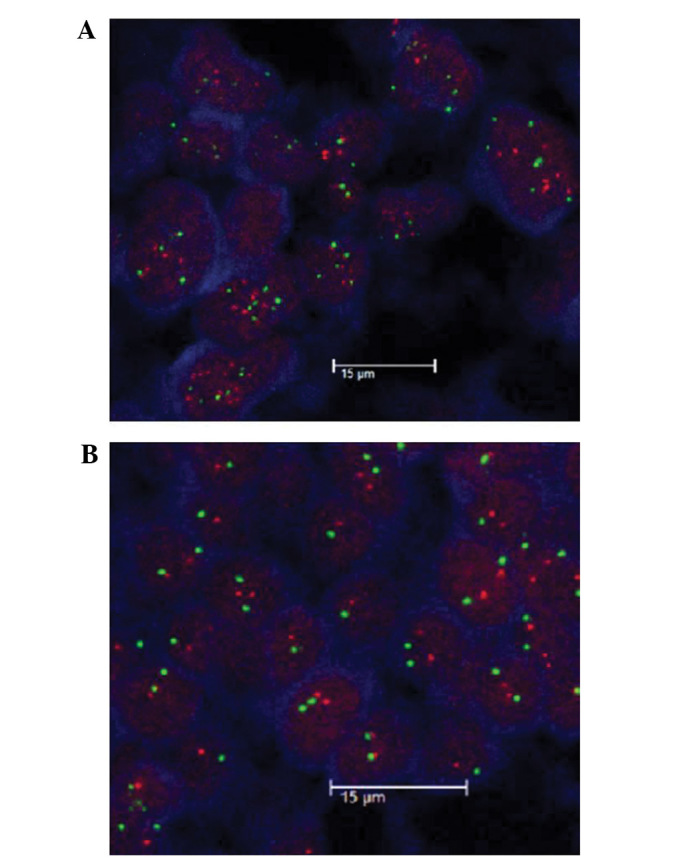
Fluorescence in situ hybridization analysis of Topo II-α amplification and chromosome 17 polyploid in laryngeal cancer tissue specimens. Blue signals represent the nucleus, green signals represent chromosome 17 and red signals represents Topo II-α. (A) Topo II-α amplification. Topo II-α gene was amplified and chromosome 17 was found to be polyploid (magnification, ×630). (B) Chromosome 17 aneuploidy. Topo II-α was not amplified and chromosome 17 was found to be diploid (magnification, ×630).
Topo II-α amplification and association with the expression of Topo II-α protein in laryngeal carcinoma tissue specimens
Topo II-α amplification was detected in 7/48 (14.58%) laryngeal carcinoma tissue specimens. The association between Topo II-α amplification and the expression of Topo II-α protein was investigated in laryngeal carcinoma tissue specimens. The results showed that Topo II-α protein was not expressed in 15 cases, whereas 12 cases expressed low levels of Topo II-α protein (+), 17 expressed moderate levels (++) and four expressed high levels (+++). With regards to Topo II-α amplification, seven cases exhibited amplification, with one case demonstrating negative Topo II-α protein expression, one case demonstrating (+) Topo II-α protein expression, and five cases demonstrating (++) Topo II-α protein expression (P>0.05; Table V). However, Topo II-α amplification was not found to be associated with any clinicopathological data from laryngeal cancer patients (data not shown; Fig. 2).
Table V.
Association between Topo II-α protein expression and Topo II-α amplification in 48 patients with laryngeal squamous cell carcinoma.
| FISH | |||
|---|---|---|---|
|
|
|||
| IHC | Patents, n | Non-amplification | Amplification |
| (−) | 15 | 14 | 1 |
| (+) | 12 | 11 | 1 |
| (++) | 17 | 12 | 5 |
| (+++) | 4 | 4 | 0 |
IHC, immunohistochemistry; FISH, fluorescence in situ hybridization.
Discussion
In the present study, the expression of Topo II-α protein, Topo II-α amplification and chromosome 17 ploidy were analyzed in laryngeal cancer tissues. In addition, the association between the expression levels of Topo II-α protein and the clinicopathological data from the patients, as well as the association between Topo II-α expression, Topo II-α amplification and chromosome 17 ploidy was analyzed. It was found that the expression of Topo II-α protein was upregulated in tumor tissues when compared with their healthy counterparts. Furthermore, the expression of Topo II-α protein was associated with tumor de-differentiation and advanced tumor T stage, although not with clinical classification or cervical lymph node metastasis of laryngeal carcinoma. The levels of Topo II-α protein expression were not found to be associated with Topo II-α amplification, however, were closely associated with the aneuploidy of chromosome 17. The findings of the current study indicate that the expression of Topo II-α protein may be used as a tumor marker and inhibition of Topo II-α activity may be further developed as a therapeutic target for laryngeal cancer patients.
At present, few studies have assessed Topo II-α expression and the association between Topo II-α protein and clinicopathological data from laryngeal cancer patients; however, the majority of these studies have reached consistent conclusions. Horibe et al (19) performed an immunohistochemical analysis of 63 cases of early glottic laryngeal carcinoma and 10 cases of normal laryngeal mucosa, and showed that the expression of Topo II-α protein was significantly increased in early glottic laryngeal carcinoma tissue when compared with the normal laryngeal mucosa. Furthermore, Shvero et al (20) immunohistochemically analyzed Topo II-α protein expression in 50 cases of laryngeal carcinoma, and demonstrated that the expression level of Topo II-α protein was associated with the pathological grade, clinical stage, postoperative survival and recurrence rate, indicating that Topo II-α protein overexpression is associated with a poor prognosis. In addition, Deng et al (21) investigated Topo II-α protein expression in 24 cases of laryngeal squamous cell carcinoma and eight cases of vocal cord polyps using immunohistochemistry and found that the positive expression of Topo II-α protein was significantly higher in patients with laryngeal squamous cell carcinoma compared with that in patients with vocal cord polyps and that the levels of Topo II-α expression were associated with the degree of tumor differentiation. Guo (22) also conducted an immunohistochemical study of Topo II-α protein expression in 50 cases of laryngeal cancer and revealed that Topo II-α protein expression correlated with the degree of differentiation, clinical stage and lymph node metastasis, furthermore, poor differentiation, higher clinical stage and the presence of lymph node metastasis was found to be associated with elevated Topo II-α protein expression. Altogether, these studies indicated that the levels of Topo II-α expression may serve to predict tumor de-differentiation or prognosis for patients with laryngeal squamous cell carcinoma. The results of the current study are consistent with these studies, however, the current study did not include survival data.
Furthermore, the potential cause of Topo II-α overexpression was assessed in laryngeal cancer tissue specimens using a fluorescence in situ hybridization technique. It was found that only seven out of 48 cases exhibited Topo II-α amplification, however, 22 cases exhibited chromosome 17 aneuploidy. In the current study, an association between the expression levels of the Topo II-α protein and Topo II-α amplification was not identified, however, chromosome 17 aneuploidy was identified to be associated with the expression levels of the Topo II-α protein. Expression of the Topo II-α protein increased in line with an increase in chromosome 17 aneuploidy, however, expression of Topo II-α protein levels in laryngeal carcinoma was not found to be associated with the Topo II-α mRNA levels for reasons that remain unclear. This indicates that chromosome 17 aneuploidy may induce the stability of the Topo II-α mRNA or protein. In conclusion, the results of the current study indicate that the aberrant expression of the Topo II-α protein may be involved in the development and progression of laryngeal cancer, and that the assessment of Topo II-α protein expression levels may provide insightful information regarding potential targets for use in laryngeal cancer treatment.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Ahmad Kiadaliri A, Jarl J, Gavriilidis G, Gerdtham UG. Alcohol drinking cessation and the risk of laryngeal and pharyngeal cancers: a systematic review and meta-analysis. PLoS One. 2013;8:e58158. doi: 10.1371/journal.pone.0058158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marioni G, Marchese-Ragona R, Cartei G, Marchese F, Staffieri A. Current opinion in diagnosis and treatment of laryngeal carcinoma. Cancer Treat Rev. 2006;32:504–515. doi: 10.1016/j.ctrv.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 4.Licitra L, Bernier J, Grandi C, Locati L, Merlano M, Gatta G, Lefebvre JL. Cancer of the larynx. Crit Rev Oncol Hematol. 2003;47:65–80. doi: 10.1016/s1040-8428(03)00017-9. [DOI] [PubMed] [Google Scholar]
- 5.Pommier Y, Leteurtre F, Fesen MR, Fujimori A, Bertrand R, Solary E, Kohlhagen G, Kohn KW. Cellular determinants of sensitivity and resistance to DNA topoisomerase inhibitors. Cancer Invest. 1994;12:530–542. doi: 10.3109/07357909409021413. [DOI] [PubMed] [Google Scholar]
- 6.Kellner U, Sehested M, Jensen PB, Gieseler F, Rudolph P. Culprit and victim - DNA topoisomerase II. Lancet Oncol. 2002;3:235–243. doi: 10.1016/s1470-2045(02)00715-5. [DOI] [PubMed] [Google Scholar]
- 7.Nitiss JL. DNA topoisomerase II and its growing repertoire of biological functions. Nat Rev Cancer. 2009;9:327–337. doi: 10.1038/nrc2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Withoff S, de Vries EG, Keith WN, Nienhuis EF, van der Graaf WT, Uges DR, Mulder NH. Differential expression of DNA topoisomerase II alpha and -beta in P-gp and MRP-negative VM26, mAMSA and mitoxantrone-resistant sublines of the human SCLC cell line GLC4. Br J Cancer. 1996;74:1869–1876. doi: 10.1038/bjc.1996.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bredel M, Slavc I, Birner P, Czech T, Haberler C, Ströbel T, Wolfsberger S, Budka H, Hainfellner JA. DNA topoisomerase IIalpha expression in optic pathway gliomas of childhood. Eur J Cancer. 2002;38:393–400. doi: 10.1016/s0959-8049(01)00387-2. [DOI] [PubMed] [Google Scholar]
- 10.Coleman LW, Bronstein IB, Holden JA. Immunohistochemical staining for DNA topoisomerase I, DNA topoisomerase II-alpha and p53 in gastric carcinomas. Anticancer Res. 2001;21:1167–1172. [PubMed] [Google Scholar]
- 11.Monnin KA, Bronstein IB, Gaffney DK, Holden JA. Elevations of DNA topoisomerase I in transitional cell carcinoma of the urinary bladder: correlation with DNA topoisomerase II-alpha and p53 expression. Hum Pathol. 1999;30:384–391. doi: 10.1016/s0046-8177(99)90112-0. [DOI] [PubMed] [Google Scholar]
- 12.Coleman LW, Perkins SL, Bronstein IB, Holden JA. Expression of DNA toposiomerase I and DNA topoisomerase II-alpha in testicular seminomas. Hum Pathol. 2000;31:728–733. doi: 10.1053/hupa.2000.8462. [DOI] [PubMed] [Google Scholar]
- 13.Rody A, Karn T, Ruckhäberle E, Müller V, Gehrmann M, Solbach C, Ahr A, Gätje R, Holtrich U, Kaufmann M. Gene expression of topoisomerase II alpha (TOP2A) by microarray analysis is highly prognostic in estrogen receptor (ER) positive breast cancer. Breast Cancer Res Treat. 2009;113:457–466. doi: 10.1007/s10549-008-9964-x. [DOI] [PubMed] [Google Scholar]
- 14.Gao XH, Yu ZQ, Zhang C, Bai CG, Zheng JM, Fu CG. DNA topoisomerase II alpha: a favorable prognostic factor in colorectal caner. Int J Colorectal Dis. 2012;27:429–435. doi: 10.1007/s00384-011-1346-x. [DOI] [PubMed] [Google Scholar]
- 15.Durbecq V, Paesmans M, Cardoso F, Desmedt C, Di Leo A, Chan S, Friedrichs K, Pinter T, Van Belle S, Murray E, et al. Topoisomerase-II alpha expression as a predictive marker in a population of advanced breast cancer patients randomly treated either with single-agent doxorubicin or single-agent docetaxel. Mol Cancer Ther. 2004;3:1207–1214. [PubMed] [Google Scholar]
- 16.Lv NL, Kong WM. Expression of multi-drug resistance genes in cervical cancer and its relationship with the effect of concurrent radio-chemotherapy. Zhong Liu Xue Za Zhi. 2006;5:385–388. (In Chinese) [Google Scholar]
- 17.Hicks DG, Tubbs RR. Assessment of the HER2 status in breast cancer by fluorescence in situ hybridization: a technical review with interpretive guidelines. Hum Pathol. 2005;36:250–261. doi: 10.1016/j.humpath.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 18.Wong SW, Rangan AM, Bilous AM, Boyages J, Gebski V, Benson EM. The value of S-phase and DNA ploidy analysis as prognostic markers for node-negative breast cancer in the Australian setting. Pathology. 1999;31:90–94. doi: 10.1080/003130299105241. [DOI] [PubMed] [Google Scholar]
- 19.Horibe Y, Murakami M, Komori K, Imaeda Y, Kasahara M. Expression of topoisomerase II alpha, Ki-67 and p53 in early stage laryngeal carcinomas not featuring vocal cord fixation. APMIS. 2000;108:689–696. doi: 10.1034/j.1600-0463.2000.d01-16.x. [DOI] [PubMed] [Google Scholar]
- 20.Shvero J, Koren R, Shvili I, Yaniv E, Sadov R, Hadar T. Expression of human DNA Topoisomerase II-alpha in squamous cell carcinoma of the larynx and its correlation with clinicopathologic variables. Am J Clin Pathol. 2008;130:934–939. doi: 10.1309/AJCPROG61USKCBEI. [DOI] [PubMed] [Google Scholar]
- 21.Deng G, Yang C, Chen W. The expression of Ki-67 and topoIIalpha in laryngeal squamous cell carcinoma. Lin Chuang Er Bi Yan Hou Ke Za Zhi. 2005;19:396–398. (In Chinese) [PubMed] [Google Scholar]
- 22.Guo YQ. Mdm2 PARP ToPoII in laryngeal carcinoma and its clinical significance. Zhong Guo Yi Yao Dao Kan. 2011;1:112–114. (In Chinese) [Google Scholar]