Abstract
BILN 2061 is a novel, specific hepatitis C virus (HCV) NS3 serine protease inhibitor discovered by Boehringer Ingelheim that has shown potent activity against HCV replicons in tissue culture and is currently under clinical investigation for the treatment of HCV infection. The poor fidelity of the HCV RNA-dependent RNA polymerase will likely lead to the development of drug-resistant viruses in treated patients. The development of resistance to BILN 2061 was studied by the in vitro passage of HCV genotype 1b replicon cells in the presence of a fixed concentration of the drug. Three weeks posttreatment, four colonies were expanded for genotypic and phenotypic characterization. The 50% inhibitory concentrations of BILN 2061 for these colonies were 72- to 1,228-fold higher than that for the wild-type replicon. Sequencing of the individual colonies identified several mutations in the NS3 serine protease gene. Molecular clones containing the single amino acid substitution A156T, R155Q, or D168V resulted in 357-fold, 24-fold, and 144-fold reductions in susceptibility to BILN 2061, respectively, compared to the level of susceptibility shown by the wild-type replicon. Modeling studies indicate that all three of these residues are located in close proximity to the inhibitor binding site. These findings, in addition to the three-dimensional structure analysis of the NS3/NS4A serine protease inhibitor complex, provide a strategic guide for the development of next-generation inhibitors of HCV NS3/NS4A serine protease.
Hepatitis C virus (HCV) infection is believed to be the leading cause of chronic hepatitis, end-stage cirrhosis, and hepatocellular carcinoma, affecting over 4 million Americans and about 170 million people worldwide. Currently, the most effective treatment of HCV infection involves a combination of the nucleoside analog ribavirin with alpha interferon (IFN-α). However, the regimen is prolonged and not well tolerated, and only approximately half of the genotype 1 HCV-infected individuals have a sustained virological response, although the response rate improves significantly (∼80%) when genotypes 2 and 3 are treated (7, 29). An oral agent that offers promise as an efficacious alternative to IFN or that may be used in IFN-containing regimens and that improves efficacy and/or the side effect profile is in great demand.
The HCV genome is a 9.6-kb single-stranded RNA of positive polarity encoding a large polyprotein that is posttranslationally cleaved into structural and nonstructural proteins (3, 12, 23). The N-terminal domain (approximately 180 amino acids) of NS3 and the small hydrophobic NS4A protein compose a heterodimeric enzyme catalyzing the posttranslational processing of the HCV nonstructural proteins (1, 2, 23). Its structure has been extensively studied by X-ray crystallography (9, 30, 31) and nuclear magnetic resonance spectroscopy (1, 6). The proteolytic activity of NS3/NS4A serine protease is known to be essential for viral RNA replication (10, 14). A recent study indicated that the NS3/NS4A serine protease also blocks activities of IFN regulatory factor 3, a key cellular antiviral signaling molecule associated with host cellular control of viral infection. Therefore, the inhibition of NS3/NS4A serine protease could potentially block HCV replication and restore IFN regulatory factor 3 control of the viral infection (11).
The development of inhibitors of the HCV NS3/NS4A serine protease has been difficult because of the smooth and shallow surface of its binding site, but significant progress has been made recently in the development of peptide inhibitors (17, 18, 22, 25, 26, 27; C. Lin, K. Lin, C. A. Gates, S. Ma, D. Brennan, J. Fulghum, H.-M. Hsiao, G. Rao, Y. Wei, J. Alford, R. B. Perni, and A. D. Kwong, Hepatology 38, Suppl. 1, Pt. 4, abstr. 1000, 2003) based on the integrated efforts of chemistry, molecular biology, enzymology, and molecular modeling. The first serine protease inhibitor to enter clinical development, BILN 2061, is a macrocyclic tripeptide that is highly potent in the HCV replicon cell model system and is a specific inhibitor of HCV serine protease (16). In both IFN treatment-experienced and naïve patients with genotype 1 infections, the administration twice daily of a 25-, 200-, or 500-mg dose of BILN 2061 resulted in a 2- to 3-log reduction of HCV RNA within 48 h after treatment (16).
Resistance to protease inhibitors has been observed both in vitro and in patients treated with human immunodeficiency virus (HIV) protease inhibitors and is due to specific mutations in the protease that lead to decreased phenotypic susceptibility to the drugs (20, 21). Because of the poor fidelity rate of HCV polymerase, drug-resistant mutations will likely develop in patients treated with HCV serine protease inhibitors as well. In fact, in vitro selection of HCV replicons resistant to an acyclic tripeptide inhibitor of the NS3/NS4A serine protease has been recently reported by Trozzi et al. (27). They found that a single amino acid substitution of NS3 aspartate 168 mediated significant HCV replicon resistance to the drug. In this study, we describe the in vitro selection of HCV replicons with decreased susceptibility to BILN 2061. In addition, we have defined the genotypic basis for the observed resistance phenotype. Finally, by using molecular modeling and site-directed mutagenesis, we have determined the critical amino acid substitutions in the NS3 serine protease conferring resistance to this antiviral agent.
MATERIALS AND METHODS
Compounds.
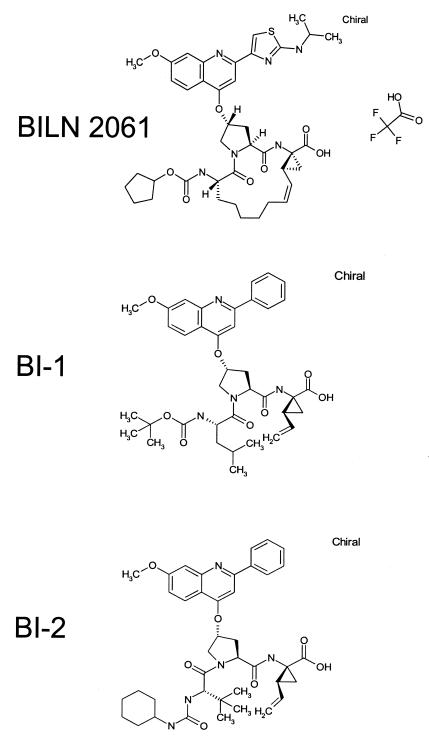
BILN 2061, BI-1, and BI-2 (acyclic tripeptide analogs of BILN 2061) (Fig. 1) were synthesized according to procedures described previously (4, 5). IFN-α was purchased from Sigma.
FIG. 1.
Chemical structures of compounds used in this study.
Cells and HCV replicons.
HCV genotype 1b strain N subgenomic replicon cell lines were obtained and licensed from Stanley Lemon (13, 32). Replicon cells were cultured in Dulbecco’s modified Eagle medium (Invitrogen) supplemented with 10% fetal bovine serum and 400 μg of G418 per ml. Nneo/3-5B(RG) replicon cells (13) were cured of the endogenous replicon by passaging cells in the presence of IFN-α (200 IU/ml) for 2 weeks at high cell confluence, followed by an additional week of passage in the absence of IFN-α. To confirm curing, cells were treated with 400 μg of G418 per ml for 3.5 weeks, after which time no G418-resistant colonies were detected. Loss of the replicon was also confirmed with a real-time reverse transcription-PCR (RT-PCR) assay for HCV RNA with a detection limit of 0.1 copy per cell.
In vitro selection of BILN 2061-resistant colonies.
The selection protocol was performed as described by Trozzi et al. (27) with the following modifications. Replicon cells (2 × 104) were plated in 10-cm-diameter tissue culture dishes and cultured in the presence of selection medium containing 400 μg of G418 per ml, 2 μg of blasticidin per ml, and 40 nM (10 times the 50% inhibitory concentration [IC50]) BILN 2061. After approximately 2 weeks, colonies of cells resistant to BILN 2061 and antibiotics became visible. Some of these resistant colonies were isolated, grown in the presence of BILN 2061 for about another 2 weeks, and subsequently characterized. Replicon cells incubated in the selection medium without BILN 2061 were used for the parental cell control.
Sequence analysis of replicon RNA.
Total RNA was extracted from parental and inhibitor-resistant cells with an RNeasy mini kit (QIAGEN). RNA was extracted according to the manufacturer's instructions. HCV replicon cDNA was synthesized with the oligo(dA)20 and SuperScript III first-strand synthesis system for RT-PCR (Invitrogen) according to the manufacturer's instructions. The cDNA coding for the HCV NS3-5B region was amplified by PCR with Platinum Pfx DNA polymerase (Invitrogen) and oligonucleotides SNS3-5-s (5′-GATAATACCATGGCGCCCATCACGGCCTAC-3′) and SNS3-5-as (5′-GGAAATGGCCTATTGGCCTGGAGTGTTTAGCTC-3′). The underlined portion of SNS3-5-s and all of SNS3-5-as correspond to nucleotides 3423 to 3440 and 9395 to 9427, respectively, of HCV strain N (GenBank accession number AF139594). At least three independent PCRs were performed for each sample, and PCR products were pooled. Amplified cDNAs were purified with the QIAquick PCR purification kit (QIAGEN), and nucleotide sequences were determined by automated sequencing with the Applied Biosystems v1.1 sequence system.
Drug susceptibility assay.
The inhibitory effects of compounds on viral replicon were tested on the inhibitor-selected colonies and parental cells by a secreted alkaline phosphatase (SEAP) assay as described previously (32). Briefly, 1,500 cells were placed into each well of a 96-well plate. The next day, cell culture supernatant was removed in order to reduce background SEAP, and fresh medium containing compound was added. After another 4 days of culture, the supernatant was assayed with the Phospha-Light SEAP reporter gene assay system (ABI) according to the manufacturer's instructions. The IC50 was then determined by nonlinear regression analysis with Prism (GraphPad Software, Inc.). Cellular toxicity was measured by the 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide colorimetric assay (24).
Construction of molecular clones containing NS3 serine protease mutants.
The replicon obtained from Stanley Lemon has HIV tat followed by the gene encoding the 2A protease of foot-and-mouth disease virus fused to the 5′ end of the neomycin resistance gene. When Huh-7 cells containing a SEAP reporter gene under the control of the HIV long terminal repeat are transfected with this replicon RNA, SEAP is produced (32). However, the SEAP reporter assay does not produce a sufficient signal in a transient-replication assay with replicons containing the NS3 serine protease mutations. Therefore, the Tat-2A-Neo region was replaced with a sequence encoding the 12 N-terminal amino acids of the HCV core fused to the firefly luciferase gene. The NS3 mutations R155Q, A156T, and D168V were separately introduced into the plasmid with the QuikChange multisite-directed mutagenesis kit (Stratagene) and the 5′-phosphorylated oligonucleotides 5′-GCTGTGGGCGTCTTCCAGGCCGCTGTATGCACCCGG-3′ (nucleotides 3870 to 3905 of HCV strain N),5′-GCTGTGGGCGTCTTCCGGACCGCTGTATGCACCCGG-3′ (nucleotides 3870 to 3905 of HCV strain N), and 5′-GGTTGCAAAGGCGGTGGTTTTTGTCCCCGTTGAGTCC-3′ (nucleotides 3908 to 3944 of HCV strain N), respectively. In each oligonucleotide, the introduced mutation is underlined.
RNA transcription and RNA transient-transfection assay.
For in vitro RNA transcription, XbaI-linearized replicon plasmids and the Megascript T7 kit (Ambion) were used according to the manufacturer's instructions. Cured Nneo/3-5B(RG) cells (107) were washed twice with Dulbecco's phosphate-buffered saline without Ca2+ and Mg2+ (Invitrogen) and then mixed with 20 μg of replicon RNA in a Gene Pulser cuvette with a 0.4-cm electrode gap (Bio-Rad). Electroporation was immediately performed at 970 V and 25 μF with two manual pulses. Transfected cells were plated into 96-well plates with 5,000 cells per well. Compounds at various concentrations were added into cells after 2 h and were cocultured for 4 days. The cells were lysed with passive lysis buffer, and luciferase activity was measured with the luciferase assay system kit (Promega) and Wallac 1420 work station (PerkinElmer Life Science) as described by the manufacturers. Luciferase activity was measured 4 h posttransfection without drug to determine the efficiency of transfection. Replication capacity was determined by measuring the luciferase activity of transfected cells after 4 days of culture in the absence of drug. IC50s were determined according to the procedure described above.
Structural analysis and modeling.
Coordinates for the three-dimensional structure of the full-length NS3 protein were taken from Protein Data Bank entry 1CU1 (31). A model of BILN 2061 was docked into the protein according to the reported binding mode of a close analog (28) with the InsightII software package (Accelrys, Inc.).
RESULTS
Resistant colonies were generated by in vitro selection.
In order to select replicons with decreased susceptibility to BILN 2061, replicon cells were treated with 40 nM (10 times the IC50) BILN 2061. Two weeks posttreatment, a total of 10 colonies were identified on the cell culture dish. Since 2 × 104 cells were used initially and the cells were not split during the selection procedure, resistance to BILN 2061 was shown by approximately 1 of every 2,000 cells. Of the 10 colonies, 4 were too small to expand. After isolation, two of the remaining six colonies did not grow well in the presence of the concentration of BILN 2061 initially used to select resistant mutants. Therefore, the remaining four colonies (SB1, SB4, SB5, and SB6) were expanded and studied in detail. Each cell line (SB1, SB4, SB5, and SB6) was cultured in the presence of different concentrations of BILN 2061 in triplicate, and IC50s were determined by nonlinear regression analysis (Table 1). All colonies had growth rates similar to those of cells that were grown in parallel but in the absence of BILN 2061 (parental cells). As measured by the SEAP assay, the IC50 for SB4 was 72-fold higher than that for the wild-type replicon cell line, while SB1, SB5, and SB6 showed much higher resistance levels (IC50s for them increased 1,228-, 635-, and 553-fold, respectively) (Table 1). However, all of the colonies maintained sensitivity to IFN-α (Table 1), indicating that the resistance was inhibitor specific.
TABLE 1.
Genotypes and phenotypes of BILN 2061-resistant coloniesa
| Replicon colony | BILN 2061
|
IFN-α IC50 (IU) | NS3 mutation(s)c | |
|---|---|---|---|---|
| SEAP IC50 (μM) | FCb | |||
| Wild type | 0.006 ± 0.002 | 1.6 ± 0.54 | ||
| SB1 | 7.37 ± 0.069 | 1,228 | 1.8 ± 0.85 | T72I, P88L, A156T |
| SB4 | 0.43 ± 0.041 | 72 | 1.5 ± 0.87 | R155Q |
| SB5 | 3.81 ± 0.231 | 635 | 1.9 ± 0.35 | I71T, A156T, T312A |
| SB6 | 3.32 ± 0.228 | 553 | 1.7 ± 0.58 | D168V, T260A |
Data shown are averages ± standard deviations of results from triplicate experiments.
FC, change in IC50 (n-fold) over that seen with the wild type.
Bold letters indicate amino acids in the protease domain. The others are in the helicase domain. The position of each mutation in the three-dimensional structure of the enzyme is shown in Fig. 2A.
The appearance of phenotypic resistance to BILN 2061 is associated with specific genotypic changes in the serine protease.
In order to detect distinct replicon mutations associated with resistance, the NS3/NS4A coding regions from resistant colonies were sequenced and compared with the same regions from parental cells. All four colonies contained mutations not observed in parental cells in the NS3 region (Table 1). These mutations appeared in both the serine protease and the helicase domains (Fig. 2A). Mutation A156T in the NS3 serine protease region was observed in two of the four colonies. However, during selection this mutation developed together with other mutations, such as T72I and P88L in SB1 and I71T and T312A in SB5. The D168V mutation found in SB6 has been identified by others as a major mutation that renders antiviral resistance in similar studies using both BILN 2061 (Lin et al., Hepatology 38, abstr. 1000) and an acyclic tripeptide inhibitor (27). This mutation developed together with T260A during the selection procedure. It is notable that SB4, which possessed only an R155Q mutation in the NS3 region, was less resistant to BILN 2061 than the other colonies. No mutations were observed in the NS4A coding region or in the NS3/NS4A serine protease cleavage site in any of the colonies.
FIG. 2.
Three-dimensional structure of the BILN 2061 binding site of HCV protease. (A) Protein backbone structure of the HCV NS3 protein showing the separate helicase and protease domains in orange. BILN 2061 is colored green, and the alpha carbons of the mutant residues are shown as large blue balls. The sequence corresponding to the NS4A peptide is colored black. (B) Close-up view showing BILN 2061 (carbon, green; oxygen, red; nitrogen, blue) within the active site of HCV NS3 protease and the three nearby mutating residues.
Modeling of NS3 mutations.
The three-dimensional locations in NS3 of the eight mutations observed in this study are shown in Fig. 2A along with the expected binding location of BILN 2061 (28). Three mutations, R155Q, A156T, and D168V, are close to the inhibitor binding site. Because of their close proximity, these three mutations are likely to have a significant impact upon the binding affinity of BILN 2061. A close-up view of the positions of these three mutations relative to BILN 2061 is shown in Fig. 2B. The side chains of residues 156 and 168 make contact with the cyclopentylcarbamate portion of BILN 2061. The side chain of residue 155 makes contact with the quinoline ring system of BILN 2061. Mutations at any of these positions are expected to disrupt Van der Waals contact between the protein and ligand and lead to decreased inhibitory potency for the mutant enzymes, in accord with the observations above. The remaining five mutations are either distal within the serine protease domain, i.e., residues 71, 72, and 88, or even further distant within the helicase domain, i.e., residues 260 and 312. How mutations at these non-active-site positions could impact BILN 2061 binding, if there is any effect, is unclear. The location of residues 71, 72, and 88 near the NS4A activation protein suggests that they may play a role in influencing enzyme activation, but further experimentation would be required to evaluate this possibility.
An amino acid alignment of the NS3 region containing these mutations revealed that R155 and A156 are conserved across HCV genotypes and subtypes (Fig. 3) but that D168 is relatively less conserved. Glutamine is found at position 168 in genotypes 3a, 3b, and 10a, and glutamic acid is seen in genotype 5a. It is not known what impact these differences will have on sensitivity to BILN 2061, since it has not been assessed in these genotypes.
FIG. 3.
Amino acid sequence alignment of HCV genotypes and subtypes. The alignment of residues 143 to 180 of HCV NS3 is shown, with the three mutant residues shaded. The GenBank accession numbers for each strain listed in this figure are as follows: for 1a-1, AAB66324; for 1a-2, AAK66555; for 1a-3, BAA01582; for 1a-4, AAA45676; for 1b-1, AAD44718; for 1b-2, CAB46677; for 1b-3, AAC15725; for 1c, BAA03581; for 2a, BAB32872; for 2b, BAA01761; for 2c, BAA08911; for 2k, BAA88057; for 3a, CAA54244; for 3b, BAA08372; for 4a, CAA72338; for 5a, CAA73640; for 6a, CAA72801; for 6b, BAA32664; for 7b, BAA32665; for 8b, BAA32666; for 9a, BAA32667; for 10a, BAA09890; and for 11a, BAA09891.
Sensitivity of single-mutation replicons to inhibitors.
To ascertain whether the NS3 mutations R155Q, A156T, and D168V are responsible for the resistance, we introduced these mutations individually into a subgenomic replicon containing a luciferase reporter gene. RNA from these replicon constructs was used to transfect cured Nneo/3-5B(RG) cells, which support replication of HCV replicons much more efficiently than Huh-7 cells (8, 27). The transfected cells were grown in the presence of different concentrations of drug for 4 days, and then the IC50s were determined as described in Materials and Methods.
All three mutations significantly reduced the potency of the serine protease inhibitor BILN 2061 (Table 2), with IC50 increases of 24-fold for R155Q, 357-fold for A156T, and 144-fold for D168V. The relative effects of these individual mutations on viral resistance to BILN 2061, A156T > D168V > R155Q, is in agreement with the observation from resistant colonies (Table 1) that SB4, which contains the R155Q mutation, had the smallest IC50 increase compared to other mutants, while SB1 and SB5, containing the A156T mutation, showed the highest resistance levels. However, the molecular clone containing the single mutation A156T was more susceptible to BILN 2061 than either SB1 or SB5 (Tables 1 and 2). These differences may be due either to compensatory mutations that developed during selection (T72I and P88L in SB1 or I71T and T312A in SB5) or to the different assays used in these experiments. The SEAP assay was used for stable replicon cell lines, whereas the luciferase reporter assay was used for molecular clones containing single mutations. Similar results were obtained when the molecular clone containing D168V was compared to SB6 (Tables 1 and 2). As was observed for the resistant colonies, these individual mutations had no effect on replicon sensitivity to IFN-α (Table 2).
TABLE 2.
Sensitivities of replicons with single NS3 mutations to HCV serine protease inhibitors and IFN-α in a transient-transfection assaya
| Replicon clone | BILN 2061
|
BI-1
|
BI-2
|
IFN-α IC50 (IU) | |||
|---|---|---|---|---|---|---|---|
| IC50 (μM) | FC | IC50 (μM) | FC | IC50 (μM) | FC | ||
| Wild type | 0.003 ± 0.00004 | 0.492 ± 0.061 | 0.239 ± 0.029 | 0.49 ± 0.063 | |||
| R155Q mutant | 0.072 ± 0.010 | 24 | 3.457 ± 0.090 | 7 | >20 | >83 | 0.42 ± 0.082 |
| A156T mutant | 1.072 ± 0.153 | 357 | >100 | >203 | >20 | >83 | 0.52 ± 0.129 |
| D168V mutant | 0.432 ± 0.013 | 144 | 10.75 ± 0.879 | 22 | >20 | >83 | 0.49 ± 0.064 |
Cells were transfected in the presence of different concentrations of each drug to measure IC50s. Data are from luciferase assays performed 4 days after transfection. Data shown are averages ± standard deviations from triplicate experiments. FC, change in IC50 (n-fold) over that seen with the wild type.
To assess cross-resistance, two acyclic tripeptide analogs of BILN 2061, BI-1 and BI-2 (Fig. 1), were used to test the wild-type replicon and replicons containing single mutations in NS3. These compounds are structurally similar to the inhibitor used by Trozzi et al. (27) to select resistant HCV replicons. The HCV inhibitory potencies of these two compounds against the wild-type replicon are about 100-fold weaker than that of BILN 2061 (Table 2). All three mutant replicons showed a high level of resistance to compound BI-2 at 20 μM, which was the highest concentration used. The R155Q mutant showed a similar magnitude of potency loss to all three compounds, which may be because all of the compounds contain the same methoxy quinoline group that makes direct contact with residue 155. The smaller effects for the acyclic analogs BI-1 and BI-2 might also be due to their intrinsic affinity for enzyme and relatively suboptimal interactions with mutant residues. The D168V replicon was more sensitive to BI-1 (the IC50 increase was 22-fold) than it was to BILN 2061 (the IC50 increase was 144-fold). The tert-butyl moiety in BI-1, instead of the cyclopentyl moiety found in BILN 2061, may account for this change by increasing the ability to bind to mutated residue 168.
Replication capacity of single-mutation replicons.
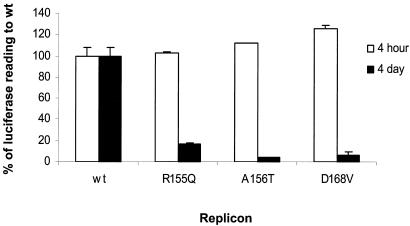
Since the copy number of the luciferase gene is determined by the replication level of the corresponding replicon RNA (15), the replication capacity of each mutant can be determined by measuring luciferase activity in a lysate of transfected cells. Each mutant replicon was transfected in the absence of BILN 2061, and luciferase activity was measured 4 days posttransfection. Luciferase activities from the mutant replicons were then compared with the luciferase activity from the wild-type replicon. Each of the replicons with a single mutation in the NS3 serine protease showed a greatly reduced level of replication (Fig. 4). All of them had very similar levels of luciferase activity at 4 h after transfection, indicating comparable levels of RNA transfection and translation ability. At 4 days after transfection, the R155Q, A156T, and D168V mutants retained only 17, 4, and 6% of wild-type replication capacity, respectively (Fig. 4). Despite the reduction in replication and the corresponding reduction in luciferase seen with the mutant replicons, the luciferase activity remained high enough for the accurate determination of IC50s (Table 2).
FIG. 4.
Comparison of the replication capacity of the wild-type replicon (wt) to those of mutant replicons engineered with single mutations. Cells were transfected either with wild-type or with mutant replicon RNA in the absence of BILN 2061, and luciferase activity was measured at 4 h and 4 days after transfection. Luciferase activities of mutants were normalized as percentages of the wild-type activity.
DISCUSSION
We report here that the in vitro selection of HCV replicon with a fixed concentration of HCV serine protease inhibitor BILN 2061 leads to the selection of specific mutations in the serine protease gene which confer reduced susceptibility to the inhibitor. Three-dimensional analysis indicates that three residues at positions 155, 156, and 168 within the active site are likely the structurally most dominant sites for conferring resistance. Site-directed mutagenesis of these individual mutations into replicons confirmed that each could play a role in the development of resistance. While all of the potency decreases for the mutations are large enough to be significant in an in vivo situation, there is the trend that the R155 mutation gives less of an effect than either the A156 or the D168 mutation. This may, in part, be due to the polarity retention in the R155Q mutation, while changes at residues 156 and 168 involve a polarity change. A complete reversal of the character of a protein residue that contacts an inhibitor could be one factor leading to a larger resistance magnitude in those cases. A detailed atomic analysis, however, would require further X-ray crystallographic studies of both wild-type and mutant enzymes.
A recent study using HCV NS3/NS4A serine protease inhibitor VX-950 (Lin et al., Hepatology 38, abstr. 1000) reported the selection of resistant colonies that contained an A156S mutation. Lin et al. showed that a replicon containing A156S was sensitive to BILN 2061. In contrast, we found that a replicon containing A156T was 357-fold-more resistant to BILN 2061. Although the reason(s) for the differences in potency loss is unclear, the additional methyl of the threonine residue relative to serine may create additional steric hindrance that might cause the A156T mutant to be more resistant than the A156S mutant. As stated above, residue 156 makes intimate contact with several atoms of the ligand, and an additional methyl group may make a significant impact. A more detailed structural analysis, including protein crystallographic studies of the mutant enzymes, would be required for a more complete understanding of this differential effect.
The polarity changes may also play a role in the lower replication capacity of the A156T and D168V replicons relative to that of the R155Q replicon. However, the effect on replication by all three mutations was significant. Considering the conserved nature of these residues in various HCV genotypes (Fig. 3) and the reduced replication of replicons containing the single mutations (Fig. 4), it is unlikely that there are preexisting mutants containing only changes at any one of these residues in the natural population.
The role of the individual mutations at NS3 positions 71, 72, 88, 260, and 312 was not studied. However, the phenotypic resistance of molecular clones containing single amino acid mutations at position 155, 156, or 168 correlates well with the phenotypic susceptibility of replicon cell line colonies that contain those mutations. Therefore, the other mutations may play a compensatory role for replication but not for resistance (19). This possibility is supported by the observation that these residues are far from the serine protease active site. It is also supported by the observations that the resistant colonies containing multiple mutations grew normally but that the replicons containing a single change at residue 155, 156, or 168 exhibited greatly reduced HCV RNA replication in the transient-replication assay. Interestingly, resistant colony SB4 had only the R155Q mutation in NS3, indicating that mutations from different gene coding regions might compensate for the defect in replication caused by this NS3 mutation (data not shown). Engineering combinations of mutations into replicons and studying their replication capacity would be helpful for understanding the interaction of the various residues.
In the region of the active-site mutations, BI-1 and BI-2 are expected to project functional groups that are either structurally identical to or very similar to BILN 2061 when it is bound to the enzyme. Thus, it is expected that the losses in potency against the mutants are similar for all three serine protease inhibitors. The accurate comparison of the decrease (n-fold) in the potency of BI-2 to that of BILN 2061 is made difficult by the dramatically different potencies against the wild-type enzyme. Our results showed that any one of several single mutations results in a high level of resistance to BILN 2061. However, replicons containing either multiple mutations or single engineered mutations were still sensitive to IFN-α (Table 1 and 2), indicating that combination therapy similar to HIV treatment using several drugs with different functions and targets may help overcome drug-resistant mutations and control HCV infection.
In this study, we modified the resistance selection protocol used by Trozzi et al. (27) so that there was no cell split during the selection procedure. Without cell splitting, a resistant colony can be assumed to grow from a single cell. Therefore, by counting the number of total visible colonies and knowing the initial cell number, we roughly calculated the frequency of resistant mutations that happened in the population under the experimental conditions used. In protocols that include splitting cells, it is possible that all colonies can arise from a single mutated cell, which raises the possibility that cells containing adaptive mutations that allow faster growth lead to selection of only the dominant mutants. Indeed, in this study, where no cell spitting occurred, a larger variety of mutations was observed than was seen in the studies by Trozzi et al. (27) and Lin et al. (Hepatology 38, abstr. 1000).
In conclusion, BILN 2061 encountered resistance from HCV replicons mediated by mutations at the serine protease active site. The resistance pattern found in vitro may be of benefit for the prediction of in vivo resistance profiles and the development of next-generation serine protease inhibitors. Finally, the sensitivity of BILN 2061-resistant mutants to IFN-α indicates that a cocktail therapy combining several drugs may be necessary to overcome drug resistance in vivo.
Acknowledgments
We thank Stanley Lemon for providing replicon cell lines. We also thank Teresa Ng for providing the replicon-cured cell line; Yupeng He, Moshe Weitzberg, Rolf Wagner, and Mike Tufano for helpful discussions; and Shing Chang and Bill Kohlbrenner for providing continuous support.
REFERENCES
- 1.Barbato, G., D. O. Cicero, M. C. Nardi, C. Steinkuhler, R. Cortese, R. De Francesco, and R. Bazzo. 1999. The solution structure of the N-terminal proteinase domain of the hepatitis C virus (HCV) NS3 protein provides new insights into its activation and catalytic mechanism. J. Mol. Biol. 289:371-384. [DOI] [PubMed] [Google Scholar]
- 2.Bartenschlager, R. 1999. The NS3/4A proteinase of the hepatitis C virus: unravelling structure and function of an unusual enzyme and a prime target for antiviral therapy. J. Viral Hepat. 6:165-181. [DOI] [PubMed] [Google Scholar]
- 3.Bartenschlager, R., L. Ahlborn-Laake, J. Mous, and H. Jacobsen. 1994. Kinetic and structural analyses of hepatitis C virus polyprotein processing. J. Virol. 68:5045-5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boehringer Ingelheim (Canada), Ltd. August2003. Macrocyclic peptides active against the hepatitis C virus. U.S. patent 6,608,027 B1.
- 5.Boehringer Ingelheim (Canada), Ltd. March2003. Hepatitis C inhibitor tri-peptides. U.S. patent 6,534,523 B1.
- 6.Cicero, D. O., G. Barbato, U. Koch, P. Ingallinella, E. Bianchi, M. C. Nardi, C. Steinkuhler, R. Cortese, V. Matassa, R. De Francesco, A. Pessi, and R. Bazzo. 1999. Structural characterization of the interactions of optimized product inhibitors with the N-terminal proteinase domain of the hepatitis C virus (HCV) NS3 protein by NMR and modelling studies. J. Mol. Biol. 289:385-396. [DOI] [PubMed] [Google Scholar]
- 7.Cornberg, M., H. Wedemeyer, and M. P. Manns. 2002. Treatment of chronic hepatitis C with PEGylated interferon and ribavirin. Curr. Gastroenterol. Rep. 4:23-30. [DOI] [PubMed] [Google Scholar]
- 8.De Francesco, R., G. Migliaccio, and G. Paonessa. August2002. Hepatitis C virus replicons and replicon enhanced cells. WO patent 0259321.
- 9.Di Marco, S., M. Rizzi, C. Volpari, M. A. Walsh, F. Narjes, S. Colarusso, R. De Francesco, V. G. Matassa, and M. Sollazzo. 2000. Inhibition of the hepatitis C virus NS3/4A protease: the crystal structures of two protease-inhibitor complexes. J. Biol. Chem. 275:7152-7157. [DOI] [PubMed] [Google Scholar]
- 10.Failla, C., L. Tomei, and R. De Francesco. 1995. An amino-terminal domain of the hepatitis C virus NS3 protease is essential for interaction with NS4A. J. Virol. 69:1769-1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foy, E., K. Li, C. Wang, R. Sumpter, Jr., M. Ikeda, S. M. Lemon, and M. G. Gale, Jr. 2003. Regulation of interferon regulatory factor-3 by the hepatitis C virus serine protease. Science 300:1145-1148. [DOI] [PubMed] [Google Scholar]
- 12.Grakoui, A., C. Wychowski, C. Lin, S. M. Feinstone, and C. M. Rice. 1993. Expression and identification of hepatitis C virus polyprotein cleavage products. J. Virol. 67:1385-1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ikeda, M., M. Yi, K. Li, and S. M. Lemon. 2002. Selectable subgenomic and genome-length dicistronic RNAs derived from an infectious molecular clone of the HCV-N strain of hepatitis C virus replicate efficiently in cultured Huh7 cells. J. Virol. 76:2997-3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kolykhalov, A. A., K. Mihalik, S. M. Feinstone, and C. M. Rice. 2000. Hepatitis C virus-encoded enzymatic activities and conserved RNA elements in the 3′ nontranslated region are essential for virus replication in vivo. J. Virol. 74:2046-2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krieger, N., V. Lohmann, and R. Bartenschlager. 2001. Enhancement of hepatitis C virus RNA replication by cell culture-adaptive mutations. J. Virol. 75:4614-4624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lamarre, D., P. C. Anderson, M. Bailey, P. Beaulieu, G. Bolger, P. Bonneau, M. Bos, D. R. Cameron, M. Cartier, M. G. Cordingley, A.-M. Faucher, N. Goudreau, S. H. Kawai, G. Kukolj, L. Lagace, S. R. LaPlante, H. Narjes, M.-A. Poupart, J. Rancourt, R. E. Sentjens, R. St. George, B. Simoneau, G. Steinmann, D. Thibeault, Y. S. Tsantrizos, S. M. Weldon, C.-L. Yong, and M. Llinas-Brunet. 2003. An NS3 protease inhibitor with antiviral effects in humans infected with hepatitis C virus. Nature 426:186-189. [DOI] [PubMed] [Google Scholar]
- 17.Llinas-Brunet, M., M. Bailey, G. Fazal, E. Ghiro, V. Gorys, S. Goulet, T. Halmos, R. Maurice, M. Poirier, M. A. Poupart, J. Rancourt, D. Thibeault, D. Wernic, and D. Lamarre. 2000. Highly potent and selective peptide-based inhibitors of the hepatitis C virus serine protease: towards smaller inhibitors. Bioorg. Med. Chem. Lett. 10:2267-2270. [DOI] [PubMed] [Google Scholar]
- 18.Llinas-Brunet, M., M. Bailey, G. Fazal, S. Goulet, T. Halmos, S. Laplante, R. Maurice, M. Poirier, M. A. Poupart, D. Thibeault, D. Wernic, and D. Lamarre. 1998. Peptide-based inhibitors of the hepatitis C virus serine protease. Bioorg. Med. Chem. Lett. 8:1713-1718. [DOI] [PubMed] [Google Scholar]
- 19.Lohmann, V., F. Körner, A. Dobierzewska, and R. Bartenschlager. 2001. Mutations in hepatitis C virus RNAs conferring cell culture adaptation. J. Virol. 75:1437-1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mo, H., L. Lu, T. Dekhtyar, K. D. Stewart, E. Sun, D. J. Kempf, and A. Molla. 2003. Characterization of resistant HIV variants generated by in vitro passage with lopinavir/ritonavir. Antivir. Res. 59:173-180. [DOI] [PubMed] [Google Scholar]
- 21.Molla, A., M. Korneyeva, Q. Gao, S. Vasavanonda, P. J. Schipper, H. M. Mo, M. Markowitz, T. Chernyavskiy, P. Niu, N. Lyons, A. Hsu, G. R. Granneman, D. D. Ho, C. A. Boucher, J. M. Leonard, D. W. Norbeck, and D. J. Kempf. 1996. Ordered accumulation of mutations in HIV protease confers resistance to ritonavir. Nat. Med. 2:760-766. [DOI] [PubMed] [Google Scholar]
- 22.Narjes, F., U. Koch, and C. Steinkuhler. 2003. Recent developments in the discovery of hepatitis C virus serine protease inhibitors—towards a new class of antivirial agents? Expert Opin. Investig. Drugs 12:153-163. [DOI] [PubMed] [Google Scholar]
- 23.Neddermann, P., L. Tomei, C. Steinkuhler, P. Gallinari, A. Tramontano, and R. De Francesco. 1997. The non-structural proteins of hepatitis C virus: structure and functions. Biol. Chem. 378:469-476. [PubMed] [Google Scholar]
- 24.Pauwels, R., J. Balzarini, M. Baba, R. Snoeck, D. Schols, P. Herdewijn, J. Desmyter, and E. De Clercq. 1988. Rapid and automated tetrazolium-based colorimetric assay for the detection of anti-HIV compounds. J. Virol. Methods 20:309-321. [DOI] [PubMed] [Google Scholar]
- 25.Pizzi, E., A. Tramontano, L. Tomei, N. La Monica, C. Failla, M. Sardana, T. Wood, and R. De Francesco. 1994. Molecular model of the specificity pocket of the hepatitis C virus protease: implications for substrate recognition. Proc. Natl. Acad. Sci. USA 91:888-892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steinkuhler, C., U. Koch, F. Narjes, and V. G. Matassa. 2001. Hepatitis C virus protease inhibitors: current progress and future challenges. Curr. Med. Chem. 8:919-932. [DOI] [PubMed] [Google Scholar]
- 27.Trozzi, C., L. Bartholomew, A. Ceccacci, G. Biasiol, L. Pacini, S. Altamura, F. Narjes, E. Muraglia, G. Paonessa, U. Koch, R. De Francesco, C. Steinkuhler, and G. Migliaccio. 2003. In vitro selection and characterization of hepatitis C virus serine protease variants resistant to an active-site peptide inhibitor. J. Virol. 77:3669-3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsantrizos, Y. S., G. Bolger, P. Bonneau, D. R. Cameron, N. Goudreau, G. Kukolj, S. R. LaPlante, M. Llinas-Brunet, H. Nar, and D. Lamarre. 2003. Macrocyclic inhibitors of the NS3 protease as potential therapeutic agents of hepatitis C virus infection. Angew. Chem. Int. Ed. Engl. 42:1356-1360. [DOI] [PubMed] [Google Scholar]
- 29.Wright, M., J. Main, and H. C. Thomas. 2001. Treatment of chronic viral hepatitis. Antivir. Chem. Chemother. 12:201-212. [DOI] [PubMed] [Google Scholar]
- 30.Yan, Y., Y. Li, S. Munshi, V. Sardana, J. L. Cole, M. Sardana, C. Steinkuehler, L. Tomei, R. De Francesco, L. C. Kuo, and Z. Chen. 1998. Complex of NS3 protease and NS4A peptide of BK strain hepatitis C virus: a 2.2 Å resolution structure in a hexagonal crystal form. Protein Sci. 7:837-847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yao, N., P. Reichert, S. S. Taremi, W. W. Prosise, and P. C. Weber. 1999. Molecular views of viral polyprotein processing revealed by the crystal structure of the hepatitis C virus bifunctional protease-helicase. Struct. Fold Des. 7:1353-1363. [DOI] [PubMed] [Google Scholar]
- 32.Yi, M., F. Bodola, and S. M. Lemon. 2002. Subgenomic hepatitis C virus replicons inducing expression of a secreted enzymatic reporter protein. Virology 304:197-210. [DOI] [PubMed] [Google Scholar]