Abstract
Aryl electrophiles containing tethered allylboronate units undergo efficient intramolecular coupling in the presence of a chiral palladium catalyst to give enantioenriched carbocyclic products. The reaction is found to be quite general, affording 5, 6, and 7-membered carbocyclic products as single regioisomers and with moderate enantioselectivities. Examination of differential coupling partners points to rapid allyl-equilibration as a key stereodefining feature.
The development of metal-catalyzed cross-coupling reactions has revolutionized the manner in which molecules are assembled, providing reliable, predictable, and versatile entry to a variety of molecular frameworks.1 Recently, allylmetal reagents have garnered significant attention as valuable partners in cross-coupling protocols. Employing nonsymmetric allyl fragments allows bond formation at one of two sites, presenting the opportunity for both regioselective and in some cases enantioselective transformations. In particular, allyl boron compounds2−4 have proven to be practical nucleophilic partners, in part due to their ease of handling, high functional group tolerance, and facile preparation.5
Recently, several reports engaging allyl boronates as partners in both branch- and linear-selective cross coupling reactions have been disclosed (Scheme 1). In 2006, Szabó achieved a highly branch-selective coupling of allylboronic acids with aryl electrophiles.2n More recently, Crudden reported the regioselective coupling of internal allyl boronates with aryl electrophiles.2f The linear-selective coupling of allyl boronates and aryl electrophiles was accomplished by Organ utilizing a bulky Pd-PEPPSI catalyst.2g Notably, Buchwald was able to achieve both branch- and linear-selective coupling with the proper choice of ligand.2h While Aggarwal and Crudden recently disclosed the enantiospecific cross-coupling of enantiomerically enriched allyl boronates with aryl electrophiles,2i the only example of enantioselective coupling of allyl boronates and aryl electrophiles with chiral catalysts remains the work of Miyaura.6 When an electron-rich Josiphos-based ligand with the use of crotyl potassium trifluoroboronates was employed, a highly branch-selective coupling was achieved with good enantioselectivity.
Scheme 1. Recent Examples of Intermolecular Allyl Boronate Aryl Coupling.
In stark contrast to the aforementioned intermolecular coupling of allyl boronates, an intramolecular version is unknown.7 In related coupling reactions,8 allylsilanes,9 allylstannanes,10 and allylindium11 reagents have all been successfully engaged in intramolecular cross coupling to forge the desired carbo- or heterocyclic products. With the notable exception of work by Tietze,9d−9f all intramolecular allyl–aryl couplings have thus far been executed in a racemic manner. Herein, we describe the use of allyl boronates as nucleophilic partners in an intramolecular coupling with aryl electrophiles to afford enantiomerically enriched carbocyclic products in a regioselective fashion.
Initial attempts to achieve the desired transformation are outlined in Table 1. The use of a variety of bidentate chiral phosphine ligands generally resulted in efficient conversion to the carbocyclic product in a regioselective fashion, albeit with low levels of enantioselectivity (entries 1–6). In contrast to the good enantioselectivities obtained by Miyaura under similar reaction conditions for intermolecular allyl–aryl coupling, employment of a Josiphos-based catalyst results in a nearly racemic reaction product (entry 1). Utilization of cesium fluoride under anhydrous conditions resulted in a slight improvement in selectivity with Me-DuPhos (entry 8); however, significantly higher enantiomeric selectivity was obtained with the use of monodentate phosphoramidite ligands utilizing a TADDOL based backbone (entries 9–11). While increasing the size of the ligand aryl groups resulted in only marginally elevated selectivities, the nature of the amino group proved more influential, with small groups yielding superior results (cf. L2 vs L3); thus, (R,R)-L2 was selected for further study.
Table 1. Survey of Chiral Ligands in Intramolecular Allyl–Aryl Couplinga.

| entry | base | solvent | ligand | conv (%) | er |
|---|---|---|---|---|---|
| 1 | KOH | THF/H2O | JosiPhos | >98 | 51:49 |
| 2 | KOH | THF/H2O | Binap | >98 | 51:49 |
| 3 | KOH | THF/H2O | QuinoxP* | >98 | 56:44 |
| 4 | KOH | THF/H2O | PhBPE | >98 | 51:49 |
| 5 | KOH | THF/H2O | iPr-DuPhos | >98 | 56:44 |
| 6 | KOH | THF/H2O | Me-DuPhos | >98 | 57:43 |
| 7 | CsF | THF/H2O | Me-DuPhos | 63 | 56:44 |
| 8 | CsF | THF | Me-DuPhos | >98 | 59:41 |
| 9 | CsF | THF | L1 | >98 | 83:17 |
| 10 | CsF | THF | L2 | >98 | 84:16 |
| 11 | CsF | THF | L3 | >98 | 71:29 |
Percent conversion determined by 1H
NMR analysis; er (enantiomer ratio) determined by GLC analysis
with a chiral stationary phase.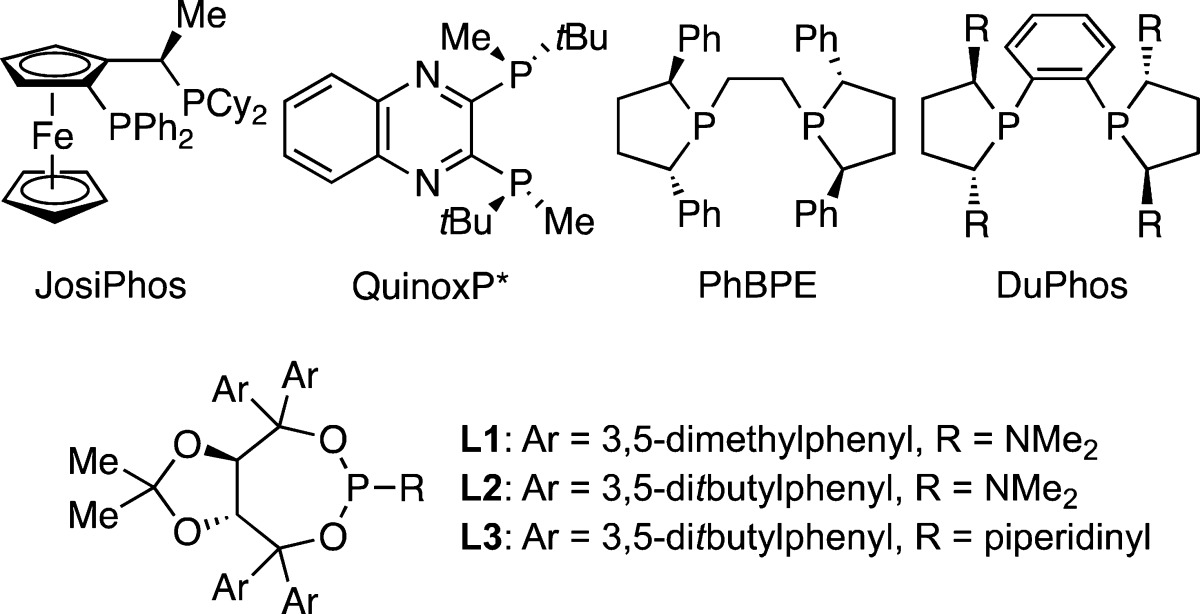
In an effort to improve reaction efficiency further, additional conditions were examined (Table 2). While promoting efficient ring closure in most cases, bases other than CsF gave inferior levels of enantioselectivity (entries 1–5). Similarly, both polar (entries 3, 6–8) and nonpolar (entries 9, 10) solvents proved to be suitable reaction media, with THF affording the best selectivity. Because of its ability to promote π–σ–π isomerization, the use of NBu4Cl was examined. In line with observations by Trost12 and Togni,13 this led to a significant increase in the levels of enantioselectivity obtained. Employing aryl chlorides (entry 12) in place of aryl bromides also resulted in greater selectivity, which can be further augmented with the use of NBu4Cl (entry 13); however, reactivity generally suffers under these conditions. Taken together, the use of aryl chlorides without additive in THF, and with CsF as the base, was found to yield the best results, achieving full conversion with good levels of enantioselectivity.
Table 2. Survey of Reaction Conditions in Intramolecular Allyl–Aryl Couplinga.
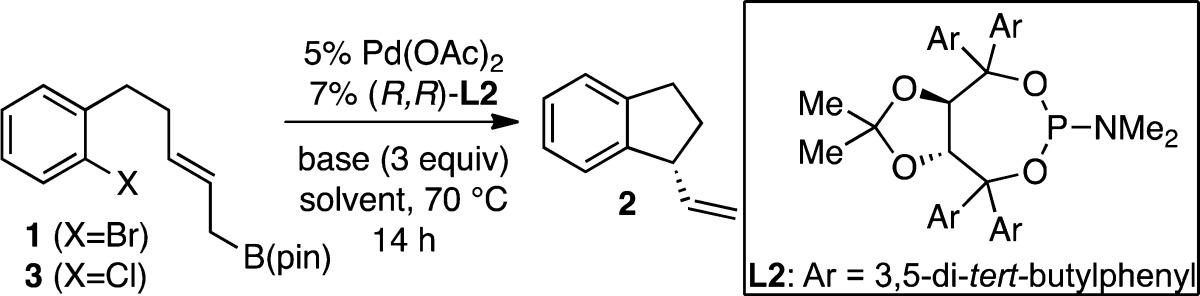
| entry | X | base | solvent | additive | conv (%) | er |
|---|---|---|---|---|---|---|
| 1 | Br | Cs2CO3 | THF | >98 | 68:32 | |
| 2 | Br | K3PO4 | THF | >98 | 56:44 | |
| 3 | Br | CsF | THF | >98 | 84:16 | |
| 4 | Br | KF | THF | 24 | 76:24 | |
| 5 | Br | TBAF | THF | >98 | 46:54 | |
| 6 | Br | CsF | MeCN | >98 | 61:39 | |
| 7 | Br | CsF | EtOAc | 74 | 83:17 | |
| 8 | Br | CsF | dioxane | >98 | 82:18 | |
| 9 | Br | CsF | toluene | >98 | 74:26 | |
| 10 | Br | CsF | hexane | >98 | 74:26 | |
| 11 | Br | CsF | THF | Bu4NCl | 70 | 90:10 |
| 12 | Cl | CsF | THF | >98 | 90:10 | |
| 13 | Cl | CsF | THF | Bu4NCl | 45 | 93:7 |
Percent conversion determined by 1H NMR analysis; er (enantiomer ratio) determined by GLC analysis with a chiral stationary phase.
With the optimal conditions in hand, the scope of the intramolecular allyl–aryl coupling was examined (Scheme 2). Compared to unsubstituted substrate 3, meta-substituted substrates exhibited enhanced selectivity (products 4, 7) with substitution in the ortho position affording lower selectivities (5). Importantly, the use of NBu4Cl can restore selectivity to achieve moderate levels of asymmetric induction for this more challenging substitution pattern, albeit with diminished yield.
Scheme 2. Survey of Reaction Conditions in Intramolecular Allyl–Aryl Coupling.
Yield refers to isolated yield of purified material and is an average of two experiments. er was determined chromatographically by either GC or SFC analysis using a chiral stationary phase.
Yield in parentheses determined by 1H NMR versus internal standard.
Selectivity obtained with NBu4Cl (1.5 equiv), < 30% conversion in both cases.
Both electron-rich (6) and electron-poor (8) substrates engage smoothly in intramolecular coupling, with the selectivity for electron-poor substrates benefiting from the use of NBu4Cl. Notably, substitution on the allyl boronate moiety is well tolerated, and 9 is produced in good yield with moderate enantioselectivity. Changing the tether length between the aryl electrophile and the allyl boronate allows efficient formation of six- and seven-membered rings (10 and 11) in moderate yield with moderate levels of enantioselectivity.
Seeking a more complete understanding of the origins of stereoinduction in the reaction, it was reasoned that selectivity could be imparted during olefin binding (formation of A), transmetalation (A →D, Scheme 3), or reductive elimination (B, C, D → product). Miyaura and co-workers found that during the related enantioselective intermolecular allyl–aryl coupling, transmetalation was the stereochemistry determining step.6b Thus, after oxidative addition with an aryl halide, the Josiphos-based catalyst effectively selected one of the prochiral faces of the crotyl boronate during SE2′ transmetalation, thereby establishing a palladium–carbon bond in an enantioselective fashion. For the Miyaura system, in order to suppress competing allyl isomerization, a subsequent rapid reductive elimination of the product was found to be essential to achieve high regio- and enantioselectivity.
Scheme 3. Proposed Catalytic Cycle.
To test whether the intramolecular reaction occurs with a similar mode of selectivity to the intermolecular variant, a series of substrates were prepared in which the nucleophile and electrophile components were altered (Scheme 4). If the stereochemistry-determining step of the intramolecular coupling followed the intermolecular precedent established by Miyaura,6 it was reasoned that changing the geometry of the allyl boronate moiety would have a significant impact on the stereochemical outcome of the coupling reaction. However, employing the Z-allyl boronate under the standard reaction conditions produces the same enantiomer of product that is observed when the E-allyl boronate is used and with nearly identical levels of selectivity (Scheme 4, eq 1 vs 2). Moreover, the use of substrates with inverse polarity coupling partners also resulted in formation of the same enantiomer of product (eqs 3 and 4). Since the likelihood of achieving similar levels of selectivity during transmetalation of vastly different species is low, it seems plausible that allyl equilibration14 of the transmetalation adducts results in stereochemical convergence to a common palladacycle intermediate, followed by stereochemistry-determining reductive elimination to give the observed carbocycle.
Scheme 4. Examination of Alternate Substrate Configurations.
Yields determined by 1H NMR versus internal standard.
This mechanistic proposal in Scheme 3 accounts for many of the observations noted earlier. In order to have the opportunity to correct for a nonselective transmetalation that gives both B and C, the rate of this isomerization must be rapid relative to reductive elimination. Consequently, any features of the reaction system which either expedite reductive elimination or slow isomerization, may ultimately result in decreased enantioselectivity. For instance, in line with experiments by Trost,12 the beneficial influence of NBu4Cl can be attributed to stabilization of 14 electron η1 allyl intermediates, which are required for stereoinvertive isomerization. Similarly, the detrimental effects of bidentate ligands could arise from accelerated reductive elimination and the suppression of isomerization by disfavoring η-bound Pd(II) intermediates required for isomerization.15 While these high energy 18-electron intermediates appear to be accessible in intermolecular systems3b,3c,3e,3f and with the use of nickel,16 in the current Pd-catalyzed intramolecular case this could be problematic.17
In conclusion, we have described a catalytic strategy for the stereoselective construction of carbocycles by intramolecular allyl aryl cross-couplings. Further exploration of the scope and applications of this process is in progress and will be reported in due course.
Acknowledgments
Support by the NIGMS (GM-64451) is gratefully acknowledged. We thank AllyChem for a donation of B2(pin)2; C.H.S. and Z.A.K. are grateful for LaMattina Graduate Fellowship and Kozarich Fellowships, respectively.
Supporting Information Available
Experimental procedures and characterization of all new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- a de Meijere A., Diederich F., Eds. Metal-Catalyzed Cross-Coupling Reactions, 2nd ed.; Wiley-VHC: Weinheim, 2004. [Google Scholar]; For selected reviews, see:; b Negishi E.Handbook of Organopalladium Chemistry for Organic Synthesis; Wiley: New York, 2002; Vol. 1. [Google Scholar]; c Miyaura N.; Suzuki A. Chem. Rev. 1995, 95, 2457. [Google Scholar]; d Jana R.; Pathak T. P.; Sigman M. S. Chem. Rev. 2011, 111, 1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For cross-coupling of aryl electrophiles and allyl boronic esters, see:; a Nilsson K.; Hallberg A. Acta Chem. Scand. 1987, 41b, 569. [Google Scholar]; b Occhiato E. G.; Trabocchi A.; Guarna A. J. Org. Chem. 2001, 66, 2459. [DOI] [PubMed] [Google Scholar]; c Kotha S.; Behera M.; Shah V. R. Synlett 2005, 1877. [Google Scholar]; d Rossi E.; Abbiati G.; Canevari V.; Celentano G.; Magri E. Synthesis 2006, 299. [Google Scholar]; e Gerbino D. C.; Mandolesi S. D.; Schmalz H.-G.; Podestá J. C. Eur. J. Org. Chem. 2009, 3964. [Google Scholar]; f Glasspoole B. W.; Ghozati K.; Moir J. W.; Crudden C. M. Chem. Commun. 2012, 48, 1230. [DOI] [PubMed] [Google Scholar]; g Farmer J. L.; Hunter H. N.; Organ M. G. J. Am. Chem. Soc. 2012, 134, 17470. [DOI] [PubMed] [Google Scholar]; h Yang Y.; Buchwald S. L. J. Am. Chem. Soc. 2013, 135, 10642. [DOI] [PMC free article] [PubMed] [Google Scholar]; i Chausset-Boissarie L.; Ghozati K.; LaBine E.; Chen J. L-Y.; Aggarwal V. K.; Crudden C. M. Chem.—Eur. J. 2013, 19, 17698. [DOI] [PubMed] [Google Scholar]; Aryl electrophiles and allyl boranes:; j Kalinin V. N.; Denisov F. S.; Bubnov Y. N. Mendeleev Commun. 1996, 6, 206. [Google Scholar]; k Fürstner A.; Seidel G. Synlett 1998, 161. [Google Scholar]; l Fürstner A.; Leitner A. Synlett 2001, 290. [Google Scholar]; m Dai Q.; Xie X.; Xu S.; Ma D.; Tang S.; She X. Org. Lett. 2011, 13, 2302. [DOI] [PubMed] [Google Scholar]; Aryl electrophiles and allyl boronic acids:; n Sebelius S.; Olsson V. J.; Wallner O. A.; Szabó K. J. J. Am. Chem. Soc. 2006, 128, 8150. [DOI] [PubMed] [Google Scholar]; Aryl electrophiles and allyl trifluoroborates:; o Yamamoto Y.; Takada S.; Miyaura N. Chem. Lett. 2006, 35, 704. [Google Scholar]; p Al-Masum M.; Alam S. Tetrahedron Lett. 2009, 50, 5201. [Google Scholar]
- For coupling with allyl electrophiles:; a Flegeau E. F.; Schneider U.; Kobayashi S. Chem.—Eur. J. 2009, 15, 12247. [DOI] [PubMed] [Google Scholar]; b Zhang P.; Brozek L. A.; Morken J. P. J. Am. Chem. Soc. 2010, 132, 10686. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Zhang P.; Le H.; Kyne R. E.; Morken J. P. J. Am. Chem. Soc. 2011, 133, 9716. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Jiménez-Aquino A.; Flegeau E. F.; Schneider U.; Kobayashi S. Chem. Commun. 2011, 47, 9456. [DOI] [PubMed] [Google Scholar]; e Brozek L. A.; Ardolino M. J.; Morken J. P. J. Am. Chem. Soc. 2011, 133, 16778. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Le H.; Kyne R. E.; Brozek L. A.; Morken J. P. Org. Lett. 2013, 15, 1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For coupling with unsaturated carbonyls:; a Sieber J. D.; Liu S.; Morken J. P. J. Am. Chem. Soc. 2007, 129, 2214. [DOI] [PubMed] [Google Scholar]; b Sieber J. D.; Morken J. P. J. Am. Chem. Soc. 2008, 130, 4978. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Zhang P.; Morken J. P. J. Am. Chem. Soc. 2009, 131, 12550. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Brozek L. A.; Sieber J. D.; Morken J. P. Org. Lett. 2011, 13, 995. [DOI] [PMC free article] [PubMed] [Google Scholar]; For coupling with propargyl electrophiles:; e Ardolino M. J.; Morken J. P. J. Am. Chem. Soc. 2012, 134, 8770. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Ardolino M. J.; Eno M. S.; Morken J. P. Adv. Synth. Catal. 2013, 355, 3413. [DOI] [PMC free article] [PubMed] [Google Scholar]; For coupling with acid chlorides:; g Al-Masum M.; Liu K.-Y. Tetrahedron Lett. 2011, 52, 5090. [Google Scholar]; For coupling with aldimines:; h Viera E. M.; Snapper M. L.; Hoveyda A. H. J. Am. Chem. Soc. 2011, 133, 3332. [DOI] [PMC free article] [PubMed] [Google Scholar]; For coupling with β-lactones:; i Niyomchon S.; Audisio D.; Luparia M.; Maulide N. Org. Lett. 2013, 15, 2318. [DOI] [PubMed] [Google Scholar]
- For synthesis from allylmagnesium compounds, see:; a Roush W. R.; Walts A. E.; Hoong L. K. J. Am. Chem. Soc. 1985, 107, 8186. [Google Scholar]; b Brown H. C.; Racherla U. S.; Pellechia P. J. J. Org. Chem. 1990, 55, 1868. [Google Scholar]; c Clary J. W.; Rettenmaier T. J.; Snelling R.; Bryks W.; Banwell J.; Wipke N. T.; Singaram B. J. Org. Chem. 2011, 76, 9602. [DOI] [PubMed] [Google Scholar]; From allyllithiums:; d Brown H. C.; Rangaishenvi M. V. Tetrahedron Lett. 1990, 31, 7113. [Google Scholar]; From vinylboron compounds:; e Matteson D. S.; Majumdar D. J. Am. Chem. Soc. 1980, 102, 7588. [Google Scholar]; f Sadhu K. M.; Matteson D. S. Organometallics 1985, 4, 1687. [Google Scholar]; g Brown H. C.; Singh S. M.; Rangaishenvi M. V. J. Org. Chem. 1986, 51, 3150. [Google Scholar]; h Althaus M.; Mahmood A.; Suárez J. R.; Thomas S. P.; Aggarwal V. K. J. Am. Chem. Soc. 2010, 132, 4025. [DOI] [PubMed] [Google Scholar]; From allyl electrophiles:; i Ishiyama T.; Ahiko T.; Miyaura N. Tetrahedron Lett. 1996, 37, 6889. [Google Scholar]; j Murata M.; Wantanabe S.; Masuda Y. Tetrahedron, Lett. 2000, 41, 5877. [Google Scholar]; k Olsson V. J.; Sebelius S.; Selander N.; Szabó K. J. J. Am. Chem. Soc. 2006, 128, 4588. [DOI] [PubMed] [Google Scholar]; l Dutheuil G.; Selander N.; Szabó K. J.; Aggarwal V. K. Synthesis 2008, 2293. [Google Scholar]; m Guzman-Martinez A.; Hoveyda A. H. J. Am. Chem. Soc. 2010, 132, 10634. [DOI] [PMC free article] [PubMed] [Google Scholar]; n Zhang P.; Roundtree I. A.; Morken J. P. Org. Lett. 2012, 14, 1416. [DOI] [PMC free article] [PubMed] [Google Scholar]; o Larsson J. M.; Szabó K. J. J. Am. Chem. Soc. 2013, 135, 443. [DOI] [PubMed] [Google Scholar]; From 1,3-dienes:; p Satoh M.; Nomoto Y.; Miyaura N.; Suzuki A. Tetrahedron Lett. 1989, 30, 3789. [Google Scholar]; q Wu J. Y.; Moreau B.; Ritter T. J. Am. Chem. Soc. 2009, 131, 12915. [DOI] [PubMed] [Google Scholar]; r Ely R. J.; Morken J. P. J. Am. Chem. Soc. 2010, 132, 2534. [DOI] [PMC free article] [PubMed] [Google Scholar]; From olefins:; s Kiesewetter E. T.; O’Brian R. V.; Yu E. C.; Meek S. J.; Schrock R. R.; Hoveyda A. H. J. Am. Chem. Soc. 2013, 135, 6026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Yamamoto Y.; Takada S.; Miyaura N. Chem. Lett. 2006, 35, 1368. [Google Scholar]; b Yamamoto Y.; Takada S.; Miyaura N. Organometallics 2009, 28, 152. [Google Scholar]
- For examples of intramolecular B-alkyl couplings, see:; a Miyaura N.; Ishiyama T.; Sasaki H.; Ishikawa M.; Sato M.; Suzuki A. J. Am. Chem. Soc. 1989, 111, 314. [Google Scholar]; b Miyaura N.; Ishikawa M.; Suzuki A. Tetrahedron Lett. 1992, 33, 2571. [Google Scholar]; c Soderquist J. A.; León G.; Colberg J. C.; Martínez I. Tetrahedron Lett. 1995, 36, 3119. [Google Scholar]; d Chemler S. R.; Danishefsky S. J. Org. Lett. 2000, 2, 2695. [DOI] [PubMed] [Google Scholar]; e Mohr P. T.; Halcomb R. L. J. Am. Chem. Soc. 2003, 125, 1712. [DOI] [PubMed] [Google Scholar]
- For a review of cyclizations featuring organosilanes and organostannanes, see:; Méndez M.; Echavarren A. M. Eur. J. Org. Chem. 2002, 15. [DOI] [PubMed] [Google Scholar]; For a review of allyltin and allylindium reagents, see:; Roy U. K.; Roy S. Chem. Rev. 2010, 110, 2472. [DOI] [PubMed] [Google Scholar]
- Selected examples involving allylsilanes:; a Moeller K. D.; Hudson C. M. Tetrahedron Lett. 1991, 32, 2307. [Google Scholar]; b Hudson C. M.; Marzabadi M. R.; Moeller K. D.; New D. G. J. Am. Chem. Soc. 1991, 113, 7372. [Google Scholar]; c Moeller K. D.; Hudson C. M.; Tinao-Wooldridge L. V. J. Org. Chem. 1993, 58, 3478. [Google Scholar]; d Tietze L. F.; Schimpf R. Angew. Chem., Int. Ed. 1994, 33, 1089. [Google Scholar]; e Tietze L. F.; Thede K.; Schimpf R.; Sannicoló F. Chem. Commun. 2000, 583. [Google Scholar]; f Tietze L. F.; Modi A. Eur. J. Org. Chem. 2000, 1959. [Google Scholar]
- Selected examples involving allylstannanes:; a Trost B. M.; Walchi R. J. Am. Chem. Soc. 1987, 109, 3487. [Google Scholar]; b Grigg R.; Sansano J. M. Tetrahedron 1996, 52, 13441. [Google Scholar]
- Selected examples involving allylindiums:; a Seomoon D.; Lee K.; Kim H.; Lee P. H. Chem.—Eur. J. 2007, 13, 5197. [DOI] [PubMed] [Google Scholar]; b Lee K.; Kim H.; Mo J.; Lee P. H. Chem.—Asian. J. 2011, 6, 2147. [DOI] [PubMed] [Google Scholar]
- Trost B. M.; Toste F. D. J. Am. Chem. Soc. 1999, 121, 4545. [Google Scholar]
- Burckhardt U.; Baumann M.; Togni A. Tetrahedron: Asymmetry 1997, 8, 155. [Google Scholar]
- For discussion of Pd(II) allyl species, see:; a Trost B. M.; Van Vranken D. L. Chem. Rev. 1996, 96, 395. [DOI] [PubMed] [Google Scholar]; b Pregosin P. S.; Salzmann R. Coord. Chem. Rev. 1996, 155, 35. [Google Scholar]
- Jolly P. W. Angew. Chem., Int. Ed. 1985, 24, 283. [Google Scholar]
- a Kurosawa H.; Ohnishi H.; Emoto M.; Chatani N.; Kawasaki Y.; Murai S.; Ikeda I. Organometallics 1990, 9, 3038. [Google Scholar]; b Rufińska A.; Goddard R.; Weidenthaler C.; Bühl M.; Pörschke K.-R. Organometallics 2006, 25, 2308. [Google Scholar]
- Employing Ni(cod)2 in place of Pd(OAc)2 with (R,R)-Me-DuPhos under the standard reaction conditions results in significantly higher levels of enantioselectivity (80:20 er and 59:41 er, respectively). While it is possible the mode of selectivity may change between the two metals, the greater ease with which Ni can form 18 electron allyl species may be a factor (ref (16)).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.







