Abstract
The association between the macrolide efflux gene mef(E) and the tet(M) gene was studied in two clinical strains of Streptococcus pneumoniae that belonged to serotypes 19F and 6A, respectively, and that were resistant to both tetracycline and erythromycin. The mef(E)-carrying element mega (macrolide efflux genetic assembly; 5,511 bp) was found to be inserted into a Tn916-like genetic element present in the chromosomes of the two pneumococcal strains. In both strains, mega was integrated at the same site, an open reading frame identical to orf6 of Tn916. The new composite element, Tn2009, was about 23.5 kb and, with the exception of the tet(M)-coding sequence, appeared to be identical in both strains. By sequencing of the junction fragments of Tn2009 at the site of insertion into the chromosome, it was possible to show that (i) the insertion site was identical in the two clinical strains and (ii) the integration of Tn2009 caused a 9.5 kb-deletion in the pneumococcal chromosome. It was not possible to detect the conjugal transfer of Tn2009 to a recipient pneumococcal strain; however, transfer of the whole element by transformation was shown to occur. It is possible to hypothesize that Tn2009 relies on transformation for its spread among clinical strains of S. pneumoniae.
In the last few years, the rates of macrolide resistance in Streptococcus pneumoniae have increased considerably in Italy, reaching a prevalence close to 30% among strains from the general population (20) and 50% among strains from children (22). In Italy the most common mechanism of macrolide resistance in pneumococci is target modification due to an erm(B)-encoded ribosome methylase (15, 18, 20). A second mechanism consists of active efflux of the antibiotic due to an efflux pump that belongs to the major facilitator superfamily and that is encoded by the mef(A) gene (6). This mechanism is prevalent or very common in macrolide-resistant pneumococci from the United States (9), Canada (13), and some European countries (23, 26), although in Italy it is the mechanism of resistance for only 25% of the resistant isolates (20). Of the two mef(A) gene subclasses described to date, Italian macrolide-resistant pneumococci more commonly harbor subclass mef(A), while subclass mef(E), considered characteristic of S. pneumoniae, is comparatively rare (7). The two subclasses share approximately 90% DNA identity (28) and are carried by distinct genetic elements. In S. pneumoniae mef(A) is part of a 7.2-kb defective transposon, designated Tn1207.1 (31), which contains eight open reading frames (ORFs), including a putative site-specific recombinase. Tn1207.1 has a unique integration site in the genome of the pneumococcus that corresponds to celB, a gene involved in competence. Insertion of Tn1207.1 disrupts celB and makes the bacterial cell unable to be transformed by exogenous DNA (7, 31). The element carrying mef(E), called mega (macrolide efflux genetic assembly), is approximately 5.5 kb and contains five ORFs but no putative transposase or recombinase (10). The mega element can be found to be integrated in different sites of the bacterial chromosome (10) and does not appear to interfere with the natural competence of S. pneumoniae (7). Both elements contain an ORF immediately downstream of the mef gene, designated orf5 in Tn1207.1 and mel in mega. The ORF has sequence homology with msr(A), an efflux gene of Staphylococcus aureus, and its contribution to macrolide resistance is unclear at present.
Strains harboring mef(A) were characterized in a previous study (7) with a large collection of Italian erythromycin-resistant pneumococci. Pneumococci carrying subclass mef(A) were found to belong to serotype 14 and to be sensitive to all the antibiotics tested except macrolides, while isolates carrying subclass mef(E) were found to belong to different serotypes and to be resistant to other antibiotics, in addition to macrolides (7).
The aim of this work was to further analyze two mef(E)-positive strains that were resistant to both erythromycin and tetracycline in order to characterize the resistance determinants carried and verify a possible linkage between them. In both strains, mega, the element carrying mef(E), was found to be inserted into a Tn916-like transposon containing tet(M), forming the new composite transposon Tn2009.
MATERIALS AND METHODS
Bacterial strains.
The two S. pneumoniae clinical isolates examined in this study were characterized previously (7). Strain PN150, serotype 19F, is resistant to erythromycin and tetracycline and is intermediately resistant to penicillin; and strain PN34, serotype 6A, is resistant to erythromycin, tetracycline, and chloramphenicol. Pneumococcal strain DP1322 was used for Tn916 mapping. DP1322 carries Tn5251, a transposon whose structure and termini are identical to those of Tn916 (25, 27, 38). In conjugation experiments, the recipient strain was FP10, which has been described previously (7). In transformation experiments, the recipient strain was DP1004, a streptomycin-resistant derivative of Rx1, with a genetic background homologous to that of strain R6; both Rx1 and R6 are derivatives of D39 (21, 34).
Detection of resistance determinants and Southern blot hybridization.
PCR experiments with the primer pairs and the protocols described previously (11) were used to detect the erythromycin resistance gene mef(E), the tetracycline resistance gene tet(M), and the chloramphenicol resistance gene cat. DNA probes for these resistance genes were prepared by PCR and were designated the MEF, TET, and CAT probes, respectively. PCR fragments were purified from agarose gels with a QIAquick Gel Extraction kit (Qiagen SpA, Milan, Italy). Genomic DNA from S. pneumoniae was prepared in agarose plugs by standard methods (16), digested with SalI or AvaI, and submitted to pulsed-field gel electrophoresis with a CHEF-Mapper system (Bio-Rad Laboratories, Milan, Italy). After electrophoresis, the DNA was transferred to nylon membranes with a vacuum system and hybridized with probes labeled using the ECL direct nucleic acid labeling and detection system (Amersham Pharmacia Biotech Italia, Milan, Italy) according to the instructions of the manufacturer.
Amplification and sequencing of mef(E) and tet(M) DNA.
To determine the linkage between mef(E) and tet(M), divergent oligonucleotides whose sequences were specific for the extremities of mef(E) (oligonucleotides LMf and LMr) and tet(M) (oligonucleotides LTf and LTr) were designed (Table 1). The four combinations of primer pairs associated with the possible reciprocal orientation of mef(E) and tet(M) were used in PCR assays with the chromosomal DNAs of strains PN150 and PN34, which were purified with a commercial kit (Blood and Cell Culture DNA kit; Qiagen), as templates and the ExTaq system (Takara Shuzo Co., Shiga, Japan). After the amplicons spanning mef(E) and tet(M) in both isolates were obtained, the PCR products were purified and submitted to sequencing analysis. Sequencing was performed with a Perkin-Elmer ABI 377 DNA sequencer and an ABI Prism BigDye Terminator Cycle Sequencing Ready Reaction kit (PE Applied Biosystems)
TABLE 1.
Oligonucleotide primers used in the study
| Primer | Oligonucleotide sequence (5′-3′) | Nucleotides | GenBank accession no. |
|---|---|---|---|
| LMf | AATAAGCATGGAACACTTTAATGGAACGCC | 1856-1885 | AF274302 |
| LMr | CACTTGATTACCCAGCTTAGGTATACGTAC | 1715-1686 | AF274302 |
| LTf | GCAGAGTATACCATTCACATCGAAGTTCCAC | 3379-3409 | X90939 |
| LTr | CAAAGTTCAGACTGACCTCGATGTGTTGATG | 2846-2816 | X90939 |
| TETM1 | TGGGCTTTTGAATGGAGG | 2072-2089 | X90939 |
| TETM2 | CTATCTCCTCCTTTACAC | 4112-4095 | X90939 |
| OM14 | TCCATACCCTATAGTCGGT | 3340-3358 | AF274302 |
| OM18a | TGCTTGCCCTGCCCATATTG | 1181-1162 | AF274302 |
| OM21 | GGCAAAATCACTGAGTATTGG | 2975-2995 | AF274302 |
| SG3 | GAATCTTTAGCCAGCGGTATC | 14435-14415 | U09422 |
| TN4 | AGGCTTTACGAGCATTTAAG | 17975-17994 | U09422 |
| TN6 | GCTGAATGAATGTTTGATGG | 307-288 | U09422 |
| GD1 | CAGGAACCATTCTAGCTTGG | 451-432 | AE008493 |
| GS13 | GACTTGACAACCTGAAGTAG | 225-244 | AE008492 |
| SG1 | CTCACTGCACCAGAGGTGTA | 5285-5304 | AF274302 |
| JL2 | GGAATCGTTGAAACAGCAAC | 4960-4941 | AF274302 |
From reference 7.
Determination of the structure of the composite element.
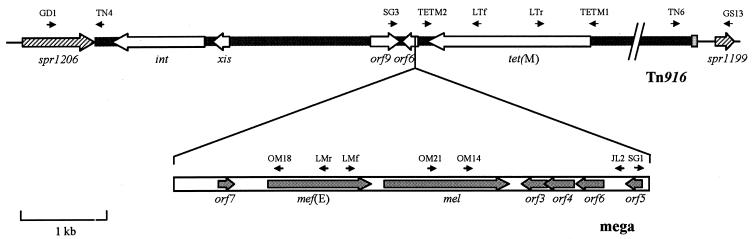
On the basis of the result of the linkage between mef(E) and tet(M), additional primers were synthesized to amplify and sequence the mega element containing mef(E) and its flanking regions. The primers used are described in Table 1 and are shown in Fig. 1.
FIG. 1.
Schematic representation of Tn2009, composed of the mega element inserted into a Tn916-like transposon. The structure of mega is boxed, and ORFs are indicated by gray arrows. Tn916 is indicated by a black bar, and white arrows represent the relevant ORFs. The exogenous sequence at the right end of Tn2009 is in gray. The flanking chromosome of S. pneumoniae is represented by a black line; striped arrows indicate ORFs, designed according to the homologous ORFs of strain R6. Short black arrows indicate the positions of the oligonucleotide primers used for PCR amplification and sequencing.
The structure of the Tn916-like element containing the mega element was examined by a series of PCRs with the primers pairs designed by Poyart et al. (24). The sizes of the fragments obtained were compared with the sizes of the fragments obtained with the chromosomal DNA of DP1322 as the template. To obtain the complete sequence of tet(M), primer pair TETM1-TETM2 (Table 1) was used to amplify a 2,041-bp fragment spanning the whole gene.
Conjugation and transformation experiments.
Mating experiments were carried out as described previously (7). Transconjugants were selected by using multilayer selection plates that contained streptomycin (500 μg/ml), chloramphenicol (10 μg/ml), and either erythromycin (1 μg/ml) or tetracycline (5 μg/ml) in the overlay layer.
Transformation was performed by previously published methods (14, 21). Briefly, transforming DNA and competence-stimulating peptide were added to precompetent pneumococcal cells that were incubated for 45 min at 37°C. Selection of the transformants was obtained by plating the transformation mixture onto multilayer plates, with tetracycline (5 μg/ml) added in the overlay. The transforming DNA was a pneumococcal crude lysate prepared essentially as described by Shoemaker and Guild (35).
Characterization of the flanking regions.
The DNA region flanking the left terminus of the composite element in transformant MF58 was explored by inverse PCR. Briefly, chromosomal DNA was digested with SacI, and the fragments were ligated under conditions favoring the production of monomeric circles. PCRs were performed with primer pair TN4-OM21 (Table 1). The PCR products were purified and submitted to DNA sequencing analysis. To confirm the result, direct amplification of the left junction was obtained with primer pair GD1-TN4 (Table 1). The right junction of the element was obtained by direct PCR amplification of the region spanning the right terminus of Tn916 and the chromosomal DNA downstream of the insertion site. Several PCRs were performed by using one forward primer that read from the right end of Tn916 (primer TN6) and different reverse primers, designed on the basis of the genomic sequence of R6 at approximately 1-kb intervals, that read from the insertion site of Tn916 up to approximately 10 kb downstream.
Nucleotide sequence accession numbers.
The partial DNA sequence of Tn2009 of strain PN150 containing the mega element and its junctions into Tn916 and tet(M) has been deposited in GenBank as an update of the record with accession no. AF376746. The sequences of the left and right junctions of Tn2009 have been assigned GenBank accession nos. AY466609 and AY466610, respectively; the complete sequence of tet(M) of strain PN34 has been assigned GenBank accession no. AY466395.
RESULTS
Detection and localization of the resistance determinants.
The mef(E) and tet(M) genes were detected in both the PN34 and the PN150 clinical isolates by PCR, while the cat gene was found only in PN34; these results are in accordance with the antibiotic resistance phenotypes of the isolates. Hybridization experiments were carried out with total DNA to localize the resistance genes. In PN150, following digestion with SalI or AvaI, both the MEF and the TET probes hybridized with bands of the same molecular mass, approximately 40 kb. In PN34, the MEF, TET, and CAT probes hybridized with the same band of approximately 76 kb following digestion with SalI. After digestion with AvaI, the MEF and the TET probes hybridized with a band of approximately 34 kb and the CAT probe hybridized with a different band of approximately 30 kb. These findings suggest that in both isolates mef(E) and tet(M) are located in proximity within a fragment of 30 to 40 kb.
The linkage between mef(E) and tet(M) was investigated by PCR. In both isolates, the combination of oligonucleotide primers LMf and LTf produced an amplicon of approximately 4.4 kb, indicating that mef(E) and tet(M) are in close proximity and are oriented in opposite directions.
DNA sequencing of the mega element and its insertion sites in Tn916.
Sequence analysis of the fragment spanning mef(E) and tet(M) revealed that mef(E) is carried by the mega element and that the mega element is flanked by sequences homologous to those of an ORF of Tn916. Amplification and sequencing of the right and left junctions of mega in both isolates confirmed that this element was integrated in orf6 of Tn916, at position 14,166 of the sequence of Flannagan et al. (GenBank accession no. U09422). The site of integration included the target sequence CACA, and the integration generated a 2-base deletion (CA), as already observed by Gay and Stephens (10) in the evaluation of mega element integration into the chromosome.
Sequence analysis of the strain PN150 mega element showed that this element presents some differences from the mega element of S. pneumoniae described by Gay and Stephens (10) (GenBank accession no. AF274302), consisting of a 16-bp deletion upstream of the mef(E) gene, 5 additional single nucleotide deletions, and 8 nucleotide substitutions. These differences were outside the coding regions of mef(E) and mel. The mega element of PN150 was found to be 100% identical to the 5,511-bp element recently described (36) in Streptococcus salivarius (GenBank accession no. AJ318993). Analysis of the sequence of the mega element of PN150 showed that, in addition to the four short ORFs following mel (orf3 to orf6), an additional ORF, designated orf7, could be recognized upstream of mef(E) (Fig. 1). This ORF was found to be homologous to a sequence present in an enterococcal plasmid carrying several putative resistance determinants, including an ABC transporter (GenBank accession no. AF408195).
As for the mega element of PN34, the sequence of a 3,201-bp fragment spanning mef(E) and mel (7), the sequences of both ends, and the size of the fragment determined by PCR were identical to those of the mega element of PN150.
Organization of the composite element and insertion in the pneumococcal chromosome.
The organization of the composite element was explored by a series of PCRs. The PCR fragments obtained from both strain PN150 and strain PN34 were of the same size as those obtained from control strain DP1322, which contains Tn5251, a transposon identical in size and structure to Tn916. The only exceptions were the PCR fragments spanning orf6, the insertion site of the mega element. The amplicon obtained with primer pair O11-O14 (24), which spanned tet(M) and orf6, was approximately 3.2 kb when it was amplified from strain DP1322, while it was about 8.7 kb when it was amplified from PN150 or PN34. The composite element including the mega element and Tn916 was designated Tn2009.
Although the structure of Tn2009 in the two isolates appeared to be the same, the sequences of tet(M) were different at 48 of 1,920 nucleotides. The tet(M) sequence of strain PN150 was almost identical to the tet(M) sequence of pneumococcal transposon Tn5251, with only 2 nucleotide changes, while the sequence of tet(M) of strain PN34 was different from those of any of the published tet(M) alleles. The tet(M) sequence of PN34 exhibited the characteristic mosaic structure described previously at the variable nucleotide positions (19).
Transferability of the element and determination of the junctions of Tn2009 in the pneumococcal chromosome.
All attempts to transfer erythromycin or tetracycline resistance by conjugation from strain PN150 or strain PN34 to a pneumococcal recipient were unsuccessful.
Transformation experiments were carried out to isolate and characterize Tn2009. A crude lysate of strain PN150 was used to transform competent cells of strain DP1004. Transformants were selected for acquisition of the tetracycline resistance phenotype. The resulting transformants, MF58 and MF59, were resistant to both tetracycline and erythromycin; and the presence of mef(E) and tet(M) in the transformants was confirmed by PCR. Tn2009 was transformed at a frequency of 4 × 10−7 transformants per total CFU.
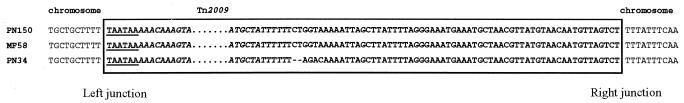
The junctions of Tn2009 in the chromosome were characterized in transformant MF58 by inverse PCR and direct sequencing. As the recipient strain DP1004 is a strain Rx1 derivative (34), the genetic background of transformant MF58 is almost identical to that of strain R6, with both strains being derivatives of strain D39. The left junction of Tn2009, obtained by inverse PCR, was found to be inside an ORF homologous to spr1206 of R6 at base 1,205,156 of the complete genome (Gene Bank accession no. NC_003098). This ORF is conserved in pneumococcal genomes and codes for a hypothetical protein. Six nucleotides that formed the coupling sequence were identified between the sequence of the protein and the left terminus of Tn916 (Fig. 2) (32).
FIG. 2.
Junctions of Tn2009 with the chromosome in clinical isolates PN150 and PN34 and transformant MF58. The sequences belonging to Tn2009 and the exogenous sequences are shown in boldface and boxed. The nucleotides at the left and right ends of Tn916 are italicized. At the left junction, the 6 nucleotides representing the coupling sequences are underlined. At the right junction, the 63- or 61-nucleotide exogenous sequences are indicated.
The right junction of Tn2009 was obtained by direct PCR amplification of chromosomal DNA with primer TN6, whose sequence is specific for inside the right end of Tn2009, and primer GS13 (Table 1), whose sequence is specific for the sequence of spr1199, an ORF located approximately 10 kb downstream of the insertion of Tn2009, in the strain R6 genome. The amplified fragment was 858 bp. The insertion of Tn2009 caused a deletion of 9,573 bp with reference to the R6 genome. The right terminus of the Tn916-like element of Tn2009 was found to be flanked by a 63-bp stretch that exhibited a G+C content of 28% and that did not show any significant homology with other sequences in the GenBank database. The junctions of Tn2009 in clinical isolates PN150 and PN34 were amplified and sequenced. The junctions in PN150, the donor strain, were identical to those in transformant MF58, including the extra 63-bp sequence at the right junction. In PN34, the left junction of Tn2009 was identical to that of MF58, while there were minor differences at the right junction, consisting of a 2-bp deletion and 3 nucleotide substitutions at the beginning of the exogenous sequence (Fig. 2).
DISCUSSION
In S. pneumoniae antibiotic resistance determinants are often carried by mobile genetic elements that reside in the chromosome, such as conjugative transposons. This is the case for the tetracycline and the erythromycin resistance genes tet(M) and erm(B), respectively, which are located on conjugative transposons and which are often linked (11, 15). tet(M) is generally associated with Tn916 or Tn5251, a transposon structurally homologous to Tn916, or with Tn1545, which also contains the erm(B) gene. These transposons are considered the prototypes of a closely related family (27).
The macrolide efflux elements recently described in S. pneumoniae, Tn1207.1, which carries mef(A) (31) and the mega element, which carries mef(E) (10), are both defective transposons. Mega has been shown to be incapable of conjugative transfer (10), and previously, mef(E) could not be transferred from our strains by conjugation (7). Differently from Tn1207.1, which has a preferential insertion site (31), the mega element can be inserted in different sites of the chromosome (7, 10).
In two clinical isolates of S. pneumoniae of different serotypes and genetic backgrounds (7), the mega element was found to be inserted in a Tn916-like element, in a sequence homologous to orf6 of Tn916. The integration site was identical in both strain PN34 and strain PN150 at the nucleotide level. The target site presented sequence similarity with other sites of integration of the mega element in the pneumococcal chromosome (10).
The integration of the mega element into a Tn916-like transposon generates a new composite element of approximately 23.5 kb that we designated Tn2009. Tn2009 carries determinants for tetracycline and erythromycin resistance and is apparently not transferable by conjugation but can be transferred by transformation to pneumococci, conferring resistance to both antibiotics. Tn2009 resembles another composite element found in S. pneumoniae, Tn3872, in which Tn917 carrying erm(B) is integrated into orf9 of Tn916 (17). orf6 and orf9 are adjacent and reside in a Tn916 region downstream of tet(M) that is involved in the regulation of transposon activity (5) and that can undergo interruptions without detrimental effects on the stability of the transposon (37). Tn3872, like Tn2009, is nonconjugative, suggesting that interruption of the contiguity between tet(M) and the int-Tn and xis-Tn genes impairs the ability of Tn916 to transpose, possibly blocking the formation of the long transcripts responsible for the regulation of transfer in the presence of tetracycline (27).
The mega element of strain PN150 was different from that of S. pneumoniae described by Gay and Stephens (10) but was identical to that of S. salivarius (36). Interestingly, in the mega sequence of PN150, we recognized an additional small ORF that was homologous to a sequence of an enterococcal plasmid carrying resistance determinants, including a putative efflux pump. Transfer of the mega element by transformation from S. salivarius to the pneumococcus has been demonstrated in vitro (36). It is possible that in vivo transformation from S. salivarius to S. pneumoniae could lead to the spread of the mega element among pneumococci. Commensal organisms that dwell in the oropharynx, like viridans group streptococci, appear to be a wide reservoir of antibiotic resistance for S. pneumoniae. Pneumococcus can exchange portions of housekeeping genes with homologous genes of viridans group streptococci, acquiring mosaic gene structures that determine lower levels of susceptibility or resistance to antibiotics like penicillin (8) or the fluoroquinolones (3). In addition, viridans group streptococci seem to represent a reservoir of resistance genes and genetic elements, including erm(B) (33), mef(E) (1), and the mega element (36), that can be horizontally transmitted, contributing greatly to the diffusion of macrolide resistance among pathogenic streptococci.
The tet(M) sequences of Tn2009 were different in the two clinical isolates. The sequence of the tet(M) allele of strain PN150 was almost identical to that of the allele present in Tn5251 (25) and can be considered a typical pneumococcal allele. The sequence of tet(M) carried by Tn2009 of strain PN34 shows a mosaic structure, with nucleotides homologous to different tet(M) alleles at the variable sites (19). In particular, variable sites were homologous either to tet(M) of Tn5251 or to the enterococcal allele, tet(M) of Tn916. We have no indication whether mega elements integrated independently into different Tn916-like transposons or the different evolutions of tet(M) occurred after integration of the mega element.
The Tn2009 insertion site in the chromosome was the same in the two clinical isolates, indicating that this is a preferential insertion site for Tn2009. A typical 6-bp coupling sequence could be recognized at the left end of the element. The coupling sequence represents the joint in the formation of the circular intermediate at transposition, is derived from the donor, and is present at only one end of the transposon (32). However, at the right end of Tn2009, between the terminus of the Tn916-like element and the pneumococcal chromosome, several exogenous nucleotides (63 nucleotides in PN150 and 61 nucleotides in PN34) were found; these likely represent the sequence adjacent to the transposon in its previous host. This short sequence shows no significant homology with other sequences in the GenBank database and has a lower G+C content (28%) relative to that of the S. pneumoniae R6 or TIGR4 genome (39.6%) (http://www.tigr.org). The genomic G+C contents of other streptococcal species range from 35 to 40%, while staphylococci and clostridia have lower G+C contents (32 and 28 to 31%, respectively). Interestingly, a G+C content similar to that of the exogenous sequence is present in S. aureus plasmids (28 to 30%). The presence of an exogenous sequence without significant homology with the bacterial genomes sequenced and with a low G+C content suggests that the original host or an intermediate host of Tn2009 was a plasmid, possibly a staphylococcal plasmid. The integration of a staphylococcal plasmid into the pneumococcal chromosome has been demonstrated for pC194, which carries the chloramphenicol resistance determinant cat (39). Strain PN34 is also resistant to chloramphenicol and was found to carry the cat gene. Although Southern hybridization experiments have shown that this gene is located in the same 76-kb SalI fragment as tet(M) and mef(E), the genetic element involved and its chromosomal site have not been investigated.
The concentration of resistance genes in particular genetic elements is an increasingly common occurrence, as exemplified by the integrons of gram-negative bacteria (29). In gram-positive bacteria such as the streptococci, transposons residing in the chromosome can exert the same function, capturing other resistance elements to form composite transposons. Such composite elements often involve a Tn916-like element and the tetracycline resistance gene tet(M) (2, 17). Tn916 is a broad-host-range transposon known to occur naturally in different species and genera of both gram-positive and gram-negative microorganisms and therefore represents a powerful vehicle for the dissemination of antibiotic resistance genes (27). Although in Italy and other European countries tetracycline has largely been replaced by other antibiotic classes in human medicine (4), it still represents the antibiotic that is the most used in the veterinary field (International Federation for Animal Health—Europe Dossier 9, Antibiotics for animals—a FEDESA perspective on antibiotics, animal health and the resistance debate, 1999). The presence of a tetracycline resistance determinant can be instrumental to the preservation of Tn916 in different environments. In addition, positive regulation of the transferability of the transposon in the presence of tetracycline has been shown (5), similarly to the stimulus on conjugative transfer exerted by tetracycline on some Bacteroides transposons (30). In addition to tetracycline, most of the composite elements in pneumococci carry an erythromycin resistance determinant, mostly erm(B) (2, 11, 17), which contributes to the spread of macrolide resistance in this group of microorganisms. We have also demonstrated that the recently described mega element, which codes for a macrolide efflux pump in pneumococci, has found a new site in a Tn916-like transposon, forming the element Tn2009. Although Tn2009 appears to be incapable of transfer by conjugation, it could disseminate to other pneumococcal isolates by transformation.
Another demonstration of the frequent association between tetracycline and macrolide resistance genes is given by the genetic linkage between mef(A) and the tetracycline resistance determinant tet(O), recently described in Streptococcus pyogenes (12). The appearance of new genetic elements carrying multiple resistance genes indicates an alarming evolution of antibiotic resistance and is contributing greatly to the spread of macrolide resistance among streptococci.
Acknowledgments
This study was supported in part by grants from the Italian Ministero della Salute (Progetti Finalizzati 1999 and Progetto “Sorveglianza della Resistenza agli Agenti Antimicrobici,” 2002), Italian MURST (COFIN 2002), and the Commission of the European Union (QLK2-CT-2000-00543).
REFERENCES
- 1.Arpin, C., M. H. Canron, J. Maugein, and C. Quentin. 1999. Incidence of mefA and mefE genes in viridans group streptococci. Antimicrob. Agents Chemother. 43:2335-2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ayoubi, P., A. O. Kilic, and M. N. Vijayakumar. 1991. Tn5253, the pneumococcal Ω(cat tet) BM6001 element is a composite structure of two conjugative transposon, Tn5251 and Tn5252. J. Bacteriol. 173:1617-1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balsalobre, L., M. J. Ferrándiz, J. Liñares, F. Tubau, and A. G. de la Campa. 2003. Viridans group streptococci are donors in horizontal transfer of topoisomerase IV genes to Streptococcus pneumoniae. Antimicrob. Agents Chemother. 47:2072-2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cars, O., S. Molstad, and A. Melander. 2001. Variation in antibiotic use in the European Union. Lancet 357:1851-1853. [DOI] [PubMed] [Google Scholar]
- 5.Celli, J., and P. Trieu-Cuot. 1998. Circularization of Tn916 is required for expression of the transposon-encoded transfer functions: characterization of long tetracycline-inducible transcripts reading through the attachment site. Mol. Microbiol. 28:103-117. [DOI] [PubMed] [Google Scholar]
- 6.Clancy, J., J. Petitpas, F. Dib-Hajj, W. Yuan, M. Cronan, A. V. Kamath, J. Bergeron, and J. A. Retsema. 1996. Molecular cloning and functional analysis of a novel macrolide-resistance determinant, mefA, from Streptococcus pyogenes. Mol. Microbiol. 22:867-879. [DOI] [PubMed] [Google Scholar]
- 7.Del Grosso, M., F. Iannelli, C. Messina, M. Santagati, N. Petrosillo, S. Stefani, G. Pozzi, and A. Pantosti. 2002. Macrolide efflux genes mef(A) and mef(E) are carried by different genetic elements in Streptococcus pneumoniae. J. Clin. Microbiol. 40:774-778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dowson, C. G., T. J. Coffey, C. Kell, and R. A. Whiley. 1993. Evolution of penicillin resistance in Streptococcus pneumoniae; the role of Streptococcus mitis in the formation of a low affinity PBP2B in S. pneumoniae. Mol. Microbiol. 9:635-643. [DOI] [PubMed] [Google Scholar]
- 9.Gay, K., W. Baughman, Y. Miller, D. Jackson, C. G. Whitney, A. Schuchat, M. M. Farley, F. Tenover, and D. S. Stephens. 2000. The emergence of Streptococcus pneumoniae resistant to macrolide antimicrobial agents: a 6-year population-based assessment. J. Infect. Dis. 182:1417-1424. [DOI] [PubMed] [Google Scholar]
- 10.Gay, K., and D. Stephens. 2001. Structure and dissemination of a chromosomal insertion element encoding macrolide efflux in Streptococcus pneumoniae. J. Infect. Dis. 184:56-65. [DOI] [PubMed] [Google Scholar]
- 11.Gherardi, G., M. Del Grosso, A. Scotto d'Abusco, F. D'Ambrosio, G. Dicuonzo, and A. Pantosti. 2003. Phenotypic and genotypic characterization of two penicillin-susceptible serotype 6B Streptococcus pneumoniae clones circulating in Italy. J. Clin. Microbiol. 41:2855-2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giovanetti, E., A. Brenciani, R. Lupidi, M. C. Roberts, and E. P. Varaldo. 2003. Presence of the tet(O) gene in erythromycin- and tetracycline-resistant strains of Streptococcus pyogenes and linkage with either the mef(A) or the erm(A) gene. Antimicrob. Agents Chemother. 47:2844-2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoban, D. J., A. K. Wierzbowski, K. Nichol, and G. G. Zhanel. 2001. Macrolide-resistant Streptococcus pneumoniae in Canada during 1998-1999: prevalence of mef(A) and erm(B) and susceptibilities to ketolides. Antimicrob. Agents Chemother. 45:2147-2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iannelli, F., and G. Pozzi. 2004. Method for introducing specific and unmarked mutations into the chromosome of Streptococcus pneumoniae. Mol. Biotechnol. 26:81-86. [DOI] [PubMed] [Google Scholar]
- 15.Leclercq, R., and P. Courvalin. 2002. Resistance to macrolides and related antibiotics in Streptococcus pneumoniae. Antimicrob. Agents Chemother. 46:2727-2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maslow, J. N., A. Slutsky, and R. D. Arbeit. 1993. Application of pulsed-field gel electrophoresis to molecular epidemiology, p. 563-572. In D. H. Persing, F. C. Tenover, and T. J. White (ed.), Diagnostic molecular microbiology: principles and applications. American Society for Microbiology, Washington, D.C.
- 17.McDougal, L. K., F. C. Tenover, L. N. Lee, J. K. Rasheed, J. E. Patterson, J. H. Jorgensen, and D. J. LeBlanc. 1998. Detection of Tn917-like sequences within a Tn916-like conjugative transposon (Tn3872) in erythromycin-resistant isolates of Streptococcus pneumoniae. Antimicrob. Agents Chemother. 42:2312-2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Montanari, M. P., M. Mingoia, I. Cochetti, and P. E. Varaldo. 2003. Phenotypes and genotypes of erythromycin-resistant pneumococci in Italy. J. Clin. Microbiol. 41:428-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oggioni, M., C. G. Dowson, J. M. Smith, R. Provvedi, and G. Pozzi. 1996. The tetracycline resistant gene tet(M) exhibits mosaic structure. Plasmid 35:156-163. [DOI] [PubMed] [Google Scholar]
- 20.Pantosti, A., D. Boccia, F. D'Ambrosio, S. Recchia, G. Orefici, M. Moro, the National Surveillance of Bacterial Meningitis, and the EARSS-Italia Groups. 2003. Inferring the potential success of pneumococcal vaccination in Italy: serotypes and antibiotic resistance of isolates from invasive diseases. Microb. Drug Resist. 9:S61-S68. [DOI] [PubMed] [Google Scholar]
- 21.Pearce, B. J., F. Iannelli, and G. Pozzi. 2002. Construction of new unencapsulated (rough) strains of Streptococcus pneumoniae. Res. Microbiol. 153:243-247. [DOI] [PubMed] [Google Scholar]
- 22.Petrosillo, N., A. Pantosti, E. Bordi, A. Spanò, M. Del Grosso, B. Tallarida, and G. Ippolito. 2002. Prevalence, determinants, and molecular epidemiology of Streptococcus pneumoniae isolates colonizing the nasopharynx of healthy children in Rome. Eur. J. Clin. Microbiol. Infect. Dis. 21:181-188. [DOI] [PubMed] [Google Scholar]
- 23.Pihlajamaki, M., J. Jalava, P. Huovinen, P. Kotilainen, and the Finnish Study Group for Antimicrobial Resistance. 2003. Antimicrobial resistance of invasive pneumococci in Finland in 1999-2000. Antimicrob. Agents Chemother. 47:1832-1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poyart, C., G. Quesne, P. Acar, P. Berche, and P. Trieu-Cuot. 2000. Characterization of the Tn916-like transposon Tn3872 in a strain of Abiotrophia defectiva (Streptococcus defectivus) causing sequential episodes of endocarditis in a child. Antimicrob. Agents Chemother. 44:790-793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Provvedi, R., R. Manganelli, and G. Pozzi. 1996. Characterization of conjugative transposon Tn5251 of Streptococcus pneumoniae. FEMS Microbiol. Lett. 135:231-236. [DOI] [PubMed] [Google Scholar]
- 26.Reinert, R. R., R. Luttichen, A. Bryskier, and A. Al-Lahham. 2003. Macrolide-resistant Streptococcus pneumoniae and Streptococcus pyogenes in the pediatric population in Germany during 2000-2001. Antimicrob. Agents Chemother. 47:489-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rice, L. B. 1998. Tn916 family conjugative transposons and dissemination of antimicrobial resistance determinants. Antimicrob. Agents Chemother. 42:1871-1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roberts, M. C., J. Sutcliffe, P. Courvalin, L. B. Jensen, J. Rood, and H. Seppälä. 1999. Nomenclature for macrolide and macrolide-lincosamide-streptogramin B resistance determinants. Antimicrob. Agents Chemother. 43:2823-2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rowe-Magnus, D., and D. Mazel. 1999. Resistance gene capture. Curr. Opin. Microbiol. 2:483-488. [DOI] [PubMed] [Google Scholar]
- 30.Salyers, A. A., N. B. Shoemaker, A. M. Stevens, and L.-Y. Li. 1995. Conjugative transposons: an unusual and diverse set of integrated gene transfer elements. Microbiol. Rev. 59:579-590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Santagati, M., F. Iannelli, M. Oggioni, S. Stefani, and G. Pozzi. 2000. Characterization of a genetic element carrying the macrolide efflux gene mef(A) in Streptococcus pneumoniae. Antimicrob. Agents Chemother. 44:2585-2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scott, J. R., F. Bringel, D. Marra, G. Van Alstine, and C. K. Rudy. 1994. Conjugative transposition of Tn916: preferred targets and evidence for conjugative transfer of a single strand and for a double-stranded circular intermediate. Mol. Microbiol. 11:1099-1108. [DOI] [PubMed] [Google Scholar]
- 33.Seppälä, H., M. Haanperä, M. Al-Juhaish, H. Järvinen, J. Jalava, and P. Huovinen. 2003. Antimicrobial susceptibility patterns and macrolide resistance genes of viridans group streptococci from normal flora. J. Antimicrob. Chemother. 52:636-644. [DOI] [PubMed] [Google Scholar]
- 34.Shoemaker, N. B., and W. R. Guild. 1974. Destruction of low-efficiency markers is a slow process occurring at a heteroduplex stage of transformation. Mol. Gen. Genet. 128:283-290. [DOI] [PubMed] [Google Scholar]
- 35.Shoemaker, N. B., and W. R. Guild. 1972. Kinetics of integration of transforming DNA in pneumococcus. Proc. Natl. Acad. Sci. USA 69:3331-3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stadler, C., and M. Teuber. 2002. The macrolide efflux genetic assembly of Streptococcus pneumoniae is present in erythromycin-resistant Streptococcus salivarius. Antimicrob. Agents Chemother. 46:3690-3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Su, Y. A., and D. B. Clewell. 1992. Characterization of the left 4 kb of conjugative transposon Tn916: determinants involved in excision. Plasmid 30:234-250. [DOI] [PubMed] [Google Scholar]
- 38.Vijayakumar, M. N., S. D. Priebe, and W. R. Guild. 1986. Structure of a conjugative element in Streptococcus pneumoniae. J. Bacteriol. 166:978-984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Widdowson, C. A., P. V. Adrian, and K. P. Klugman. 2000. Acquisition of chloramphenicol resistance by the linearization and integration of the entire staphylococcal plasmid pC194 into the chromosome of Streptococcus pneumoniae. Antimicrob. Agents Chemother. 44:393-395. [DOI] [PMC free article] [PubMed] [Google Scholar]