Abstract
An efficient combination between the Passerini three-component reaction and aldol condensation has been developed for the synthesis of bicyclic isocoumarins with different substituted patterns via solvent-dependent domino pathways. These two operationally friendly methods simultaneously install C–O and C–C bonds in a one-pot manner, allowing the utilization of low-cost and readily accessible 2-formylbenzoic acid, isocyanides, and arylglyoxals. Mechanisms of formation of different substituted isocoumarin derivatives are also proposed.
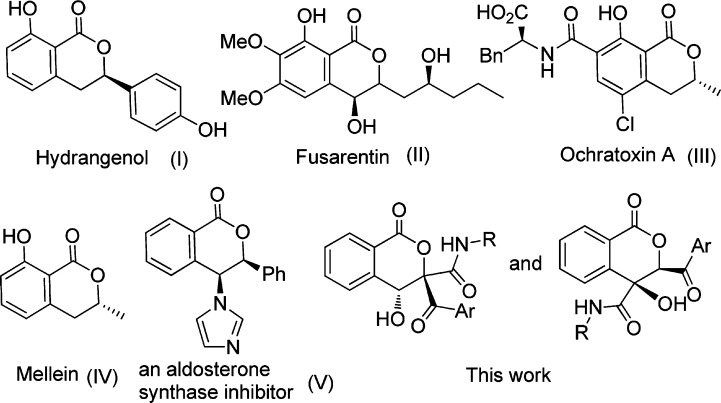
Functional isocoumarins, due to their unique and intriguing chemical and medicinal properties, have been widely applied in organic and medicinal research over the past decades.1,2 As important structural motifs, 3,4-dihydroisocoumarins were omnipresent in many natural products, in which hydrangenol3 (Figure 1, type I), fusarentin4 (Figure 1, type II), ochratoxin A5 (Figure 1, type III), and mellein6 (Figure 1, type IV) are most representative. Of particular interest, synthetic 3,4-dihydroisocoumarins have also become attractive targets for chemists as they were found to possess a wide spectrum of biological activities and constitute a great number of important pharmaceuticals.7 Among them, significantly useful imidazol-1-yl substituted 3,4-dihydroisocoumarin has acted as an aldosterone synthase inhibitor8 (Figure 1, type V). Therefore, significant efforts have been devoted to powerful and reliable access to isocoumarin derivatives. Their metal-catalyzed variants have taken a pivotal position, and a variety of transition-metal species including rhodium,9 palladium,10 ruthenium,11 and copper,12 have been utilized in isocoumarin syntheses. Despite these useful isocoumarin syntheses, an exploration of a metal-free synthetic strategy for the direct construction of an isocoumarin skeleton and its multifunctionalization would be highly favorable.
Figure 1.
Biologically important 3,4-dihydroisocoumarins.
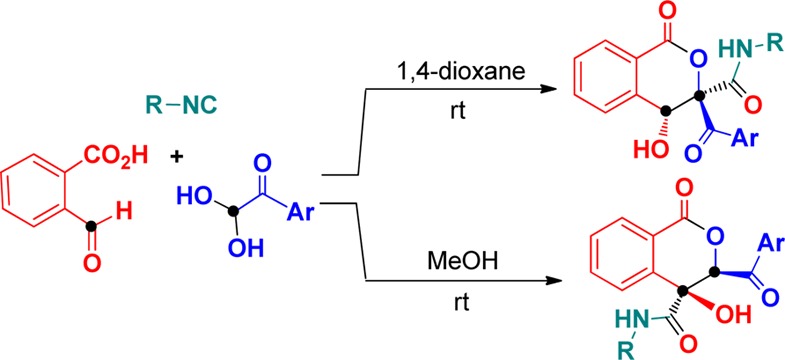
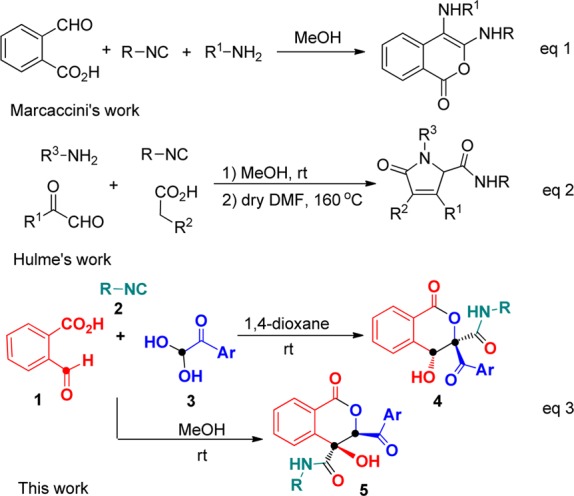
Isocyanide-based multicomponent reactions (IMCRs) have emerged as a powerful tool in organic and medical chemistry, allowing the formation of complex molecular architectures from low-cost and readily accessible precursors in a convergent manner.13 Due to their atom economy, bond-forming efficiency, functional group tolerance, and high levels of selectivity, IMCRs have become a special and interesting research topic, enabling their rapid development over the past several decades.14 Recently, Marcaccini and co-workers reported an Ugi reaction of 2-formylbenzoic acid and aromatic amine with isocyanides affording 1H-isochromen-1-ones (isocoumarins) (Scheme 1, eq 1).15 Hulme and co-workers combined an Ugi four-component reaction with aldol condensation to synthesize pyrrolinones from accessible glyoxaldehydes, isonitriles, carboxylic acids, and amines (Scheme 1, eq 2).16 Inspired by these reactions and our recent findings on the applicability of multicomponent reactions of arylglyoxals,17 we envisioned that a domino strategy encompassing a Passerini reaction and an aldol condensation could pave the way to the collection of isocoumarin derivatives. Here, we report a novel and challenging three-component annulation of 2-formylbenzoic acid 1 and isocyanides 2 with arylglyoxals 3, providing straightforward domino accesses to isocoumarins with different substituted patterns 4 and 5 by solvent-dependent Passerini–Aldol sequence (Scheme 1, eq 3). The present method would enable direct C–C formation between two electrophilic sites of both 2-formylbenzoic acid and arylglyoxal monohydrates without the use of any carbene catalysts, thus allowing efficient generation of functionalized isocoumarins by continuous Passerini–Aldol sequence in a one-pot fashion.
Scheme 1. Synthesis of Compounds 3.
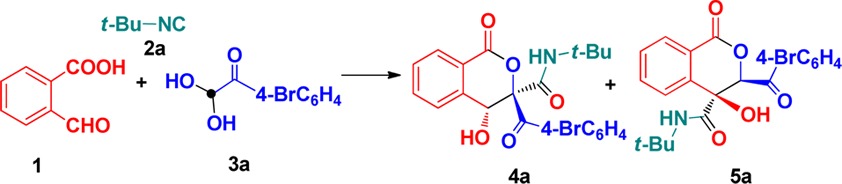
Our initial studies focused on optimizing the reaction conditions by treatment with 2-formylbenzoic acid (1), tert-butyl isocyanide (2a), and 1-(4-bromophenyl)-2,2-dihydroxyethanone (3a). The results are listed in Table 1. The three-component reaction of 1 with 2a and 3a was carried out in MeOH at room temperature for 24 h, and the expected functionalized isocoumarin 4a was afforded in 33% chemical yield. Interestingly, another different substituted isocoumarin product 5a with 41% yield was unexpectedly obtained in this reaction system after flash column chromatography. Enticed by the potential of these results, the identical reactions were further investigated in different solvents such as EtOH, N,N-dimethylformamide (DMF), 1,2-dichloroethane (DCE), CH3CN, toluene, tetrahydrofuran (THF), and 1,4-dioxane. Experimental results showed that the solvent has significant influence on the reaction selectivity. For example, no product 4a or 5a was produced using DMF as a solvent. The increase in yield of 4a and the decrease in yield of 5a were observed when EtOH, DCE, CH3CN, toluene, and THF were employed as reaction media. 1,4-Dioxane gave the highest yield of 4a (74%), accompanied by very poor yield of 5a (<5%). Raising the reaction temperature to 60 °C failed to improve the reaction yield of 4a and 5a. Therefore, we decided to conduct the subsequent reactions in 1,4-dioxane and MeOH at room temperature to construct products 4a and 5a, respectively.
Table 1. Optimization for the Synthesis of 4 and 5.
| entry | solvent | t (°C) | time/h | (4a/5a) yield (%)a |
|---|---|---|---|---|
| 1 | MeOH | rt | 24 | 33/41 |
| 2 | EtOH | rt | 24 | 62/19 |
| 3 | DMF | rt | 24 | trace/trace |
| 4 | DCE | rt | 24 | 57/13 |
| 5 | CH3CN | rt | 24 | 48/23 |
| 6 | toluene | rt | 24 | 72/<5 |
| 7 | THF | rt | 24 | 62/10 |
| 8 | 1,4-dioxane | rt | 24 | 74/<5 |
| 9 | MeOH | 60 | 1 | 39/34 |
| 10 | 1,4-dioxane | 60 | 1 | 68/<5 |
Isolated yield.
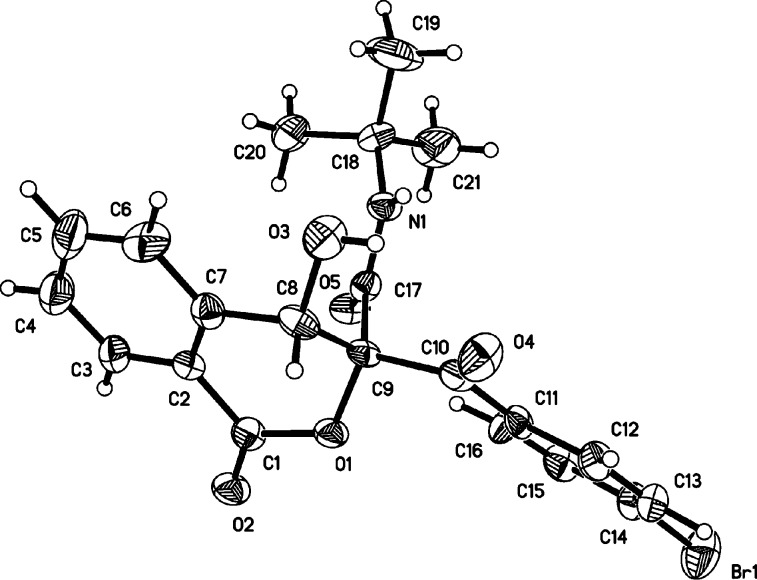
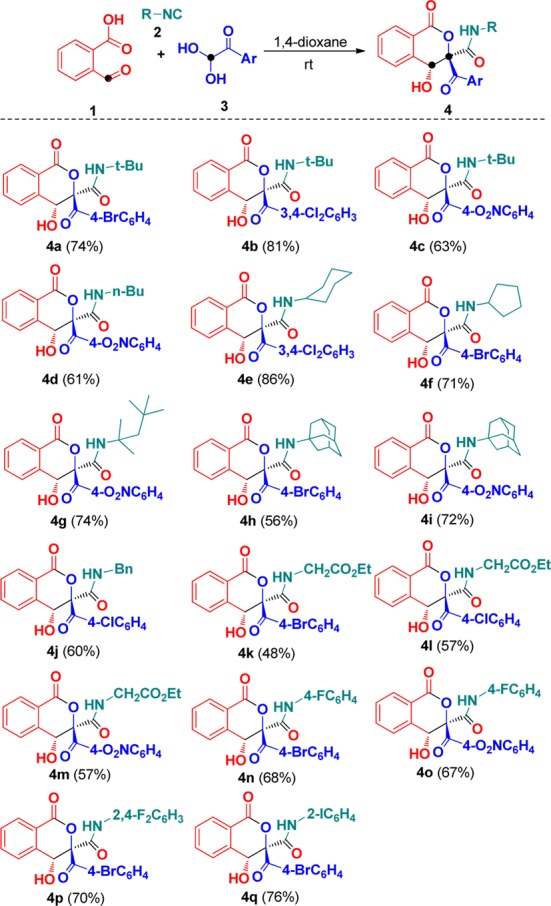
With the optimal conditions established above, (Table 1, entry 8), the substrate scope of the synthesis was then explored. Results in Scheme 2 clearly revealed that all reactions worked well to afford the expected isocoumarins 4 with moderate to good yields. First, the use of various arylglyoxals was investigated in the reaction with 1 and 2a. The arylglyoxals carrying electron-withdrawing groups could be smoothly converted to the desired isocoumarin products 4a–c. However, the coupling of arylglyoxals containing electron-donating methoxy and methyl groups did not afford isocoumarins 4. Next, we systematically varied the substituents of the isocyanides and employed these derivatives to the treatment with 1 and diverse arylglyoxals 3. A variety of functional groups in substituted isocyanides, such as n-butyl, cyclohexyl, cyclopentyl, 1,1,3,3-tetramethylbutyl, adamantanyl, and benzyl groups, were well tolerated to give the corresponding isocoumarins 4 with good yields. To probe the feasibility of the reaction, ethyl isocyanoacetate was employed as a potential component, participating in current Passerini–Aldol reaction successfully. Similarly, aryl isocyanides were also smoothly transformed into the desired isocoumarins 4n–q with 67–76% yields. Although isocoumarins 4 were fully characterized by their NMR spectroscopy and HRMS, their structures were determined by X-ray diffraction of compound 4a (Figure 2).
Scheme 2. Domino Synthesis of Isocoumarins 4.
Isolated yield.
Figure 2.
ORTEP drawing of 4a.
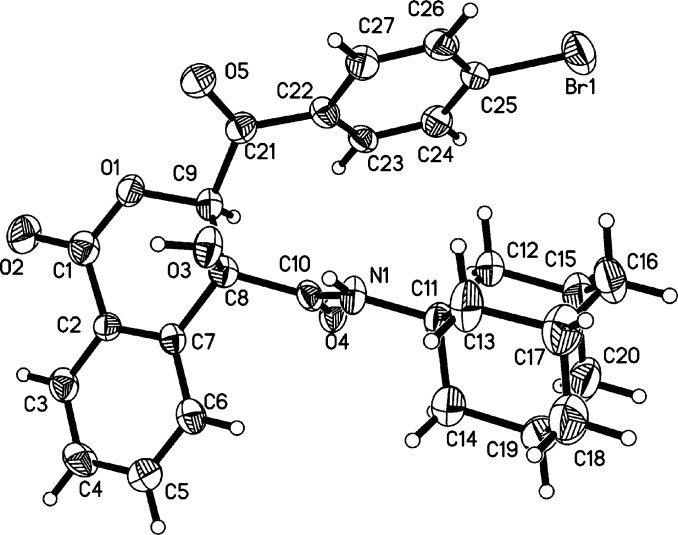
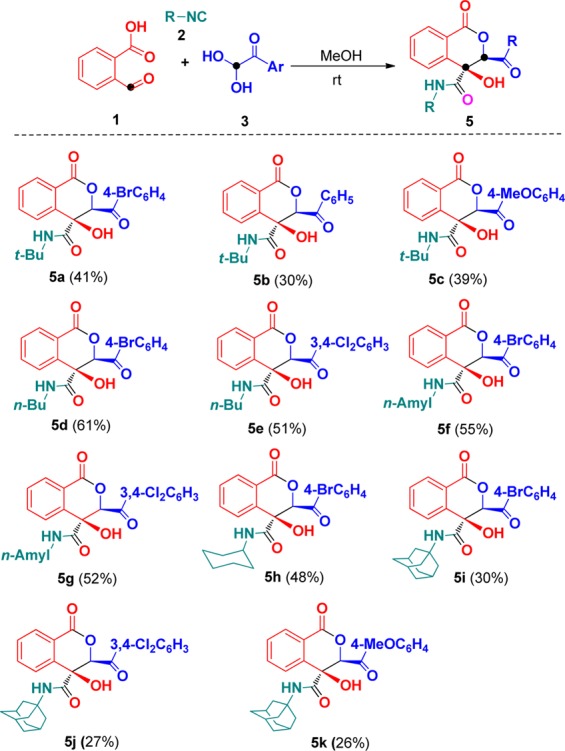
After successfully synthesizing isocoumarins 4, we turned our attention to evaluate the formation of isocoumarins 5 under optimal conditions (Table 1, entry 1). It was found that the substituents on the arylglyoxals had slight influence on the yields of products 5 (Scheme 3). Generally, the arylglyoxals bearing electron-withdrawing groups such as bromo and chloro groups showed higher reactivity and gave higher yields in comparison to those containing electron-neutral or electron-donating groups like the methoxyl group. As we expected, a set of diverse substituted isocyanides, such as tert-butyl-, n-butyl-, cyclohexyl-, n-amyl-, and adamantanyl isocyanides, were well incorporated in these current domino reactions. In general, these domino reactions give new examples for synthesizing richly decorated isocoumarins, which are widespread structural cores in a large number of bioactive compounds. The structure of 5i was unambiguously confirmed by X-ray diffraction analysis (Figure 3).
Scheme 3. Domino Synthesis of Isocoumarins 5.
Isolated yield.
Figure 3.
ORTEP Drawing of 5i.
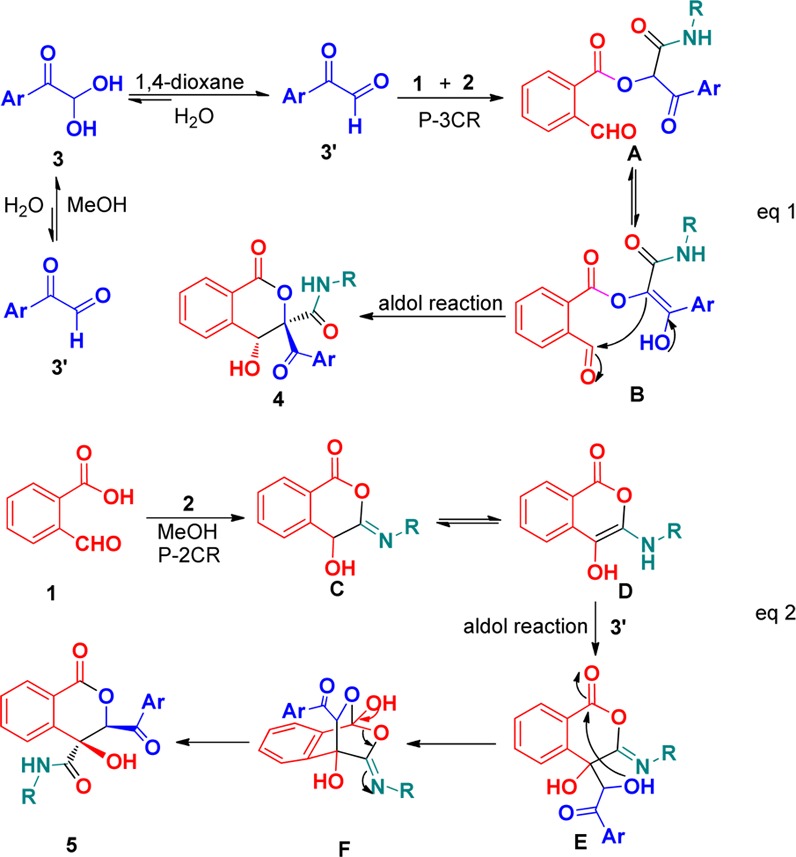
We believed that the reaction mechanism is a special case of a multicomponent domino reaction involving a Passerini reaction and aldol condensation. On the basis of literature reports and our experimental results, reasonable mechanisms for two different domino reactions are postulated in Scheme 4. The reason for the discrimination between 1 to A and 1 to C may depend on the solvent used. In dioxane, arylglyoxals 3 are readily converted into the corresponding aldehyde form in the presence of acid, whereas 3 mostly exists as hydrate form in MeOH. Therefore, the former undergoes an initial Passerini three-component reaction (P-3CR) of 1, 2, and 3′,18 followed by tautomerization and aldol condensation to yield isocoumarins 4 (Scheme 4, eq 1). Similar to the former reaction, the latter involves a Passerini two-component reaction (P-2CR) of 1 and 2, providing intermediate D. The aldol condensation between intermediate D and 3′ gives intermediate E, which undergoes intramolecular nucleophilic addition and subsequent ring-opening of intermediate F to form final isocoumarins 5 (Scheme 4, eq 2).
Scheme 4. Mechanism Hypothesis for Forming 4 and 5.
In conclusion, we have demonstrated the sequential combination of the Passerini three-component reaction with aldol condensation from low-cost and readily accessible arylglyoxals and isocyanides with 2-formylbenzoic acid. Two straightforward and operationally friendly methods enable selective accesses to isocoumarins with different substituted patterns by varying reaction solvents. These reactions also feature wide substrate scope, reliable scalability, and flexibility of structural modification as well as mild reaction conditions, making these strategies highly attractive. Further investigations on exploring the mechanism of these transformations and evaluating their biological activity are in progress.
Acknowledgments
We are grateful for financial support from the NSFC (Nos. 21332005, 21232004, 21272095, and 21102124), PAPD of Jiangsu Higher Education Institutions, Jiangsu Science and Technology Support Program (No. BE2011045), the Qing Lan Project (12QLG006), Robert A. Welch Foundation (D-1361, U.S.A.), and NIH (R33DA031860, U.S.A.).
Supporting Information Available
Experimental procedures and spectroscopic data for all new compounds 4a–4q, 5a–5k, and X-ray crystal data (CIF) for 4a, 5i. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- a Lehmann F.; Currier E. A.; Olsson R.; Ma J.-N.; Burstein E. S.; Hacksell U.; Luthman K. Bioorg. Med. Chem. 2010, 18, 4844. [DOI] [PubMed] [Google Scholar]; b Paraschos S.; Magiatis P.; Kalpoutzakis E.; Harvala C.; Skaltsounis A.-L. J. Nat. Prod. 2001, 64, 1585. [DOI] [PubMed] [Google Scholar]; c Saeed A. J. Asian Nat. Prod. Res. 2006, 8, 417. [DOI] [PubMed] [Google Scholar]; d Zhang H.; Matsuda H.; Kumahara A.; Ito Y.; Nakamura S.; Yoshikawa M. Bioorg. Med. Chem. Lett. 2007, 17, 4972. [DOI] [PubMed] [Google Scholar]; e Li Y.; Plitzko I.; Zaugg J.; Hering S.; Hambuger M. J. Nat. Prod. 2010, 73, 768. [DOI] [PubMed] [Google Scholar]; f Haritakun R.; Sappan M.; Suvannakad R.; Tasanathai K.; Isaka M. J. Nat. Prod. 2010, 73, 75. [DOI] [PubMed] [Google Scholar]; g Xu L.; He Z.; Xue J.; Chen X.; Wei X. J. Nat. Prod. 2010, 73, 885. [DOI] [PubMed] [Google Scholar]
- a Yoshikawa M.; Harada E.; Naitoh Y.; Inoue K.; Matsuda H.; Shimoda H.; Yamahara J.; Murakami N. Chem. Pharm. Bull. 1994, 42, 2225. [DOI] [PubMed] [Google Scholar]; b Weigele M.; Czajkowski R.; Blount J. F.; De Bernardo S.; Tengi J. P.; Leimgruber W. J. Org. Chem. 1976, 41, 390. [Google Scholar]; c Weigele M.; Blount J. F.; Tengi J. P.; Czajkowski R. C.; Leimgruber W. J. Am. Chem. Soc. 1972, 94, 4052. [Google Scholar]; d Devienne K. F.; Calgaro-Helena A. F.; Dorta D. J.; Prado I. M. R.; Raddi M. S. G.; Vilegas W.; Uyemura S. A.; Santos A. C.; Curti C. Phytochemistry 2007, 68, 1075. [DOI] [PubMed] [Google Scholar]; e Engelmeier D.; Hadacek F.; Hofer O.; Lutz-Kutschera G.; Nagl M.; Wurz G.; Greger H. J. Nat. Prod. 2004, 67, 19. [DOI] [PubMed] [Google Scholar]
- a Hashimoto T.; Tori M.; Asakawa Y. Phytochemistry 1988, 27, 109. [Google Scholar]; b Watanabe M.; Sahara M.; Kubo M.; Furukawa S.; Billedeau R. J.; Snieckus V. J. Org. Chem. 1984, 49, 742. [Google Scholar]; d Yasuda T.; Kayaba S.; Takahashi K.; Nakazawa T.; Ohsawa K. J. Nat. Prod. 2004, 67, 1604. [DOI] [PubMed] [Google Scholar]; e Mandal S. K.; Roy S. C. Tetrahedron Lett. 2007, 48, 4131. [Google Scholar]; f Bader A.; De Tommasi N.; Cotugno R.; Braca A. J. Nat. Prod. 2011, 74, 1421. [DOI] [PubMed] [Google Scholar]
- a Trotter T. N.; Albury A. M. M.; Jennings M. P. J. Org. Chem. 2012, 77, 7688. [DOI] [PubMed] [Google Scholar]; b Fang B.; Xie X.; Zhao C.; Jing P.; Li H.; Wang Z.; Gu J.; She X. J. Org. Chem. 2013, 78, 6338. [DOI] [PubMed] [Google Scholar]; c Dillon M. P.; Simpson T. J.; Sweeney J. B. Tetrahedron Lett. 1992, 33, 7569. [Google Scholar]; d Fujita M.; Mori K.; Shimogaki M.; Sugimura T. Org. Lett. 2012, 14, 1294. [DOI] [PubMed] [Google Scholar]; e Zhang W.; Krohn K.; Draeger S.; Schulz B. J. Nat. Prod. 2008, 71, 1078. [DOI] [PubMed] [Google Scholar]; f Tianpanich K.; Prachya S.; Wiyakrutta S.; Mahidol C.; Ruchirawat S.; Kittakoop P. J. Nat. Prod. 2011, 74, 79. [DOI] [PubMed] [Google Scholar]
- a van der Merwe K. J.; Steyn P. S.; Fourie L.; Scott D. B.; Theron J. J. Nature 1965, 205, 1112. [DOI] [PubMed] [Google Scholar]; b de Jesus A. E.; Steyn P. S.; Vleggaar R.; Wessels P. L. J. Chem. Soc., Perkin Trans. 1 1980, 52. [Google Scholar]; c Gillman I. G.; Yezek J. M.; Manderville R. A. Chem. Commun. 1998, 647. [Google Scholar]; d Dai J.; Park G.; Perry J. L.; Il’Ichev Y. V.; Bow D. A. J.; Pritchard J. B.; Faucet V.; Pfohl-Leszkowicz A.; Manderville R. A.; Simon J. D. Acc. Chem. Res. 2004, 37, 874. [DOI] [PubMed] [Google Scholar]; d Bouisseau A.; Roland A.; Reillon F.; Schneider R.; Cavelier F. Org. Lett. 2013, 15, 3888. [DOI] [PubMed] [Google Scholar]
- Georgiev M. I.; Ali K.; Alipieva K.; Verpoorte R.; Choi Y. H. Phytochemistry 2011, 72, 2045. [DOI] [PubMed] [Google Scholar]; b Alipieva K. I.; Orhan I. E.; Cankaya I. I. T.; Kostadinova E. P.; Georgiev M. I. Phytochem. Rev. 2014, 13, 417. [Google Scholar]
- a Endringer D. C.; Guimaraes K. G.; Kondratyuk T. P.; Pezzuto J. M.; Braga F. C. J. Nat. Prod. 2008, 71, 1082. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Hobson S. J.; Parkin A.; Marquez R. Org. Lett. 2008, 10, 2813. [DOI] [PubMed] [Google Scholar]; c Yang J. X.; Chen Y.; Huang C.; Shu Z.; Lin Y. Chem. Nat. Compd. 2011, 47, 13. [Google Scholar]; d Egan B. A.; Paradowski M.; Thomas L. H.; Marquez R. Org. Lett. 2011, 13, 2086. [DOI] [PubMed] [Google Scholar]; e Dey D.; Neogi P.; Sen A.; Sharma S. D.; Nag B. Patent WO/02/30888 A2, 2002.
- Adams C.; Papillon J.; Ksander G. M. Patent WO/2007/117982, 2007.
- a Shimizu M.; Hirano K.; Satoh T.; Miura M. J. Org. Chem. 2009, 74, 3478. [DOI] [PubMed] [Google Scholar]; b Ueura T.; Satoh T.; Miura M. Org. Lett. 2007, 9, 1407. [DOI] [PubMed] [Google Scholar]; c Ueura K.; Satoh T.; Miura M. J. Org. Chem. 2007, 72, 5362. [DOI] [PubMed] [Google Scholar]
- a Zhao P.; Chen D.; Song G.; Han K.; Li X. J. Org. Chem. 2012, 77, 1579. [DOI] [PubMed] [Google Scholar]; b Zeni G.; Larock R. C. Chem. Rev. 2004, 104, 2285. [DOI] [PubMed] [Google Scholar]; c Larock R. C.; Doty M. J.; Han X. J. Org. Chem. 1999, 64, 8770. [DOI] [PubMed] [Google Scholar]; d Larock R. C.; Yum E. K.; Doty M. J.; Sham K. K. C. J. Org. Chem. 1995, 60, 3270. [Google Scholar]
- a Chinnagolla R. K.; Jeganmohan M. Chem. Commun. 2012, 2030. [DOI] [PubMed] [Google Scholar]; b Ackermann L.; Pospech J.; Graczyk K.; Rauch K. Org. Lett. 2012, 14, 930. [DOI] [PubMed] [Google Scholar]
- a Guo X.-X. J. Org. Chem. 2013, 78, 1660. [DOI] [PubMed] [Google Scholar]; b Kavala V.; Wang C.-C.; Barange D. K.; Kuo C.-W.; Lei P.-M.; Yao C.-F. J. Org. Chem. 2012, 77, 5022. [DOI] [PubMed] [Google Scholar]
- a Isocyanide Chemistry; Nenajdenko V. G., Ed.; Wiley-VCH: Weinheim, Germany, 2012. [Google Scholar]; b Multicomponent Reactions; Zhu J., Bienaymé H., Eds.; Wiley-VCH: Weinheim, Germany, 2005. [Google Scholar]
- a Vlaar T.; Ruijter E.; Maes B. U. W.; Orru R. V. A. Angew. Chem., Int. Ed. 2013, 52, 7084. [DOI] [PubMed] [Google Scholar]; b Doemling A. Chem. Rev. 2006, 106, 17. [DOI] [PubMed] [Google Scholar]; c Soeta T.; Matsuzaki S.; Ukaji Y. Chem.—Eur. J. 2014, 20, 5007. [DOI] [PubMed] [Google Scholar]; d Akbarzadeh R.; Amanpour T.; Khavasi H. R.; Bazgir A. Tetrahedron 2014, 70, 169. [Google Scholar]; e Wang X.; Xu X.-P.; Wang S.-Y.; Zhou W.; Ji S.-J. Org. Lett. 2013, 15, 4246. [DOI] [PubMed] [Google Scholar]; f Wen L.-R.; Lan M.-C.; Yuan W.-K.; Li M. Org. Biomol. Chem. 2014, 12, 4628. [DOI] [PubMed] [Google Scholar]; g Li M.; Lv X.-L.; Wen L.-R.; Hu Z.-Q. Org. Lett. 2013, 15, 1262. [DOI] [PubMed] [Google Scholar]; h Su S.; Li C.; Jia X.; Li J. Chem.—Eur. J. 2014, 20, 5905. [DOI] [PubMed] [Google Scholar]
- Faggi C.; Garcia-Valverde M.; Marcaccini S.; Menchi G. Org. Lett. 2010, 12, 788. [DOI] [PubMed] [Google Scholar]
- Xu Z.; De Moliner F.; Cappelli A. P.; Hulme C. Org. Lett. 2013, 15, 2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Jiang B.; Ye Q.; Fan W.; Wang S.-L.; Tu S.-J.; Li G. Chem. Commun. 2014, 6108. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Jiang B.; Fan W.; Sun M.-Y.; Ye Q.; Wang S.-L.; Tu S.-J.; Li G. J. Org. Chem. 2014, 79, 5258. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Fan W.; Ye Q.; Xu H.-W.; Jiang B.; Wang S.-L.; Tu S.-J. Org. Lett. 2013, 15, 2258. [DOI] [PubMed] [Google Scholar]; d Bossio R.; Marcaccini S.; Pepino R.; Torroba T. Synthesis 1993, 783. [Google Scholar]; e Marcaccini S.; Pepino R.; Marcos C. F.; Polo C.; Torroba T. J. Heterocycl. Chem. 2000, 37, 1501. [Google Scholar]; f Ayaz M.; Dietrich J.; Hulme C. Tetrahedron Lett. 2011, 38, 4821. [DOI] [PMC free article] [PubMed] [Google Scholar]; g Xu Z.; De Moliner F.; Cappelli A. P.; Hulme C. Angew. Chem., Int. Ed. 2012, 51, 8037. [DOI] [PMC free article] [PubMed] [Google Scholar]; h Shaw A. Y.; Denning C. R.; Hulme C. Tetrahedron Lett. 2012, 53, 4151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Jee J.-A.; Spagnuolo L. A.; Rudick J. G. Org. Lett. 2012, 14, 3292. [DOI] [PubMed] [Google Scholar]; b Okandeji B. O.; Sello J. K. J. Org. Chem. 2009, 74, 5067. [DOI] [PubMed] [Google Scholar]; c Brioche J.; Masson G.; Zhu J. Org. Lett. 2010, 12, 1432. [DOI] [PubMed] [Google Scholar]; d Wang Q.; Zhu J.; Wang M.-X. In Asymmetric Synthesis II; Christmann M., Brase S., Eds.; 2012; pp 95–101. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.