Abstract
The aim of the present study was to assess the pharmacokinetic behavior of atazanavir-ritonavir when it is coadministered with tenofovir disoproxil fumarate (DF) in human immunodeficiency virus (HIV)-infected patients. Eleven patients enrolled in Agence Nationale de Recherche sur le SIDA (National Agency for AIDS Research, Paris, France) trial 107 were included in this pharmacokinetic study. They received atazanavir at 300 mg and ritonavir at 100 mg once a day (QD) from day 1 to the end of study. For the first 2 weeks, their nucleoside analog reverse transcriptase inhibitor (NRTI) treatments remained unchanged. Tenofovir DF was administered QD from day 15 to the end of the study. Ongoing NRTIs were selected according to the reverse transcriptase genotype of the HIV isolates from each patient. The values of the pharmacokinetic parameters for atazanavir and ritonavir were measured before (day 14 [week 2]) and after (day 42 [week 6]) initiation of tenofovir DF and are reported for the 10 patients who completed the study. There was a significant decrease in the area under the concentration-time curve from 0 to 24 h (AUC0-24) for atazanavir with the addition of tenofovir DF (AUC0-24 ratio, 0.75; 90% confidence interval, 0.58 to 0.97; P = 0.05). There was a trend for a decrease in the minimum concentrations of atazanavir and ritonavir in plasma when they were combined with tenofovir, but none of the differences reached statistical significance. The median decreases in the HIV RNA loads at week 2 and week 6 were 0.1 and 0.2 log copies/ml, respectively. In summary, our data are consistent with the existence of a significant interaction between atazanavir and tenofovir DF.
Human immunodeficiency virus (HIV) type 1 (HIV-1) protease inhibitor (PI)-containing regimens are highly effective in previously untreated patients. Despite the improved outcomes, problems such as failure and toxicity are apparent. The management of patients experiencing failure of different highly active antiretroviral treatment regimens represents a major clinical challenge. Present guidelines recommend that, whenever possible, patients in whom several lines of antiretroviral therapy have failed should receive agents to which they have not previously been exposed (15). Most clinical trials have evaluated the impacts of salvage regimens containing only one investigational drug, but these regimens rarely drive the viral load below the detection limits of current assays. A currently accepted approach to the management of antiviral treatment failure in patients already receiving a PI-containing regimen is to include at least two new antiretroviral agents as part of the salvage regimen. Furthermore, the use of ritonavir-boosted PIs is recommended for these patients (11). Finally, daily dosing can improve adherence (12). In this setting, we hypothesized that combining two newly marketed antiretroviral drugs, ritonavir-boosted atazanavir and tenofovir disoproxil fumarate (tenofovir DF), could improve the outcome. Tenofovir DF and atazanavir appear to be promising because their pharmacokinetic profiles, activities, safety, and resistance development properties allow once-daily (QD) dosing (12). A phase IIb clinical trial (the Puzzle 2 study, Agence Nationale de Recherche sur le SIDA [ANRS; National Agency for AIDS Research, Paris, France] clinical trial 107) was conducted to evaluate the efficacy of tenofovir DF plus atazanavir-ritonavir in combination with recycled nucleoside reverse transcriptase inhibitors (NRTIs) as salvage therapy in patients who previously failed PI- and non-NRTI (NNRTI)-containing regimens.
Atazanavir is a novel azapeptide PI with a distinct resistance profile (6). The recommended dose of atazanavir is 400 mg QD (7). Like other PIs, atazanavir is a CYP3A substrate, and pharmacokinetic data from studies with healthy volunteers suggest that atazanavir concentrations could be boosted by the addition of ritonavir at a low dose (E. O'Mara, V. Mummaneni, M. Bifano, D. Randall, H. Uderman, L. Knox, and M. Geraldes, Abstr 8th Conf. Retrovir. Opportunistic Infect., abstr. 740, 2001; S. Agarwala, R. Russo, V. Mummaneni, D. Randall, M. Geraldes, E. O'Mara, Abstr. 42nd Intersci. Conf. Antimicrob. Agents. Chemother., abstr H1716, 2002). Thus, one may speculate that the pharmacokinetics and antiviral activity of atazanavir could be optimized by the addition of ritonavir at a low dose to the treatment regimens for patients infected with HIV-1 isolates exhibiting high rates of PI mutations. Tenofovir DF is a prodrug of tenofovir, an NRTI. The recommended dose is 300 mg QD. Tenofovir is eliminated unchanged through the kidney (3).
Although these two drugs do not share the same elimination pathways, pharmacokinetic interactions at other sites cannot be excluded (1).
The aim of the present ANRS trial 107 substudy was to assess the pharmacokinetic behaviors of atazanavir and ritonavir before and after the initiation of tenofovir DF treatment in HIV-infected patients.
(This study was presented in part at the 10th Conference on Retrovirus and Opportunistic Infections, Boston, Mass., 10 to 14 February 2003 [A. M. Taburet et al., 10th Conf. Retrovir. Opportunistic Infect., abstr. 537, 2003].)
MATERIALS AND METHODS
Study design.
ANRS trial 107 was a randomized open-label, multiple-dose study with HIV-infected patients who had failed previous antiretroviral therapy. Patients were randomized to receive for the first 2 weeks either unchanged treatments with PIs and NRTIs (group 1) or unchanged treatments with NRTIs in combination with atazanavir (300 mg QD) and ritonavir (100 mg QD) as substitutes for the failing PI therapy (group 2). From week 3 (day 15) to week 26, patients from either group switched to ritonavir-boosted atazanavir plus tenofovir DF at 300 mg QD and NRTIs selected according to the baseline reverse transcriptase genotype of the HIV isolate infecting each patient. Because of potential pharmacokinetic interactions, patients who were receiving an NNRTI at screening stopped taking the drug at least 2 weeks before inclusion in the study.
The pharmacokinetic substudy of ANRS trial 107 was conducted with 11 HIV-infected patients in group 2 at four selected centers.
The objective of the study was to measure the pharmacokinetic parameters of atazanavir when it was administered with ritonavir either before (day 14 [week 2]) or after (day 42 week 6) initiation of tenofovir DF in HIV-infected patients in order to detect pharmacokinetic interactions between the two drugs.
Blood samples for atazanavir and ritonavir pharmacokinetic assessments were collected on days 14 (period 1 [week 2]) and 42 (period 2 [week 6]) from a subgroup of patients in group 2. Samples were drawn prior to drug intake in the morning and then after dosing at times of 1, 2, 3, 5, 8, and 24 h. Plasma samples were kept at −20°C until analysis.
Drugs were administered in the morning with a light continental breakfast. The actual times of drug administration and samplings were recorded.
Study population.
All patients gave written informed consent to participate in this pharmacokinetic study, which was approved by the Institutional Review Board of Hôpital Saint-Antoine, Paris VI University.
HIV-infected adults were eligible for inclusion if they met the following criteria: no change in antiretroviral treatment within the last month before inclusion in the study, plasma HIV-1 RNA loads of ≥10 000 copies/ml, documented failure of previous treatment with at least two PIs and one NNRTI, and the absence of cardiomyopathy or conduction system disease, defined as QTc interval, >450 ms; pause length, >3 s on screening electrocardiography; heart rate, <40 beats/min; third-degree heart blockage; and clinical symptoms potentially related to heart blockage.
Adverse events were recorded and graded according to the ANRS scale (10).
Drug assays.
Plasma samples were assayed for their atazanavir contents at Bristol-Myers Squibb, Saint Nazaire, France, by a validated liquid chromatography-mass spectrometry-mass spectrometry assay (13). The lower limit of quantification of the plasma atazanavir concentration was 1 ng/ml. Day-to-day variability for the quality control samples was 5.7% for the three concentrations included in each analytical run.
Plasma was assayed for ritonavir concentrations by a validated reverse-phase high-pressure liquid chromatography method with UV detection, as described by the manufacturer (R. Wiebolt, Abbott Laboratories, Abbott Park, Ill.). The lower limit of quantification of the plasma ritonavir concentration was 25 ng/ml. Day-to-day variability for the quality controls was <5% at the three concentrations included in each analytical run.
Pharmacokinetic analysis.
The pharmacokinetic parameters for atazanavir and ritonavir were assessed by noncompartmental methods (WinNonlin; Pharsight Corporation, Mountain View, Calif.). The linear-log trapezoidal method was used to calculate the areas under the concentration-versus-time curves (AUCs) during a dosing interval at steady state (AUC0-t), where t is the time that the last sample was taken, close to 24 h postdosing. The AUCs from 0 to 24 h (AUC0-24s) were calculated by extrapolation, whenever the last sample was drawn before 24 h after drug intake and determination of the terminal rate constant (λz) was possible. Average concentrations at steady state (Caves) and oral clearances (CLos) were calculated by the following standard equations: Cave = AUC0-24/τ, where τ is the dosing interval, and CLo = dose/AUC0-24. The maximum concentration observed in plasma (Cmax), the observed predose concentration (C0), the lowest quantifiable observed concentration before Cmax (Cmin) or just before the next dose if the time to Cmin was <24 h, and the time to the first occurrence of Cmax (Tmax) were obtained visually from the plasma concentration-time curves determined on the last day of each study period.
Statistical analysis.
This was an observational pharmacokinetic study that assessed for the first time the concentrations of atazanavir when it was combined with ritonavir in the plasma of HIV-infected patients. When this pilot study was designed, there were no data to provide estimates for sample size calculation. Therefore, it was decided that 10 patients would be included to detect important impairments of atazanavir pharmacokinetics.
All statistical analyses were carried out with SAS (version 8.2) software. The pharmacokinetic parameters for atazanavir and ritonavir were summarized by study day by using descriptive statistics. The values of AUC0-24, Cmax, and Cmin for atazanavir and ritonavir at week 2 and week 6 were compared by a one-sample t test for paired data. When appropriate, the values of AUC0-24, Cmax, and Cmin were log transformed. Two-sided 90% confidence intervals (CIs) were constructed for the ratios of the geometric mean values (day 42 [week 6] versus day 14 [week 2]) of AUC0-24, Cmax, and Cmin for atazanavir and ritonavir. For these, the values of AUC0-24, Cmax, and Cmin for atazanavir and ritonavir were log transformed; and the resulting points and interval estimates of the means and the mean differences were exponentiated to express the results as geometric means and the ratios of the geometric means on the original scale of measurement.
RESULTS
Patient characteristics.
A total of 11 male patients (median age, 46 years) from group 2 of the trial (staggered initiation of atazanavir and tenofovir DF) gave their informed consent to participate to the pharmacokinetic study. Their baseline characteristics are summarized in Table 1, and the changes in their nucleoside analog treatments are presented in Table 2. One patient did not complete the study because of the occurrence of asymptomatic ventricular bigeminy during sampling for the pharmacokinetic study on day 14. The plasma drug concentrations for this patient were excluded from the analysis. Ten patients completed the study. The median baseline HIV RNA load was 5.1 log10 copies/ml. Alanine aminotransferase (ALT) levels were within the normal range in 8 of the 10 patients, and 2 of the 10 patients had mild abnormalities in ALT levels (53 and 70 IU/liter). Aspartate aminotransferase (AST) levels were within the normal range in 6 of the 10 patients, and 4 patients had mild abnormalities in ALT levels (44, 48, 49, and 58 IU/liter, respectively). All patients had normal serum bilirubin levels at the time of inclusion in the study. Plasma creatinine levels were within the normal range (56 to 97 μmol/liter) in all patients. The patients' characteristics did not change significantly during the 6-week study period.
TABLE 1.
Patient demographic and clinical characteristics at the baseline
| Characteristic | Values for the 10 patients who completed the study
|
Values for the patient excluded | ||
|---|---|---|---|---|
| Median | Minimum | Maximum | ||
| Age (yr) | 46.4 | 33.3 | 59.3 | 42.2 |
| HIV RNA level (log10 copies/ml) | 5.1 | 4.1 | 5.7 | 5 |
| CD4 count (no. of cells/μl) | 117.0 | 19.0 | 328.0 | 48 |
| ALT level (IU/liter) | 36.0 | 21.0 | 70.0 | 22 |
| AST (IU/liter) | 34.0 | 23.0 | 58.0 | 32 |
| Bilirubin concn (μmol/liter) | 7.5 | 4.0 | 14.0 | 17 |
TABLE 2.
Changes in nucleoside analog treatments
| Time | No. of patients receivinga:
|
|||||
|---|---|---|---|---|---|---|
| Abacavir | Didanosine | Lamivudine | Stavudine | Zalcitabine | Zidovudine | |
| Inclusion | 3 | 4 | 5 | 2 | 1 | 1 |
| Wk 2 to 6 | 7 | 4 | 7 | 1 | 2 | 4 |
| Introduced at wk 2 | 5 | 2 | 5 | 1 | 2 | 4 |
The patient excluded from the study received lamivudine.
Atazanavir and ritonavir pharmacokinetics.
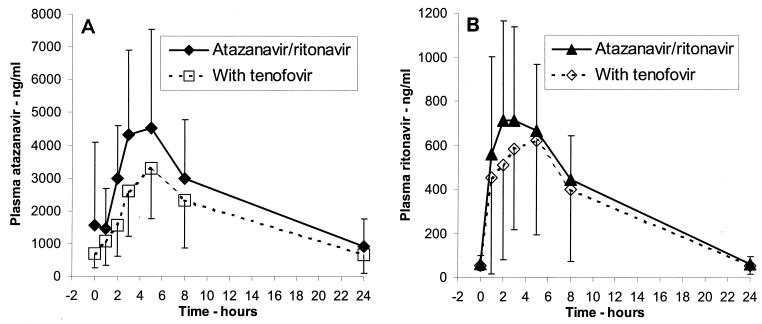
Individual plots of concentrations versus time are shown in Fig. 1. As expected, the concentrations of ritonavir were lower than those of atazanavir. Before dosing the atazanavir trough concentrations were greater than 300 ng/ml in all patients at week 2. Interestingly, the atazanavir AUCs were higher for the four patients who were receiving didanosine at week 2 (AUC0-24s, 70,676 versus 31,840 ng · h/ml; P = 0.02). The pharmacokinetic parameters measured for atazanavir and ritonavir at weeks 2 and 6, in the absence and presence of tenofovir, respectively, are compared in Table 3. The mean Cmax, Cmin, and AUC0-24 values for atazanavir at week 2 were 5,233 ng/ml, 862 ng/ml, and 53,761 ng · h/ml, respectively; those for ritonavir were 940 ng/ml, 50 ng/ml, and 7,546 ng · h/ml, respectively. There was a correlation between the atazanavir Cmin and the ritonavir Cmin at week 2 (r2 = 0.74; P = 0.01). There was a significant decrease in the atazanavir AUC0-24 with the addition of tenofovir DF (AUC0-24 ratio, 0.75; 90% CI, 0.58 to 0.97; P = 0.05). The number of additional NRTIs introduced at week 2 had no effect on this decrease; however, the number of patients enrolled in this pilot study was small. The decrease in the atazanavir Cmin observed with the addition of tenofovir DF was not significant (Cmin ratio, 0.77; 90% CI, 0.54 to 1.1; P = 0.22), but the interindividual variability was high and the study was underpowered to detect a difference. There was a trend for a decrease in ritonavir concentrations when ritonavir was combined with tenofovir, but none of the differences reached significance.
FIG. 1.
Mean ± standard deviation atazanavir (A) and ritonavir (B) concentration-time profiles for patients who received atazanavir at 300 mg QD plus ritonavir at 100 mg QD in the absence (close symbols and solid lines) or the presence (open symbols and dotted lines) of tenofovir DF at 300 mg QD.
TABLE 3.
Pharmacokinetic parameters for atazanavir and ritonavir on week 2 (atazanavir-ritonavir) and week 6 (with addition of tenofovir)a
| Drug and time | Cmax (ng/ml) | Tmax (h)b | C0 (ng/ml) | Clast (ng/ml) | Cmin (ng/ml) | AUC0-t (ng · h/ml) | AUC0-24 (ng · h/ml) | Carc (ng/ml) | CLo (ml/min) | t1/2 (h) |
|---|---|---|---|---|---|---|---|---|---|---|
| Atazanavir | ||||||||||
| Wk 2 | 5,233 ± 3,033 | 3 (2-5) | 1,571 ± 2,524 | 924 ± 829 | 862 ± 838 | 53,634 ± 35,255 | 53,761 ± 35,294 | 2,240 ± 1,471 | 123 ± 61 | 8.6 ± 2.3 |
| Wk 6 | 3,443 ± 1,412 | 5 (1-5) | 685 ± 417 | 665 ± 559 | 577 ± 367 | 39,231 ± 23,035 | 39,276 ± 23,034 | 1,636 ± 960 | 164 ± 84 | 8.6 ± 3.1 |
| Rc (90% CI) | 0.72 (0.50-1.05) | 0.77 (0.54-1.10) | 0.75 (0.58-0.97) | |||||||
| P | 0.06 | 0.22 | 0.05 | |||||||
| Ritonavir | ||||||||||
| Wk 2 | 940 ± 336 | 3 (2-8) | 60 ± 37 | 59 ± 37 | 50 ± 31 | 7,541 ± 3,226 | 7,546 ± 3,226 | 314 ± 134 | 255 ± 98 | 5.1 ± 0.7 |
| Wk 6 | 812 ± 447 | 3 (0-5) | 52 ± 22 | 53 ± 39 | 42 ± 19 | 6,575 ± 4,041 | 6,603 ± 3,999 | 275 ± 167 | 507 ± 737 | 5.3 ± 0.7 |
| R (90% CI) | 0.72 (0.43-1.21) | 0.91 (0.73-1.13) | 0.75 (0.44-1.24) | |||||||
| P | 0.38 | 0.42 | 0.41 |
Data are expressed as means ± standard deviations unless indicated otherwise.
Data are expressed as medians (ranges).
R is the ratio of the geometric means for the parameters at week 6 to those at week 2.
Virologic parameters.
At week 2 and week 6, the median decreases in HIV RNA levels from the baseline were not significant and were 0.1 (95 CI, −0.11 to 0.25) and 0.2 (95 CI, −0.02 to 0.90), respectively.
Safety.
One patient did not complete the study because of the occurrence of asymptomatic ventricular bigeminy during sampling for the pharmacokinetic study on day 14. This patient had the highest ritonavir Cmax (1,721 ng/ml) and AUC0-24 (18,794 ng · h/ml), and the atazanavir concentrations were in the upper range (Cmax, 5,300 ng/ml; AUC0-24, 98,217 ng · h/ml), with a slow decline in concentrations versus time (half-life [t1/2], 26.7 h). The episode was unrelated to study drug administration, given the absence of a significant increase in the QT interval (<400 ms) following drug administration and the intermittent recurrence of the anomaly 1 month after discontinuation of the study therapy.
DISCUSSION
In patients who have failed therapy with multiple lines of antiretroviral drugs, there is a need to increase PI concentrations to overcome the increase in viral activity. The concentrations of atazanavir obtained when it was combined with ritonavir were two- to fivefold higher than those obtained when atazanavir was administered alone at 400 mg QD (O'Mara et al., Abstr. 8th Conf. Retrovir. Opportunistic Infect., abstr. 740, 2001). The effect of ritonavir on the disposition of atazanavir was studied in healthy volunteers who first received atazanavir at 300 mg QD, followed by atazanavir-ritonavir at 300/100 mg QD (Agarwala et al., 42nd ICAAC). It has been demonstrated that ritonavir increases the level of exposure to atazanavir (AUC) by three- to fourfold; the t1/2 is increased from 6.5 h (with atazanavir alone) to 15 to 18 h (with the addition of ritonavir), suggesting that the decrease in the hepatic clearance of atazanavir by ritonavir is the major mechanism of the interaction. The values of the pharmacokinetic parameters obtained for the HIV-infected patients in this study are in agreement with those data, although the values for the patients in the present study were slightly lower than those reported for healthy volunteers (Agarwala et al., 42nd ICAAC). The geometric mean AUC0-24s for patients after 14 days of therapy and volunteers after 20 days of therapy were 46,073 and 57,039 ng · h/ml, respectively. The t1/2s measured during a dosing interval were shorter in patients than in volunteers (8.6 and 16 h, respectively). Several reasons can explain this discrepancy. First, a meal can have an effect, as atazanavir absorption is sensitive to the fat content of meals (for a summary of atazanavir characteristics, see http://www.fda.gov/cder/approval/index.htm) and a standard breakfast administered to healthy volunteers in the United States is not the same as the continental breakfast administered in our hospital settings. Second, the duration of therapy can have an effect. Autoinduction was reported with PIs (1) and could explain the lower concentrations after 14 days of therapy compared with those after 10 days of therapy. In keeping with this assumption is the shorter t1/2s measured in this study compared to those measured in healthy volunteers (8.6 and 18.1 h, respectively). However, it has been demonstrated in healthy volunteers that the atazanavir steady state is reached within 4 days of therapy when atazanavir is administered either alone or in combination with ritonavir (6). Finally, the rate and extent of absorption could be impaired in HIV-infected patients. Of note, as previously demonstrated with other CYP3A substrates, the plasma atazanavir and ritonavir concentrations were highly variable among the patients included in this study.
Ritonavir concentrations were low, in keeping with the 100-mg QD dosing regimen (4). Cmaxs ranged from 454 to 1,592 ng/ml on week 2 and from 73 to 1,558 ng/ml on week 6. Such concentrations are below those reported to be efficient against susceptible HIV-1 clinical isolates. Comparison with the data obtained for healthy volunteers (Agarwala et al., 42nd ICAAC) indicates that the ritonavir concentrations were twice those observed in the patients in this study (AUC0-24s, 14,844 and 7,011 ng · h/ml, respectively, on week 2), although t1/2s remained unchanged (5.1 and 5.1 h, respectively). The nonreliable absorption of ritonavir in patients or the effect of a meal, as mentioned above for atazanavir, could explain this difference. Despite the low ritonavir concentrations, inhibition of atazanavir metabolism appeared to be sustained during a 24-h dosing interval as a consequence of the high affinity of ritonavir for CYP3A (8).
After the addition of tenofovir DF, there was a trend for lower atazanavir and ritonavir concentrations, but the decrease in the atazanavir AUC0-24 was the only difference that reached statistical significance. Interestingly, a significant 25 to 40% decrease in atazanavir concentrations after the addition of tenofovir DF was recently reported from a study with healthy subjects (S. Kaul, K. Bassi, B. Damle, J. Xie, J. Gale, B. Kearne, G. Hanna, Abstr. 43rd Intersci. Conf. Antimicrob. Agents. Chemother., abstr A-1616, 2003). The mechanism of such an interaction remains to be elucidated. Tenofovir is eliminated unchanged via the kidneys; therefore, an interaction at the biotransformation level is very unlikely (3). Furthermore, it should be pointed out that the atazanavir and ritonavir t1/2s remained unchanged throughout the study (8.6 ± 2.3 and 8.6 ± 3.1 h for atazanavir on week 2 and week 6, respectively, and 5.1 ± 0.7 and 5.3 ± 0.7 h for ritonavir on week 2 and week 6, respectively), suggesting that tenofovir has no effect on atazanavir clearance. Therefore, this interaction likely occurs at the gut level. HIV PIs are substrates of P-glycoprotein (1, 9). The human multidrug resistance P-glycoprotein (ABCB1), which is localized in epithelial cells of the intestine, transports a wide variety of structurally diverse hydrophobic compounds and plays a major role in reducing their bioavailabilities (9). Absorption of the prodrug of tenofovir, tenofovir DF, was reported to involve P-glycoprotein (14); whether modulation of this transporter could explain this interaction remains to be established. It has previously been demonstrated that adefovir, which is closely related to tenofovir, decreased saquinavir concentrations by 50% in HIV-infected patients receiving the combination saquinavir-ritonavir-adefovir compared to those in patients receiving saquinavir-ritonavir-delavirdine (5). Although the exact mechanism underlying this interaction was not elucidated, an induction of P-glycoprotein was hypothesized. Whether adefovir and tenofovir are P-glycoprotein inducers remains to be clearly established. A physicochemical interaction between atazanavir and tenofovir DF when they are simultaneously present in the gut is another postulated hypothesis. The lack of interaction of tenofovir DF with another PI, such as the coformulation of lopinavir-ritonavir, supports the latter assumption (B. P. Kearney, A. Mittan, J. Sayre, J. F. Flaherty, L. Zhong, J. J. Toole, and A. K. Cheng, Abstr. 43rd Intersci. Conf. Antimicrob. Agents. Chemother., abstr. A-1617, 2003). One may therefore speculate that staggered administration of atazanavir-ritonavir and tenofovir DF would avoid such an interaction. However, clinicians are discouraged from using such an approach until data are available to support this hypothesis.
Our data failed to demonstrate a significant correlation between the pharmacokinetic parameters and the viral response. However, this could be explained by the small sample size and the poor overall virologic responses at week 2 and week 6 due to very high levels of resistance mutations among the isolates from the study population at the baseline. The clinical significance of the decrease in atazanavir concentrations when atazanavir treatment was boosted with ritonavir and coadministered with tenofovir DF is unknown. First, atazanavir trough concentrations remained threefold higher than those obtained with atazanavir at 400 mg without ritonavir, and second, data from a recent randomized trial showed that through 16 weeks there was no difference in virologic response whether patients who all received tenofovir DF in combination with one NRTI and one boosted PI were in the arm with the lopinavir-ritonavir coformulation or the arm with the atazanavir-ritonavir coformulation (R. Badaro, E. DeJesus, A. Lazzarin, J. Jemsek, B. Clotet, A. Rightmire, A. Thiry, and R. Wilber, Abstr. 2nd IAS Conf. HIV Pathogenesis Treatment, abstr. 118, 2003). It is now recognized that PI concentrations should be compared and discussed in terms of the concentrations necessary to inhibit the viral replication (2), especially in heavily pretreated patients infected with less susceptible mutant viruses.
One may speculate that the coadministration of tenofovir DF and atazanavir without ritonavir may result in a significant impairment of the virologic response. Further studies are needed to better understand the clinical relevance of this pharmacokinetic interaction in HIV-infected patients.
Acknowledgments
We thank Gilead Sciences and Bristol Myers Squibb for helpful discussions and support for carrying out this trial, Hélène Meytraud (CLD) for monitoring the trial, and the following investigators who participated in the ANRS trial 107 Puzzle 2 substudy: E. Oksenhendler, L. Gérard, D Séréni, C Lascoux-Combe, and P. Palmer (Hôpital Saint-Louis, Paris); J.-F. Delfraissy, C. Goujard, Y. Quertainmont, and N. Idri (Hôpital Bicêtre, Kremlin-Bicêtre); M. Kazatchkine, C. Piketty, A. Aouba, N. Bengrait, and A. Si-Mohamed (Hôpital Européen Georges Pompidou, Paris); and P.-M. Girard, D. Bollens, F. Bess, L. Morand-Joubert, A. Charrois, and E. Dasque with the support of the Clinical Investigation Center INSERM-AP-HP (Hôpital Saint-Antoine, Paris).
This study was supported by ANRS.
REFERENCES
- 1.Barry, M., F. Mulcahy, C. Merry, S. Gibbons, and D. Back. 1999. Pharmacokinetics and potential interactions amongst antiretroviral agents used to treat patients with HIV infection. Clin. Pharmacokinet. 36:289-304. [DOI] [PubMed] [Google Scholar]
- 2.Becker, S., A. Fisher, C. Flexner, J. G. Gerber, R. Haubrich, A. D. Kashuba, A. D. Luber, and S. C. Piscitelli. 2001. Pharmacokinetic parameters of protease inhibitors and the Cmin/IC50 ratio: call for consensus. J. Acquir. Immune Defic. Syndr. 27:210-211. [DOI] [PubMed] [Google Scholar]
- 3.Chapman, T. M., J. K. McGavin, and S. Noble. 2003. Tenofovir disoproxil fumarate. Drugs 63:1597-1608. [DOI] [PubMed] [Google Scholar]
- 4.Cooper, C. L., R. P. G. van Heeswijk, K. Gallicano, and D. W. Cameron. 2003. A review of low-dose ritonavir in protease inhibitor combinaison therapy. Clin. Infect. Dis. 36:1585-1592. [DOI] [PubMed] [Google Scholar]
- 5.Fletcher, C. V., E. P. Acosta, H. Cheng, R. Haubrich, M. Fischl, R. Raasch, C. Mills, X. J. Hu, D. Katzenstein, R. P. Remmel, R. M. Gulick, and the ACTG Protocol Team. 2000. Competing drug-drug interactions among multidrug antiretroviral regimens used in the treatment of HIV-infected subjects: ACTG 884. AIDS 14:2495-2501. [DOI] [PubMed] [Google Scholar]
- 6.Goldsmith, D. R., and C. M. Perry. 2003. Atazanavir. Drugs 63:1679-1693. [DOI] [PubMed] [Google Scholar]
- 7.Gong, Y. F., B. S. Robinson, R. E. Rose, C. Deminie, T. P. Spicer, D. Stock, R. J. Colonno, and P. F. Lin. 2000. In vitro resistance profile of the human immunodeficiency virus type 1 protease inhibitor BMS-232632. Antimicrob. Agents Chemother. 44:2319-2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumar, G. N., J. Dykstra, E. M. Roberts, V. K. Jayanti, D. Hickman, J. Uchic, Y. Yao, B. Surber, S. Thomas, and G. R. Granneman. 1999. Potent inhibition of the cytochrome P-450 3A-mediated human liver microsomal metabolism of a novel HIV protease inhibitor by ritonavir: a positive drug-drug interaction. Drug Metab. Dispos. 27:902-908. [PubMed] [Google Scholar]
- 9.Lin, J. H., and M. Yamazaki. 2003. Role of P-glycoprotein in pharmacokinetics; clinical implication. Clin. Pharmacokinet. 42:59-98. [DOI] [PubMed] [Google Scholar]
- 10.Molina, J. M., G. Chêne, F. Ferchal, V. Journot, M. N. Sombardier, I. Pellegrin, C. Rancinan, L. Cotte, I. Madeleine, R. Roué, and J. M. Decazes for the ALBI Study Group. 1999. The ALBI trial: a randomized controlled trial comparing stavudine plus didanosine with zidovudine plus lamivudine and a regimen alternating both combinations in previously untreated patients infected with human immunodeficiency virus. J. Infect. Dis. 180:351-358. [DOI] [PubMed] [Google Scholar]
- 11.Moyle, G. J., and D. Back. 2001. Principle and practice of HIV-protease inhibitor pharmacoenhancement. HIV Med. 2:105-113. [DOI] [PubMed] [Google Scholar]
- 12.Rosenbach, K. A., R. Allison, and J. P. Nadler. Daily dosing of highly active antiretroviral therapy. Clin. Infect. Dis. 34:686-692. [DOI] [PubMed]
- 13.Schuster, A., S. Burzawa, M. Jemal, E. Loizillon, P. Couerbe, and D. Whigan. 2003. Quantitative determination of the HIV protease inhibitor atazanavir (BMS-232632) in human plasma by liquid chromatography-tandem mass spectrometry following automated solid-phase extraction. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 788:377-386. [DOI] [PubMed] [Google Scholar]
- 14.van Gelder, J., S. Deferme, L. Naesens, E. De Clercq, G. van den Mooter, R. Kinget, and P. Augustijns. 2002. Intestinal absorption enhancement of the ester prodrug tenofovir disoproxil fumarate through modulation of the biochemical barrier by defined ester mixtures. Drug Metab. Dispos. 30:924-930. [DOI] [PubMed] [Google Scholar]
- 15.Yeni, P., S. M. Hammer, C. J. Carpenter, D. A. Cooper, M. A. Fischl, J. M. Gatell, B. G. Gazzard, M. S. Hirsch, D. M. Jacobsen, D. A. Katzenstein, J. S. Montaner, D. D. Richman, M. S. Saag, M. Schechter, R. T. Schooley, M. A. Thompson, S. Vella, and P. A. Volberding. 2002. Antiretroviral treatment for adult HIV infection: updated recommendations of the International AIDS Society—USA Panel. JAMA 288:222-235. [DOI] [PubMed] [Google Scholar]