Abstract
In Enterobacter aerogenes, β-lactam resistance often involves a decrease in outer membrane permeability induced by modifications of porin synthesis. In ATCC 15038 strain, we observed a different pattern of porin production associated with a variable antibiotic susceptibility. We purified Omp35, which is expressed under conditions of low osmolality and analyzed its pore-forming properties in artificial membranes. This porin was found to be an OmpF-like protein with high conductance values. It showed a noticeably higher conductance compared to Omp36 and a specific location of WNYT residues in the L3 loop. The importance of the constriction region in the porin function suggests that this organization is involved in the level of susceptibility to negative large cephalosporins such as ceftriaxone by bacteria producing the Omp35 porin subfamily.
The bacterial porins are major outer membrane proteins that form water-filled channels, allowing the diffusion across the outer membrane of small polar molecules, amino acids, and nutrients (14, 18, 23). General nonspecific porins, such as OmpF and OmpC from Escherichia coli, form homotrimers in the outer membrane. These porin trimers are constituted by large β-barrel monomer subunits which are constricted about halfway through the membrane by an internal loop, loop 3 (L3) (18). Resolution of the three-dimensional structure of the E. coli OmpF and Klebsiella pneumoniae OmpK36 has led to identification of the functional domains of the enterobacterial channels (5, 8, 13, 17).
Enterobacter aerogenes is one of the most frequently described gram-negative pathogens responsible for nosocomial respiratory tract infections in France and Belgium (2, 6, 10). This bacterial pathogen harbors a variety of antibiotic resistance mechanisms (7, 9, 19, 24, 30). Among them, the modification of outer membrane permeability, a porin deficiency associated with the expression of cephalosporinase activity, is frequently detected in clinical resistant isolates (7, 19). We recently isolated a strain with an unusual porin phenotype: this EA3 strain synthesized a mutated Omp36 porin containing a G112D substitution in the L3 domain (19). Despite this prominent role of porins in drug susceptibility, only one porin, Omp36, is today functionally characterized in E. aerogenes (19).
The aim of this work was to investigate the structural and functional properties of the second major pore-forming protein produced by the E. aerogenes strain. The sequence and the channel properties of the new porin Omp35 were determined and agree with the biological differences concerning the β-lactam susceptibility, observed between the bacteria producing either Omp35 or Omp36.
MATERIALS AND METHODS
Bacterial strains, growth conditions, and antibiotic susceptibility tests.
The E. aerogenes ATCC 15038 strain was used as the standard strain. E. aerogenes EAEP289 is a Kans derivative of the previously described clinical strain EA27 (24). Bacteria were routinely grown in Luria-Bertani (LB) medium or nutrient broth (NB). For the determination of MICs, NB broth was prepared according to Difco recommendations using an NB preparation containing Bacto beef extract (3 g/liter) and Bacto Peptone (5 g/liter) (Difco Laboratories, Detroit, Mich.). E. aerogenes cells were grown in NB broth (51 mOsm/kg) or in NB broth containing 20% sorbitol (1.5 Osm/kg) as previously reported (12, 13). The MICs were determined with twofold serial dilutions in adequate broth. Approximately 106 cells were inoculated into 1 ml of medium with various concentrations of antibiotics, and the results were read after 18 h at 37°C.
Separation and purification of E. aerogenes major outer membrane protein.
Outer membranes were extracted using the spheroplast procedure (9), and the final membrane fractions were obtained by centrifugation (180,000 × g) at 4°C for 1 h. The pellet was resuspended in a solution containing 20 mM NaPi (pH 7.6), 10 mM NaCl, and 1% octylpolyoxyethylene (octyl-POE; Bachem, Bubendorf, Switzerland), and the suspension was homogenized and stirred for 30 min at 4°C. The suspension was centrifuged (180,000 × g) for 1 h at 4°C, and the pellet was resuspended in 20 mM NaPi (pH 7.6), 10 mM NaCl and 3% octyl-POE. Three successive extractions with 3% octyl-POE were performed, leading to a specific recovery of outer membrane proteins. The first step of purification involved sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) with a 4% polyacrylamide stacking gel (pH 6.8) and a 12.5% polyacrylamide resolving gel (pH 8.8). The gel was stained (Coomassie brilliant blue G), and the band corresponding to the major protein was excised and electroeluted with a solution containing 192 mM glycine, 25 mM Tris-base, and 0.2% Triton X-100. We checked the purity of the preparation by SDS-PAGE (7-to-12.5% gel system) with silver staining.
Reconstitution into planar lipid bilayers.
Virtually solvent-free planar lipid bilayers were formed over a hole (diameter, 100 to 150 μm) in a polytetrafluoroethylene film (10 μm thick) pretreated with a mixture of 1:40 (vol/vol) hexadecane-hexane and sandwiched between two half cells. Lipids, phosphatidylcholine from soy beans (asolectin [type IV S] from Sigma), dissolved in hexane (0.5%) were spread on the top of electrolyte solution (1 M KCl, 10 mM HEPES [pH 7.4]) in both compartments. Bilayer formation was achieved by lowering and raising the level in one or both compartments and monitoring capacity responses (21). Voltage was applied through an Ag/AgCl electrode in the cis side, and the trans side was grounded. The purified Omp35 was added in the cis compartment (1 to 10 ng/ml). Current fluctuations were recorded with an RK 300 amplifier (Bio-Logic, Claix, France) and stored on a CD recorder (DRA 200, Bio-Logic France) for off-line analysis. CD data were then analyzed by the windac32 (ShareIt!, Inc., Greensburg, Pa.) and Biotools (Bio-Logic) softwares. For voltage gating measurements, the doped membranes were subjected to slow ramps of potential (10 mV/s) and transmembrane currents were fed into an amplifier (BBA-01; Eastern Scientific, Rockville, Md.). Current-voltage curves were stored on a computer and analyzed with Scope software (Bio-Logic). For ion selectivity measurements, zero-current potentials were determined by establishing a 10-fold KCl gradient (1 M:100 mM cis/trans) across the bilayer. The ion selectivity was characterized by the ratio PC/PA (i.e., the ratio of the permeability for cations and the permeability for anions) calculated according to the Goldman-Hodgkin-Katz equation. All experiments were performed at room temperature.
Reconstitution into liposomes and patch-clamp recording.
Purified porin in Triton X-100 was incubated with small unilamellar vesicles of asolectin (type IV S; Sigma) at a protein-to-lipid ratio of 1:2,000 to 1:4,000 (wt/wt) for 30 min at room temperature before the addition of Bio-Beads (80 mg/ml; Bio-Rad, Ivry sur Seine, France) to get rid of detergent. After 4 h of incubation at room temperature, the beads were discarded, and the suspension was centrifuged for 20 min at 337,000 × g. The pellet was resuspended in 30 μl of 10 mM HEPES (pH 7.4), and aliquots of the suspension were subjected to a dehydration-rehydration procedure to obtain giant liposomes (11). For each experiment 1 to 2 μl of proteoliposomes was placed in a 1-ml chamber containing recording solution (200 mM KCl, 10 mM HEPES, pH 7.4). Membrane patches, obtained from unilamellar blisters from collapsing liposomes, were examined using the standard patch-clamp technique (10). Micropipettes used were borosilicate glass capillaries (Harvard Apparatus, Kent, United Kingdom) filled with recording solution. The single-channel currents were visualized with the Visual Patch 500 amplifier (Bio-logic), and recorded data were analyzed with Biotools software (Bio-logic).
SDS-PAGE and immunoblotting.
Bacteria in the exponential growth phase in Luria-Bertani broth or in NB broth in the absence or in the presence of 20% sorbitol were pelleted and solubilized at 96°C as described previously (19). Samples (equivalent to 0.02 optical density units at 600 nm) were loaded on SDS-polyacrylamide gels (11% polyacrylamide, 0.1% SDS) and subjected to electrophoresis. The resulting bands were electrotransferred to nitrocellulose membranes in the presence of 0.05% SDS. The membranes were saturated by incubation overnight with Tris-buffered saline (50 mM Tris-HCl, 150 mM NaCl [pH 8]) containing 10% skim milk powder at 4°C. They were then incubated in the same buffer supplemented with 0.2% Triton X-100 for 2 h at room temperature in the presence of polyclonal antibodies (at a dilution of 1/500 to 1/1,000) directed against denatured porins (19). The membrane was washed four times and incubated with alkaline phosphatase-conjugated AffinitiPure goat anti-rabbit immunoglobulin G antibodies (Jackson ImmunoResearch). The polyclonal antibodies directed against denatured porins have been described and recognized the E. aerogenes porins, as previously reported (19).
Determination of N-terminal amino acid sequences.
To determine the N-terminal sequence of the major outer membrane protein of E. aerogenes, the 3% octyl-POE membrane preparations were resolved by SDS-PAGE (7-to-15% discontinuous gel system) and electrotransferred onto an Immobilon membrane (Millipore, St Quentin en Yvelines, France). The major protein band was excised, and its N-terminal sequence was determined by Edman degradation (492A protein sequencer; Applied Biosystems, Courtaboeuf, France).
Omp35 cloning and sequencing.
DNA isolation, PCR, and transformations of plasmids were performed as described previously (3). The primers were selected from the sequence of K. pneumoniae ompK35 gene (AJ303057 and AJ011501). PCR was conducted at 52°C for 35 cycles, using P351 (5′ ATA ACA TAT GAT GAA GCG CAA TAT TCT 3′) and P352 (5′ TAT ACT CGA GGA ACT GGT AAA CGA TAC CAA 3′) probes. PCR products were cloned into a plasmid conferring kanamycin resistance, pDrive Cloning Vector (QIAGEN). Plasmids were first transferred into a susceptible E. coli host, strain JM109, and after that were transferred into EAEP289, a strain of E. aerogenes lacking porin. pCB35 plasmid encoded the omp35 of E. aerogenes.
Nucleotide sequence accession numbers.
The complete omp35 sequences were deposited in the GenBank database under the accession numbers AY487900 to AY487904.
RESULTS
β-Lactam susceptibility and antigenic profile.
Strain ATCC 15038 was assayed for its β-lactam susceptibilities in different media supplemented or not with sorbitol (20%). The results are presented in Table 1. A noticeable difference was observed when the assay was carried out at high osmolarity, i.e., in the presence of sorbitol. Under this condition, higher alterations of susceptibility to negatively charged cephalosporin were observed corresponding to 8- to 16-fold increase of MICs for aztreonam and ceftriaxone, respectively (Table 1). Smaller changes were also found for ceftazidime, cefotaxime, ticarcillin, and cefepime, while imipenem seemed to be unaffected.
TABLE 1.
Antibiotic susceptibilities of E. aerogenes ATCC 15038 strain
| Drug | Charge | Molecular mass (Da) | MICa (μg/ml) for ATCC 15038 as determined in:
|
|
|---|---|---|---|---|
| NB | NB + sorbitol (20%) | |||
| Imipenem | +, − | 317 | 0.25 | 0.25 |
| Cephaloridine | +, − | 415 | 16 | 16 |
| Cefepime | +, − | 480 | 0.5 | 1 |
| Cefotaxime | − | 455 | 0.125 | 2 |
| Ceftazidime | +, 2− | 546 | 0.5 | 4 |
| Ticarcillin | 2− | 384 | 4 | 16 |
| Aztreonam | 2− | 435 | 0.5 | 4 |
| Ceftriaxone | 2− | 554 | 1 | 16 |
Values are means of three independent determinations.
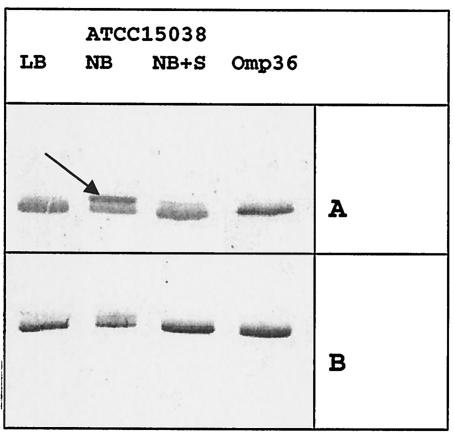
The porin contents were checked with specific antibodies (Fig. 1). The sorbitol has been reported to efficiently downregulate OmpK35 porin expression in K. pneumoniae (12). In Fig. 1, in low-osmotic medium (NB), strain ATCC 15038 expressed two proteins which are recognized by the polyclonal antiserum directed against porin. The synthesis of proteins that migrate at high molecular weights was strongly reduced in the presence of sorbitol, and we concomitantly observed an increasing expression of lower-molecular-weight product (Fig. 1). Interestingly, the sorbitol-sensitive protein presented a more intense signal with antiserum directed against the OmpF denatured monomer, while the antiserum directed against the OmpC monomer strongly recognizes the lower molecular weight product. The migration of the latter corresponded to the Omp36 (Fig. 1) previously identified in strain ATCC 13048 (9). This results suggest that an osmotically sensitive protein, immunorelated to E. coli OmpF porin, was expressed in E. aerogenes under conditions of low osmolality. This porin was called Omp35.
FIG. 1.
Antigenic porin profiles of the E. aerogenes strains. Total bacterial proteins were resolved by SDS-PAGE (19). The proteins were electrotransferred to membranes and immunodetected with polyclonal antibodies directed against denatured OmpF porin (A) and denatured OmpC porin (B). Only the relevant part of the blot is shown. The arrow indicates the migration of Omp35. Abbreviations: LB, Luria-Bertani broth; S, sorbitol (20%); Omp36, purified Omp36; ATCC 15038, E. aerogenes ATCC 15038.
Omp35 sequence and expression.
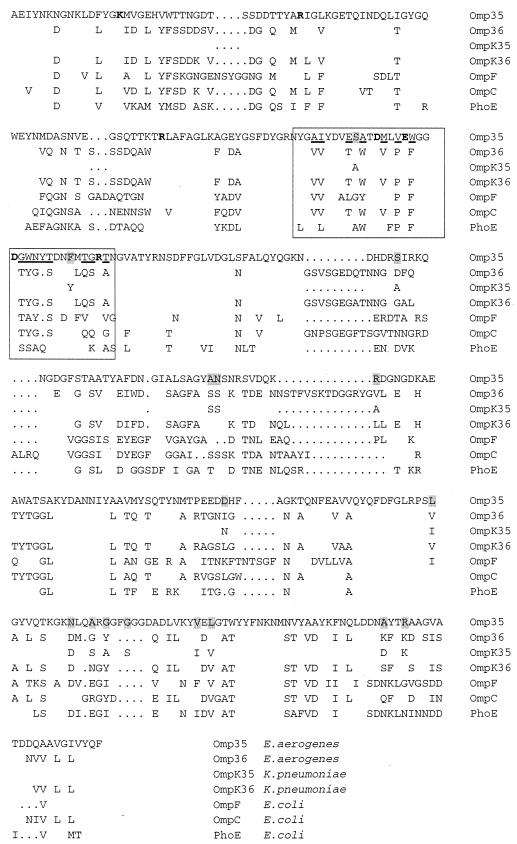
In order to characterize the porin produced in ATCC 15038, we sequenced the gene in the ATCC 15038 strain and in the ATCC 13048 type strain. The sequence was presented in Fig. 2 and compared to other porins. The sequence of the Omp35 shows a large homology with OmpK35 (95.3%). Comparison of the sequence indicates that Omp35 belongs to the enterobacterial porin family described by Jeanteur et al. (15). The alignment showed that the L3 region exhibited a close similarity with the OmpK35. In this L3 domain, the MLVEWGGDGWNYT sequence is conserved in two porins (with underlined residues) and absent in other sequences (Fig. 2). It is important to note the presence of the N and Y residues adding polar groups in the carboxyl negatively charged region of the constriction area of the channel. This is a characteristic of the Omp35 and OmpK35, suggesting a special conformation of this eyelet region. Interestingly, we also note the presence of several polar residues in this PhoE eyelet part. In addition, we sequenced Omp35 gene issued from five clinical strains (data not shown) and alignment showed complete conservation of the Omp35 sequence. This special sequence MLVEWGGDGWNYT, included in the putative internal loop, seemed to emerge as an L3 marker of this porin class.
FIG. 2.
Alignment of E. aerogenes Omp35 with OmpF, OmpC, and PhoE of E. coli and OmpK35 and OmpK36 of K. pneumoniae. Boldface characters indicate charged residues (K, R, D, and E) involved in the OmpF constriction area (17); the L3 region is boxed, and underlined residues indicate the conserved positions in Omp35 and OmpK35 in this region. The variations between Omp35 and OmpK35 are shaded. Only variant amino acids and gaps used for alignment (dots) are indicated.
Omp35 purification and functional analyses.
To express Omp35, we transformed EAEP289 strain, a porin deficient strain with pCB35 and the resulting cells were assayed for drug susceptibilities (19, 24). The synthesis of Omp35, verified by immunoanalysis (data not shown), restored a noticeable β-lactam susceptibility in bacterial producing cells (Table 2). Concerning the ceftazidime, the low susceptibility observed in the present study was probably associated with the presence in EAEP289 of TEM24, which has a peculiar activity against this cephalosporin (4; www.lahey.org/studies).
TABLE 2.
Antibiotic susceptibilities of E. aerogenes EAEP289 strain
| Drug | MICa (μg/ml) for EAEP289
|
|
|---|---|---|
| − | +pCB35 | |
| Imipenem | 4 | 0.25 |
| Cefepime | 32 | 1 |
| Cefotaxime | 256 | 4 |
| Aztreonam | 256 | 4 |
| Ceftazidime | >512 | 128 |
MICs were determined in MH broth with parental strain (−) or with cells transformed with pCB35 encoding Omp35 (+pCB35). Values are means of three independent determinations.
We tested for the presence of Omp35 during the separation and purification steps described in Materials and Methods by immunoblotting. SDS-PAGE analysis of the purified Omp35 showed a band corresponding to the trimer at about 120 kDa after migration at 20°C, whereas monomeric forms comigrating at about 38 kDa were detected after heating at 96°C. These two forms were recognized by antiporin antiserum (data not shown). The N terminus of the protein was analyzed by microsequencing and the first 20 residues were identical to the OmpK35 sequence and to the protein sequence deduced from omp35 gene.
Channel formation by Omp35 in E. aerogenes was studied by single-channel conductance measurements after reconstitution of the purified proteins into planar lipid bilayers or into giant liposomes for examination by the patch-clamp procedure. Independently of the technique used, Omp35 formed stable ion channels characterized by stepwise current increment as observed previously for Omp36 (9). The single channel conductances measured in both artificial membrane systems are presented in Table 3. In 1 M potassium chloride, the single channel conductance of Omp35, 1,430 ± 90 pS, is higher than that of Omp36 of E. aerogenes (i.e., 1,000 ± 10 pS). This tendency is also observed in the patch-clamp at a lower salt concentration (0.2 M KCl) used for the reconstitution into giant liposomes, 400 ± 30 pS for Omp35 compared to 260 ± 10 pS for Omp36.
TABLE 3.
Ion channel properties of E. aerogenes porinsa
| Porin | Conductance (pS) in:
|
Critical voltage(s) (mV) | Ion selectivity | |
|---|---|---|---|---|
| 1 M KCl | 0.2 M KClb | |||
| Omp 35 | 1,430 ± 90 | 400 ± 30 | 130 ± 9, −150 ± 6 | 4 ± 0.5 |
| Omp 36 | 1,000 ± 10 | 260 ± 10 | ±170c | 4 ± 0.8c |
The conductance properties, ion selectivity (PK/PCl) and voltage sensitivity in asolectin lipid bilayers of the two MOMPs were determined. Selectivity ratios were calculated for an electrolyte gradient of 1 M/0.1 M (cis/trans).
Data obtained by the patch-clamp procedure.
Data obtained from reference 9.
The ion selectivity of Omp35, expressed as the ratio of cation to anion permeability (PK+/PCl−) was determined with zero-current membrane potential measurements, after applying a 10-fold salt gradient (1 M KCl cis side/0.1 M KCl trans side) across the planar lipid bilayer. The PK+/PCl− ratio we calculated for Omp35 (Table 3) is identical to the one previously determined for Omp36 (9). These results indicate that both of these porins are cation selective.
Current voltage relationships for porin molecules are linear (i.e., ohmic) until a critical voltage (Vc) is reached. To measure Vc of the Omp35, membranes containing the porin were continuously subjected to a slow increase in voltage, 10 mV/s, from −150 to 150 mV. After equilibration with 10 ng of purified porin, the conductance of macroscopic I/V curves were ohmic in the range from 130 ± 9 mV to −150 ± 6 mV. Further increases in voltage led to channel closures. The Omp35 Vc values we measured, especially the ones at positive voltage ramps (Table 3) are significantly lower than the critical voltages determined in similar conditions for Omp36 (9), indicating a higher sensitivity to voltage of Omp35 channels in planar lipid bilayers.
DISCUSSION
We previously studied the channel characteristics of the Omp36 of E. aerogenes ATCC 13048 and showed that this porin belongs to the group of OmpC-like porin (9). In this work, we studied another major E. aerogenes porin, Omp35. This porin is synthesized under conditions of low osmolarity, and we purified the protein under conditions which preserve the trimeric integrity, allowing the study of channel parameters (9). Omp35 was reconstituted into artificial lipid bilayers and showed specific porin characteristics. Omp35 forms cation selective channels in planar lipid bilayers, similarly to Omp36 (9). The Omp35 channel tended to close at a lower critical voltage than Omp36 channels and behaved more like OmpF and PhoE (28, 29). It had a high channel conductance, 1,430 pS in 1 M KCl for the monomer, quite similar to the OmpF channel (26). Note that Omp35 exhibits a higher conductance, 140% in the planar bilayer and 150% in the patch-clamp, respectively, than Omp36 (9). This difference may support a better efficiency in the translocation of large cephalosporin as reflected by the drug susceptibility.
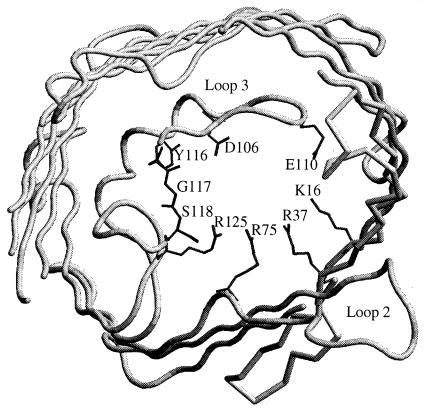
The sequence alignment showed that Omp35 of E. aerogenes and OmpK35 of K. pneumoniae are identical in the L3 domain in contrast to other porins (15). They contain a specific sequence MLVEWGGDGWNYT (underlined residues) in the L3 domain. This side of the pore constriction region exhibits negative charges (D106, E110, and D114) facing K16, R37, R75, and R125 group in the OmpK36 (Fig. 3). In OmpF, the corresponding residues play a critical role in determining the characteristics of the pore (4, 16, 17). Various mutations located in these residues which perturb the electrostatic field acting in the eyelet strongly change the physicochemical and biological channel properties (4, 26, 28, 29). The representation of the OmpK36, based on crystallographic structure (13), shows that the three residues Y116, G117, and S118, polar-apolar-polar residues, participate in the hydrogen-bond network in the eyelet due to their special location and could be involved in the porin properties. Consequently, the corresponding WNY (position 116 to 118; apolar, polar, and polar residues, respectively) in Omp35 may induce a reorganization of this domain which participates to the functional divergences between Omp36 and Omp35 pore characteristics.
FIG. 3.
Representation of the Omp36 eyelet from the crystallographic structure (1). The residues involved in the pore eyelet—K16, D106, E110, R37, R75, and R125—are indicated. Y116, G117, and S118 are also represented. The channel eyelet is viewed from external side.
E. aerogenes ATCC 15038 displays a marked difference in cephalosporin susceptibility when osmolarity conditions are modified. Interestingly, a recent study reported by Doménech-Sanchez et al. (12) described the incidence of OmpK35 and OmpK36 expression on the K. pneumoniae susceptibility towards various antibiotics. Similar observations have been previously reported in K. pneumoniae isolates and generated an interesting debate concerning the selectivity of OmpK35 (10, 25, 27). We show here that in E. aerogenes, the activities of imipenem and cefepime, two zwitterionic molecules, are only weakly affected compared to large negatively charged drugs, and similar observations were reported with OmpK35 and OmpK36 (12, 20, 25). Interestingly, an S residue is located in the PhoE eyelet and has been reported to be involved in the rate of uptake of large negatively charged cephalosporins (29). Taking into account the determination of Omp35 channel properties, the characteristics of Omp36 previously determined (9), and the analyses of β-lactam drug diffusions through the E. coli porins (31), it appears that the WNY sequence could be involved in cephalosporin uptake. These residues could modulate the diffusion of charged compound through the porin eyelet, as was previously reported for other residues located in the OmpF channel (22).
The key location of the MLVEWGGDGWNYT peptide in the constriction area of Omp35 and OmpK35 associated with its corresponding new polarity and its absence in Omp36, in OmpK36, and in E. coli porins is of particular importance in the context of bacterial drug susceptibility. The efficiency of penetration of hydrophilic antibiotics in infecting bacterial strains is strongly associated with physicochemical properties of porin expressed and the sequence identified here play important role in this process. We propose that this original Omp35 channel organization, recovered in OmpK35, is responsible for the higher susceptibility to large negatively charged cephalosporins by bacteria producing this porin subfamily.
Acknowledgments
We thank J.-M. Bolla, J. Chevalier, G. Labesse, G. Molle, and E. Pradel for fruitful discussions.
This work was supported by the Assistance Publique de Marseille (Recherche Clinique) and the Université de la Méditerranée.
REFERENCES
- 1.Alberti, S., F. Rodriquez-Quinones, T. Schirmer, G. Rummel, J. M. Tomas, J. P. Rosenbusch, and V. Benedi. 1995. A porin from Klebsiella pneumoniae: sequence homology, three-dimensional model, and complement binding. Infect. Immun. 63:903-910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arpin, C., C. M. Coze, A. Rogues, J. P. Gachie, C. Bebear, and C. Quentin. 1996. Epidemiological study of an outbreak due to multidrug-resistant Enterobacter aerogenes in a medical intensive care unit. J. Clin. Microbiol. 34:163-2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ausubel, F. M., R. E. Brent, D. D. Kingston, J. G. Moor, J. A. Seidman, and K. Struhl (ed.). 1995. Current protocols in molecular biology. John Wiley & Sons, New York, N.Y.
- 4.Bradford, P. A. 2001. Extended-spectrum β-lactamases in the 21st century: characterization, epidemiology, and detection of this important threat. Clin. Microbiol. Rev. 14:933-951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bredin, J., N. Saint, M. Malléa, E. Dé, G. Molle, J.-M. Pagès, and V. Simonet. 2002. Alteration of pore properties of Escherichia coli OmpF induced by mutation of key residues in anti-loop 3 region. Biochem. J. 363:521-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bosi, C., A. Davin-Regli, C. Bornet, M. Malléa, J.-M. Pagès, and C. Bollet. 1999. Most Enterobacter aerogenes strains in France belong to a prevalent clone. J. Clin. Microbiol. 37:2165-2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Charrel, R. N., J.-M. Pagès, P. De Micco, and M. Malléa. 1996. Prevalence of outer membrane porin alteration in β-lactam-antibiotic-resistant Enterobacter aerogenes. Antimicrob. Agents Chemother. 40:2854-2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cowan, S. W., T. Schirmer, G. Rummel, M. Steiert, R. Ghosh, R. A. Pauptit, J. A. Jansonius, and J. P. Rosenbusch. 1992. Crystal structures explain functional properties of two E. coli porins. Nature 358:727-733. [DOI] [PubMed] [Google Scholar]
- 9.Dé, E., A. Baslé, M. Jacquinod, N. Saint, M. Malléa, G. Molle, and J.-M. Pagès. 2001. A new mechanism of antibiotic resistance in Enterobacteriaceae induced by a structural modification of the major porin. Mol. Biol. 41:189-198. [DOI] [PubMed] [Google Scholar]
- 10.de Gheldre, Y., N. Maes, F. Rost, R. De Ryck, P. Clevenbergh, J. L. Vincent, and M. J. Struelens. 1997. Molecular epidemiology of an outbreak of multidrug-resistant Enterobacter aerogenes infections and in vivo emergence of imipenem resistance. J. Clin. Microbiol. 35:152-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delcour, A. H., B. Martinac, J. Adler, and C. Kung. 1989. Modified reconstitution method used in patch-clamp studies of Escherichia coli ion channels. Biophys. J. 56:631-636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doménech-Sanchez, A., L. Martinez-Martinez, S. Hernandez-Allés, M. del Carmen Conejo, A. Pascual, J. M. Tomas, S. Alberti, and V. J. Benedi. 2003. Role of Klebsiella pneumoniae OmpK35 porin in antimicrobial resistance. Antimicrob. Agents Chemother. 47:3332-3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dutzler, R., G. Rummel, S. Alberti, S. Hernandez-Alles, P. S. Phale, J. P. Rosenbusch, V. Benedi, and T. Schirmer. 1999. Crystal structure and functional characterization of OmpK36, the osmoporin of Klebsiella pneumoniae. Structure 7:425-434. [DOI] [PubMed] [Google Scholar]
- 14.Hancock, R. E. W. 1997. The bacterial outer membrane as a drug barrier. Trends Microbiol. 5:37-42. [DOI] [PubMed] [Google Scholar]
- 15.Jeanteur, D., J. H. Lakey, and F. Pattus. 1991. The bacterial porin superfamily: sequence alignment and structure prediction. Mol. Microbiol. 5:2153-2164. [DOI] [PubMed] [Google Scholar]
- 16.Jeanteur, D., T. Schirmer, D. Fourel, V. Simonet, G. Rummel, J. P. Rosenbusch, and J.-M. Pagès. 1994. Structural and functional alterations of a colicin-resistant mutant of OmpF from E. coli. Proc. Natl. Acad. Sci. USA 91:10675-10679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karshikoff, A., S. W. Cowan, V. Spassov, R. Ladenstein, and T. Schirmer. 1994. Electrostatic properties of two porin channels from E. coli. J. Mol. Biol. 240:372-384. [DOI] [PubMed] [Google Scholar]
- 18.Koebnik, R., K. P. Locher, and P. van Gelder. 2000. Structure and function of bacterial outer membrane proteins: barrels in a nutshell. Mol. Microbiol. 37:239-253. [DOI] [PubMed] [Google Scholar]
- 19.Malléa, M., J. Chevalier, C. Bornet, A. Eyraud, A. Davin-Regli, C. Bollet, and J.-M. Pagès. 1998. Porin alteration and active efflux: two in vivo drug resistance strategies used by Enterobacter aerogenes. Microbiology 144:3003-3009. [DOI] [PubMed] [Google Scholar]
- 20.Martinez-Martinez, L., S. Hernandez-Allés, S. Alberti, J. M. Tomas, V. J. Benedi, and G. A. Jacoby. 1996. In vivo selection of porin-deficient mutants of Klebsiella pneumoniae with increased resistance to cefotixin and expanded-spectrum cephalosporins. Antimicrob. Agents Chemother. 40:342-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Montal, M., and P. Mueller. 1972. Formation of biomolecular membrane from monolayers and study of their properties. Proc. Natl. Acad. Sci. USA. 69:35561-35566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nestorovich, E. M., C. Danelon, M. Winterhalter, and S. M. Bezrukov. 2002. Designed to penetrate: time-resolved interaction of single antibiotic molecules with bacterial pores. Proc. Natl. Acad. Sci. USA 99:9789-9794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nikaido, H. 1994. Prevention of drug access to bacterial targets: permeability barriers and active efflux. Science 264:382-388. [DOI] [PubMed] [Google Scholar]
- 24.Pradel, E., and J.-M. Pagès. 2002. The AcrAB-TolC efflux pump contributes to multidrug resistance in the nosocomial pathogen Enterobacter aerogenes. Antimicrob. Agents Chemother. 46:2640-2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rasheed, J. K., G. J. Anderson, H. Yigit, A. M. Queenan, A. Domenech-Sanchez, J. M. Swenson, J. W. Biddle, M. J. Ferraro, G. A. Jacoby, and F. C. Tenover. 2000. Characterization of the extended-spectrum β-lactamase reference strain, Klebsiella pneumoniae K6 (ATCC 700603), which produces the novel enzyme SHV-18. Antimicrob. Agents Chemother. 44:2381-2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saint, N., K.-L. Lou, C. Widmer, M. Luckey, T. Schirmer, and J. P. Rosenbusch. 1996. Structural and functional characterization of OmpF porin mutants selected for larger pore size. II. Functional characterization. J. Biol. Chem. 271:20676-20680. [PubMed] [Google Scholar]
- 27.Siu, L. 2001. Is OmpK35 specific for ceftazidime penetration? Antimicrob. Agents Chemother. 45:1601-1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Gelder, P., N. Saint, P. Phale, E. F. Eppens, A. Prilipov, R. van Boxtel, J. P. Rosenbusch, and J. Tommassen. 1997. Voltage sensing in the PhoE and OmpF outer membrane porins of Escherichia coli: role of charged residues. J. Mol. Biol. 269:468-472. [DOI] [PubMed] [Google Scholar]
- 29.van Gelder, P., N. Saint, R. van Boxtel, J. P. Rosenbusch, and J. Tommassen. 1997. Pore functioning of outer membrane protein PhoE Escherichia coli: mutagenesis of the constriction loop L3. Protein Eng. 10:699-706. [DOI] [PubMed] [Google Scholar]
- 30.Yigit, H., G. J. Anderson, J. W. Biddle, C. D. Steward, J. K. Rasheed, L. V. L. Valera, J. E. McGowan Juniorperiod, and F. C. Tenover. 2002. Carbapenem resistance in a clinical isolate of Enterobacter aerogenes is associated with decreased expression of OmpF and OmpC porin analogs. Antimicrob. Agents Chemother. 46:3817-3822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoshimura, F., and H. Nikaido. 1985. Diffusion of β-lactam antibiotics through the porin channels of Escherichia coli. Antimicrob. Agents Chemother. 27:84-92. [DOI] [PMC free article] [PubMed] [Google Scholar]