Abstract
In vitro transcorneal permeability studies are an important screening tool in drug development. The objective of this research is to examine the feasibility of using corneas isolated from preserved rabbit eyes as a model for permeability evaluation. Eyes from male New Zealand White rabbits were used immediately or were stored overnight in PBS or HBSS over wet ice. Integrity of isolated corneas was evaluated by measuring the TEER and by determining the permeability of paracellular and transcellular markers. Active transport was assessed by measuring transcorneal permeability of selected amino acids. Esterase activity was estimated using p-nitrophenyl assay. In all cases, corneas from freshly enucleated eyes were compared to those isolated from the day-old preserved eyes. Transcellular and paracellular passive diffusion was not affected by the storage medium and observed to be similar in the fresh and preserved eye models. However, amino acid transporters demonstrated lower functional activity in corneas excised from eyes preserved in PBS. Moreover, preserved eyes displayed almost 1.5-fold lower esterase activity in the corneal tissue. Thus, corneas isolated from day-old eyes, preserved in HBSS, closely mimics freshly excised rabbit corneas in terms of both active and passive transport characteristics but possesses slightly reduced enzymatic activity.
Keywords: in vitro models, paracellular transport, permeability, tight junction, transcellular transport, transporters
INTRODUCTION
Transcorneal absorptive pathway is the most important route of absorption for drugs applied topically to the eye1. A major challenge for pharmaceutical scientists attempting drug delivery to the anterior segment of the eye, however, is overcoming the epithelial tight junction barriers2 and also tailoring the pharmaceutical properties of the compound to meet the diffusional restrictions imposed by the unique corneal structure comprising of both hydrophilic and lipophilic layers. To exert therapeutic activity, a topically administered agent must demonstrate sufficient corneal permeability so as to generate adequate drug concentrations at the site of action in the anterior ocular chamber. Thus, in vitro transcorneal permeability studies play a critical role in the screening and selection of drug molecules and formulation components during the design phase.
Compared to in vivo experiments, in vitro experiments avoid and isolate confounding physiological mechanisms from transmembrane diffusion; are less time consuming; amenable to high throughput screening; and are thus preferred. Currently, the most favored model for in vitro corneal permeability determinations are freshly excised animal corneas3.
Rabbit eyes are physiologically very similar to the human eyes and have been extensively used to study corneal permeation of therapeutic moieties and to optimize topical formulations before preclinical and clinical testing. Unfortunately, these experiments require sacrificing of animals procured specifically for the purpose of in vitro testing and also incur high costs associated with the caring for laboratory animals4. In some cases investigators use corneas isolated from rabbits used in other protocols. This practice does reduce the number of animals required but leaves the investigators unsure about the number and time of availability of the tissues and suffers from a lack of control on parameters such as age and sex. Moreover, the experiments carried out in the original protocol may affect ocular permeability characteristics. It would be a significant step towards reducing the number of animals required for research purpose if the pressing need to specifically sacrifice rabbits for the purpose of carrying out in vitro transcorneal experiments could be eliminated.
Cell cultures, as in vitro models, have not met with much success with respect to projection of corneal permeability rates. This is primarily because of the lack of tight junction expression, variability in the expression and polarization of transporter proteins5 and difficulty in accurately mimicking the multi-layered and multi-component corneal structure in the currently available cell culture models. The cell lines also exhibit time dependent TEER values, have a high cost associated with producing and maintaining the cultures and are susceptible to microbial contaminations6. Becker et al. have compared different epithelial cell culture models available as well as corneal constructs for in-vitro drug permeation7. The authors compared Statens Serum Institute Rabbit corneal cells (SIRC), transformed human corneal epithelial cells (HCE-T) cell lines as well as commercially available SkinEthic reconstituted human corneal epithelium (HCE-S) and Clonetrics cultured human corneal epithelium (HCE-C). The results indicated that SIRC, HCE-S and HCE-T could not differentiate between the permeabilities of molecules with different physicochemical properties. Only HCE-C remained a viable option but has to be used within 24 hours of receipt from the manufacturer. Since 14[C] mannitol permeability across HCE-C was not reported a direct comparison cannot be made between the corneal tissue and the HCE-C cell line in terms of the paracellular diffusional barrier properties. Moreover, the authors did not evaluate the cell culture models with respect to molecular expression and functional activity of carrier-mediated systems, which plays a critical role in transcorneal diffusion.
Alternatively, eyes obtained from local abattoirs have been evaluated for studying transcorneal permeability8-10. Various means to transport the excised eyes from the slaughterhouse to the laboratory have been reported. Eyes have been transported directly on ice or have been placed in solutions and transported over ice8-10. In all studies, the eyes were used within a couple of hours post isolation and knowledge about the effect of prolonged storage is currently lacking. Considering that the abattoirs would sacrifice the animals only once or twice a week, an option for preserving the procured eyes overnight in specified solutions, would significantly help in the research process as well as bring down the need for sacrificing laboratory animals. Storage may, however, lead to alterations in the corneal epithelial structure and protein expression which would impact permeability of drug molecules. To date, the effect of the storage conditions and storage solutions on corneal integrity and permeability characteristics has not been fully investigated.
Literature indicates that eyes stored in phosphate buffered saline (PBS) and hanks balanced salt solution (HBSS) have been successfully used for the preparation of primary cell cultures11-13, indicating that corneal cells remain viable in these medium for the duration tested. However, for these studies, maintenance of the integrity of the corneal epithelial barriers was not important and was thus not tested. Corneas stored in unfavorable media would swell, display decreased integrity due to the loss of tight junction proteins and may also demonstrate diminished functional activity of transporters expressed on the cornea. As a result studies with these tissues may predict erroneous permeability values for the test compounds.
Incidentally, a lot of research has been focused on developing media for the storage and transportation of human corneas. Preserved corneas have been evaluated with respect to biological and histological characteristics14-17. Although corneas are shipped for transplantation in human subjects, the storage / transport solutions available are very expensive and the sheer volume required for shipping whole animal globes makes these media even less attractive for in vitro corneal permeability studies in the drug development process. Moreover, surprisingly, the storage solutions currently used have never been evaluated with respect to the preservation of the corneal epithelial barrier and transporter characteristics.
In this study, we have evaluated PBS and HBSS, two commonly used cell culture media, as storage solutions for whole rabbit eye globes. Mannitol and diazepam have been routinely used as markers for passive paracellular and transcellular pathways and were thus selected for this study. Additionally, corneal permeability of the hydrophilic nucleoside antiviral agent, acyclovir, known to diffuse across the cornea by passive diffusion mechanisms18, was also studied. Quinidine, a lipophilic compound traversing biological membranes by the transcellular route using a combination of passive and active diffusional processes19, was also included as a marker compound. Moreover, the functional activity of the three amino acid transporters previously reported on the corneal epithelium: the B0.+, ASCT1 and L-type amino acid transporters were also tested20-22. In addition to passive and active transport processes, activity of esterase enzymes was also compared since these enzymes regulate bioreversion of ester prodrugs in the corneal tissue and, thus, can impact net corneal permeability. It was assumed that since the eyes would generally be preserved over wet ice, the metabolic rate would be minimal. Thus, addition of nutrients, e.g. essential amino acids, would not be necessary. Also, since whole animal globes, rather than isolated corneas, would generally be preserved, no osmotic agent such as dextran was added to the medium.
EXPERIMENTAL SECTION
Materials
Acyclovir was obtained from Hawkins Inc. (Minneapolis, USA) and quinidine from Sigma Chemical Co. (St. Louis, USA). Radiolabelled amino acids were obtained from Moravek Biochemicals, Inc. (Califonia, USA) and [14C]mannitol and [3H]diazepam were from Perkin Elmer Life and Analytical Sciences (Boston, USA) respectively. All other solvents and chemicals were obtained from Fisher Scientific (Fair Lawn, NJ), and used as such.
Animals
Male, albino, New Zealand rabbits weighing between 2 to 2.5 kg were procured from Myrtles’ Rabbitry (Thompson Station, TN). Animal experiments conformed to the tenets of Association for Research in Vision and Ophthalmology (ARVO) statement on the Use of Animals in Ophthalmic and Vision Research.
Methods
Corneal Permeation Studies
Animals were anesthetized with ketazime/xylazine given intramuscularly and euthanized by an excess of pentobarbital injected through the marginal ear vein. Some of the globes so obtained were either stored in PBS or HBSS, over wet ice, or were taken for immediate isolation of the corneas and further experimentation. Corneas from the preserved globes were isolated 24 hours after storage initiation. Corneas were excised, following previously published protocols 23, with approximately 1 mm scleral portions remaining for ease of mounting. The corneas were mounted between standard, 9 mm, side-by-side diffusion cells (PermeGear Inc., Bethlehem, PA) with the epithelial layer facing the donor side. Temperature was maintained at 34°C during the transport studies with the help of a circulating water bath. Dulbeccos phosphate buffered saline (DPBS) was used as the transport medium. Volume of the receiver solution (3.2 mL DPBS) was slightly higher than that of the donor solution (3 mL drug solution) to maintain the natural curvature of the cornea. Contents of both chambers were stirred continuously. Aliquots, 200 μL, were withdrawn at appropriate time intervals and immediately replaced with an equal volume of DPBS and stored at -80° C until further analysis. Unlabeled samples were analyzed using an HPLC system.
To the radioactive aliquots five milliliters of scintillation cocktail (Scintisafe Econo 2, Fisher Scientific, USA) was added and the radioactivity was measured using a Liquid Scintillation Analyzer (Perkin Elmer Life and Analytical Sciences, Model TriCarb 2900TR, CT, USA).
Light Microscopy
Corneas were stained with hematoxylin-eosin using previously published procedures7. Briefly the corneas were dehydrated in increasing ethanol concentration, embedded in paraffin wax and then cut into 4μM sections. These sections were stained with hematoxylin and eosin solutions and used for light microscopy.
Trans-epithelial Electrical Resistance (TEER)
Ag/AgCl electrodes 2 mm in diameter were shaped in the form of circular rings and placed approximately 2 mm from the cornea in both the donor and the receiver compartments and the chambers were filled with DPBS solution. The electrical resistance across the corneas was measured, every hour for a period of three hours, using an experimental setup consisting of a waveform generator and digital multimeter (Agilent Technologies, Santa Clara, CA).
Paracellular and Transcellular Permeability
Tight-junction characteristics was compared using acyclovir (1mM) and [14C]mannitol (0.5 μCi/ml, specific radioactivity 55 mCi/mM) in DPBS, as paracellular diffusion markers. The transcellular permeability markers quinidine (0.5μM), and [3H]diazepam (0.5 μCi/ml, specific radioactivity 70 mCi/mM) in DPBS, were used to compare the properties of the lipoidal cell membrane. The studies compared fresh versus preserved corneas.
Transport Activity of Amino Acid Transporters
To compare functional activity of corneal amino acid transporters, transcorneal permeability of [14C]L-Arginine (specific activity 57 mCi/mM), [14C]L-Phenylalanine (specific activity 391 mCi/mM) and [14C]L-Alanine (specific activity 162 mCi/mM) was determined in both freshly excised and preserved rabbit corneas. L-Arginine and L-phenylalanine are known to be transported solely by the ATB0,+ and LAT120,21 transporters, respectively. Although multiple systems are involved in the transport of L-alanine, it permeates across the rabbit cornea primarily through the ASCT122 transporter. Procedures as described under corneal permeation studies were followed. The donor solutions contained 0.5 μCi/mL of the radioactive agents.
Choline chloride and K2HPO4 were used in equimolar quantities to replace NaCl and Na2HPO4, respectively, in the transport medium to study sodium dependency of the transport process. 2-Amino-2-norbornanecarboxylic acid (BCH) was used as a specific L-amino acid transporter inhibitor.
Enzymatic (esterase) Activity
A method described by Armstrong et al. was modified to determine the esterase activity in the ocular tissues obtained from fresh and preserved rabbit eyes24. Eyes were carefully dissected and the isolated tissues were stored at -80°C. Aqueous humor and vitreous humor were used after centrifugation at 13,000 rpm at 4°C for 5 minutes. Cornea, iris-cilliary body and retina-choroid were homogenized over an ice bath by using methods described elsewhere25. The protein content of the tissue homogenates was standardized to 1 mg/mL using the method of Bradford26. Total esterase activity was determined spectrophotometrically using p-nitrophenyl acetate (3 mM) as a substrate. In 3 mL acetone, 54.3 mg p-nitrophenylacetate was dissolved and the volume was made up to 100 mL using 10 mM phosphate buffer (pH 7.2) to obtain a final concentration of 1mM p-nitrophenylacetate. To 1 mL of this solution, 1.9 mL of buffer and 100 μL of the tissue homogenate were added in a quartz cell. The contents were mixed by briefly inverting the cell and the kinetics of hydrolysis was evaluated at 348 mμ, using a Thermo Scientific GENESYS 6™ UV-Vis spectrophotometer, for 5 mins. For these studies, p-nitrophenyl acetate in the buffer solution, in the absence of the enzyme, was used as a blank.
HPLC Analytical Method
Samples were analyzed using an HPLC system consisting of a Waters 600 pump controller, a refrigerated Waters 717 plus autosampler, a 2475 Multi λ Fluorescence detector and a Agilent 3295 integrator. A Waters C18 Symmetry column, 4.6 × 250 mm, was used. Mobile phase for acyclovir analysis consisted of 15 mM phosphate buffer (pH 2.5) containing 1% acetonitrile. Detection was carried out at excitation wavelength of 270 nm and emission wavelength of 380 nm. Quinidine was analyzed at an excitation wavelength of 250 nm and a quantification wavelength of 440 nm, using a mobile phase consisting of 20 mM phosphate buffer (pH adjusted to 2.5) and acetonitrile (88:12).
Data Analysis
Flux and permeability calculations were performed as previously reported23,27. Briefly, steady state flux values were calculated from the plot of cumulative amount of drug in the receiver phase (Ccum) with respect to time (Eq. 1). Steady flux values were normalized to donor concentration (Cd) to calculate drug permeability (Eq. 2).
| (1) |
| (2) |
All experiments were carried out at least in triplicate. Student’s t test for unpaired sample was used for statistical analysis. Data obtained for multiple groups was subjected to statistical analysis using One Way Analysis of Variance (ANOVA). Variation between the groups was checked using Levenes’ test and significant difference between group means was determined using Tukeys HSD test. Results were considered statistically significant if p-value was < 0.05.
RESULTS
Light Microscopy
Hematoxylin-eosin stained cross sections for corneas extracted from ocular globes stored in PBS/HBSS did not show any significant difference from the fresh corneas (Fig. 1).
Figure 1.
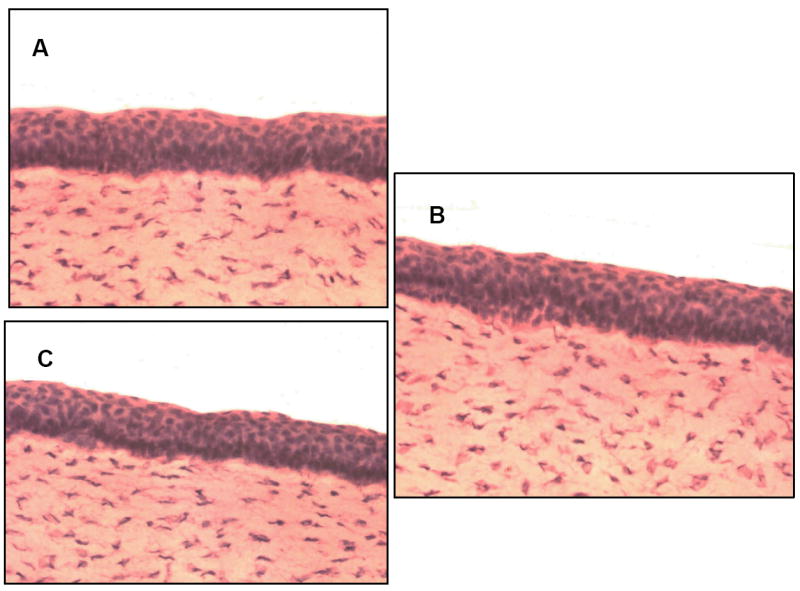
Hematoxylin-eosin stained cross section of rabbit cornea. (A) Freshly excised rabbit cornea. (B) Cornea extracted from eyes preserved in PBS over wet ice for 24h. (C) Cornea extracted from eyes preserved in HBSS over wet ice for 24 hours.
Trans-epithelial Electrical Resistance
TEER values represented in Fig. 2 for corneas from eyes preserved in PBS (4.7 ± 0.3 KΩ.cm2) and HBSS (4.8 ± 0.25 KΩ.cm2) were slightly lower but not significantly different from corneas from freshly isolated eyes (5.2 ± 0.3 KΩ.cm2). The TEER values remained constant throughout the three hour duration of the experiment.
Figure 2.
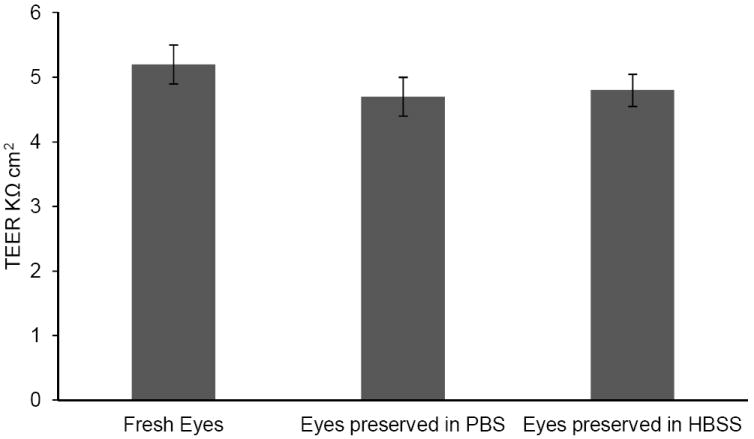
Trans-epithelial electrical resistance (TEER) values across freshly excised corneas and corneas obtained from eyes preserved in PBS/HBSS over wet ice for 24h. Results are depicted as mean ± S.D (n=4).
Paracellular and Transcellular transport
Permeability of the paracellular marker [14C]mannitol was found to be approximately three times higher across corneas preserved in PBS or HBSS compared to freshly excised corneas (3.96 ± 1.44 × 10-6 cm/sec). Ca+2 plays an important role in maintaining the integrity of tight junctions28. Since Ca+2 is present in HBSS it was thought that their presence would help maintain the integrity of the tight junctions. However, significant difference was not observed (Fig. 3) in the permeability of [14C]mannitol between eyes preserved in PBS (14.88 ± 1.69 × 10-6 cm/sec) and HBSS (13.6 ± 0.26 × 10-6 cm/sec).
Figure 3.
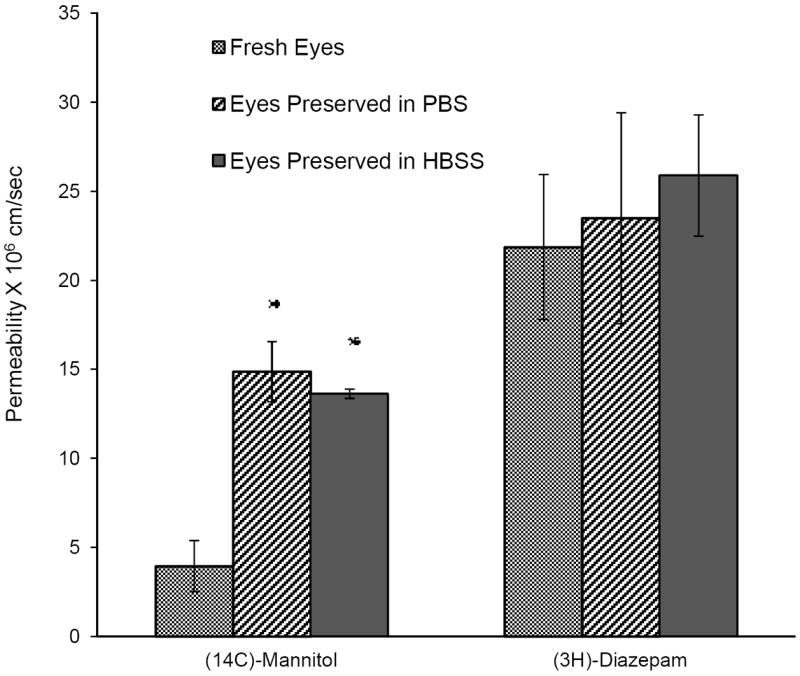
Transcorneal permeability of [14C]Mannitol (0.5 μCi/ml) and [3H]Diazepam (0.5 μCi/ml), across corneas from fresh or preserved (in PBS or HBSS over wet ice for 24h) rabbit eyes. Results are depicted as mean ± SD (n=3). * p<0.05
Corneal diffusion of acyclovir (Fig. 4), however, was not affected by the storage medium. Corneal permeability of acyclovir across corneas from fresh eyes (3.25 ± 0.11 × 10-6 cm/sec) was not significantly different from those stored in PBS (4.01 ± 0.38 × 10-6 cm/sec).
Figure 4.
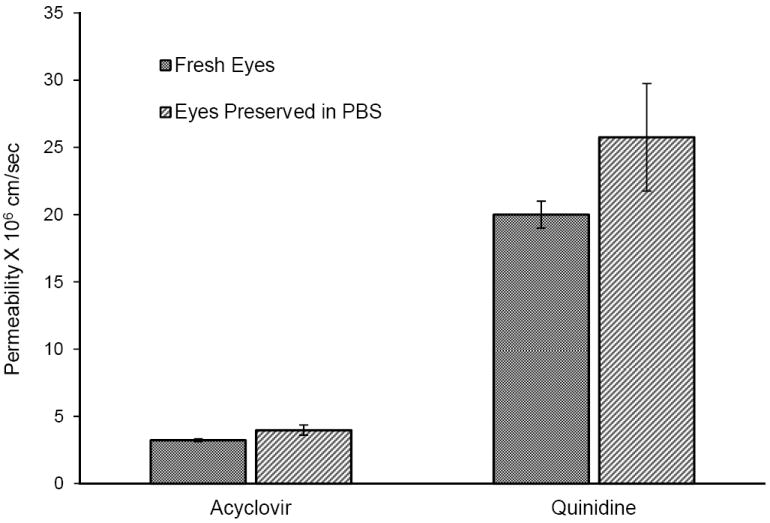
Transcorneal permeability of acyclovir and quinidine across corneas from eyes preserved in phosphate buffered saline (PBS) over wet ice for 24h compared with freshly excised rabbit corneas. The studies were conducted at 34°C. Results are depicted a mean ± SD (n=3). * p<0.05
Permeability of the transcellular marker, [3H]diazepam, in fresh corneas (2.19 ± 0.4 × 10-5 cm/sec) was not significantly different from corneas extracted from eyes preserved in PBS (2.35 ± 0.59 × 10-5 cm/sec) or HBSS (2.59 ± 0.34 × 10-5 cm/sec)(Fig. 3). Also permeability of quinidine (2.0 ± 0.1 × 10-5 cm/sec) was not affected when eyes were preserved in PBS (2.5 ± 0.4 × 10-5 cm/sec) as depicted in Figure 4.
Amino Acid Transporter Activity
Eyes preserved in PBS and HBSS exhibited functional activity of the amino acid transporters. In the case of eyes preserved in PBS, transport of [14C]L-Arginine (Fig. 5) across fresh eyes (6.46 ± 2 × 10-6 cm/sec) was not significantly different from those obtained from eyes preserved in PBS (6.59 ± 2.2 × 10-6 cm/sec) or HBSS (7.06 ± 1.68 × 10-6 cm/sec). [14C]L-Arginine transport was inhibited significantly by the specific L-amino acid transporter inhibitor, BCH, in eyes preserved in both PBS and HBSS (Fig.6A & B).
Figure 5.
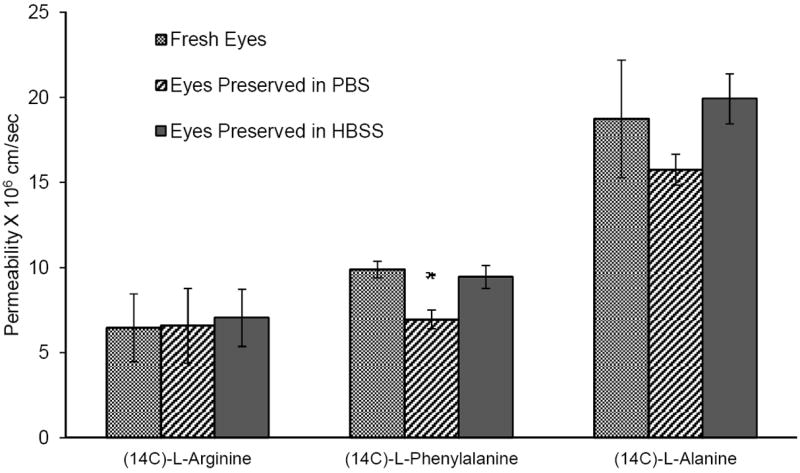
Transcorneal permeability of [14C]L-Arginine (0.5 μCi/ml), [3H]L-Phenylalanine (0.5 μCi/ml) and [14C]L-Alanine (0.5 μCi/ml) across corneas from fresh or preserved (in PBS or HBSS over wet ice for 24h) rabbit eyes. Results are depicted as mean ± SD (n=3). * p<0.05
Figure 6.
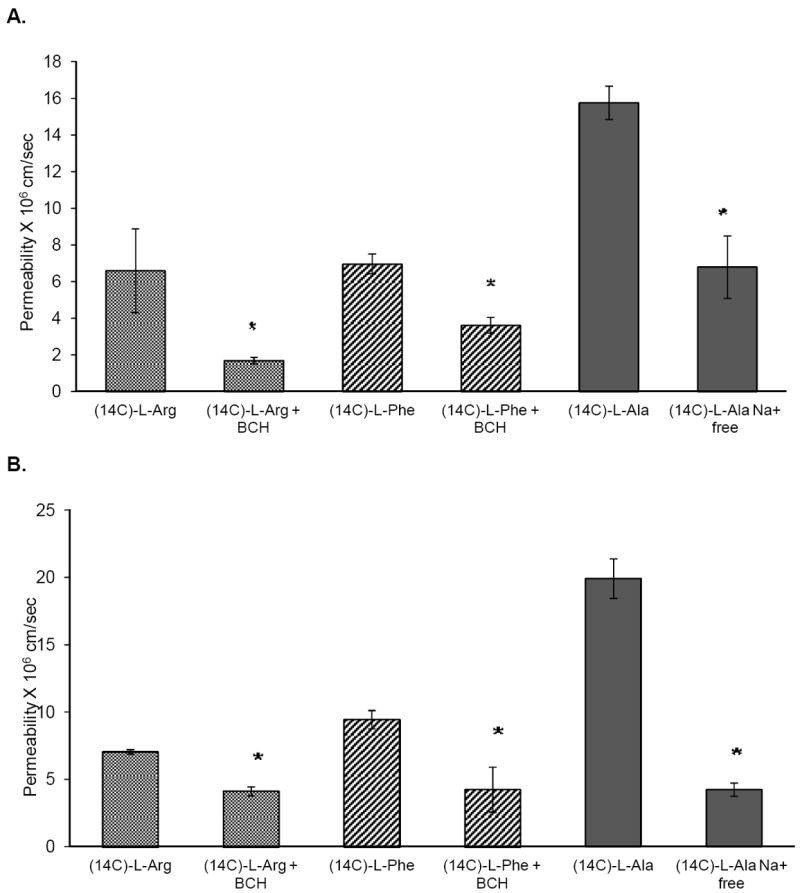
Permeability of [14C]L-Arginine (0.5 μCi/ml), [14C]L-Arginine (0.5 μCi/ml) in the presence of BCH (5mM), [14C]L-Phenylalanine(0.5 μCi/ml), [14C]L-Phenylalanine(0.5 μCi/ml) in the presence of BCH (5mM), [14C]L-Alanine (0.5 μCi/ml) and [14C]L-Alanine (0.5 μCi/ml) in Na+ free medium, across corneas from eyes stored for 24h in A) phosphate buffered saline (PBS) and B) hanks balanced salt solution (HBSS) over wet ice, at 34°C. Results are depicted as mean ± SD (n=3). * p<0.05
Corneal permeability of [14C]phenylalanine (9.89 ± 0.49 × 10-6 cm/sec, freshly excised corneas) was found to be significantly lower when the eyes were preserved in PBS (6.9 ± 0.54 × 10-6 cm/sec). However, permeability values across the corneas from eyes that were preserved in HBSS (9.45 ± 0.67 × 10-6 cm/sec) (Fig. 5) were not significantly different from the fresh corneas. In all cases transport of [14C]phenylalanine was significantly inhibited in the presence of the inhibitor, BCH (Fig. 6A & B).
Active transport of [14C]L-Alanine across corneas that were freshly excised (1.87 ± 0.35 × 10-5 cm/sec) was found to be similar to those from eyes preserved in PBS (1.57 ± 0.90 × 10-5 cm/sec) or HBSS (1.99 ± 0.15 × 10-5 cm/sec)(Fig. 5). When the studies were carried out in sodium free medium permeability was significantly reduced (Fig. 6A & B) in all cases.
The observed corneal permeability values (from fresh eyes) of the amino acids are about 2 to 2.5 fold lower than literature reports 20-22. This difference may be attributed to use of a different radiolabel (14C instead of 3H) being used in this study.
Enzymatic Activity
Non-specific esterase activity was assessed using p-nitrophenyl acetate assay protocols. Esterase activity in preserved corneas was observed to be about 1.5-fold lower compared to the freshly excised corneas. There was, however, no significant difference in the esterase activity of corneas obtained from eyes preserved in PBS or HBSS (Table 1). Iris-ciliary tissues from eyes stored in PBS exhibited an almost 2-fold decrease in the esterase activity. However, esterase activity was preserved at normal levels in the iris-ciliary tissues when the eyes were stored in HBSS. The other ocular tissues tested did not demonstrate any significant difference between fresh or preserved eyes.
Table 1.
Esterase activity in fresh eyes compared to eyes preserved in PBS/HBSS over wet ice for 24h using p-nitrophenyl acetate as substrate. Results are depicted as a mean ± SD (n=3).
| Rate of hydrolysis of p-nitrophenol acetate [μM/(min.mg protein)] | |||
|---|---|---|---|
| Fresh Eyes | Eyes Preserved in PBS | Eyes Preserved in HBSS | |
| Cornea | 2.78 ± 0.19 | *1.79 ± 0.11 | *1.79 ± 0.11 |
| Aqueous Humor | 2.59 ± 0.19 | 3.09 ± 0.39 | 2.78 ± 0.32 |
| Iris-Ciliary Body | 14.01 ± 0.11 | *8.46 ± 0.11 | 13.77 ± 0.11 |
| Vitreous Humor | 4.26 ± 0.19 | 5.06 ± 0.11 | 4.63 ± 0.19 |
| Retina-Choroid | 3.33 ± 0.19 | 4.51 ± 0.11 | 4.51 ± 0.11 |
p<0.05
DISCUSSION
Drug molecules can permeate across the cornea by passive or active transport mechanisms. Passive diffusion across the cornea involves the paracellular or transcellular pathway. In transcellular diffusion the drug molecules partition into the lipoidal cell membrane, but permeability depends on the molecular size, charge and lipophilicity29. Paracellular diffusion, wherein the drug molecules move through the intercellular spaces, is limited by the tight junctions present on the superficial epithelial cells 30. Several carrier systems, including the amino acid transporters ASCT1, LAT1 and Bo,+, have been reported on the corneal epithelium of rabbits 20,22,31. These transporters are responsible for nutrient transport, but are being frequently targeted for drug delivery across biological barriers 31,32. Thus, when transcorneal permeability of a therapeutic candidate is being evaluated in vitro, it is vital that these passive and active transport mechanisms remain unaltered in the model.
The objective of this study was to examine the utility of corneas isolated from preserved rabbit eyes for transcorneal permeability studies and to identify a media which would be economical, simple to prepare and would maintain the barrier and transport mechanisms of the cornea intact. A lot of work has been carried out in the field of preserving human corneas for transplantation. The cornea is either stored in organ culture media at physiological temperatures (31-37°C) or stored in serum free media at hypothermic temperatures (4°C)33. Organ culture media are expensive and calls for complex preparation steps. Moreover, the corneas have to be de-swelled in dextran before use. This would be a laborious process for preserving corneas for in vitro transport experiments. Hypothermic storage media are commercially available but are extremely expensive.
Spencer et. al. compared storage of isolated corneas in balanced salt solution(BSS) to McCarey Kaufman (MK) media for a period of four days in the presence or absence of the steroid hydrocortisone34. They reported that there was no significant difference if the eyes were stored in BSS or M-K media at 4°C. Also there was no significant difference on storage even in the presence of hydrocortisone. That study, however, was limited to the examination of autolysis using the marker enzyme, acid phosphatase.
In view of the earlier reports, HBSS was chosen as one of the storage media. Since the eyes were to be stored in this study for a relatively short duration (not more than 24 hours), PBS, the most common storage medium was also selected. Both HBSS and PBS buffers are easy to prepare, very economical and use chemicals readily available in most laboratories.
Currently, two methods are used to transport corneal tissues. Either the whole eyeball is transported in a moist chamber, or, the cornea is isolated in situ and transported in a solution. In situ isolation has been reported to be better at preserving the cornea compared to transporting the whole eye globes35. The primary focus in preserving human corneas is to maintain endothelial cell viability and to prevent autolytic changes after death which may occur due to contact with stagnant aqueous humor. Thus, the cornea needs to be separated from the rest of the globe. However, for the purpose of drug permeation experiments the endothelium is a very weak barrier. Rather, an intact epithelium is essential for in vitro experiments.
TEER is an electrophysiological technique to measure the integrity of the paracellular pathway 36. Although the TEER values of corneas excised from preserved eyes were observed to be higher compared to fresh corneas, the difference was not statistically significant, indicating no change in the epithelial barrier properties. However, TEER values alone cannot be used as a measure for assessing structural integrity. Permeability of [14C]mannitol, a paracellular marker, was found to be three times higher in corneas from preserved eyes compared to freshly excised corneas. However, there was no significant difference in permeability of acyclovir, another hydrophilic molecule also known to permeate through the paracellular route37. The slightly lower TEER values and higher mannitol permeability of corneas from preserved eyes, compared to fresh corneas, indicates that the tight junctions are disrupted to an extent that small linear molecules like mannitol can pass through in greater quantities. A more branched molecular structure like acyclovir, however, shows no difference in corneal permeability in all models.
A significant alteration of the tight junction integrity would also have an impact on the permeability of lipophilic molecules which diffuse transcellularly. These lipophilic agents can diffuse through the paracellular route to a greater extent than in the presence of intact tight junctions. Lack of any changes in the transcorneal permeation values of the lipophilic, transcellular markers, diazepam and quinidine, indicates that the integrity of the junction proteins is not significantly disrupted, and permeability of only very small molecules, such as mannitol, is affected. Moreover, the observed transcellular permeability values for these lipophilic agents were statistically similar in the corneal tissues obtained from both fresh and preserved rabbit eyes.
Transporter targeted prodrugs have been designed to enhance transcorneal permeation of the parent moiety using a piggy-banking approach, where the prodrug is translocated by the carrier system. In-vitro transport experiments are thus often carried out to determine specific transporter involvement in the translocation process of a new therapeutic agent or to optimize a transporter targeted prodrug design approach. Preservation of the active transport mechanisms is thus essential for accurate permeation predictions or for the purpose of ranking various candidates. In this study we evaluated the permeability characteristics of three amino acid transporters reported on the rabbit corneal epithelium. Results indicate that the amino acid transporters, LAT1, ASCT1 and B0,+, remain functionally active in the corneas extracted from the eyes preserved in PBS or HBSS as the presence of BCH or lack of sodium significantly decrease the transport of their substrates. However, for corneas extracted from whole globes preserved in PBS, the permeability of L-Phenylalanine was significantly lower compared to that across corneas from eyes that were freshly excised or were preserved in HBSS. Thus, corneas from rabbit eyes stored in HBSS exhibited functional activity of all three amino acid transporters at levels similar to that in freshly excised corneas. Both PBS and HBSS are isotonic but HBSS has added glucose and metal ions. Thus, even at low temperatures where metabolic activity is minimal some form of nutrition needs to be added to the medium for amino acid transporters to remain fully functional.
Esterase activity of corneas from eyes preserved in PBS and HBSS was observed to be lower compared to freshly excised cornea. The esterase activity in the other tissues, from eyes stored in HBSS, was not significantly different. The lower esterase activity in the cornea, compared to other tissues, can probably be explained by the fact that the cornea is exposed to the storage conditions (solution and period) to a greater extent in comparison to the other tissues which remain relatively protected inside the ocular globe, deriving nutrition from the vitreous humor. The reduced enzyme activity may impact bioreversion and thus permeability rates. However, considering the extremely rapid enzymatic degradation rates observed with most ester prodrugs and the low drug concentrations used in general, the impact of this 1.5-fold reduction in enzymatic hydrolysis rate may not be very significant with respect to relative permeability evaluation.
In conclusion, the results from this study indicates that storage of the extracted whole rabbit eye in HBSS, over wet ice, for a period of up to 24 hours, preserves the active and passive transport mechanisms in the cornea. Eyes shipped from abattoirs in this manner, within this timeframe, would serve as an excellent in vitro model for transcorneal permeation studies and would significantly decrease the need for laboratory animal sacrifice. Further studies evaluating means to extend the period of storage are currently underway.
Acknowledgments
This project was supported by NIH Grant Number EY018426-02 from the National Eye Institute and P20RR021929 from the National Center For Research Resources.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Eye Institute or the National Center for Research Resources.
References
- 1.Hamalainen KM, Kananen K, Auriola S, Kontturi K, Urtti A. Characterization of paracellular and aqueous penetration routes in cornea, conjunctiva, and sclera. Invest Ophthalmol Vis Sci. 1997;38(3):627–634. [PubMed] [Google Scholar]
- 2.Yi X-j, Wang Y, Yu F-SX. Corneal Epithelial Tight Junctions and Their Response to Lipopolysaccharide Challenge. Invest Ophthalmol Vis Sci. 2000;41(13):4093–4100. [PubMed] [Google Scholar]
- 3.Reichl S, Dohring S, Bednarz J, Muller-Goymann CC. Human cornea construct HCC-an alternative for in vitro permeation studies? A comparison with human donor corneas. Eur J Pharm Biopharm. 2005;60(2):305–308. doi: 10.1016/j.ejpb.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 4.Reichl S, Muller-Goymann CC. The use of a porcine organotypic cornea construct for permeation studies from formulations containing befunolol hydrochloride. Int J Pharm. 2003;250(1):191–201. doi: 10.1016/s0378-5173(02)00541-0. [DOI] [PubMed] [Google Scholar]
- 5.Reichl S, Becker U. Cell Culture Models of the Corneal Epithelium and Reconstructed Cornea Equivalents for In Vitro Drug Absorption Studies. Drug Absorption Studies. 2008:291–294. [Google Scholar]
- 6.Pels L. Organ culture: the method of choice for preservation of human donor corneas. Br J Ophthalmol. 1997;81(7):523–525. doi: 10.1136/bjo.81.7.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Becker U, Ehrhardt C, Schneider M, Muys L, Gross D, Eschmann K, Schaefer UF, Lehr CM. A comparative evaluation of corneal epithelial cell cultures for assessing ocular permeability. Altern Lab Anim. 2008;36(1):33–44. doi: 10.1177/026119290803600106. [DOI] [PubMed] [Google Scholar]
- 8.Aggarwal D, Garg A, Kaur IP. Development of a topical niosomal preparation of acetazolamide: preparation and evaluation. J Pharm Pharmacol. 2004;56(12):1509–1517. doi: 10.1211/0022357044896. [DOI] [PubMed] [Google Scholar]
- 9.Babiole M, Wilhelm F, Schoch C. In vitro corneal permeation of unoprostone isopropyl (UI) and its metabolism in the isolated pig eye. J Ocul Pharmacol Ther. 2001;17(2):159–172. doi: 10.1089/10807680151125492. [DOI] [PubMed] [Google Scholar]
- 10.Scholz M, Lin JE, Lee VH, Keipert S. Pilocarpine permeability across ocular tissues and cell cultures: influence of formulation parameters. J Ocul Pharmacol Ther. 2002;18(5):455–468. doi: 10.1089/10807680260362731. [DOI] [PubMed] [Google Scholar]
- 11.Lozano JS, Chay EY, Healey J, Sullenberger R, Klarlund JK. Activation of the epidermal growth factor receptor by hydrogels in artificial tears. Exp Eye Res. 2008;86(3):500–505. doi: 10.1016/j.exer.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsumoto S, Stern ME. Effect of anti-infective ophthalmic solutions on corneal cells in vitro. Adv Ther. 2000;17(3):148–151. doi: 10.1007/BF02853156. [DOI] [PubMed] [Google Scholar]
- 13.Panjwani N, Michalopoulos G, Song J, Zaidi TS, Yogeeswaran G, Baum J. Neutral glycolipids of migrating and nonmigrating rabbit corneal epithelium in organ and cell culture. Invest Ophthalmol Vis Sci. 1990;31(4):689–695. [PubMed] [Google Scholar]
- 14.Greenbaum A, Hasany SM, Rootman D. Optisol vs Dexsol as storage media for preservation of human corneal epithelium. Eye. 2004;18(5):519–524. doi: 10.1038/sj.eye.6700693. [DOI] [PubMed] [Google Scholar]
- 15.Hasany SM, Basu PK. Changes of MK medium during storage of human cornea. Br J Ophthalmol. 1987;71(6):477–483. doi: 10.1136/bjo.71.6.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lindstrom RL, Kaufman HE, Skelnik DL, Laing RA, Lass JH, Musch DC, Trousdale MD, Reinhart WJ, Burris TE, Sugar A, et al. Optisol corneal storage medium. Am J Ophthalmol. 1992;114(3):345–356. doi: 10.1016/s0002-9394(14)71803-3. [DOI] [PubMed] [Google Scholar]
- 17.Stoiber J, Ruckhofer J, Lametschwandtner A, Muss W, Hitzl W, Weikinger K, Grabner G. Eurosol versus fetal bovine serum-containing corneal storage medium. Cornea. 2001;20(2):205–209. doi: 10.1097/00003226-200103000-00020. [DOI] [PubMed] [Google Scholar]
- 18.Anand BS, Mitra AK. Mechanism of corneal permeation of L-valyl ester of acyclovir: targeting the oligopeptide transporter on the rabbit cornea. Pharm Res. 2002;19(8):1194–1202. doi: 10.1023/a:1019806411610. [DOI] [PubMed] [Google Scholar]
- 19.Shirasaki Y. Molecular design for enhancement of ocular penetration. J Pharm Sci. 2008;97(7):2462–2496. doi: 10.1002/jps.21200. [DOI] [PubMed] [Google Scholar]
- 20.Jain-Vakkalagadda B, Dey S, Pal D, Mitra AK. Identification and functional characterization of a Na+-independent large neutral amino acid transporter, LAT1, in human and rabbit cornea. Invest Ophthalmol Vis Sci. 2003;44(7):2919–2927. doi: 10.1167/iovs.02-0907. [DOI] [PubMed] [Google Scholar]
- 21.Jain-Vakkalagadda B, Pal D, Gunda S, Nashed Y, Ganapathy V, Mitra AK. Identification of a Na+-dependent cationic and neutral amino acid transporter, B(0,+), in human and rabbit cornea. Mol Pharm. 2004;1(5):338–346. doi: 10.1021/mp0499499. [DOI] [PubMed] [Google Scholar]
- 22.Katragadda S, Talluri RS, Pal D, Mitra AK. Identification and characterization of a Na+-dependent neutral amino acid transporter, ASCT1, in rabbit corneal epithelial cell culture and rabbit cornea. Curr Eye Res. 2005;30(11):989–1002. doi: 10.1080/02713680500306439. [DOI] [PubMed] [Google Scholar]
- 23.Majumdar S, Hingorani T, Srirangam R, Gadepalli RS, Rimoldi JM, Repka MA. Transcorneal permeation of L- and D-aspartate ester prodrugs of acyclovir: delineation of passive diffusion versus transporter involvement. Pharm Res. 2009;26(5):1261–1269. doi: 10.1007/s11095-008-9730-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Armstrong JM, Myers DV, Verpoorte JA, Edsall JT. Purification and Properties of Human Erythrocyte Carbonic Anhydrases. J Biol Chem. 1966;241(21):5137–5149. [PubMed] [Google Scholar]
- 25.Dias CS, Anand BS, Mitra AK. Effect of mono- and di-acylation on the ocular disposition of ganciclovir: physicochemical properties, ocular bioreversion, and antiviral activity of short chain ester prodrugs. J Pharm Sci. 2002;91(3):660–668. doi: 10.1002/jps.10072. [DOI] [PubMed] [Google Scholar]
- 26.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 27.Majumdar S, Srirangam R. Solubility, stability, physicochemical characteristics and in vitro ocular tissue permeability of hesperidin: a natural bioflavonoid. Pharm Res. 2009;26(5):1217–1225. doi: 10.1007/s11095-008-9729-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brown RC, Davis TP. Calcium Modulation of Adherens and Tight Junction Function: A Potential Mechanism for Blood-Brain Barrier Disruption After Stroke. Stroke. 2002;33(6):1706–1711. doi: 10.1161/01.str.0000016405.06729.83. [DOI] [PubMed] [Google Scholar]
- 29.Touitou E, Barry BW. Enhancement in Drug Delivery. CRC Press; 2006. pp. 530–533. [Google Scholar]
- 30.Cereijido M, Anderson J. Tight junctions. 2. CRC Press; 2001. p. 772. [Google Scholar]
- 31.Hatanaka T, Haramura M, Fei YJ, Miyauchi S, Bridges CC, Ganapathy PS, Smith SB, Ganapathy V, Ganapathy ME. Transport of amino acid-based prodrugs by the Na+- and Cl(-) -coupled amino acid transporter ATB0,+ and expression of the transporter in tissues amenable for drug delivery. J Pharmacol Exp Ther. 2004;308(3):1138–1147. doi: 10.1124/jpet.103.057109. [DOI] [PubMed] [Google Scholar]
- 32.Gynther M, Laine K, Ropponen J, Leppanen J, Mannila A, Nevalainen T, Savolainen J, Jarvinen T, Rautio J. Large neutral amino acid transporter enables brain drug delivery via prodrugs. J Med Chem. 2008;51(4):932–936. doi: 10.1021/jm701175d. [DOI] [PubMed] [Google Scholar]
- 33.Jeng BH. Preserving the cornea: corneal storage media. Curr Opin Ophthalmol. 2006;17(4):332–337. doi: 10.1097/01.icu.0000233950.63853.88. [DOI] [PubMed] [Google Scholar]
- 34.Spencer JA, Dixon WS, Ranadive NS, Basu PK. Factors in the survival of stored corneas. Can J Ophthalmol. 1977;12(2):123–127. [PubMed] [Google Scholar]
- 35.Rootman DB, Wankiewicz E, Sharpen L, Baxter SA. In situ versus whole-globe harvesting of corneal tissue from remote donor sites: effects on initial tissue quality. Cornea. 2007;26(3):270–273. doi: 10.1097/ICO.0b013e31802c9e05. [DOI] [PubMed] [Google Scholar]
- 36.Nakamura T, Yamada M, Teshima M, Nakashima M, To H, Ichikawa N, Sasaki H. Electrophysiological characterization of tight junctional pathway of rabbit cornea treated with ophthalmic ingredients. Biol Pharm Bull. 2007;30(12):2360–2364. doi: 10.1248/bpb.30.2360. [DOI] [PubMed] [Google Scholar]
- 37.Majumdar S, Hippalgaonkar K, Repka MA. Effect of chitosan, benzalkonium chloride and ethylenediaminetetraacetic acid on permeation of acyclovir across isolated rabbit cornea. Int J Pharm. 2008;348(1-2):175–178. doi: 10.1016/j.ijpharm.2007.08.017. [DOI] [PubMed] [Google Scholar]


