Abstract
Objective
The aim of our study was to investigate the association between the TNF-α-308G/A polymorphism and obstructive sleep apnea syndrome (OSAS).
Method
The Medline, Web of Science, EMBASE, Chinese National Knowledge Infrastructure (CNKI), and Cochrane Central Register of Controlled Trials were searched. Pooled odds ratios (ORs) and 95% confidence intervals (CIs) were calculated to study TNF-α-308G/A polymorphism and risk of OSAS.
Result
10 case-control studies were included in our meta-analysis. The results from our study showed that the TNF-α-308G/A polymorphism was significantly associated with risk of OSAS (A vs. G: OR = 1.67, 95% CI = 1.43–1.95). In the subgroup analysis by ethnicity, the statistical similar results were observed both in European (A vs. G: OR = 1.68, 95% CI = 1.35–2.08) and Asian population (A vs. G: OR = 2.02, 95% CI = 1.50–2.71). When stratified by age, a significantly increased risk was observed in adult carries A allele compared with G allele (OR = 1.79, 95% CI = 1.50–2.13), whereas no association was found in children (OR = 1.09, 95% CI = 0.70–1.69).
Conclusion
Our study suggested that the TNF-α- 308G/A polymorphism contributed to the susceptibility to the risk of OSAS. Additional well-designed large studies are needed to validate our findings.
Introduction
Obstructive sleep apnea syndrome (OSAS) is a common sleep disorder characterized by repetitive partial or complete obstruction of the upper respiratory tract during sleep, resulting in apnea or hypopnea [1]. Due to obesity and aging population, the OSAS has undergone an increasing prevalence all over the world. It was reported that more than 5% of the general population has been affected [2]. According to the National Sleep Foundation (NSF) Sleep in America, there were 1/4 Americans at high risk of suffering sleep apnea on the basis of the Berlin Questionnaire [3]. OSAS has been reported to be associated with various health related consequences, including cardiovascular disease, hypertension, stroke, insulin resistance and all-cause mortality [4]. OSAS represents a vital public health concern and should be given much more attention because of the high prevalence and its enormous negative consequences. In consequence, improving our understanding of the pathogenesis of OSAS is essential for the development of effective and safe therapies.
Tumor necrosis factor (TNF)-α, a member of the TNF/TNFR cytokine family, is an intercellular communicating molecule involved in a wide variety of human diseases. Krueger et al. [5] has pointed out that TNF-α is one of the most important pleiotropic proinflammatory cytokines involved in sleep regulation. Raised levels of circulating TNF-α in patients with OSAS have been reported in previous studies [6]–[8]. The synthesis of TNF-α has been suggested to be mostly regulated at the transcriptional level [9]. The DNA variations in the promoter region of the TNF-α gene may directly influence the transcription of the TNF gene. The TNF-α gene is located within the highly polymorphic major histocompatibility complex (MHC) region on the short arm of chromosome 6p21.3 [10]. Several polymorphisms in the promoter region of the TNF-α gene have been identified. Among which, polymorphism at position −308 in the promoter region, consisting of a guanine (G) to adenine (A) substitution, has been reported to be associated with increased production of TNF-α levels both in vitro and in vivo [11], [12]. There is evidence that A allele is associated with increased level of TNF-α in plasma compared with G allele [13]. As the TNF-α-308G/A polymorphism is strongly associated with circulating TNF-α concentrations, it is assumed that it might be closely related to the OSAS risk. To investigate a possible association between TNF-α polymorphism and risk of OSAS, we conducted a meta-analysis from all available relevant studies.
Materials and Methods
Literature search strategy and eligibility criteria
The Medline, Web of Science, EMBASE, Chinese National Knowledge Infrastructure (CNKI) and Cochrane Central Register of Controlled Trials were searched. A broad search strategy was used for optimum sensitivity. Using Medical Subject Headings (MeSH) and text words, we adopted the following terms to search the databases: (“obstructive sleep apnea–hypopnea syndrome” OR “obstructive sleep apnea” OR “sleep apnea” OR “apnea” OR “OSAS” OR “OSA”) AND (“single nucleotide polymorphisms” OR “SNP” OR “polymorphism” OR “gene variant” OR “mutation”) AND (“tumor necrosis factor α” OR “tumor necrosis factor-α” OR “tumor necrosis factor” OR “TNF-α” OR “TNF”). The search was restricted to humans. Titles and abstracts were screened up to 31 March 2014 were retrieved. Articles were screened at the title and abstract phase by two authors (Yanping Wu and Chao Cao).
Inclusion and exclusion criteria
Inclusion criteria for studies included: (a) evaluation of the relationship between TNF-α- 308G/A polymorphism and OSAS susceptibility; (b) case–control study; (c) validated genotyping methods were used; (d) detailed genotype frequencies in cases and controls for the calculation. Major reasons for exclusion of studies were: (a) no control group, case reports or review articles; (b) duplicate data; (c) enrolled patients were using TNF inhibitors.
Data extraction and quality assessment
Information was carefully extracted from all eligible studies independently by two of the authors, according to the inclusion criteria listed above. From each study, data on general study characteristics was extracted: first author's surname, year of publication, ethnic descent of the study population (European, Asian or mixed), genotyping methods, numbers of eligible cases and controls, genotype distributions in cases and controls and available subgroups. Disagreements were resolved by discussion and consensus.
The Newcastle-Ottawa Scale (NOS), which was developed to assess the quality of non-randomised studies, was performed to assess the quality of included studies [14]. The system included three broad perspectives: the selection of the study groups; the comparability of the groups; and the ascertainment of the outcome. And the scores of 0–3, 4–6, and 7–9 indicated low, moderate, and high quality of studies, respectively.
Statistical analysis
All analyses were performed on Review Manager version 5.0.16 and Stata version 12.0. The Hardy-Weinberg equilibrium (HWE) was utilized to compare the observed genotype frequencies with expected genotype frequencies in controls. The association between risk of OSAS and TNF-α-308G/A polymorphism was estimated for each study using the odds ratio (OR) and 95% confidence interval (CI). Between-study heterogeneity was assessed with the χ2-based Q statistical test [15]. We also used the statistic of I2 to efficiently test for the heterogeneity, with I2<25%, 25–75% and >75% to represent low, moderate and high degree of inconsistency, respectively [16], [17]. When P>0.1 or I2<50%, indicating a lack of heterogeneity, and for these analyses, the fixed-effect model was used; otherwise, the random-effect model was applied [16]–[19]. The significance of the combined OR was determined by the Z-test, in which P<0.05 was considered significant. Effects were tested in models as follows: allele comparison (A vs. G), homozygote comparison (GG vs. AA), heterozygote comparison (AG vs. AA), dominant model (AG+GG vs. AA), and recessive model (GG vs. AA+AG). Stratified analysis was performed by ethnicity and age. Sensitivity analysis was performed to evaluate the effect of each individual study on the pooled ORs. Funnel plots and Egger's linear regression test were used to evaluate the potential publication bias [20].
Results
Characteristics of studies
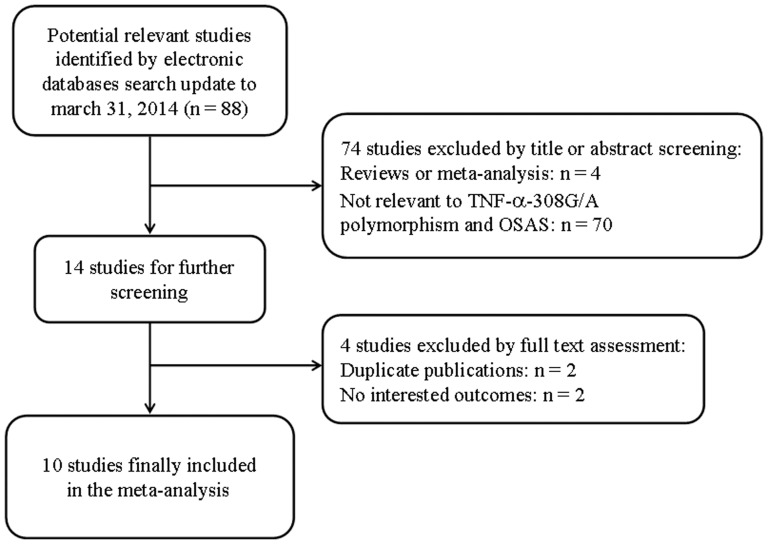
The search strategy retrieved 88 potentially relevant studies. After initial search by the titles and abstracts, 74 studies were excluded. Among the excluded studies, 4 were reviews or meta-analysis and 70 were not relevant to TNF-α-308G/A polymorphism and OSAS. By further full text screening, 2 studies were duplicate publications and 2 had no interested outcomes. Finally, 10 full-text articles, with a total of 1522 OSAS cases and 1234 controls, which definitely addressed the association between TNF-α-308G/A polymorphism and OSAS risk were enrolled in our study [21]-[30] (Figure 1). According to the NOS quality assessment, all included studies achieved a score of moderate to high quality (table S1). The genotype distribution in each study was extracted and detailed information in one study was referred to published data. Of all eligible studies for TNF-α-308G/A polymorphism, four were performed in European population [23], [24], [27], [30], five in Asian descent [21], [22], [26], [28], [29] and one in mixed populations (European and Asian descents) [25]. In terms of age, nine studies were conducted in adults [21]–[24], [26]–[30] and one in children [25]. Polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) [22]–[24], [26]–[28] and TaqMan assay [21], [25], [29], [30] were used to analyze the genotyping. The genotype distributions of all control samples except one study were in HWE [26]. The general characteristics and genotype information of each study were summarized in Table 1 and Table 2.
Figure 1. Flow chart of literature search and study selection.
Table 1. Study characteristics from published studies on the relation of the TNF-α-308G/A polymorphism to OSAS risk in this meta-analysis.
| Author | Year | Country | Ethnicity | Cases | Controls | Male (%) | Age, Mean(SD) [Range], y | BMI(kg/m2) | Source of control | Controls matched for | Methods | |||
| Cases | Controls | Cases | Controls | Cases | Controls | |||||||||
| Popko et al 27 | 2008 | Poland | European | 102 | 77 | 72.6 | 50.7 | [21.0–77.0] | [17.0–65.0] | NA | NA | Population based | BMI | PCR-RFLP |
| Khalyfa et al 25 | 2011 | United States | mixed | 138 | 151 | 50 | 50 | (7.2±0.2) | (7.2±0.3) | NA | NA | Population based | age, sex, ethnicity and BMI | TaqMan |
| Riha et al 30 | 2005 | United Kingdom | European | 103 | 190 | 80.6 | NA | (52.0±9.0) | NA | 30.0±6.0 | NA | Population based | not describled | TaqMan |
| Liu et al 28 | 2006 | China | Asian | 76 | 42 | 88.2 | 88.1 | (44.3±9.8) | (41.7±10.1) | 26.3±3.5 | 25.7±3.3 | Hospital based | BMI | PCR-RFLP |
| Karkucak et al 23 | 2012 | Turkish | European | 69 | 42 | 75.4 | 69.1 | (49.8±11.3) | (50.6±10.7) | 31.1±4.1 | 28.5±4.3 | Hospital based | age, gender and BMI | PCR-RFLP |
| Almpanidou et al 24 | 2012 | Greece | European | 220 | 319 | 90 | 87.1 | (51.0±12.4) | (50.6±13.8) | 31.4±5.2 | 29.6±4.3 | Population based | age, race and gender | PCR-RFLP |
| Li et al 21 | 2013 | China | Asian | 155 | 100 | 100 | 100 | (45.0±9.0) | (46.3±8.0) | 29.4±2.1 | 24.1±2.3 | Population based | not describled | TaqMan |
| Bhushan et al 26 | 2009 | Indians | Asian | 104 | 103 | 80.8 | 63.1 | (46.2±10.7) | (44.0±10.0) | 31.5±4.3 | 30.9±4.3 | Hospital based | age and BMI | PCR-RFLP |
| Guan et al 22 | 2013 | China | Asian | 531 | 162 | 85.1 | 77.8 | (43.6±11.7) | (42.6±13.1) | 27.4±3.4 | 26.9±3.7 | Hospital based | age, gender and BMI | PCR-RFLP |
| Li et al 29 | 2006 | China | Asian | 24 | 48 | 91.7 | 87.5 | (39.7±7.9) | (38.3±9.2) | 30.6±4.6 | 29.7±4.6 | Hospital based | age, race, gender and BMI | TaqMan |
NA: Not applicable; BMI: Body mass index; PCR-RFLP: Polymerase chain reaction–restriction fragment length polymorphism.
Table 2. Genotype distribution in included studies.
| Author | Case | Control | G allele frequency (%) | HWE(P) | ||||
| AA | AG | GG | AA | AG | GG | |||
| Popko et al 27 | 0 | 29 | 73 | 0 | 18 | 59 | 88.3 | 0.25 |
| Khalyfa et al 25 | 7 | 33 | 98 | 5 | 38 | 108 | 84.1 | 0.47 |
| Riha et al 30 | 7 | 44 | 52 | 8 | 52 | 130 | 82.1 | 0.34 |
| Liu et al 28 | 8 | 23 | 45 | 1 | 6 | 35 | 90.5 | 0.27 |
| Karkucak et al 23 | 0 | 18 | 51 | 0 | 9 | 33 | 89.3 | 0.44 |
| Almpanidou et al 24 | 19 | 83 | 118 | 14 | 84 | 221 | 82.4 | 0.11 |
| Li et al 21 | 0 | 18 | 137 | 0 | 12 | 88 | 94.0 | 0.52 |
| Bhushan et al 26 | 8 | 22 | 74 | 3 | 10 | 90 | 92.2 | <0.01 |
| Guan et al 22 | 6 | 95 | 430 | 1 | 18 | 143 | 93.8 | 0.60 |
| Li et al 29 | 3 | 10 | 11 | 1 | 12 | 35 | 85.4 | 0.98 |
HWE: Hardy-Weinberg equilibrium.
Quantitative synthesis
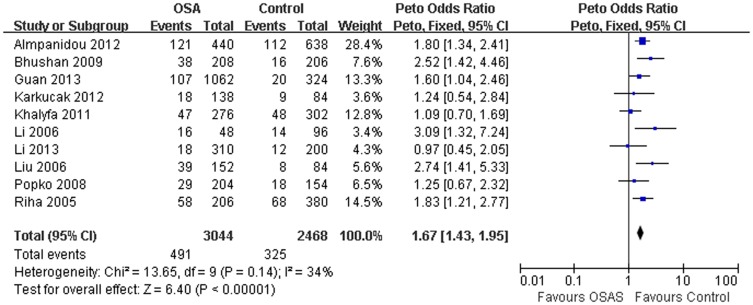
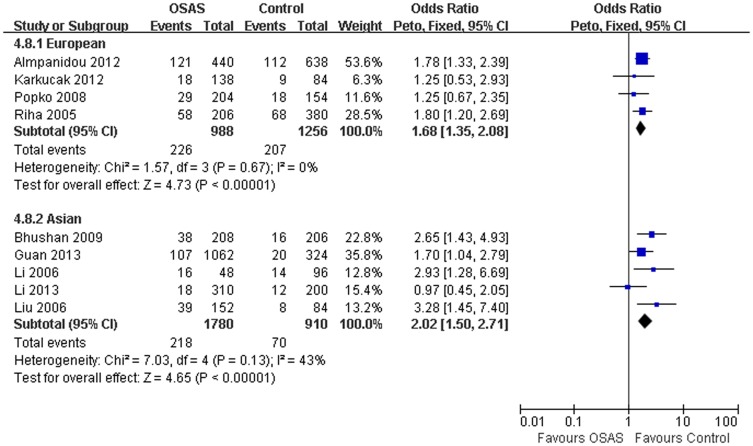
Our study suggested a significant association of the TNFα-308G/A polymorphism with OSAS risk (A vs. G: OR = 1.67, 95% CI = 1.43–1.95, P<0.00001; homozygote comparison: OR = 2.62, 95% CI = 1.68–4.09, P<0.0001; dominant model: OR = 1.74, 95% CI = 1.36–2.22, P<0.00001; recessive model: OR = 2.15, 95% CI = 1.39–3.32, P = 0.0006, Table 3; Figure 2). However, no significant association between this polymorphism and OSAS risk was observed in heterozygote comparison (OR = 1.38, 95% CI = 0.86–2.21, P = 0.18). In the subgroup analysis by ethnicity, similar results were observed in European (A vs. G: OR = 1.68, 95% CI = 1.35–2.08, P<0.00001) and Asian population (A vs. G: OR = 2.02, 95% CI = 1.50–2.71, P<0.00001; Figure 3). When stratified according to age, significant association between the TNFα-308G/A polymorphism and risk of OSAS was observed in adults (A vs. G: OR = 1.79, 95% CI = 1.50–2.13, P < 0.00001), but not for children (A vs. G: OR = 1.09, 95% CI = 0.70–1.69, P = 0.71).
Table 3. Stratified analysis of the TNF-α-308G/A polymorphism on OSAS risk.
| Variables | No.a | Cases/Controls | A vs G | AA vs GG | AA vs AG | AA vs AG+GG | AA+AG vs GG | ||||||||||
| OR(95%CI) | P b | P | OR(95%CI) | P b | P | OR(95%CI) | P b | P | OR(95%CI) | P b | P | OR(95%CI) | P b | P | |||
| Total | 10 | 1522/1234 | 1.67[1.43,1.95] | 0.14 | 0.00 | 2.62[1.68,4.09] | 0.79 | 0.00 | 1.38[0.86,2.21] | 0.98 | 0.18 | 2.15[1.39,3.32] | 0.91 | 0.00 | 1.74[1.36,2.22] | 0.14 | 0.00 |
| Ethnicities | |||||||||||||||||
| European | 4 | 494/628 | 1.68[1.35,2.08] | 0.67 | 0.00 | 2.42[1.33,4.41] | 0.82 | 0.00 | 1.25[0.67,2.33] | 0.67 | 0.48 | 1.92[1.07,3.46] | 0.74 | 0.03 | 1.83[1.41,2.36] | 0.57 | 0.00 |
| Asian | 5 | 890/455 | 2.02[1.50,2.71] | 0.13 | 0.00 | 3.95[1.56,10.00] | 0.76 | 0.00 | 1.60[0.59,4.33] | 0.87 | 0.36 | 3.28[1.30,8.26] | 0.84 | 0.01 | 2.01[1.45,2.80] | 0.16 | 0.00 |
| mixed | 1 | 138/151 | 1.09[0.70,1.69] | NA | 0.71 | 1.54[0.47,5.02] | NA | 0.47 | 1.61[0.47,5.56] | NA | 0.45 | 1.56[0.48,5.04] | NA | 0.46 | 1.03[0.62,1.71] | NA | 0.92 |
| Age | |||||||||||||||||
| Adults | 9 | 1384/1083 | 1.79[1.50,2.13] | 0.30 | 0.00 | 2.80[1.69,4.63] | 0.85 | 0.00 | 1.34[0.79,2.27] | 0.96 | 0.27 | 2.24[1.37,3.69] | 0.87 | 0.00 | 1.89[1.55,2.32] | 0.36 | 0.00 |
| Children | 1 | 138/151 | 1.09[0.70,1.69] | NA | 0.71 | 1.54[0.47,5.02] | NA | 0.47 | 1.61[0.47,5.56] | NA | 0.45 | 1.56[0.48,5.04] | NA | 0.46 | 1.03[0.62,1.71] | NA | 0.92 |
number of studies.
P value of Q-test for heterogeneity test.
0.00 means value<0.01.
Figure 2. Meta-analysis with a fixed model for the ORs of OSAS risk associated with TNF-α-308G/A polymorphism (A vs. G).
Figure 3. Meta-analysis with a fixed model for the ORs of OSAS risk in subgroup analysis by ethnicity (A vs. G).
Sensitivity analysis
Sensitivity analysis was carried out to assess the influence of each individual study on the pooled ORs. By removing each study, the pooled ORs were not altered significantly, which indicated that no individual study significantly affected the pooled results. When one HWE-violating study was excluded, the corresponding pooled ORs were still meaningful, showing the results were robust (OR = 1.62, 95% CI = 1.37–1.91, P<0.00001).
Publication bias
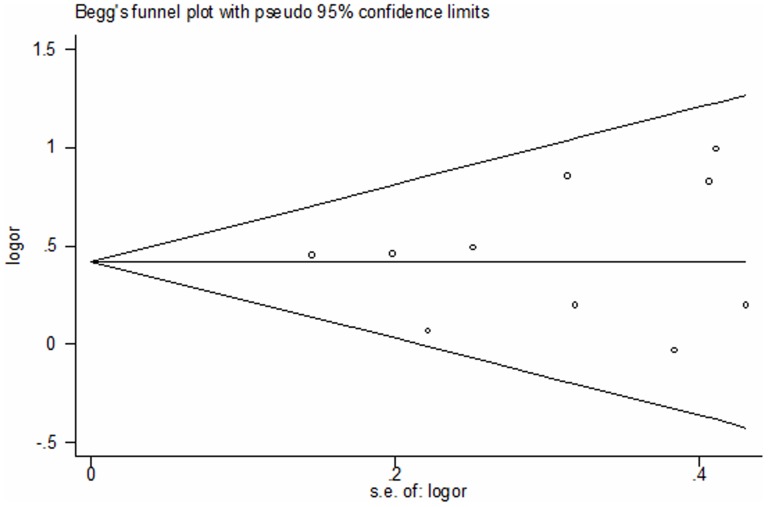
Begg's test and funnel plot were performed to verify the publication bias of the literature. The shapes of the funnel plots did not show obvious asymmetry (Figure 4). The asymmetry of the funnel plot was further tested by Egger's regression (t = 0.37, P = 0.724), which showed no significant publication bias.
Figure 4. Funnel plot for publication bias of the meta-analysis of OSAS risk and TNF-α-308G/Apolymorphism (A vs. G).
Discussion
In this meta-analysis, we concluded the existing data on the association of TNFα-308G/A polymorphism and OSAS risk. 10 studies involving 1,522 cases and 1,234 controls met the eligibility criteria. Our results demonstrated that the TNF-α-308G/A polymorphism was associated with OSAS risk (A vs. G: OR = 1.67, 95% CI = 1.43–1.95, P<0.00001). The A allele, compared with the G allele, was more likely to promote the risk of OSAS.
In stratified analyses, we observed that the association between TNFα-308G/A polymorphism and OSAS risk remained significant in Europeans (A vs. G: OR = 1.68, 95% CI = 1.35–2.08) and Asians (A vs. G: OR = 2.02, 95% CI = 1.50–2.71). Genetic background might have significant influence on the susceptibility of OSAS. However, up to date, no studies were performed in Africans, which limited our further analysis in those populations. At the same time, it was worth emphasizing that TNFα-308G/A polymorphism was contributed to the increase of OSAS susceptibility in adults (A vs. G: OR = 1.79, 95% CI = 1.50–2.13), but not for children (A vs. G: OR = 1.09, 95% CI = 0.70–1.69). This might reflect the fact that increased age modified the associations and might helpful to understand the pathogenesis of adults' OSAS.
The results from our study showed that TNFα-308G/A polymorphism associated with risk of OSAS. Compared with G allele, A allele was closely related to increased susceptibility to OSAS. The significance of this polymorphism could be explained by its possible influence on the expression of TNF-α protein. It was reported that the A allele of TNF-α gene increased TNF-α expression and resulted in higher TNF-α production by means of regulating adipose tissue metabolism [31], [32]. Moreover, TNF-α played an important role in regulating the pro-inflammatory cytokines during sleep and closely related to the level of high sensitivity C-reactive protein (hsCRP) [33]. Shamsuzzaman et al. demonstrated that the plasma C-reactive protein was elevated in OSAS patients and the magnitude of CRP elevation was remarkably related to the severity of OSAS [34].
Compared with the previous meta-analysis performed by Huang et al [35], our study had several superiorities. Firstly, the previous study identified less than half the number of studies as compared with the present study. More subjects were included in our study to provide a more comprehensive result. Secondly, in the previous meta-analysis, the authors did not evaluate some clinically significant outcomes and clinically relevant subgroups. This is first meta-analysis showed TNF-α-308 polymorphism increased susceptibility to the OSAS in both in European and Asian population. In addition, we firstly observed TNF-α-308 polymorphism increased the risk of OSAS in Adults, but not for children. Thirdly, no significant between-study heterogeneity and publication bias were observed in our study. Moreover, we carried out sensitivity analyses to evaluate the effect of each individual study on the pooled ORs.
Some limitations of this meta-analysis should be addressed. First, the number of published studies, especially for African population and children, was not large enough. Second, one study did not agreed with HWE in our analysis [26]. However, pooled ORs were not significant affect when the study was excluded. Third, in most studies detail information such as the co-morbidities were not available, which limited our further performed a risk-adjusted analysis.
Conclusion
In summary, this meta-analysis suggested that the A allele of the TNF-α-308 polymorphism increased susceptibility to the OSAS. These results provided the important role of the TNF-α-308G/A polymorphisms in the development of OSAS. Additional well-designed large studies were needed to validate our findings.
Supporting Information
(DOCX)
Quality assessment of case-control studies.
(DOC)
Acknowledgments
This work was supported by grant of National Natural Science Foundation of China (No. 81170037 and No. 81370126) and Natural Science Foundation of Ningbo (No. 2012A610257). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by grant of National Natural Science Foundation of China (No. 81170037 and No. 81370126) and Natural Science Foundation of Ningbo (No. 2012A610257). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Eckert DJ, Malhotra A (2008) Pathophysiology of adult obstructive sleep apnea. Proc Am Thorac Soc 5: 144–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Young T, Peppard PE, Gottlieb DJ (2002) Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med 165: 1217–1239. [DOI] [PubMed] [Google Scholar]
- 3. Hiestand DM, Britz P, Goldman M, Phillips B (2006) Prevalence of symptoms and risk of sleep apnea in the US population: Results from the national sleep foundation sleep in America 2005 poll. Chest 130: 780–786. [DOI] [PubMed] [Google Scholar]
- 4. Kielb SA, Ancoli-Israel S, Rebok GW, Spira AP (2012) Cognition in obstructive sleep apnea-hypopnea syndrome (OSAS): current clinical knowledge and the impact of treatment. Neuromolecular Med 14: 180–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Krueger JM (2008) The role of cytokines in sleep regulation. Curr Pharm Des 14: 3408–3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ciftci TU, Kokturk O, Bukan N, Bilgihan A (2004) The relationship between serum cytokine levels with obesity and obstructive sleep apnea syndrome. Cytokine 28: 87–91. [DOI] [PubMed] [Google Scholar]
- 7. Li NF, Yao XG, Zhu J, Yang J, Liu KJ, et al. (2010) Higher levels of plasma TNF-alpha and neuropeptide Y in hypertensive patients with obstructive sleep apnea syndrome. Clin Exp Hypertens 32: 54–60. [DOI] [PubMed] [Google Scholar]
- 8. Zamarron C, Garcia Paz V, Riveiro A (2008) Obstructive sleep apnea syndrome is a systemic disease. Current evidence. Eur J Intern Med 19: 390–398. [DOI] [PubMed] [Google Scholar]
- 9. Raabe T, Bukrinsky M, Currie RA (1998) Relative contribution of transcription and translation to the induction of tumor necrosis factor-alpha by lipopolysaccharide. J Biol Chem 273: 974–980. [DOI] [PubMed] [Google Scholar]
- 10. Wilson AG, Symons JA, McDowell TL, McDevitt HO, Duff GW (1997) Effects of a polymorphism in the human tumor necrosis factor alpha promoter on transcriptional activation. Proc Natl Acad Sci U S A 94: 3195–3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Louis E, Franchimont D, Piron A, Gevaert Y, Schaaf-Lafontaine N, et al. (1998) Tumour necrosis factor (TNF) gene polymorphism influences TNF-alpha production in lipopolysaccharide (LPS)-stimulated whole blood cell culture in healthy humans. Clin Exp Immunol 113: 401–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kroeger KM, Steer JH, Joyce DA, Abraham LJ (2000) Effects of stimulus and cell type on the expression of the −308 tumour necrosis factor promoter polymorphism. Cytokine 12: 110–119. [DOI] [PubMed] [Google Scholar]
- 13. Wilson AG, di Giovine FS, Blakemore AI, Duff GW (1992) Single base polymorphism in the human tumour necrosis factor alpha (TNF alpha) gene detectable by NcoI restriction of PCR product. Hum Mol Genet 1: 353. [DOI] [PubMed] [Google Scholar]
- 14.Wells GA, Shea B, O'Connell D, Peterson J, Welch V, et al.. (2010) The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses.
- 15. Lau J, Ioannidis JP, Schmid CH (1997) Quantitative synthesis in systematic reviews. Ann Intern Med 127: 820–826. [DOI] [PubMed] [Google Scholar]
- 16. Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21: 1539–1558. [DOI] [PubMed] [Google Scholar]
- 18. Mantel N, Haenszel W (1959) Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 22: 719–748. [PubMed] [Google Scholar]
- 19. DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 20. Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li T, Qian X, Guo R, Sun B, Liu C (2013) Relation between TNF-α polymorphism and obstructive sleep apnea syndrome accompanying hypertension. Acad J Chinese PLA Med Sch 34.. [Google Scholar]
- 22.Guan Jian YH, Meng L, Su K, Yin S (2013) Study of association of TNF-alpha gene Polymorphism with OSAHS and metabolic syndrome CHlN ARCH OTOLARYNGOL HEAD NECK SURG 20.
- 23. Karkucak M, Ursavas A, Ocakoglu G, Görükmez O, Yakut T, et al. (2012) Analysis of TNF-alpha G308A and C857T gene polymorphisms in Turkish patients with obstructive sleep apnea syndrome. Turkiye Klinikleri Journal of Medical Sciences 32: 1368–1373. [Google Scholar]
- 24. Almpanidou P, Hadjigeorgiou G, Gourgoulianis K, Papadimitriou A (2012) Association of tumor necrosis factor-alpha gene polymorphism (−308) and obstructive sleep apnea-hypopnea syndrome. Hippokratia 16: 217–220. [PMC free article] [PubMed] [Google Scholar]
- 25. Khalyfa A, Serpero LD, Kheirandish-Gozal L, Capdevila OS, Gozal D (2011) TNF-alpha gene polymorphisms and excessive daytime sleepiness in pediatric obstructive sleep apnea. J Pediatr 158: 77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bhushan B, Guleria R, Misra A, Luthra K, Vikram NK (2009) TNF-alpha gene polymorphism and TNF-alpha levels in obese Asian Indians with obstructive sleep apnea. Respir Med 103: 386–392. [DOI] [PubMed] [Google Scholar]
- 27. Popko K, Gorska E, Potapinska O, Wasik M, Stoklosa A, et al. (2008) Frequency of distribution of inflammatory cytokines IL-1, IL-6 and TNF-alpha gene polymorphism in patients with obstructive sleep apnea. J Physiol Pharmacol 59 Suppl 6607–614. [PubMed] [Google Scholar]
- 28. Liu HG, Guan P, Lin M, Xu YJ, Zhang ZX (2006) The relationship between tumor necrosis factor-alpha gene promoter polymorphism and obstructive sleep apnea-hypopnea syndrome. Zhonghua Jie He He Hu Xi Za Zhi 29: 596–599. [PubMed] [Google Scholar]
- 29.Li F, Xue Y (2006) TUMOR NECROSIS FACTOR-a(−308)GENE AND SEROTONIN TRANSPORIER GENE POLYMORPHISM WITH OBSTRUCTIVE APNEA SYNDROME.
- 30. Riha RL, Brander P, Vennelle M, McArdle N, Kerr SM, et al. (2005) Tumour necrosis factor-alpha (−308) gene polymorphism in obstructive sleep apnoea-hypopnoea syndrome. Eur Respir J 26: 673–678. [DOI] [PubMed] [Google Scholar]
- 31. Hoffstedt J, Eriksson P, Hellstrom L, Rossner S, Ryden M, et al. (2000) Excessive fat accumulation is associated with the TNF alpha-308 G/A promoter polymorphism in women but not in men. Diabetologia 43: 117–120. [DOI] [PubMed] [Google Scholar]
- 32. Brand E, Schorr U, Kunz I, Kertmen E, Ringel J, et al. (2001) Tumor necrosis factor-alpha-−308 G/A polymorphism in obese Caucasians. Int J Obes Relat Metab Disord 25: 581–585. [DOI] [PubMed] [Google Scholar]
- 33. Teramoto S, Yamamoto H, Ouchi Y (2003) Increased C-reactive protein and increased plasma interleukin-6 may synergistically affect the progression of coronary atherosclerosis in obstructive sleep apnea syndrome. Circulation 107: E40–40. [DOI] [PubMed] [Google Scholar]
- 34. Shamsuzzaman AS, Winnicki M, Lanfranchi P, Wolk R, Kara T, et al. (2002) Elevated C-reactive protein in patients with obstructive sleep apnea. Circulation 105: 2462–2464. [DOI] [PubMed] [Google Scholar]
- 35. Huang J, Liao N, Huang QP, Xie ZF (2012) Association between tumor necrosis factor-alpha-308G/A polymorphism and obstructive sleep apnea: a meta-analysis. Genet Test Mol Biomarkers 16: 246–251. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Quality assessment of case-control studies.
(DOC)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.