Abstract
The dimorphic fungus Candida albicans excretes farnesol, which is produced enzymatically from the sterol biosynthetic intermediate farnesyl pyrophosphate. Inhibition of C. albicans by four azole antifungals, fluconazole, ketoconazole, miconazole, and clotrimazole, caused elevated farnesol production (10- to 45-fold). Furthermore, farnesol production occurs in both laboratory strains and clinical isolates (J. M. Hornby et al., Appl. Environ. Microbiol. 67:2982-2992, 2001) of C. albicans.
The inoculum size effect in Candida albicans results from the natural production and excretion of an extracellular quorum-sensing molecule (QSM) which our laboratory identified as farnesol (4). Exogenous farnesol, purified from spent media or supplied commercially, prevented mycelial development in both a growth morphology assay and a differentiation assay. The differentiation assay used three chemically distinct triggers for germ tube formation: l-proline, N-acetylglucosamine, and serum. In all cases, the presence of farnesol prevented the yeast-to-mycelium conversion, resulting in actively budding yeasts without influencing cellular growth rates (4). We later showed that only the (E,E)-isomer of farnesol was biologically active (11) and that C. albicans cell extracts contained enzymatic activity to convert [3H]farnesyl pyrophosphate (FPP) to [3H]farnesol (5).
Because FPP is the biosynthetic precursor of both farnesol (5) and ergosterol (10), we reasoned that drugs which block the sterol biosynthetic pathway after FPP might lead to the accumulation of FPP which would, in turn, lead to enhanced farnesol production. This reasoning proved correct; treatment of C. albicans with 0.5 μM zaragozic acid B, a potent inhibitor of squalene synthase (2), led to an eightfold increase in the amount of farnesol produced by C. albicans (5). At that time our group predicted that all drugs which blocked carbon flow through the sterol pathway would also cause enhanced production of farnesol. The present paper tests that generality by showing that four azole antifungals, fluconazole, clotrimazole, ketoconazole, and miconazole, also boost farnesol production by C. albicans. Additionally, because the farnesol-stimulating activity of zaragozic acid B had only been shown with strain A72, we tested the generality of farnesol production in C. albicans by analyzing five other strains, including recent clinical isolates.
Growth, sample preparation, and determination of extracellular farnesol by gas chromatography-mass spectroscopy were as previously described (4, 5). Table 1 displays the levels of extracellular farnesol produced by seven strains of C. albicans. The four clinical isolates displayed similar levels of farnesol production as the lab strains A72 and CAI-4. No significant differences were observed. In past work (4), our investigators reported quorum-sensing activity in five other laboratory strains (MEN, SC5314, LGH1095, SG3314, and ATCC10261). Thus, this study has shown a general theme of farnesol production by the majority of C. albicans strains used here. At this time we also clarified the distinction between our work, showing that farnesol is the QSM in C. albicans, and that of Oh et al. (9), who described the production of farnesoic acid as the QSM in C. albicans 10231. C. albicans 10231 produced no detectable farnesol (Table 1), thereby revealing a significant metabolic modification in this strain. To date, C. albicans 10231 is the only strain tested that does not produce farnesol. Direct comparison of the quorum-sensing activity of farnesoic acid and farnesol showed that farnesoic acid displays only 3.3% of the activity exhibited by (E,E)-farnesol (11).
TABLE 1.
Farnesol production by laboratory and clinical isolates of C. albicans
| Strain of C. albicans | Farnesol produced (mg/g [dry wt])a |
|---|---|
| A72 | 0.120-0.133 |
| CAI-4 | 0.120-0.133 |
| ATCC 10231 | <0.005 |
| Clinical isolate 4b | 0.125 ± 0.005 |
| Clinical isolate 6 | 0.107 ± 0.012 |
| Clinical isolate 11 | 0.137 ± 0.003 |
| Clinical isolate 12 | 0.131 ± 0.007 |
Cell dry weights were determined for each sample in Tables 1 and 2. Values for A72 and CAI-4 are the average of six or more determinations conducted over a 2-year time span. For C. albicans 10231, no farnesol was detected in two independent studies (detection limit of 0.005 mg/g).
The four clinical isolates examined were randomly chosen from 12 isolates provided by Thomas Stalder (Lincoln, Nebr.). Values represent the average and variation of duplicate independent measurements.
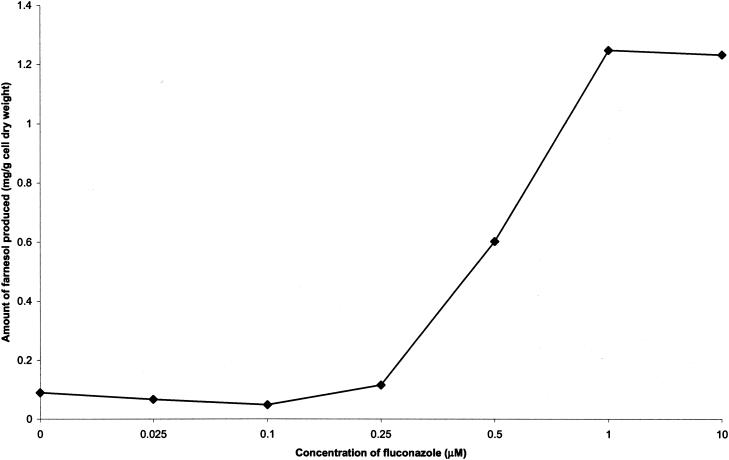
Following our group's work showing increased farnesol production after treatment of C. albicans with the squalene synthase inhibitor zaragozic acid B (5), additional sterol inhibitors were studied. Treatment with fluconazole resulted in the dose-response curve shown in Fig. 1. The farnesol excreted increased roughly 13-fold, reaching a maximum of ca. 1.24 mg of farnesol per gram (cell dry weight) at 1 μM fluconazole (Fig. 1 and Table 2). Intracellular farnesol increased in a proportional manner (data not shown). The fluconazole concentration needed for maximal farnesol production (1 μM) corresponded well with the MIC of fluconazole which, for C. albicans A72, is ca. 1 μM, although even at 10 μM fluconazole complete inhibition of growth is not achieved. This effect, known as trailing growth, is common in many strains of C. albicans (6).
FIG. 1.
Increased farnesol production by fluconazole-treated cells. Each point is the average of at least two separate experiments agreeing within ±10%.
TABLE 2.
Enhanced farnesol production by C. albicans A72 treated with drugs which target sterol biosynthesis
| Antibiotic (concn [μM]) | Farnesol produced (mg/g [dry wt]) | Fold increase |
|---|---|---|
| None | 0.120-0.133a | |
| Zaragozic acid (0.5) | 1.0b | 8 |
| Fluconazolec (1) | 1.24 | 10 |
| Clotrimazole (1) | 5.44 | 45 |
| Ketoconazole (1) | 5.43 | 45 |
| Miconazole (0.5) | 5.31 | 44 |
| Tolnaftate (1) | 0.132d |
From Table 1.
From reference 5.
Fluconazole was kindly provided by Pfizer Pharmaceuticals. Tolnaftate, ketoconazole, clotrimazole, and miconazole were purchased from Sigma. Values for farnesol production are averages of two independent experiments agreeing within ± 10%.
Value in the presence of tolnaftate is an average of two independent experiments agreeing within ±3%.
Table 2 illustrates the commonality of increased farnesol production due to sterol inhibition by azole antifungals. Treatment with 1 μM ketoconazole, 1 μM clotrimazole, or 0.5 μM miconazole led to farnesol production increases of 45-, 45-, and 44-fold, respectively. Dose-response curves were not conducted for these three azoles. With the doses chosen, very little growth was observed over 24 h (cell dry weights were ca. 2% of those of the untreated controls). Therefore, the higher levels of farnesol produced with clotrimazole, ketoconazole, and miconazole (Table 2) probably reflected how little growth occurred rather than any mechanistic differences between fluconazole and the other azoles. Significantly, tolnaftate, an inhibitor of squalene epoxidation added as a control because it is known to be inactive versus C. albicans (1), did not increase farnesol production above wild-type levels (Table 2).
Our investigators previously proposed the general theme that inhibition of the sterol pathway would lead to increased production of farnesol (5). This idea was based on our work with squalene synthase inhibition by zaragozic acid B (5). This theme has now been confirmed for the inhibition of lanosterol 14α-demethylase by four cytochrome P450-inhibitory azoles (Table 2). For most strains of C. albicans, any drug that inhibits a step in the sterol biosynthetic pathway, thus inhibiting carbon flow to ergosterol, may also increase farnesol production.
Increased farnesol could also be used to screen drugs that target the sterol biosynthetic pathway and to examine the sensitivity of clinical isolates to known sterol-inhibitory drugs. While the hypothesis that azoles promote farnesol production via inhibition of the ergosterol pathway is attractive, at this time we cannot exclude the possibility that pleiotropic effects of the azole family affect farnesol independently of sterol biosynthesis.
Elevated production of farnesol at sublethal levels of fluconazole (Fig. 1), coupled with the known ability of farnesol to cause a shift from mycelia to yeast in C. albicans (4), provides an attractive explanation for past work which noted that subinhibitory concentrations of azole drugs inhibited hyphal development and maintained the cells in the yeast morphology. Interestingly, this inhibitory effect was independent of any inhibition on growth of the fungus (3, 8). Odds (7) also mentioned in his comprehensive review of Candida that “most azoles are able to prevent or greatly perturb hyphal growth of C. albicans. The drugs retard or annul the initial outgrowth of germ tubes and entirely prevent hyphal branching, thus leading to cultures of largely or entirely yeast-form cells, even on media that normally support development of long hyphae.”
Acknowledgments
We thank Sara Basiaga for her assistance with gas chromatography-mass spectroscopy, Thomas Stalder for providing the clinical isolates used in this study, and Ted White for his scientific commentary related to the history of sublethal concentrations of azoles inhibiting hyphal development in C. albicans. We also thank Pfizer Pharmaceuticals, Sandwich, United Kingdom, for providing the fluconazole used in this study.
This work was supported by grants from the National Science Foundation (MCB-0110999) and the University of Nebraska Tobacco Settlement Biomedical Research Enhancement Fund.
REFERENCES
- 1.Barrett-Bee, K. J., A. C. Lane, and R. W. Turner. 1986. The mode of antifungal action of tolnaftate. J. Med. Vet. Mycol. 24:155-160. [DOI] [PubMed] [Google Scholar]
- 2.Bergstrom, J. D., C. Dufresne, G. F. Bills, M. Nallin-Omstead, and K. Byrne. 1995. Discovery, biosynthesis, and mechanism of action of the zaragozic acids: potent inhibitors of squalene synthase. Annu. Rev. Microbiol. 49:607-639. [DOI] [PubMed] [Google Scholar]
- 3.Ha, K. C., and T. C. White. 1999. Effects of azole antifungal drugs on the transition from yeast cells to hyphae in susceptible and resistant isolates of the pathogenic yeast Candida albicans. Antimicrob. Agents Chemother. 43:763-768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hornby, J. M., E. C. Jensen, A. D. Lisec, J. J. Tasto, B. Jahnke, R. Shoemaker, P. Dussault, and K. W. Nickerson. 2001. Quorum sensing in the dimorphic fungus Candida albicans is mediated by farnesol. Appl. Environ. Microbiol. 67:2982-2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hornby, J. M., B. W. Kebaara, and K. W. Nickerson. 2003. Farnesol biosynthesis in Candida albicans: cellular response to sterol inhibition by zaragozic acid B. Antimicrob. Agents Chemother. 47:2366-2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marr, K. A., T. R. Rustad, J. H. Rex, and T. C. White. 1999. The trailing end point phenotype in antifungal susceptibility testing is pH dependent. Antimicrob. Agents Chemother. 43:1383-1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Odds, F. C. 1988. Candida and candidosis, 2nd ed. Bailliere Tindall, London, England.
- 8.Odds, F. C., A. Cockayne, J. Hayward, and A. B. Abbott. 1985. Effects of imidazole- and triazole-derivative antifungal compounds on the growth and morphological development of Candida albicans hyphae. J. Gen. Microbiol. 131:2581-2589. [DOI] [PubMed] [Google Scholar]
- 9.Oh, K.-.B, H. Miyazawa, T. Naito, and H. Matsuoka. 2001. Purification and characterization of an autoregulatory substance capable of regulating the morphological transition in Candida albicans. Proc. Natl. Acad. Sci. USA 98:4664-4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parks, L. W., and W. M. Casey. 1995. Physiological implications of sterol biosynthesis in yeast. Annu. Rev. Microbiol. 49:95-116. [DOI] [PubMed] [Google Scholar]
- 11.Shchepin, R., J. M. Hornby, E. Burger, T. Niessen, P. Dussault, and K. W. Nickerson. 2003. Quorum sensing in Candida albicans: probing farnesol's mode of action with 40 natural and synthetic farnesol analogs. Chem. Biol. 10:743-750. [DOI] [PubMed] [Google Scholar]