Abstract
Voriconazole is a broad-spectrum triazole that offers extended activity against molds and yeasts that are not susceptible to earlier azole-type drugs. Recent studies indicate that melanization can severely reduce the susceptibility of certain antifungal drugs, but there is no information as to whether voriconazole is vulnerable to this effect. The activity of voriconazole on C. neoformans was assessed by MIC analysis and time-kill assays for melanized and nonmelanized cells. Cell morphology, capsule release, and phagocytosis of C. neoformans were studied in the presence or absence of subinhibitory concentrations of voriconazole. Voriconazole was fungicidal at concentrations of ≥8 μg/ml in vitro against the strains of C. neoformans examined, and its efficacy was not diminished by melanization. Cells grown in subinhibitory concentrations of voriconazole had smaller cellular and capsular volumes than cells grown in the absence of drug. The induction of the capsule by serum was not affected by voriconazole. Cells grown in subinhibitory concentrations of voriconazole released their capsule and were phagocytosed at rates comparable with yeast grown without the antifungal. The high activity of voriconazole against both melanized and nonmelanized cells results suggest that voriconazole may be a particularly valuable drug for cryptococcosis.
Cryptococcus neoformans is a relatively frequent cause of serious fungal infections in immunocompromised patients. The prevalence in the United States of cryptococcal meningoencephalitis in patients with AIDS receiving retroviral therapy is currently estimated to be <2% (23) but is >30% in areas of South East Asia and Sub-Saharan Africa (29). Patients with AIDS complicated by cryptococcosis often respond poorly to treatment and, in the setting of continued immunosuppression, require lifelong maintenance therapy since currently available antifungal agents seldom eradicate these fungal pathogens in the setting of severe immune suppression (16, 41).
Voriconazole, a synthetic derivative of fluconazole, is a broad-spectrum triazole antifungal that inhibits cytochrome P450-dependent 14α-lanosterol demethylation, which is a critical step in fungal cell membrane ergosterol synthesis. Voriconazole demonstrates excellent in vitro activity against C. neoformans (17, 31) and achieves good levels in cerebrospinal fluid (35). Voriconazole is not currently licensed for use in cryptococcosis and no clinical trials have evaluated its efficacy for cryptococcal disease. There is limited published information regarding the clinical use of voriconazole for cryptococcosis (12, 30). In a study by Perfect et al., voriconazole therapy resulted in a 39% response rate in 18 patients with refractory cryptococcal meningoencephalitis (30).
Given recent evidence that melanization can significantly reduce the efficacy of certain antifungal drugs against C. neoformans (36), we evaluated the activity of voriconazole against both melanized and nonmelanized yeast cells. Although we previously did not see a protective effect of melanin on the activity of fluconazole at three times the MIC (36), Ikeda et al. (18) found that 65% of nonmelanized yeast cells were viable after incubation with fluconazole at four times the MIC, whereas 100% of melanized yeast were viable. To gain further insight into the effect of voriconazole on C. neoformans capsular metabolism, we also evaluated the effects of the antifungal on cell morphology, capsule release, and phagocytosis.
MATERIALS AND METHODS
Chemicals.
Glucose, MgSO4, and KH2PO4 were purchased from J. T. Baker, Inc. (Philipsburg, N.J.). Glycine, NaCl, RPMI 1640 medium (with l-glutamine and without sodium bicarbonate), vitamin B1, synthetic melanin (prepared by the oxidation of tyrosine with hydrogen peroxide), morpholinepropanesulfonic acid (MOPS), and L-dopa were purchased from Sigma Chemical Corp. (Cleveland, Ohio). Voriconazole was provided by Pfizer (Sandwich, England). Amphotericin B was purchased from Gibco (Invitrogen Corp., Carlsbad, Calif.) and used as a positive control for MIC and time-kill assays (36).
C. neoformans.
C. neoformans strain 24067 was obtained from the American Type Culture Collection (Rockville, Md.). C. neoformans H99 (serotype A) was obtained from J. Perfect (Durham, N.C.). We have used strains ATCC 24067 and H99 to study the effects of melanin on fungal viability (Van Duin et al., Abstr. 102nd Gen. Meet. Am. Soc. Microbiol. 2002) and to examine cell morphology and phagocytosis after exposure to antifungal drugs (27). Yeast strains were maintained at −80°C then grown in Sabouraud (SAB) dextrose medium (Becton Dickinson, Sparks, Md.) for 36 h at 30°C with shaking at 150 rpm. For MIC determinations and time-kill assays, melanized and nonmelanized yeast cells were used. Melanization was induced by growing cells on defined minimal medium agar plates (15 mM glucose, 10 mM MgSO4, 29.4 mM KH2PO4, 13 mM glycine, 3.0 μM vitamin B1, 2% agar), with the addition of 1 mM L-dopa for 10 days. Nonmelanized controls were obtained by growing yeast cells on minimal medium agar plates without L-dopa for 10 days at 30°C.
MIC determination.
MICs were determined by the standardized protocol for yeasts developed by the National Committee for Clinical Laboratory Standards (24). Briefly, melanized and nonmelanized C. neoformans strain ATCC 24067 and H99 yeast cells were suspended in sterile normal saline and diluted to a concentration of 2 × 106 cells/ml. Cell counts were determined by using a hemocytometer. The suspensions were diluted 1:1,000 in RPMI 1640 medium with l-glutamine, without bicarbonate, and buffered to pH 7.0 with 0.165 M MOPS. Polystyrene tubes containing 0.1-ml aliquots of an antifungal at 10 times the final drug concentration were inoculated with 0.9 ml of the diluted suspensions. Final drug concentrations ranged from 0.007 to 4 μg/ml for voriconazole and from 0.063 to 4 μg/ml for amphotericin B. The MICs were recorded after incubation at 35°C for 72 h. MICs were defined as the lowest concentration at which there was an absence of growth.
Time-kill assays.
Yeast cells were suspended in sterile normal saline at a density of 2.2 × 103 cells/ml. Cell counts were determined by hemocytometer. Microcentrifuge tubes containing 0.1-ml aliquots of an antifungal at 10 times the final concentration were inoculated with 0.9 ml of the yeast suspensions. Final drug concentrations ranged from 8 to 32 μg/ml for voriconazole and from 0.125 to 0.5 μg/ml for amphotericin B. After incubation at 35°C for 2 h, aliquots were plated on SAB agar to determine their viabilities as measured by CFU. Survival was compared to that of fungal cells incubated in phosphate-buffered saline (PBS; 0.137 M NaCl, 0.003 M sodium phosphate [pH 7.4]).
The term melanin “ghosts” refers to the acid-resistant structures containing melanin from melanized C. neoformans which resemble cells in size and morphology. The ghosts were obtained from melanized C. neoformans cells as described previously (33). Briefly, melanized cryptococcal cells were serially treated with 10 mg of cell wall-lysing enzymes (from Trichoderma harzianum; Sigma)/ml, 4.0 × guanidine thiocyanate, and 1.0 mg of proteinase K/ml; extracted three times with chloroform; and boiled in 6.0 M HCl for 1 h. The resulting black debris was then dialyzed against distilled H2O (dH2O) for 10 days. Nonmelanized cryptococcal cells that are subjected to this protocol are completely solubilized (33).
A volume of 2 ml of stock solution of voriconazole and amphotericin B (in dH2O at 5 mg/ml) was incubated with cryptococcal melanin (109 particles/ml) and synthetic melanin (20 mg) for 2 h at 30°C. The melanins were removed by centrifugation. The supernatants of the voriconazole and amphotericin B solutions were used in the killing assay with C. neoformans yeast cells. The melanin pellets from the incubated solutions were washed extensively with dH2O and lyophilized with a Flexi-Dry microprocessor (FTS Systems, Inc., Stone Ridge, N.Y.). As controls, cryptococcal melanin and synthetic melanin in dH2O were lyophilized. Quantitative elemental analysis of the melanins was performed by Quantitative Technologies, Inc. (Whitehouse, N.J.). Briefly, melanin samples were converted into gases by combustion, and product gases were separated under steady-state conditions. The percentage of each element was measured as a function of thermal conductivity. Elemental ratios were calculated by dividing the percentage of each element measured by its respective atomic weight.
Electron microscopy.
C. neoformans yeast cells were grown in SAB with or without voriconazole at a concentration corresponding to 0.5 times the MIC for 36 h. The cells were collected, washed three times with PBS, and then incubated in 2.5% glutaraldehyde for 1 h at room temperature. The samples were then applied to a polylysine-coated coverslip and serially dehydrated in alcohol. The samples were fixed in a critical-point drier (Samdri-790; Tousimis, Rockville, Md.), coated with gold-palladium (Desk-1; Denton Vacuum, Inc., Cherry Hill, N.J.), and viewed with a JEOL (Tokyo, Japan) JSM-6400 scanning electron microscope. Two separate sets of cultures were prepared.
Cell and capsule measurements.
Light microscopy was used to evaluate cells grown in SAB with or without 0.5 times the MIC of voriconazole. India ink preparations were made and viewed with an Olympus AX70 (Melville, N.Y.) microscope under oil immersion at a magnification of ×1,000. Pictures were taken with a QImaging Retiga 1300 digital camera by using the QCapture Suite V2.46 software (QImaging, Burnaby, British Columbia, Canada) and processed with Adobe Photoshop 7.0 for windows (San Jose, Calif.). Capsule thickness was defined as the distance from the cell wall to the outer capsular border, and organism size was defined as the cell diameter inclusive of the polysaccharide capsule. To calculate the capsule volume, the diameter of the whole cell and the cell body were each measured, with capsule volume defined as the difference between the volume of the whole cell (yeast cell plus capsule) and the volume of the cell body (no capsule). Volumes were determined by using the equation for the volume of a sphere as 4/3 × π × (D/2)3, where D is the diameter of the cell. The measurements for the capsule and the organism were averaged (n = 20 to 40 cells).
Capsule induction.
We have previously shown that growth of C. neoformans in mammalian serum can induce capsule production (39). We therefore investigated whether the induction of capsule in melanized and nonmelanized cells could be altered by exposure to voriconazole or amphotericin B. C. neoformans strain H99 yeast cells were incubated at 37°C in SAB broth for 24 h and then transferred into PBS with 10% heat-inactivated fetal calf serum (FCS). The yeast cells (2 × 106 to 4 × 106) were placed in six-well plates containing 2 ml of medium, with or without voriconazole or amphotericin B at various concentrations, and then incubated at 37°C for 24 h. Cells were also grown for 24 h in SAB medium and transferred to PBS with or without (starvation) 10% heat-inactivated FCS overnight prior to incubation with or without the antifungal drugs. To compare the effects of the antifungal drugs on capsule induction with melanized and nonmelanized cells, strain H99 yeast cells were grown in defined chemical medium with or without L-dopa as described above prior to incubation in PBS with 10% heat-inactivated serum with or without voriconazole or amphotericin B.
Measurement of capsular polysaccharide.
Supernatants from 36 h cultures of C. neoformans grown in SAB medium with or without 0.5 times the MIC of voriconazole were analyzed by enzyme-linked immunosorbent assay (ELISA) for glucuronoxylomannan (GXM). GXM is the major component of C. neoformans polysaccharide (6). The cell density for each culture was determined with a hemocytometer. Samples were spun to pellet the cells, and a 1/50 dilution of supernatant was prepared in Tris-buffered saline (25 mM Tris, 126 mM NaCl, 2.6 mM KCl [pH 7.2]). GXM levels in the sample supernatants were determined by capture ELISA relative to the levels in the supernatant of strain ATCC 24067 GXM or H99 GXM standards (3). The GXM determinations were normalized by dividing the GXM concentrations by cell density.
Phagocytosis assays.
J774.16 is a well-characterized murine macrophage-like cell line (5) that has been extensively used to study C. neoformans-macrophage interactions. The J774.16 cells were maintained at −80°C prior to use and were prepared for the phagocytosis assays as described previously (7). C. neoformans ATCC 24067 and H99 yeast cells were grown in SAB medium with or without 0.5 times the MIC of voriconazole, collected, and washed three times in 10% heat-inactivated FCS. Cells were added to the J774.16 monolayer in a macrophage/yeast ratio of 1:1. The plates were incubated for 2 h at 37°C with either 20% FCS (not heat inactivated) or 10 μg of monoclonal antibody (MAb) 18B7/ml. MAb 18B7 binds to cryptococcal GXM (2). The monolayer was washed three times with PBS to remove nonadherent cells, fixed with cold methanol, and stained with Giemsa (Sigma). The phagocytic index is the number of internalized yeast cells per number of macrophages per field. Internalized cells were differentiated from attached cells by their presence in a well-defined phagocytic vacuole. These measurements were determined by light microscopy (Nicon Diaphot; Nikon, Inc., Instrument Division, Garden City, N.J.) at a magnification of ×600. For each experiment, three wells were examined, and the numbers of ingested cryptococcal cells and macrophages in three fields were counted, with approximately 200 macrophages per field.
Statistics.
Data were analyzed by using analysis of variance, independent Student t test, and chi-square test with Primer for Statistics (version 3.0; McGraw-Hill, Inc., New York, N.Y.). All data are expressed as averages ± the standard deviations.
RESULTS
MIC.
Table 1 summarizes the in vitro susceptibilities of melanized versus nonmelanized C. neoformans ATCC 24067 and H99. The MICs were within values previously reported for C. neoformans (1, 9, 17, 22, 31). There were no significant differences in MICs for melanized and nonmelanized cells of the same strain for either voriconazole or amphotericin B.
TABLE 1.
MICs for melanized (with L-dopa) and nonmelanized (without L-dopa) C. neoformans strains ATCC 24067 and H99
| Drug | MIC (μg/ml) for strain: |
|||
|---|---|---|---|---|
| 24067 |
H99 |
|||
| With L-dopa | Without L-dopa | With L-dopa | Without L-dopa | |
| Voriconazole | 0.015 | 0.015 | 0.03 | 0.03 |
| Amphotericin B | 0.125 | 0.125 | 0.125 | 0.125 |
Time-kill assay.
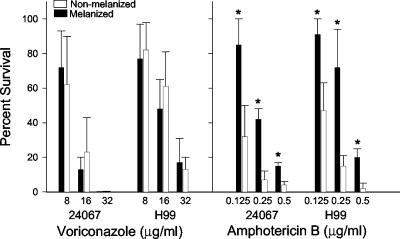
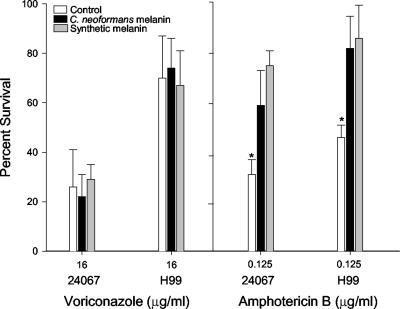
Melanization did not protect C. neoformans strains ATCC 24067 or H99 from killing by voriconazole (Fig. 1). In contrast, melanized cells were significantly less susceptible to amphotericin B, which is consistent with previously reported results (18, 36). Since prior data showed that melanins bound amphotericin B but not fluconazole or itraconazole, we investigated whether voriconazole was bound by melanin. Incubation of voriconazole with either C. neoformans or synthetic melanin prior to use did not alter killing of the yeast strains (Fig. 2). In contrast, incubation of the melanins with amphotericin B significantly reduced its efficacy compared to assays performed with the antifungals without preincubation with melanin (Fig. 2). Elemental analysis of melanins incubated with voriconazole showed that the C/N ratio of the compounds were not altered, a finding consistent with no absorption of drug by melanin (Table 2). The analysis was performed twice on melanins from different experiments with similar results. Incubation of melanin with amphotericin B resulted in an alteration in the C/N ratios of the melanins, a finding consistent with the fact that this drug binds melanins (18, 36). These results indicate that voriconazole dose not bind melanin and that the melanization state of C. neoformans does not alter the efficacy of the antifungal.
FIG. 1.
Time-kill assay. The rates of survival of melanized versus nonmelanized C. neoformans strain ATCC 24067 and H99 after exposure to various concentrations of voriconazole or amphotericin for 2 h compared to those of fungal cells incubated in PBS. Values are averages ± the standard errors of the means for four measurements. P values were calculated by comparing melanized and nonmelanized cells by using the Student t test. ✽, P < 0.001. Similar results were obtained in three separate experiments.
FIG. 2.
Time-kill assay with melanins. The rates of survival of nonmelanized C. neoformans strain ATCC 24067 and H99 exposed to voriconazole or amphotericin B with or without preincubation of the antifungal agents with C. neoformans melanin or synthetic melanin were compared. Values are averages ± the standard errors of the means for four measurements. P values refer to differences between the rates of survival for yeast cells after preincubation of the antifungal agents with either type of melanin and those for cells not preincubated with melanin (control). ✽, P < 0.001 (Student t test). Similar results were obtained in two independent experiments.
TABLE 2.
Elemental analysis of melanins
| Sample | C/N ratio |
|---|---|
| Control fungal melanin | 8:1 |
| Voriconazole plus fungal melanin | 8:1 |
| Amphotericin B plus fungal melanin | 11:1 |
| Control synthetic melanin | 9:1 |
| Voriconazole plus synthetic melanin | 9:1 |
| Amphotericin B plus synthetic melanin | 10:1 |
Microscopy.
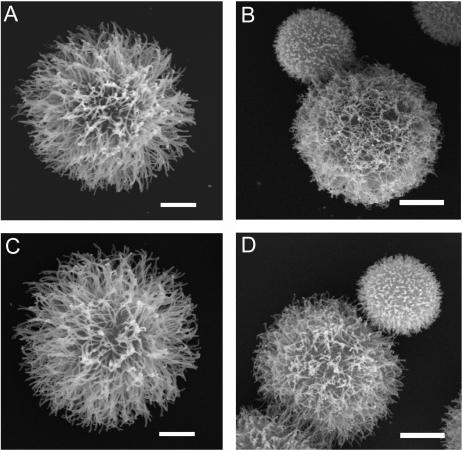
Scanning electron microscopy revealed that C. neoformans yeast cells grown in 0.5 times the MIC voriconazole had different capsule morphology compared to cells grown in medium alone (Fig. 3). Examination of cells grown in SAB medium revealed typical globular cells covered with a loose fibrillar network (Fig. 3A and C) (8, 27). The polysaccharide fibrils of yeast grown in 0.5 times the MIC of voriconazole appeared to be shorter, matted to the cell surface, and more entwined with adjacent fibrils (Fig. 3B and D).
FIG. 3.
Scanning electron microscopy of C. neoformans yeast cells. Cryptococcal cells were grown in SAB medium (ATCC 24067 [A] and H99 [C]) or SAB medium with 0.5 times the MIC of voriconazole (ATCC 24067 [B] and H99 [D]). The yeast cells shown are representative of those seen for each condition. The experiment was performed twice with similar results. Scale bars represent 2 μm.
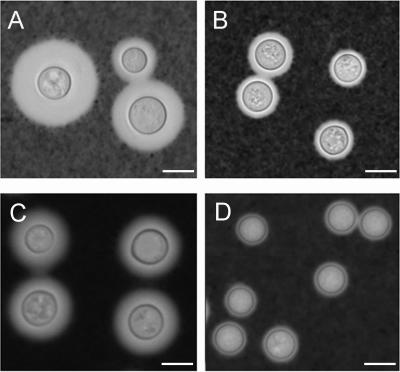
India ink analysis supported and extended the observations made by electron microscopy. Cryptococcal cells grown in SAB medium were significantly larger than yeast cells grown in medium with 0.5 times the MIC of voriconazole (Fig. 4). Measurements of the yeast cell and capsule size were performed. For both ATCC 24067 and H99, C. neoformans had larger cellular and capsular volumes when grown without antifungals relative to yeast cells grown with voriconazole (Table 3). For example, the average capsular volume of ATCC 24067 was six times greater for yeast grown without voriconazole than for cells grown with 0.5 times the MIC of the antifungal. The average H99 cell volume exclusive of the polysaccharide capsule was reduced in half when yeast cells were grown with voriconazole.
FIG. 4.
India ink analysis of C. neoformans yeast cells. Cryptococcal cells were grown in SAB medium (ATCC 24067 [A] and H99 [C]) or SAB medium with 0.5 MIC voriconazole (ATCC 24067 [B] and H99 [D]). The yeast cells shown are representative of those seen for each condition. Similar results were obtained in three separate experiments. Scale bars represent 5 μm.
TABLE 3.
Volume measurements for C. neoformans strains ATCC 24067 and H99 grown in medium alone or in medium containing 0.5 times the MIC of voriconazole
| Strain | Cell |
Capsule |
||
|---|---|---|---|---|
| Mean vol ± SDa | Pb | Mean vol ± SDc | Pb | |
| 24067 | 132.1 ± 62.9 | 635.5 ± 225.7 | ||
| 24067 + voriconazole | 89.5 ± 38.5 | <0.001 | 137.8 ± 59.4 | <0.001 |
| H99 | 184.4 ± 52.4 | 181.0 ± 78.3 | ||
| H99 + voriconazole | 109.0 ± 35.3 | <0.001 | 145.1 ± 53.2 | <0.001 |
Cell volume is the measurement of the cell excluding the capsule.
P values were calculated by using the Student's t test (n = 50) comparing the results for an individual strain with or without voriconazole.
Capsule volume was determined by subtracting the cell volume size from the total cell size inclusive of the polysaccharide capsule.
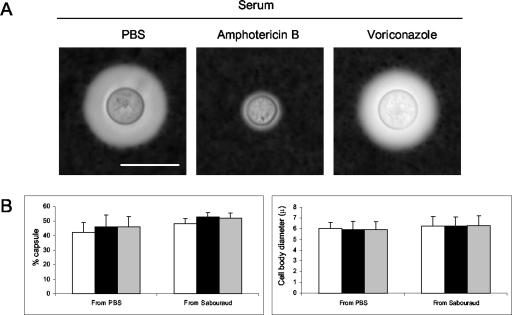
In contrast, capsule induction in the presence of serum was not inhibited in the presence of voriconazole at 0.5 or 1 times the MIC (Fig. 5). Voriconazole did not reduce capsule growth in serum even when C. neoformans was starved in PBS to deplete stored sterols (Fig. 5B). We measured both capsule size and cell size, and we did not observe any effect on any of these parameters. Similar to prior published results with cryptococcal cells grown in 10% FCS (27), the capsules of cells grown with amphotericin B remained small (Fig. 5A). Amphotericin B blocked capsular growth of melanized and nonmelanized cells, whereas the sizes of the capsules of cells grown with voriconazole were similar to that of cells grown in the absence of antifungals (results not shown).
FIG. 5.
Effect of amphotericin B and voriconazole on capsule growth. (A) C. neoformans strain H99 cells grown to logarithmic phase in SAB medium were transferred to PBS with 10% serum in the absence or presence of amphotericin B (0.067 times the MIC) or voriconazole (1 timers the MIC). Large capsules were seen with cells grown without antifungal agents (PBS) and voriconazole. The capsules of cells grown with amphotericin B were small. Representative images were taken after suspension of cells in India ink. Scale bar, 10 μm. (B) Cells were grown as in panel A but were subsequently transferred to fresh SAB medium or starved in PBS for 24 h and then incubated in 10% serum in PBS (□) or in the same medium containing voriconazole at 0.5 (▪) or 1 (░⃞) times the MIC. The cells were incubated overnight prior to determinations of capsule and cell size (n = 20 to 40 cells). No differences were seen between cells grown in either PBS or SAB medium with or without voriconazole. The experiment was repeated twice with similar results.
Measurement of capsular polysaccharide.
The concentration of soluble GXM was measured from cultures of ATCC 24067 and H99 grown with or without 0.5 times the MIC of voriconazole. Growth determinations were performed for the cultures, and no differences in growth rates were seen (data not shown). No differences in GXM concentration were found after we normalized the measurements of GXM by the number of organisms per milliliter for each condition (ATCC 24067 at 3.7 × 10−7 μg of GXM/cell density versus voriconazole at 3.4 × 10−7 μg of GXM/cell density; H99 at 5.0 × 10−7 μg of GXM/cell density versus with voriconazole at 5.1 × 10−7 μg of GXM/cell density).
Phagocytosis assays.
No differences in the phagocytosis of between C. neoformans yeast grown with or without 0.5 times the MIC of voriconazole were found (Table 4). Significantly more cells were phagocytosed in the presence of MAb 18B7 than with complement-derived opsonins. Complement was a less effective opsonin compared to MAb in these experiments.
TABLE 4.
Phagocytic index for strains of C. neoformans grown with or without 0.5 times the MIC of voriconazolea
| Strain | Drugb MICb | Mean phagocytic index ± SD |
||
|---|---|---|---|---|
| Controlc | MAb 18B7 | Complementd | ||
| ATCC 24067 | - | 0.027 ± 0.01 | 3.6 ± 0.3 | 0.14 ± 0.07 |
| 0.5 | 0.034 ± 0.01 | 3.2 ± 0.4 | 0.16 ± 0.03 | |
| H99 | - | 0 | 3.5 ± 0.7 | 0.18 ± 0.10 |
| 0.5 | 0.08 ± 0.01 | 4.0 ± 0.4 | 0.18 ± 0.02 | |
Each value represents the results from three wells. The experiment was performed three times with similar results. The phagocytic index is the number of organisms engulfed divided by number of macrophages.
Indicates whether voriconazole was absent (-) or present at a concentration of 0.5 times the MIC for the specific strain.
The phagocytosis assay was performed in the absence of opsonins.
Complement from 20% FCS (not heat inactivated).
DISCUSSION
Current treatments for many fungal diseases are inadequate. Voriconazole has recently been used for the treatment of several difficult-to-treat fungal infections, including refractory cryptococcal meningitis (19, 30). Although the overall success rate for voriconazole against refractory cryptococcosis was only 39%, the antifungal resulted in stabilization of the patients, as demonstrated by serology studies, and >90% of patients were alive at the 3-month follow-up (30). In comparison to amphotericin B, fluconazole, and itraconazole, voriconazole has the lowest MIC for C. neoformans (9, 17, 22, 25, 31, 38). Limited animal studies have shown that voriconazole is effective in reducing C. neoformans CFU in lung and brain tissues in pulmonary and intracranial models, respectively (C. Hitchcock, A. Rj, B. Lewis, and P. Troke, Abstr. 35th, Intersci. Conf. Antimicrob. Agents Chemother., abstr. F75, 1995).
In contrast to our previous studies that showed itraconazole and fluconazole were fungistatic (Van Duin et al., Abstr. 102nd Gen. Meet. Am. Soc. Microbiol. 2002), our time-kill assays demonstrated that voriconazole was fungicidal. The concentration of voriconazole required for cidality was 2 logs greater than the MIC for the strains tested, whereas amphotericin B killed >80% of nonmelanized cells when used at twice the MIC (Table 1 and Fig. 1). Melanization of C. neoformans did not affect the activity of voriconazole. This is important since melanization significantly attenuates the effectiveness of amphotericin B and caspofungin, an echinocandin, against both C. neoformans and Histoplasma capsulatum (18, 36). The production of melanin-like pigments in vitro is associated with virulence in C. neoformans (reviewed in reference 26), and melanin synthesis occurs during human infection (28). Cryptococcal melanin protects the organism against damage by microbicidal peptides, UV light, oxidants, extremes in temperature, and macrophages in vitro (reviewed in reference 4). Preincubation of voriconazole with melanin did not affect its fungicidal activity, but preincubation significantly reduced the efficacy of amphotericin B. Elemental analysis of the melanins incubated with the antifungals showed that exposure of the polymer to voriconazole had no effect on the C/N ratio of melanin, whereas incubation with amphotericin B altered the C/N ratio. The preincubation time-kill experiments and chemical analysis of the melanins demonstrate that amphotericin B is bound by melanin, whereas voriconazole is not. Hence, voriconazole, like fluconazole and itraconazole (18, 36), is not bound by melanin, suggesting that absence of melanin binding may be a property of azole-type drugs.
Although exposure of C. neoformans to subinhibitory concentrations of voriconazole did not affect the growth rate in SAB medium, voriconazole altered cellular and capsular size (Table 3). Scanning electron microscopy revealed that the appearance of the polysaccharide capsule of the fungus was significantly altered by growth in the presence of voriconazole. Similar effects have been reported after the growth of C. neoformans with fluconazole and amphotericin B (27). Although the mechanism for this effect with voriconazole at 0.5 times the MIC is unknown, exposure of C. neoformans to subinhibitory concentrations of fluconazole has been shown to alter the organism's lipid profile (13). Given that capsule synthesis is associated with the accumulation of vesicles in the cell wall (34), it is possible that inhibition of ergosterol synthesis by voriconazole translates into membrane deficits that interfere with the vesicular trafficking required for capsule synthesis.
Although no differences in phagocytosis between C. neoformans grown in SAB medium with or without voriconazole were seen in vitro, the reduction in capsule size in these cells may be important during infection. It is also important to note that even though the capsules of C. neoformans grown in SAB medium and exposed to voriconazole were significantly reduced in size, the anticapsular MAb was an effective opsonin, indicating that the epitopes recognized by the MAb are expressed. MAb 18B7 has completed phase I testing as an adjunct to antifungal therapy, using amphotericin B, in patients with cryptococcosis (2). Cryptococcal cells in lung tissue typically exhibit large capsules (32). Capsule regulation has been associated with virulence for C. neoformans (11), and loss of the ability to increase capsular size results in attenuation (15). Furthermore, complement deposition in cells with large capsules occurs in the internal regions of the capsule where it is a less-effective opsonin (40). Several conditions induce capsule growth in vitro (for a review, see reference 21). In a previous study, we demonstrated that serum was effective in inducing capsule growth (39). Prior induction of C. neoformans strain H99 capsule by growth in 10% FCS inhibited the inhibitory activity of voriconazole in vitro. The importance of this phenomenon is unclear since induction occurs in cryptococcal cells grown with serum under nutrient-limited conditions but not when cryptococcal cells are grown in rich media (39). The clinical relevance of the lack of capsular inhibition by voriconazole in cells grown in PBS with 10% FCS is therefore uncertain. Future studies with voriconazole in animal models developed to study capsule size in vivo (32) may provide additional information.
The polysaccharide capsule is a major virulence factor of C. neoformans that has both antiphagocytic and immunosuppressive properties (reviewed in reference 37). The observation that cells grown with voriconazole had smaller capsules when grown in SAB medium raised the possibility that the drug would enhance release of capsular polysaccharide. However, the fact that supernatants of cultures grown with or without voriconazole had similar concentrations of GXM suggests that the antifungal agent does not cause increased capsular release. During infection, cryptococcal polysaccharide accumulates in tissues (14). Released polysaccharide is a potent immunomodulator (37) and toxic to phagocytic cells (10). Previous studies have shown that subinhibitory concentrations of amphotericin B and fluconazole increase capsular release (27). Hence, the lack of increased polysaccharide release during voriconazole treatment may have an additional benefit to the host compared to amphotericin B and fluconazole.
Our results indicate that voriconazole compares very favorably relative to amphotericin B for several in vitro parameters that could affect the outcome of therapy. Whereas amphotericin B poorly penetrates into brain tissue (20) and fluconazole is generally fungistatic to C. neoformans, voriconazole penetrates into the cerebrospinal fluid (34) and can be cidal to C. neoformans. The present study shows that voriconazole is active against C. neoformans and that this effect is not affected by melanization provides another advantage of voriconazole compared to amphotericin B and caspofungin. In addition, we have shown that voriconazole can reduce cellular and capsular volume and does not increase capsular release, which may translate into an improved host cell response to the fungus. The effects of voriconazole on C. neoformans viability, melanin production, polysaccharide release, and cell and capsule size are areas ripe for future studies in animal models of cryptococcosis. Furthermore, these results support continued clinical investigations of the use of voriconazole for cryptococcosis.
Acknowledgments
A.C. was supported in part by National Institute of Health (NIH) grants AI33774, AI13342, AI52733, and HL59842. J.D.N. was supported in part by NIH grant AI52733 and AI01489. This study was also supported by an unrestricted grant from Pfizer Pharmaceuticals Group, New York, N.Y.
We thank Leslie Cummins of the Albert Einstein College of Medicine Analytical Imaging Center for technical assistance.
REFERENCES
- 1.Barry, A., S. Brown, and M. Traczewski. 2002. Broth medium for microdilution susceptibility tests of fluconazole and voriconazole. Eur. J. Clin. Microbiol. Infect. Dis. 21:407-410. [DOI] [PubMed] [Google Scholar]
- 2.Casadevall, A., W. Cleare, M. Feldmesser, A. Glatman-Freedman, D. L. Goldman, T. R. Kozel, N. Lendvai, J. Mukherjee, L.-A. Pirofski, J. Rivera, A. L. Rosas, M. D. Scharff, P. Valadon, K. Westin, and Z. Zhong. 1998. Characterization of a murine monoclonal antibody to Cryptococcus neoformans polysaccharide that is a candidate for human therapeutic studies. Antimicrob. Agents Chemother. 42:1437-1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Casadevall, A., J. Mukherjee, and M. D. Scharff. 1992. Monoclonal antibody based ELISA for cryptococcal polysaccharide. J. Immunol. Methods 154:27-35. [DOI] [PubMed] [Google Scholar]
- 4.Casadevall, A., A. L. Rosas, and J. D. Nosanchuk. 2000. Melanin and virulence in Cryptococcus neoformans. Curr. Opin. Microbiol. 3:354-358. [DOI] [PubMed] [Google Scholar]
- 5.Chan, J., Y. Xing, R. S. Magliozzo, and B. R. Bloom. 1992. Killing of virulent Mycobacterium tuberculosis by reactive nitrogen intermediates produced by activated murine macrophages. J. Exp. Med. 175:1111-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cherniak, M. R., and J. B. Sundstrom. 1994. Polysaccharide antigens of the capsule of Cryptococcus neoformans. Infect. Immunol. 62:1507-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cleare, W., and A. Casadevall. 1998. The different binding patterns of two immunoglobulin M monoclonal antibodies to Cryptococcus neoformans serotype A and D strains correlate with serotype classification and differences in functional assays. Clin. Diagn. Lab. Immunol. 5:125-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cleare, W., and A. Casadevall. 1999. Scanning electron microscopy of encapsulated and non-encapsulated Cryptococcus neoformans and the effect of glucose on capsular polysaccharide release. Med. Mycol. 37:235-243. [PubMed] [Google Scholar]
- 9.Espinel-Ingroff, A. 1998. In vitro activity of the new triazole voriconazole (UK-109,496) against opportunistic filamentous and dimorphic fungi and common and emerging yeast pathogens. J. Clin. Microbiol. 36:198-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feldmesser, M., Y. Kress, P. Novikoff, and A. Casadevall. 2000. Cryptococcus neoformans is a facultative intracellular pathogen in murine pulmonary infection. Infect. Immun. 68:4225-4237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fries, B. C., D. L. Goldman, R. Cherniak, R. Ju, and A. Casadevall. 1999. Phenotypic switching in Cryptococcus neoformans results in changes in cellular morphology and glucuronoxylomannan structure. Infect. Immun. 67:6076-6083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friese, G., T. Discher, R. Fussle, A. Schmalreck, and J. Lohmeyer. 2001. Development of azole resistance during fluconazole maintenance therapy for AIDS-associated cryptococcal disease. AIDS 15:2344-2345. [DOI] [PubMed] [Google Scholar]
- 13.Ghannoum, M. A., B. J. Spellberg, A. S. Ibrahim, J. A. Ritchie, B. Currie, E. D. Spitzer, J. E. J. Edwards, and A. Casadevall. 1994. Sterol composition of Cryptococcus neoformans in the presence and absence of fluconazole. Antimicrob. Agents Chemother. 38:2029-2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldman, D. L., S. C. Lee, and A. Casadevall. 1995. Tissue localization of Cryptococcus neoformans glucuronoxylomannan in the presence and absence of specific antibody. Infect. Immun. 63:3448-3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Granger, D. L., J. R. Perfect, and D. T. Durack. 1985. Virulence of Cryptococcus neoformans. Regulation of capsule synthesis by carbon dioxide. J. Clin. Investig. 76:508-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Graybill, J. R. 1988. Histoplasmosis and AIDS. J. Infect. Dis. 158:623-626. [DOI] [PubMed] [Google Scholar]
- 17.Hoban, D. J., G. G. Zhanel, and J. A. Karlowsky. 1999. In vitro susceptibilities of Candida and Cryptococcus neoformans isolates from blood cultures of neutropenic patients. Antimicrob. Agents Chemother. 43:1463-1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ikeda, R., T. Sugita, E. S. Jacobson, and T. Shinoda. 2003. Effects of melanin upon susceptibility of Cryptococcus to antifungals. Microbiol. Immunol. 47:271-277. [DOI] [PubMed] [Google Scholar]
- 19.Johnson, L. B., and C. A. Kauffman. 2003. Voriconazole: a new triazole antifungal agent. Clin. Infect. Dis. 36:630-637. [DOI] [PubMed] [Google Scholar]
- 20.Louria, D. B. 1957. Some aspects of the absorption, distribution, and excretion of amphotericin B in man. AM CT. 5:295-301. [PubMed] [Google Scholar]
- 21.McFadden, D. C., and A. Casadevall. 2001. Capsule and melanin synthesis in Cryptococcus neoformans. Med. Mycol. 39:19-30. [PubMed] [Google Scholar]
- 22.McGinnis, M. R., L. Pasarell, D. A. Sutton, A. W. Fothergill, C. R. Cooper, Jr., and M. G. Rinaldi. 1997. In vitro evaluation of voriconazole against some clinically important fungi. Antimicrob. Agents Chemother. 41:1832-1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mirza, S. A., M. Phelan, D. Rimland, E. Graviss, R. Hamill, M. E. Brandt, T. Gardner, M. Sattah, G. P. de Leon, W. Baughman, and R. A. Hajjeh. 2003. The changing epidemiology of cryptococcosis: an update from population-based active surveillance in 2 large metropolitan areas, 1992-2000. Clin. Infect. Dis. 36:789-794. [DOI] [PubMed] [Google Scholar]
- 24.National Committee for Clinical Laboratory Standards. 1997. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard M27-A. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 25.Nguyen, M. H., and C. Y. Yu. 1998. In vitro comparative efficacy of voriconazole and itraconazole against fluconazole-susceptible and -resistant Cryptococcus neoformans isolates. Antimicrob. Agents Chemother. 42:471-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nosanchuk, J. D., and A. Casadevall. 2003. The contribution of melanin to microbial pathogenesis. Cell Microbiol. 5:203-223. [DOI] [PubMed] [Google Scholar]
- 27.Nosanchuk, J. D., W. Cleare, S. P. Franzot, and A. Casadevall. 1999. Amphotericin B and fluconazole affect cellular charge, macrophage phagocytosis, and cellular morphology of Cryptococcus neoformans at subinhibitory concentrations. Antimicrob. Agents Chemother. 43:233-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nosanchuk, J. D., A. L. Rosas, S. C. Lee, and A. Casadevall. 2000. Melanisation of Cryptococcus neoformans in human brain tissue. Lancet 355:2049-2050. [DOI] [PubMed] [Google Scholar]
- 29.Perfect, J. R., and A. Casadevall. 2002. Cryptococcosis. Infect. Dis. Clin. N. Am. 16:837-874. [DOI] [PubMed] [Google Scholar]
- 30.Perfect, J. R., K. A. Marr, T. J. Walsh, R. N. Greenberg, B. DuPont, J. de la Torre-Cisneros, G. Just-Nubling, H. T. Schlamm, I. Lutsar, A. Espinel-Ingroff, and E. Johnson. 2003. Voriconazole treatment for less-common, emerging, or refractory fungal infections. Clin. Infect. Dis. 36:1122-1131. [DOI] [PubMed] [Google Scholar]
- 31.Pfaller, M. A., J. Zhang, S. A. Messer, M. E. Brandt, R. A. Hajjeh, C. J. Jessup, M. Tumberland, E. K. Mbidde, and M. A. Ghannoum. 1999. In vitro activities of voriconazole, fluconazole, and itraconazole against 566 clinical isolates of Cryptococcus neoformans from the United States and Africa. Antimicrob. Agents Chemother. 43:169-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rivera, J., M. Feldmesser, M. Cammer, and A. Casadevall. 1998. Organ-dependent variation of capsule thickness in Cryptococcus neoformans during experimental murine infection. Infect. Immun. 66:5027-5030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosas, A. L., J. D. Nosanchuk, B. L. Gomez, W. A. Edens, J. M. Henson, and A. Casadevall. 2000. Isolation and serological analyses of fungal melanins. J. Immunol. Methods 244:69-80. [DOI] [PubMed] [Google Scholar]
- 34.Sakaguchi, N., T. Baba, M. Fukuzawa, and S. Ohno. 1993. Ultrastructural study of Cryptococcus neoformans by quick-freezing and deep-etching method. Mycopathologia 121:133-141. [DOI] [PubMed] [Google Scholar]
- 35.Schwartz, S., D. Milatovic, and E. Thiel. 1997. Successful treatment of cerebral aspergillosis with a novel triazole (voriconazole) in a patient with acute leukaemia. Br. J. Hematol. 97:663-665. [DOI] [PubMed] [Google Scholar]
- 36.Van Duin, D., A. Casadevall, and J. D. Nosanchuk. 2002. Melanization of Cryptococcus neoformans and Histoplasma capsulatum reduces their susceptibility to amphotericin B and caspofungin. Antimicrob. Agents Chemother. 46:3394-3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vecchiarelli, A. 2000. Immunoregulation by capsular components of Cryptococcus neoformans. Med. Mycol. 38:407-417. [DOI] [PubMed] [Google Scholar]
- 38.Yildiran, S. T., A. W. Fothergill, D. A. Sutton, and M. G. Rinaldi. 2002. In vitro susceptibilities of cerebrospinal fluid isolates of Cryptococcus neoformans collected during a ten-year period against fluconazole, voriconazole and posaconazole (SCH56592). Mycoses 45:378-383. [DOI] [PubMed] [Google Scholar]
- 39.Zaragoza, O., B. C. Fries, and A. Casadevall. 2003. Induction of capsule growth in Cryptococcus neoformans by mammalian serum and CO2. Infect. Immun. 71:6155-6164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zaragoza, O., C. P. Taborda, and A. Casadevall. 2003. The efficacy of complement-mediated phagocytosis of Cryptococcus neoformans is dependent on the location of C3 in the polysaccharide capsule and involves both direct and indirect C3-mediated interactions. Eur. J. Immunol. 33:1957-1967. [DOI] [PubMed] [Google Scholar]
- 41.Zuger, A., E. Louie, R. S. Holzman, M. S. Simberkoff, and J. J. Rahal. 1986. Cryptococcal disease in patients with the acquired immunodeficiency syndrome: diagnostic features and outcome of treatment. Ann. Intern. Med. 104:234-240. [DOI] [PubMed] [Google Scholar]