Summary
Background
Diffusion-weighted imaging (DWI) is an MRI modality using strong bipolar gradients to create a sensitivity of the signal to the thermally-induced Brownian motions of water molecules and in vivo measurement of molecular diffusion. The apparent diffusion coefficient (ADC) is a quantitative parameter calculated from DWI images which is used as a measure of diffusion. DWI allows to obtain comprehensive information on morphological and functional state of the kidney during a single examination without contrast medium administration. The purpose of the study was to evaluate the value of DWI in differentiating benign and malignant solid kidney tumors based on the initial stage of the study.
Material/Methods
The study included 19 adult patients with pathologically verified renal tumors: 9 patients with clear cell subtype of the renal cell carcinoma, 5 patients with oncocytoma and 5 patients with angiomyolipoma (AML). In addition, 5 healthy volunteers with completely normal findings according to kidney ultrasound were included into this study and set as reference. All patients underwent renal MR imaging which included DWI with subsequent ADC measurement. MR imaging was performed with a 1.5 T body scanner using an eight-channel phased-array body coil.
Results
The mean ADC value of ccRCC was significantly lower than that of normal renal parenchyma (2.11±0.25×10−3 mm2/s vs. 3.36±0.41×10−3 mm2/s, p<0.01). There was a significant difference in ADC between the malignant and benign renal lesions: in patients with angiomyolipoma the ADC value was 2.36±0.32×10−3 mm2/s vs. 2.11±0.25×10−3 mm2/s; p<0.05 and in patients with oncocytoma – 2.75±0.27×10−3 mm2/s vs. 2.11±0.25×10−3 mm2/s; p<0.05. The difference in ADC values in patients with high and low ccRCC grades was observed.
Conclusions
DWI can be used to characterize renal lesions; the ADC of a renal lesion can be potentially used as an additional parameter to help determine the appropriate clinical management.
MeSH Keywords: Angiomyolipoma, Kidney Neoplasms, Magnetic Resonance Imaging
Background
Renal cell carcinoma (RCC) is the most common malignant epithelial tumor of the kidney, accounting for 85–90% of all solid renal tumors in adults and comprising 1–3% of all malignant visceral neoplasms [1]. Accurate evaluation of the renal masses is essential to ensure an appropriate case management and to assist in staging and prognosis. Currently, computed tomography (CT) and magnetic resonance (MR) imaging are the primary investigative tools for diagnosing, evaluation, and staging renal masses. The density or intensity on unenhanced imaging and the enhancement characteristics have been used in determining the nature of renal masses. For cystic renal lesions, the Bosniak classification system stratifies the CT or MR appearances with the risk of malignancy [2]. More recently, differences in enhancement characteristics of clear cell renal cancer and papillary renal cell cancer have been reported [3]. With the introduction of multichannel coils and parallel imaging, providing excellent temporal and spatial resolution, functional analysis has become possible. But despite the whole development, there remain many cases for which imaging tests cannot easily differentiate benign from malignant lesions. Recent studies have shown that 16–33% of nephrectomies are performed on benign lesions [4]. The most common renal benign lesions encountered in clinical practice are angiomyolipoma (AML) and oncocytoma. AML accounts for less than 10% of renal tumors, with autopsy series and ultrasound-screened populations showing a 0.3% incidence in the general population. Renal oncocytoma is the most common benign tumor, it accounts for 3% to 7% of kidney tumors [5,6].
Diffusion-weighted imaging (DWI) is an MR modality using strong bipolar gradients to create a sensitivity of the signal to the thermally-induced Brownian (or random walk) motion of water molecules and in vivo measurement of molecular diffusion [7]. The apparent diffusion coefficient (ADC) is a quantitative parameter calculated from DWI images which is used as a measure of diffusion. The ADC depends mainly on the choice of the underlying b-values. The lower the b-value applied, the higher the resulting ADC value. Image interpretation can be performed qualitatively by visual assessment of the DWI images and the corresponding ADC map, and quantitatively by measuring the ADC value of the lesion. This ADC combines the effects of capillary perfusion and water diffusion in the extracellular extravascular space, providing simultaneous information on perfusion and diffusion in any organ [8]. Diffusion-weighted imaging has been extensively used in neuroradiology for differentiating benign from malignant brain tumors and determining the grade of astrocytoma [9]. Recent studies assessed the value of DWI in renal mass evaluation [10,11].
Currently, nearly all available clinical systems (1.5 T and 3 T) have the capability of performing DWI examinations in addition to morphological/anatomical imaging. For genitourinary tumors, most DWI acquisitions are performed in the axial plane either in “free-breathing” or with “respiratory triggering” in addition to the conventional MRI sequences, with an extra time of approximately 4 min for the former and 10 min for the latter. DWI allows to obtain comprehensive information on morphological and functional state of the kidney during a single examination without contrast medium administration [12].
Taking into account recently reported concerns regarding the development of nephrogenic systemic fibrosis in patients with renal insufficiency who undergo contrast-enhanced MR imaging and given the risk of contrast material-induced nephropathy with contrast-enhanced CT, there is a growing interest to assessment of the nonenhanced imaging modalities that might be useful for characterizing renal lesions [13–15]. The purpose of our study was to evaluate the value of DWI in differentiating benign and malignant solid kidney tumors.
Material and Methods
This retrospective study was conducted in accordance with the guidelines of the local ethics committee that had approved it. Written informed consent for participation in this study was signed by all included patients.
The study included 19 adult patients aged 42 to 73 years (7 women and 12 men; mean age: 59.5 years, creatinine <1.5 mg/dL) with renal tumors previously diagnosed by US and/or CT. All patients underwent renal MR imaging which included diffusion-weighted imaging between February 2012 and January 2014. In patients diagnosed with malignant renal tumors, a consecutive partial or radical nephrectomy was performed, pathologically described as clear cell subtype of the RCC (n=9). Two tumors in grade I, 3 tumors in grade II, 2 tumors in grade III and 3 tumors in grade IV (according to the Fuhrman gradation system) in patients with clear cell RCC (ccRCC) were diagnosed by pathological examination. In patients with radiological signs suggesting presence of benign renal lesions consecutive percutaneous puncture biopsy was performed, revealing AML (n=5) and oncocytoma (n=5) evidenced by pathological examination. In addition, 5 healthy volunteers (2 women, 3 men; mean age: 58.6 years) with completely normal findings according to kidney US were included into this study and set as reference.
The imaging results were taken from the database of the Urology Department of Lviv National Medical University and from the database of the Euroclinic Medical Center, Lviv, Ukraine. The study included the examination results of all participants meeting the inclusion and exclusion criteria from these two medical facilities. Excluded criterions were as follows: metal parts in the patient’s body; other than ccRCC histological subtype; multifocal and/or cystic renal lesion; no DWI series; poor quality of DW image with obvious artifacts. Anti-tumor therapy and biopsy were not performed prior to MRI and surgery, for all patients.
MR imaging was performed with a 1.5 T body scanner (Signa HDxt, General Electric, USA) using an eight-channel phased-array body coil. MR Imaging Protocol for renal masses included such series:
Coronal T2-weighted single-shot fast spin-echo (SSFSE), repetition time (TR)=2625 ms, echo time (TE)=90 ms, flip angle=90°, field of view=40×40 cm, matrix=200×192, breath-hold, supplying valuable T2-weighted information;
Axial 2D fast imaging employing steady-state acquisition with fat saturation (FIESTA FAT SAT), TR=4.1 ms, TE=1.8 ms, flip angle=90°, field of view=40×40 cm, matrix=224×320, – ultrafast pulse sequence that provides high-resolution images with outstanding image contrast and high signal-to-noise ratio (SNR) relative to the SSFSE. Compared with other steady-state pulse sequences, the FIESTA sequence does not subject to excessive signal saturation or motion artifacts and offers an excellent image;
Axial DWI with the following parameters: TR=12000 ms, TE=90 ms, field of view=40×40 cm; matrix=200×192; NEX=3; bandwidth=250 kHz, diffusion direction=slice, slice thickness=6.0 mm, interscan gap=1.0 mm with b-value=600 s/mm2), acquisition time=17 s. DWI was conducted before contrast media administration, using single-shot echo-planar imaging sequence with parallel imaging technique and fat saturation during one breath-hold;
Axial T1-weighted fast spoiled gradient-recalled echo dual-echo (FSPGR-DE), TR=130 ms, TE=2.1 ms and 4.3 ms, flip angle=70°, field of view=43×43 cm, matrix=320×192, breath-hold;
Axial T2-weighted fast-recovery fast spin-echo (FRFSE), TR=8750 ms, TE=78 ms and 132 ms, flip angle=90°, field of view=44×44 cm, matrix=384×192;
Sagital T2-weighted SSFSE, TR=1760 ms, TE=87.4 s, flip angle=90°, field of view=37×37 cm, matrix=384×256;
Axial 3D fat-saturated T1-weighted spoiled gradient echo liver acquisition with volume acquisition (LAVA), TR=4.5 ms, TE=2.2 ms, flip angle=15°, field of view=38×38 cm, matrix=320×192, during, and following administration of gadopentetate dimeglumine, in a dose of 0.1 mmol/kg of body weight as a bolus injection with 20 s between each breath-hold acquisition. This technique combines contrast-enhanced, multi-phase imaging of the abdomen with high resolution, large coverage and uniform fat suppression. In one breath hold, LAVA acquires a stack of overlapping thin slices with high in-plane resolution. The usual protocol repeats this acquisition three or more times. In this way, LAVA produces images of the arterial and venous phases that not only precisely depict anatomy and contrast uptake, but also contain vascular information, easily revealed by a maximum intensity projection post-processing.
The signal intensity of the tumors on DWI was classified as high, iso-, and low signal intensity when compared with contralateral parenchyma. Color ADC map was generated automatically at the workstation (Advantage Windows, GE Healthcare). The ADC was calculated with linear regression analysis of the function S=S0 * exp(–b * ADC), where S is the signal intensity after application of the diffusion gradient and S0 is the signal intensity on the DW image acquired at b=0 sec/mm2. The region of interest (ROI) was placed within a portion of the solid area where the minimum ADC value on the ADC map was registered according to the color by visual inspection. An average of two to three measurements per lesion were performed, depending on the lesion size. The ROI was either circular or elliptical with the area of 60–250 mm2. Necrotic regions were identified with conventional MRI sequences and were avoided for ROI placement. For comparison, the ROI placed in the tumor was copied and then placed on the normal parenchyma of the contralateral kidney in the same site in relation to tumor, and in the corresponding upper or lower pole if the tumor was remarkably bulgy outside the contour of the kidney. The mean ADC value was recorded within ROI.
Fat-containing AMLs were diagnosed by using established criteria based on findings of in-phase and out-of-phase T1-weighted imaging with and without frequency-selective fat suppression.
Functool software was used for ADC map generation and measurements, SPSS 22.0 software was used for data processing. The ADC value was expressed as mean + standard deviation. Statistical significance was considered when P value was <0.05.
Results
All renal lesions had the maximal diameter greater than 3 cm, with median size of 5.6±2.2 cm (range from 3.0 to 13.5 cm). In 2 patients with ccRCC the size of the tumor was <4 cm, in 4 patients the tumor size was from 4 to 7 cm, in 2 patients it was from 7 to 10 cm and in one – exceeded 10 cm. On MRI images tumors showed round, oval or large and irregular shape. All DW images clearly demonstrated tumor lesions without obvious magnetic susceptibility artifacts. In patients with ccRCC in 6 cases homogenous signal intensity was observed and in the remaining 3 patients heterogeneous signal intensity was stated due to the necrotic components of the tumor. Lesions of ccRCC mainly showed high signal intensity on DWI contributed by solid components. Those regions had low signal intensity on ADC maps (Figures 1 and 2).
Figure 1.
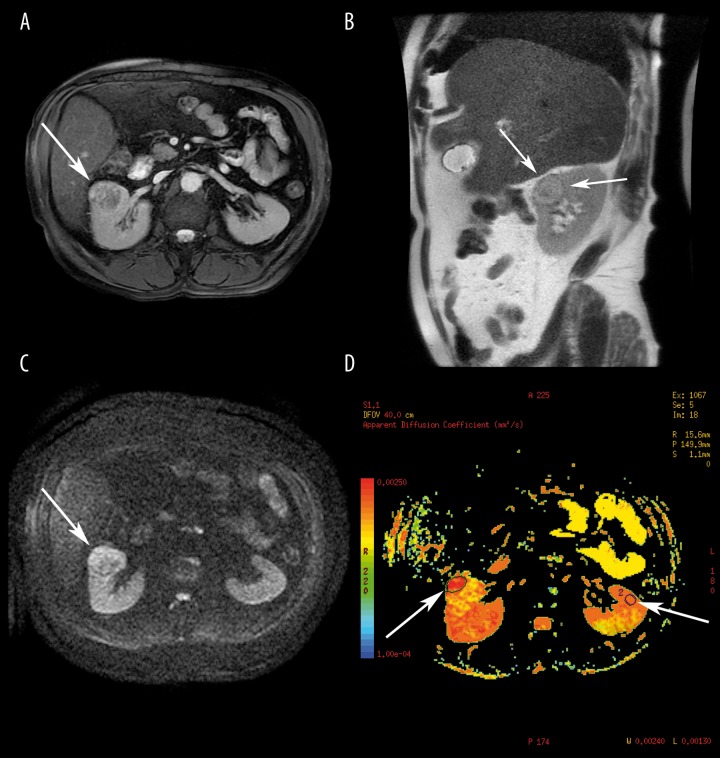
Abdominal MRI of a 65-year-old man with pathologically proven clear cell renal cell carcinoma, Fuhrman grade II. (A) Definite heterogeneous lesion of the anterior part of the right kidney (arrow) on axial FIESTA scan with fat saturation with areas of iso- and hypointensity. (B) On sagittal T2-weighted SSFSE, an isointense lesion of the medium renal segment with well-defined hypointense pseudocapsule (arrows) and central zone. (C) Diffusion-weighted MR, b-value=600 s/mm2, inhomogeneous area with peripheral zone of hyperintesity (arrow). (D) ADC map with hypointense area in the right kidney (left arrow) corresponding to the hyperintese zone on DWI image, ADC=2.19×10−3 mm2/s. The right arrow is pointing to ROI with normal left kidney parenchyma, ADC=3.32×10−3 mm2/s.
Figure 2.
Abdominal MRI of a 73-year-old woman with pathologically proven clear cell renal cell carcinoma, Fuhrman grade III. (A) Axial T2-weighted FRFSE, a large inhomogeneous lesion of the right kidney with area of hyperintensity in the posterior segment (arrow). (B) Diffusion-weighted MR, b-value=600 s/mm2, area of defined hyperintesity in the posterior segment of the right kidney (arrows). (C) ADC map with ROI over the hypointense area in the posterior segment of the right kidney (arrow) corresponding to hyperintense zone on DWI image, in that area ADC had the lowest value: 2.13×10−3 mm2/s.
The mean ADC value of ccRCC was significantly lower than that of normal renal parenchyma (2.11±0.25×10−3 mm2/s vs. 3.36±0.41×10−3 mm2/s; p<0.01). There was a significant difference in ADC between the malignant and benign renal lesions: in patients with angiomyolipoma the ADC value was 2.36±0.32×10−3 mm2/s vs. 2.11±0.25×10−3 mm2/s; p<0.05 and in patients with oncocytoma – 2.75±0.27×10−3 mm2/s vs. 2.11±0.25×10−3 mm2/s; p<0.05. A slight difference was observed in ADC values among patients with different ccRCC grades, though it appeared unreliable (p>0.05). Table 1 shows the ADC values of normal renal parenchyma in comparison with ccRCC (overall and according to Fuhrman gradation system), AML and oncocytoma (Table 1).
Table 1.
Mean ADC values of normal renal parenchyma, ccRCC, angiomyolipoma and oncocytoma.
| Pathologic types/grades (cases) | Mean tumor size, cm | Mean ADC value (×10−3 mm2/s) |
|---|---|---|
| Normal renal parenchyma (n=5) | – | 3.36±0.41 |
|
| ||
| Clear cell RCC (n=9) | 7.3 | 2.11±0.25 |
| Grade I (n=2) | 4.6 | 2.26±0.42 |
| Grade II (n=3) | 5.2 | 2.20±0.39 |
| Grade III (n=2) | 13.5 | 2.15±0.52 |
| Grade IV (n=2) | 6.1 | 2.09±0.45 |
|
| ||
| Angiomyolipoma (n=5) | 4.3 | 2.36±0.32 |
|
| ||
| Oncocytoma (n=5) | 5.1 | 2.75±0.27 |
Discussion
Solid renal masses, which consist of predominantly enhancing tissue, may be either benign or malignant. Benign lesions encountered in the clinical practice include angiomyolipoma and oncocytoma. RCC is the most common malignant tumor of the kidney, but other lesions such as transitional cell carcinoma and lymphoma might also present as solitary solid primary renal masses [16,17].
To determine whether the solid or cystic renal mass is benign or malignant, it is initially examined with the use of US, CT, MRI, or a combination of these imaging modalities. MRI offers a valuable alternative to US and CT in the evaluation of renal masses. The emerging use of nephron-sparing surgery and laparoscopic surgery for renal cancer has put more demands on the preoperative imaging work-up of kidney tumors. The application of DWI in assessing tumor grade has been extensively reported on in the neurological literature [18].
Although conventional cross-sectional imaging identifies a malignant renal lesion with high accuracy, in specific cases the differentiation between renal cell carcinoma and benign solid lesions remains challenging or even impossible. In such cases, DW-MRI might be useful due to different ADC values observed in benign and malignant lesions as well as in tumor subtypes due to their different cellularity [19].
Muller et al. [20] reported an ADC value for normal renal parenchyma between 2.88±0.65×10−3 mm2/s and 3.56±0.32×10−3 mm2/s; Cova et al. [21] reported a mean ADC value of 2.19±0×10−3 mm2/s. In our study we achieved a mean ADC value of 3.36±0.41×10−3 mm2/s.
Doganay et al. [19] reported mean ADC levels in patients with RCC at 2.21±0.63×10−3 mm2/s and 2.55±0.49×10−3 mm2/s in patients with oncocytoma. In our study, the mean ADC value of renal cell carcinomas was the lowest among all renal lesions: patients with RCC – 2.11±0.25×10−3 mm2/s vs. 2.75±0.27×10−3 mm2/s for oncocytoma and 2.36±0.32×10−3 mm2/s – for angiomyolipoma. We observed a slight difference in ADC values between low-grade and high-grade ccRCCs, though statistically insignificant, probably due to a small number of cases included. The obtained results correlate with the data available from other researchers [18,22].
This study had several potential limitations: patient population of subgroups was relatively small, there were no other histological subtypes of RCC (e.g. papillary, chromophobic) presented in the study. DWI should be assessed with larger groups with other types of focal lesions including cystic lesions, mixed and complex lesions.
Conclusions
DWI can be used to characterize renal lesions; the ADC of a renal lesion can be potentially used as an additional parameter to help determine the appropriate clinical management. Our study demonstrates that ADC value of the normal renal parenchyma is significantly higher compared to the tissues of kidney neoplasm. Malignant renal tumors had significantly lower ADC values in contrast to benign tumors. A difference in ADC values in various ccRCC grades was also observed. Longer examination time with larger groups of patients including histological subtypes of RCC are necessary to obtain reliable data.
References
- 1.Pantuck AJ, Zisman A, Belldegrun AS. The changing natural history of RCC. J Urol. 2001;166:1611–23. [PubMed] [Google Scholar]
- 2.Bosniak MA. The current radiological approach to renal cysts. Radiology. 1986;158:1–10. doi: 10.1148/radiology.158.1.3510019. [DOI] [PubMed] [Google Scholar]
- 3.Sun MR, Ngo L, Genega EM, et al. Renal cell carcinoma: dynamic contrast-enhanced MR imaging for differentiation of tumor subtypes – correlation with pathologic findings. Radiology. 2009;250:793–802. doi: 10.1148/radiol.2503080995. [DOI] [PubMed] [Google Scholar]
- 4.Kutikov A, Fossett LK, Ramchandani P, et al. Incidence of benign pathologic findings at partial nephrectomy for solitary renal mass presumed to be renal cell carcinoma on preoperative imaging. Urology. 2006;68:737–40. doi: 10.1016/j.urology.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 5.Dechet CB, Bostwick DG, Blute ML, et al. Renal oncocytoma: multifocality, bilateralism, metachronous tumor development and coexistent renal cell carcinoma. J Urol. 1999;162(1):40–42. doi: 10.1097/00005392-199907000-00010. [DOI] [PubMed] [Google Scholar]
- 6.Eble J, Amin MB, Young RH. Epithelioid angiomyolipoma of the kidney: a report of five cases with a prominent and diagnostically confusing epithelioid smooth muscle component. Am J Surg Pathol. 1997;21(10):1123–30. doi: 10.1097/00000478-199710000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Le Bihan D, Turner R, Douek P, et al. Diffusion MR imaging: clinical applications. Am J Roentgenol. 1992;159(3):591–99. doi: 10.2214/ajr.159.3.1503032. [DOI] [PubMed] [Google Scholar]
- 8.Le Bihan D, Breton E, Lallemand D, et al. MR imaging of intravoxel incoherent motions: application to diffusion and perfusion in neurologic disorders. Radiology. 1986;161(2):401–7. doi: 10.1148/radiology.161.2.3763909. [DOI] [PubMed] [Google Scholar]
- 9.Kono K, Inoue Y, Nakayama K, et al. The role of diffusion-weighted imaging in patients with brain tumors. Am J Neuroradiol. 2001;22:1081–88. [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang J, Tehrani YM, Wang L, et al. Renal masses: characterization with diffusion-weighted MR imaging – a preliminary experience. Radiology. 2008;247:458–64. doi: 10.1148/radiol.2472070823. [DOI] [PubMed] [Google Scholar]
- 11.Taouli B, Thakur RK, Mannelli L, et al. Renal lesions: characterization with diffusion-weighted imaging versus contrast-enhanced MR imaging. Radiology. 2009;251:398–407. doi: 10.1148/radiol.2512080880. [DOI] [PubMed] [Google Scholar]
- 12.Kim S, Jain M, Harris AB, et al. T1 hyperintense renal lesions: characterization with diffusion-weighted MR imaging versus contrast-enhanced MR imaging. Radiology. 2009;251:796–807. doi: 10.1148/radiol.2513080724. [DOI] [PubMed] [Google Scholar]
- 13.Grobner T. Gadolinium: a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol Dial Transplant. 2006;21:1104–8. doi: 10.1093/ndt/gfk062. [DOI] [PubMed] [Google Scholar]
- 14.Morcos SK, Thomsen HS, Webb JA. Contrast-media-induced nephrotoxicity: a consensus report—Contrast Media Safety Committee, European Society of Urogenital Radiology (ESUR) Eur Radiol. 1999;9:1602–13. doi: 10.1007/s003300050894. [DOI] [PubMed] [Google Scholar]
- 15.Mehran R, Nikolsky E. Contrast-induced nephropathy: definition, epidemiology, and patients at risk. Kidney Int Suppl. 2006;100:S11–15. doi: 10.1038/sj.ki.5000368. [DOI] [PubMed] [Google Scholar]
- 16.Razek AA, Farouk A, Mousa A, et al. Role of diffusion-weighted magnetic resonance imaging in characterization of renal tumors. J Comput Assist Tomogr. 2011;35:332–36. doi: 10.1097/RCT.0b013e318219fe76. [DOI] [PubMed] [Google Scholar]
- 17.Inci E, Hocaoglu E, Aydin S, et al. Diffusion-weighted magnetic resonance imaging in evaluation of primary solid and cystic renal masses using the Bosniak classification. Eur J Radiol. 2012;81(5):815–20. doi: 10.1016/j.ejrad.2011.02.024. [DOI] [PubMed] [Google Scholar]
- 18.Rosenkrantz AB, Niver BE, Fitzgerald EF, et al. Utility of the apparent diffusion coefficient for distinguishing clear cell renal cell carcinoma of low and high nuclear grade. Am J Roentgenol. 2010;195:W344–51. doi: 10.2214/AJR.10.4688. [DOI] [PubMed] [Google Scholar]
- 19.Doganay S, Kocakocë E, Cicëkci M. Ability and utility of diffusion-weighted MRI with different b values in the evaluation of benign and malignant renal lesions. Clin Radiol. 2011;66:420–25. doi: 10.1016/j.crad.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 20.Muller MF, Prasad PV, Bimmler D. Functional imaging of the kidney by means of measurement of the apparent diffusion coefficient. Radiology. 1994;193:711–15. doi: 10.1148/radiology.193.3.7972811. [DOI] [PubMed] [Google Scholar]
- 21.Cova M, Squillaci E, Stacul F, et al. Diffusion-weighted MRI in the evaluation of renal lesions: preliminary results. Br J Radiol. 2004;77:851–57. doi: 10.1259/bjr/26525081. [DOI] [PubMed] [Google Scholar]
- 22.Sandrasegaran K, Sundaram CP, Ramaswamy R, et al. Usefulness of diffusion-weighted imaging in the evaluation of renal masses. Am J Roentgenol. 2010;194:438–45. doi: 10.2214/AJR.09.3024. [DOI] [PubMed] [Google Scholar]