Abstract
Background
Despite much epidemiological research on brain cancer in the United States, the etiology for the various subtypes remains elusive. The black population in the United States currently experiences lower incidence but higher survival rates when compared to other races. Thus, the aim of this study is to analyze the trends in incidence and survival for the 6 most common primary brain tumors in the black population of the United States.
Material/Methods
The Surveillance, Epidemiology, and End Results (SEER) database was utilized in this study to analyze the incidence and survival rates for the 6 most common brain tumor subtypes. Joinpoint 3.5.2 software was used to analyze trends in the incidence of diagnosis from 1973 to 2008. A Kaplan-Meier curve was generated to analyze mean time to death and survival at 60 months.
Results
Joinpoint analysis revealed that per year the incidence of brain cancer in the U.S. black population increased by 0.11 between 1973 and 1989. After this period, a moderate decrease by 0.06 per annum was observed from 1989 to 2008. Lymphoma was the most common primary tumor subtype for black individuals ages 20–34, and glioblastoma was identified as the most common tumor subtype for black individuals in the age groups of 35–49, 50–64, 65–79, and 80+.
Conclusions
This population-based retrospective study of brain cancer in black adults in the United States revealed significant sex and age differences in the incidence of the 6 most common brain tumor subtypes from 1973 to 2008.
MeSH Keywords: African Americans, Brain Neoplasms, Epidemiology
Background
In the United States, 15–20 individuals for every 100 000 annually receive a diagnosis of brain cancer [1]. The primary brain tumors that constitute this cancer category continue to gain the attraction of biomedical investigators and clinicians for a multitude of reasons. Much of the current interest in primary brain tumors stems from past endeavors that focused on identifying overall incidence and survival rates [2–7] as well as race-associated incidence and survival [8–12]. Although ambiguity exists among past reports as to the precise incidence trends, evidence regarding the stability of a dismal prognosis for brain cancer is both overwhelming and unsettling.
The overall most common histological subtypes of primary brain cancer have been identified as follows, in order of decreasing magnitude: glioblastoma, lymphoma, non-specific astrocytoma, glioma, anaplastic astrocytoma, and meningioma [13]. Not only are the prognoses of the aforementioned subtypes alarmingly poor, but their elusive etiologies and risk factors are cause for concern. Only recently have genetic studies begun to surface that attempt to explain the molecular characteristics of certain histological subtypes of brain cancer [14–20]. Prior to discovering specific molecular differences between subtypes, knowledge regarding genetic associations of other forms of cancer [1] guided several studies to retrospectively examine relationships between the incidence of primary brain tumors and different races and ethnicities [2,13].
The results of racially-based incidence and survival data are contrary to the normal health disparity profile of the United States. Strong evidence shows that populations of white individuals experience higher incidence and death due to brain cancer in relation to Hispanic and black individuals [2,8,9,11,13]. Despite the compelling evidence of a racial disparity in primary brain tumors, incidence studies often preclude minority groups from sub-analysis [21] and many prospective studies fail to recruit multiple racial demographics or plan for sub-analysis of data within and between populations of different races [10,14,16,20].
Furthermore, past investigations of the effect of socioeconomic, genetic, or other factors on the overall prognosis of primary brain tumors [22] have been rather limited in resolution of analysis. Specifically, the incidence and survival trends within the black population with respect to factors such as sex, age, and tumor histology, are sparsely described in the literature. The purpose of this study is, therefore, to determine the 6 most common primary brain tumors specific to the U.S. black population and to analyze the incidence and survival trends within this population from 1973 to 2008, with respect to sex and age of diagnosis.
Material and Methods
The Surveillance, Epidemiology, and End Results (SEER) Program of the National Cancer Institute was utilized for this retrospective study. SEER is a program that collects data regarding cancer incidence and survival from 17 cancer registries, encompassing roughly 26% of the U.S. population [23]. Case ascertainment is estimated to be 98% [24]. Data was collected for this report via SEER*Stat 7.0.5 software. Overall incidence rates were obtained using parameters for year of diagnosis, age, sex, and major histological subtype. Inclusion criteria included black racial demographic, adults older than 20 years of age, and a confirmed diagnosis received between 1973 and 2008. Individuals meeting these specified parameters were then grouped into 15-year age groups; 20–34, 35–49, 50–64, 65–79, and 80+. Racial demographic was specifically defined by SEER as code race 02 (black), which included a stated-race of ‘African-American’, ‘black’, or ‘negro’ [25]. Exclusion criteria were specified as diagnosis of anything other than first primary brain tumor and diagnosis at autopsy. Based on these parameters, 4704 cases met the inclusion criteria; of these cases, 3272 remained after screening for exclusion criteria. Incidence and survival analyses were performed for the 6 most common histological subtypes of primary brain cancer (n=3272). All of the tumor subtypes compared in our study have been previously defined and published as standards by the Central Brain Tumor Registry of the United States (CBTRUS) [26]. Incidence was defined in terms of 1 per 100 000. Joinpoint 3.5.2 software was used to analyze trends in the incidence of diagnosis during the period 1973–2008 using a weighted mean squares methodology with random permutations to fit a model of a 0–3 joinpoints via a grid search method [23]. The year of diagnosis was plotted against the natural log-transformation of age-adjusted incidence rate for specified adult brain tumors to obtain average annual increase in incidence [23]. Kaplan-Meier (KM) survival analysis was performed with an end-point defined as death, for a time frame limited to 60 months post-diagnosis, as this was found to be the convention for brain cancer statistics (Figure 1). Log-rank analysis was performed and a Cox proportional hazards model was generated to calculate hazard ratios (HR). Survival data was calculated regardless of treatment decisions, to reflect the reality of variable treatment regimens and variations in patient compliance. All statistical analyses were performed with 95% confidence intervals (CI); statistical significance was determined at p<0.05. Cox proportional hazards model was generated via GraphPad Prism Version 5.04 (GraphPad Software Inc., 2012, La Jolla, CA, www.graphpad.com/prism).
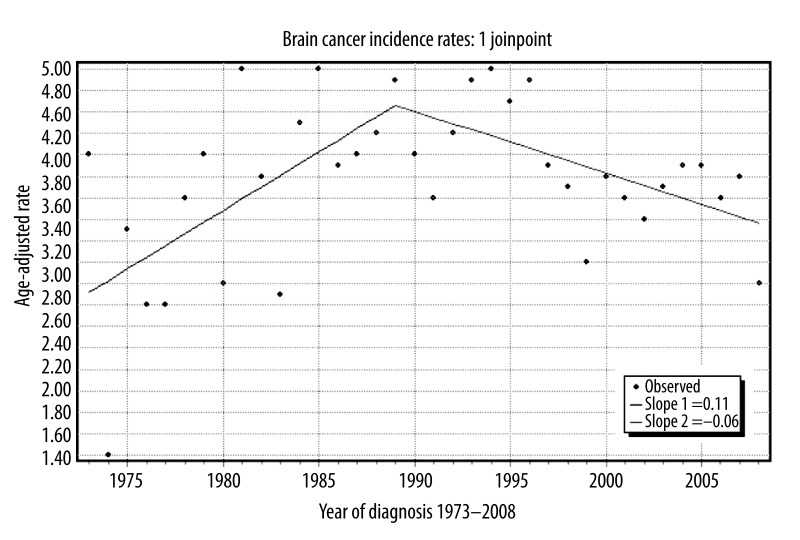
Figure 1.
Age adjusted brain cancer incidence rates per 100,000 in Black adults in the United States from 1973–2008.
Results
The 6 most common tumor subtypes for the overall black population were in agreement with previous publications. However, upon sub-analysis within the black population, differences were identified that would have been masked if we not investigated further into this population.
Incidence
Joinpoint analysis revealed that the annual rate of change for incidence of brain cancer in the U.S. black population increased by a factor of 0.11 each year between 1973 and 1989. Following this period of increasing incidence rates, a moderate decrease by a factor of 0.06 per annum was observed from 1989 to 2008 (Figure 1). Sub-analysis of tumor histology incidence uncovered unique distributions of the 6 most common brain cancer subtypes by age (Figure 2).
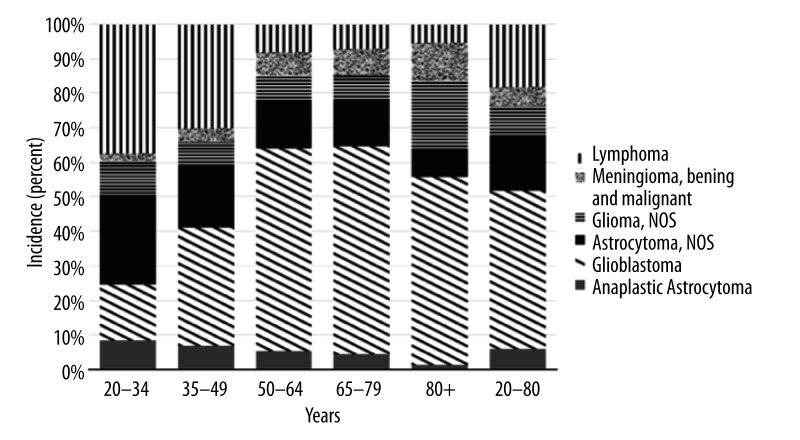
Figure 2.
Percent histology by age strata for Black adults in the United States diagnosed with brain cancer.
Incidence trends
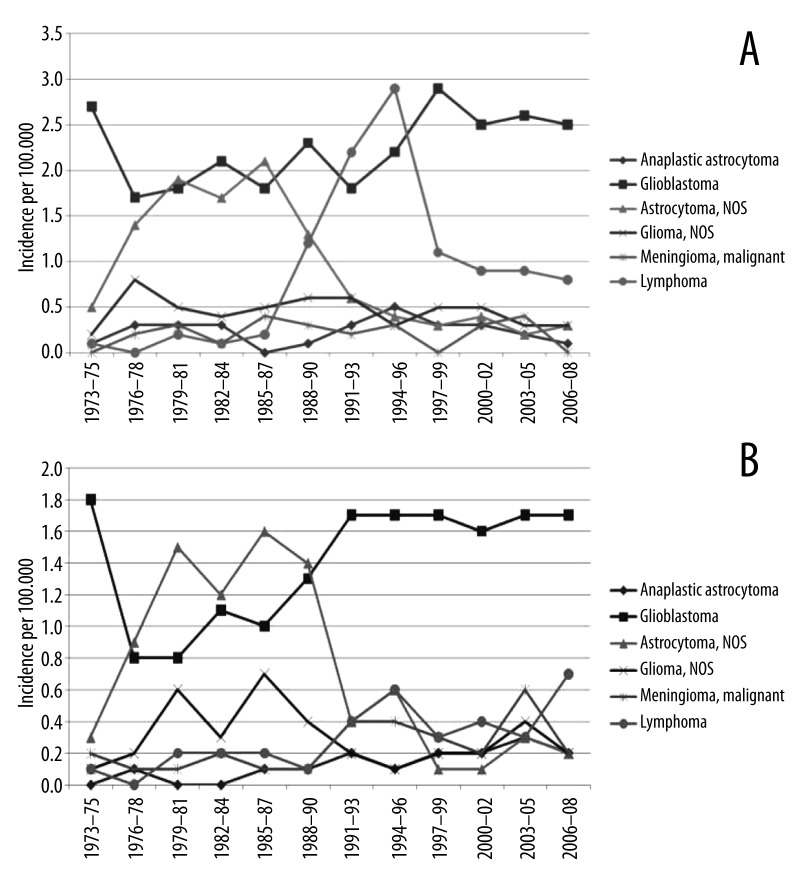
Lymphoma was found to be the most common primary tumor subtype for black individuals ages 20 to 34 years (37% of cases). In contrast, glioblastoma was identified as the most common tumor subtype for black individuals in the age groups of 35–49, 50–64, 65–79, and 80+, accounting for 34%, 58%, 60%, and 54% of cases, respectively. Further differences in the distribution of the 6 most common brain cancer subtypes by age were identified (Figure 2). Sub-analysis of incidence by sex revealed that black men had higher incidence of each brain cancer subtype except for malignant meningioma (0.19 vs. 0.34; men vs. women, respectively). Sizable differences in incidence between black men and women are those for glioblastoma (2.61 vs. 1.65), lymphoma (0.81 vs. 0.47), and non-specific astrocytoma (0.31 vs. 0.18) (Table 1). A marked increase in primary lymphomas occurred among men in the late 1980s through the 1990s when compared to women (Figure 3A, 3B; Table 1). Data also revealed a decrease in the incidence of astrocytoma during the same time period for both black men and women (Figure 3A, 3B; Table 1).
Table 1.
Brain cancer incidence rates for Black patients in the United States by gender; separate analysis was performed for time ranges 1973–2008 and 1998–2008 to account for difference in annual rate of change in incidence.
| Tumor histology | Men | Women | Total | |||
|---|---|---|---|---|---|---|
| 1973–2008 | 1998–2008 | 1973–2008 | 1998–2008 | 1973–2008 | 1998–2008 | |
| Meningioma, malignant | 0.20 | 0.19 | 0.30 | 0.34 | 0.20 | 0.28 |
| Glioma, NOS | 0.50 | 0.36 | 0.30 | 0.25 | 0.40 | 0.31 |
| Lymphoma | 1.00 | 0.81 | 0.30 | 0.47 | 0.70 | 0.65 |
| Astrocytoma, NOS | 0.80 | 0.31 | 0.60 | 0.18 | 0.70 | 0.23 |
| Anaplastic astrocytoma | 0.20 | 0.24 | 0.10 | 0.22 | 0.20 | 0.22 |
| Glioblastoma | 2.30 | 2.61 | 1.50 | 1.65 | 1.80 | 2.08 |
Figure 3.
(A) Incidence of the six most common brain cancer histological subtypes in Black men in the United States. (B) Incidence of the six most common brain cancer histological subtypes in Black women in the United States.
Survival
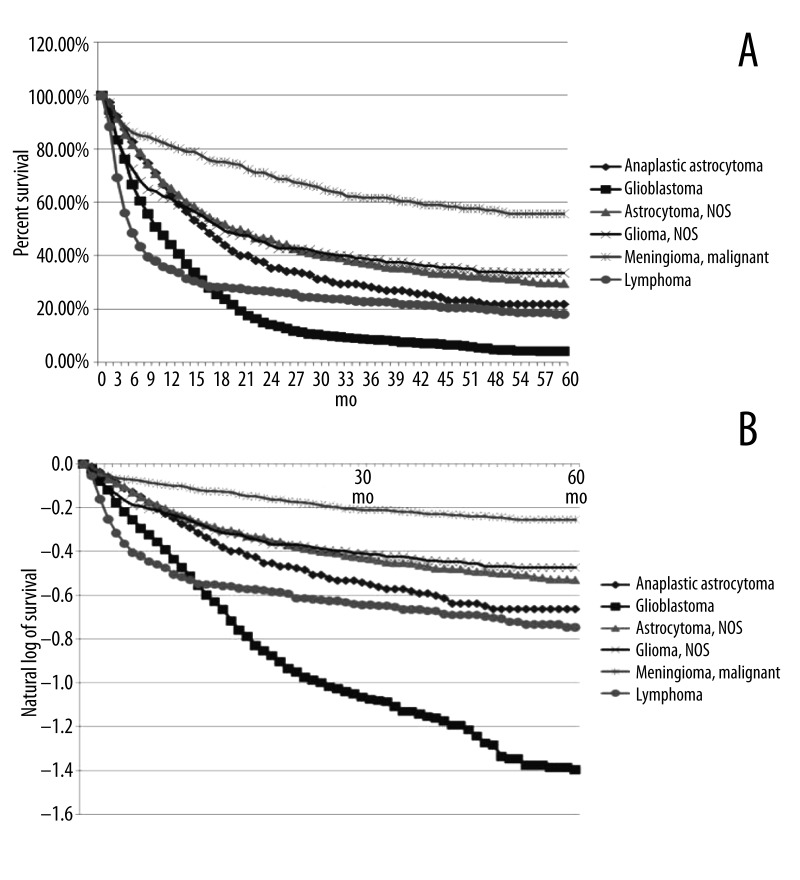
The Kaplan-Meier curve reveals differences in median time to death between the brain cancer subtypes. Our analysis showed that more than half of the patients diagnosed with lymphoma and glioblastoma died prior to 1 year after diagnosis. Half of the patients diagnosed with anaplastic astrocytoma, non-specific astrocytoma, and non-specific Glioma had died by 18 months. Interestingly, a narrow majority of the patients diagnosed with malignant meningioma survived longer than 60 months after diagnosis (Figure 4A). Survival at 60 months after diagnosis differed from the trends identified for median time to death. Our analysis shows that glioblastoma patients reached far greater mortality during the 60 months after diagnosis than any other subtype analyzed; the population of patients diagnosed with lymphoma and anaplastic astrocytoma had a similar and higher proportion of survivors at 60 months relative to glioblastoma; the population of patients diagnosed with non-specific astrocytoma and non-specific glioma attained a similar and higher proportion of survivors than the aforementioned; and the favorable survival profile of malignant meningioma patients remained at 60 months after diagnosis (Figure 4A). Meningioma cases demonstrated the most favorable long-term survival when compared to all other brain cancer subtypes; conversely, glioblastoma cases demonstrated the most dismal long-term prognosis (Figure 4A, 4B).
Figure 4.
(A) Kaplan-Meier survival curve for the six most common sub-types of brain cancer in the Black population of the United States. (B) Natural log transformed Kaplan-Meier survival curve for the six most common sub-types of brain cancer in the Black population of the United States.
Survival trends
Hazard ratios in relation to malignant meningioma were calculated to be 1.65, 2.01, 2.06, 2.79, and 5.81 for glioma, lymphoma, astrocytoma, anapestic astrocytoma, and glioblastoma, respectively (p<0.0001) (Table 2).
Table 2.
Hazard ratios of the six most common brain cancer subtypes of Black patients in the United States, as compared to malignant meningioma.
| Tumor histology | Hazard ratio | C.I. upper limit | C.I. lower limit | P value | R2 |
|---|---|---|---|---|---|
| Meningioma, malignant | 1.00 | .94 | |||
| Glioma, NOS | 1.66 | 1.72 | 1.58 | <.0001 | .85 |
| Lymphoma | 2.01 | 2.18 | 1.81 | <.0001 | .73 |
| Astrocytoma, NOS | 2.06 | 2.10 | 2.02 | <.0001 | .90 |
| Anaplastic astrocytoma | 2.79 | 2.84 | 2.73 | <.0001 | .90 |
| Glioblastoma | 5.81 | 5.83 | 5.80 | <.0001 | .94 |
Discussion
Our review of 3272 cases of primary malignant brain tumors in black individuals in the United States reveals interesting results that would have been masked had we not conducted sub-analysis within this population. Additionally, the steady increase in incidence of brain cancer that we observed in the overall U.S. black population from 1973 to 1989, followed by a moderate decrease to present day (Figure 2), is congruent with previous studies that had differing aims [2,9,11]. Past attempts to explain this peak in incidence have pointed to various associations, including: changes in histological classification of brain tumors [27], an interaction between HIV/AIDS and brain lymphoma [28–31], a transient spike in the magnitude of the elderly population, as well as increased use and quality of advanced imaging modalities [2].
Changes in histological classification and subsequent deviations in data recording is a likely contributing factor to the drop in non-specific astrocytoma diagnoses observed in our results (Figure 4A). The coincidence of the AIDS epidemic with the rise in lymphoma diagnosis that we report (Figures 1 and 4A, 4B) has been previously described [28,30,31]. Nevertheless, we do not find satisfaction in attempting to fully explain these phenomena with covariates that were not specified in our analysis. Furthermore, we do not find these associations to provide adequate explanations to the current disparities within and between racially-based sub-groups.
The contribution of increased quality of care in the United States is unlikely to be a major influence on the decline in incidence of brain cancer for the black population. Rather, it is well-documented that black patients are still at a great disadvantage in terms of access to care, which has been identified as strong evidence of the higher incidence and poorer prognoses in this population for almost all other types of cancer [22,32,33]. Although access to care is certainly a problem that warrants great resources and attention, the reversed disparity profile for brain cancer may suggest that the mechanisms behind the different primary brain tumors depend more strongly on other factors. Alternatively, the reversal of the typical disparity profile may reflect a negative, unintended, consequence of the overuse of imaging by those with high access to healthcare [34–36], which results in an increase in the only well-known risk factor for brain cancer – ionizing radiation [37].
Recent research has identified several molecular alterations that are consistent with incidence risk [14–16,19,20,38], as well as treatment response and survival [18,38–41] of certain brain cancers. None of these recent genetic studies regarding brain cancer implemented sub-analysis of the black population. The 2 studies that did compare racial differences limited their analysis to the Caucasian and Asian populations and were not conducted with patients from the United States [16,20].
Performing sub-analysis and molecular level comparisons between populations with lower incidence and higher survival (i.e., black brain cancer patients) and those with particularly high incidence and low survival (i.e., white brain cancer patients) may increase the speed at which important etiological discoveries are made. For instance, a recent study found an interesting occurrence of a 42% reduction of risk for glioma in patients with a history of diabetes [38]. Not only do blacks have lower incidence and higher survival in brain cancer, it has also been well established that the higher trend of diabetes in blacks in the U.S. continues [42]. Therefore, it would be beneficial to identify and compare polymorphisms between blacks and whites in an attempt to uncover possible genetic alterations and variations in signaling pathways that may guide future research and treatments.
Furthermore, recently identified germ-line and gene-gene interactions in brain cancers [15,19] may explain the racial clustering of incidence. We believe that it would be wise to begin exploring the genetic mechanisms of the 6 most common brain cancer subtypes in black adults in the United States, by age and sex, using results from our study as a guide for comparisons. Variations between these subtypes according to age and sex may help to identify characteristics that influence etiology, lower risk, and improved responses to treatments, as the black population has lower incidence and higher survival of brain cancer.
From our data it is apparent that differences in brain cancer incidence and survival exist beyond the level of race alone. Important differences have been identified within the black population that should be used to direct future research regarding brain cancer. It is likely that comparisons between the black and white populations in the United States at various levels (e.g., population, molecular) will yield interesting results that will enhance our understanding and treatment of brain cancer.
The present study has several limitations, including limited data available from the SEER database and the limitations inherent in all retrospective and exploratory investigations. Also, despite the high regard given to the SEER database due to its quality and breadth of representation, it is not without limitation. Those limitations that may have influenced this study include the coding for risk factors associated with primary central nervous system lymphoma (PCNSL), including congenital disorders, iatrogenic immunosuppression, HIV status, incomplete data, and the lack of central pathogenic review [23,28]. National and state population estimates are provided by the Census Bureau to estimate the years between actual censuses. Inter-census population estimates are based on numbers from updated death and birth data and are subject to increased inaccuracies compared to estimates of actual counts. Although these population estimates are thought to be the most precise way of generating these data, errors in estimates have the potential to amplify over time [9]. Nonetheless, SEER provides the largest national cancer database for obtaining the large sample sizes required to supply population-based estimates of the end results of research, clinical trials, incidence, and survival information [2].
There was a visible discrepancy in meningioma incidence rates between CBTRUS and this study. CBTRUS reported the incidence of meningioma as 7.5 per 100 000 in the adult black population [13], whereas our analysis calculated an incidence of 0.2. This discrepancy can be explained by the requirement for SEER mandatory reporting to only include malignant tumors, whereas the higher rate published by CBTRUS also takes into account benign meningiomas [25]. Malignant meningiomas have been estimated as accounting for only 1–3% of the total cases of meningiomas elsewhere [43].
Conclusions
In response to our results, as well as new molecular-level research regarding brain cancer, we believe it would be wise to conduct prospective studies that incorporate racial sub-analysis and comparisons. This population-based retrospective study of brain cancer in black adults in the United States revealed sex and age differences in the incidence of the 6 most common brain tumor subtypes from 1973 to 2008. Each histological subtype also exhibited unique survival outcomes. We feel as though the paradoxical relationship between health care access and brain cancer incidence, on the population-level, warrants quantitative investigation into imaging exposures and the incidence of brain cancer sub-types. Furthermore, we find the recent data regarding molecular alterations in certain brain tumor subtypes to be an exciting new avenue for brain cancer research – one that may benefit from our data regarding differences found within the black population – for future study design. Ultimately, we believe that identifying the differences among cancer subtypes within the black population will help to elucidate etiologies for certain brain cancers through further comparison studies, which will influence the development of more targeted treatments, identification of better predictors of prognosis, and discovery of preventable risks that will be of benefit to all individuals, regardless of race.
Footnotes
Source of support: Departmental sources
References
- 1.Freedman J. Brain Cancer: Current and Emerging Trends in Detection and Treatment. The Rosen Publishing Group; 2009. [Google Scholar]
- 2.Deorah S, Lynch C, Sibenaller Z, Ryken T. Trends in brain cancer incidence and survival in the United States: Surveillance, Epidemiology, and End Results Program, 1972 to 2001. Neurosurg Focus. 2006;20:1–7. doi: 10.3171/foc.2006.20.4.E1. [DOI] [PubMed] [Google Scholar]
- 3.Johannesen TB, Angell-Anderson E, Tretli S, et al. Trens in incidence of brain and central nervous system tumors in Norway, 1970–1999. Neuroepidemiology. 2004;23(3):101–9. doi: 10.1159/000075952. [DOI] [PubMed] [Google Scholar]
- 4.Legler JM, Ries LAG, Smith MA, et al. Cancer surveillance series [corrected]: brain and other central nervous system cancers: recent trends in incidence and mortality. J Natl Cancer Inst. 1999;91(16):1382–90. doi: 10.1093/jnci/91.16.1382. [DOI] [PubMed] [Google Scholar]
- 5.McKinley BP, Michalek AM, Fenstermaker RA, Plunkett RJ. The impact of age and sex on the incidence of glial tumors in New York state from 1976 to 1995. J Neurosurg. 2000;93(6):932–39. doi: 10.3171/jns.2000.93.6.0932. [DOI] [PubMed] [Google Scholar]
- 6.Modan B, Wagener DK, Feldman JJ, et al. Increased Mortality from Brain Tumors: A Combined Outcome of Diagnostic Technology and Change of Attitude toward the Elderly. Am J Epidemiol. 1992;135(12):1349–57. doi: 10.1093/oxfordjournals.aje.a116246. [DOI] [PubMed] [Google Scholar]
- 7.Polednak AP. Re: “Increased mortality from brain tumors: a combined outcome of diagnostic technology and change of attitude toward the elderly”. Am J Epidemiol. 1994;140:1138–43. doi: 10.1093/oxfordjournals.aje.a117214. [DOI] [PubMed] [Google Scholar]
- 8.Mariotto AB, Yabroff KR, Shao Y, et al. Projections of the Cost of Cancer Care in the United States: 2010–2020. J Natl Cancer Inst. 2010;103(2):117–28. doi: 10.1093/jnci/djq495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kohler BA, Ward E, McCarthy BJ, et al. Annual Report to the Nation on the Status of Cancer, 1975–2007, Featuring Tumors of the Brain and Other Nervous System. J Natl Cancer Inst. 2011;103(9):714–36. doi: 10.1093/jnci/djr077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inskip PD, Hoover RN, Devesa SS. Brain cancer incidence trends in relation to cellular telephone use in the United States. Neurooncology. 2010;12(11):1147–51. doi: 10.1093/neuonc/noq077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. Cancer J Clin. 2013;63(1):11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 12.Barnholtz-Sloan JS, Sloan AE, Schwartz AG. Racial Differences in Survival after Diagnosis with Primary Malignant Brain Tumor. Cancer. 2003;98(3):603–9. doi: 10.1002/cncr.11534. [DOI] [PubMed] [Google Scholar]
- 13.Central Brain Tumor Registry of the United States (CBTRUS) CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States 2004–2007. Hinsdale, IL: Central Brain Tumor Registry of the United States; Feb, 2011. [Google Scholar]
- 14.Egan KM, Thompson RC, Nabors LB, et al. Cancer susceptibility variants and the risk of adult glioma in the US case-control study. J Neurooncol. 2011;104(2):535–42. doi: 10.1007/s11060-010-0506-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sima X, Xu J, Li Q, et al. Gene-Gene Interactions Between Interleukin-12A and Interleukin-12B with the Risk of Brain Tumor. DNA Cell Biol. 2012;31(2):219–23. doi: 10.1089/dna.2011.1331. [DOI] [PubMed] [Google Scholar]
- 16.Xu C, Yuan L, Tian H, et al. Association of the MTHFR C677T polymorphism with primary brain tumor risk. Tumor Biol. 2013;34(6):3457–64. doi: 10.1007/s13277-013-0922-9. [DOI] [PubMed] [Google Scholar]
- 17.van den Bent MJ, Gravendeel LA, Gorlia T, et al. A Hypermethylated Phenotype is a Better Predictor of Survival than MGMT Methylation in Anaplastic Oligodendroglial Brain Tumors: A Report from EORTC Study 26951. Clin Cancer Res. 2011;17(22):7148–55. doi: 10.1158/1078-0432.CCR-11-1274. [DOI] [PubMed] [Google Scholar]
- 18.Wang X, Chen J, Liu J, et al. Gain of Function of Mutant TP53 in Glioblastoma: Prognosis and Response to Temozolomide. Ann Surg Oncol. 2014;21(4):1337–44. doi: 10.1245/s10434-013-3380-0. [DOI] [PubMed] [Google Scholar]
- 19.Melin B, Jenkins R. Genetics in glioma: lessons learned from genome-wide association studies. Curr Opin Neurol. 2013;26(6):687–92. doi: 10.1097/WCO.0000000000000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang F, Li D, Li Y, et al. Quantitative assessment of the association between TP53 Arg72Pro polymorphism and risk of glioma. Tumor Biol. 2014;35:747–51. doi: 10.1007/s13277-013-1101-8. [DOI] [PubMed] [Google Scholar]
- 21.Legler J, Gloeckler Ries L, Smith M, et al. Brain and Other Central Nervous System Cancers: Recent trens in Incidence and Mortality. J Natl Cancer Inst. 1999;91(16):1382–90. doi: 10.1093/jnci/91.16.1382. [DOI] [PubMed] [Google Scholar]
- 22.Curry W, Barker F. Racial, ethnic and socioeconomic disparities in the treatment of brain tumors. J Neurooncol. 2009;93:25–39. doi: 10.1007/s11060-009-9840-5. [DOI] [PubMed] [Google Scholar]
- 23.Bishop A, McDonald M, Chang A, Esiashvili N. Infant Brain Tumors: Incidence, Survival, and the Role of Radiation Based on Surveillance, Epidemiology, and End Results (SEER) Data. Int J Radiat Oncol Biol Phys. 2012;82(1):341–47. doi: 10.1016/j.ijrobp.2010.08.020. [DOI] [PubMed] [Google Scholar]
- 24.Kadan-Lottick N, Skluzacek M, Gurney J. Decreasing Incidence Rates of Primary Central Nervous System Lymphoma. Cancer. 2002;95(1):193–202. doi: 10.1002/cncr.10643. [DOI] [PubMed] [Google Scholar]
- 25.SEER Program Coding and Staging Manual 2011. Surveillance Systems Branch, Surveillance Research Program. Division of Cancer and Population Sciences, National Institutes ofHeath, Public Health Service, US Department of Health and Human Services; Effective Date: Cases Diagnosed January 1, 2011. NIH Publication Number 11–5581. http://seer.cancer.gov/manuals/2011/SPCSM_2011_maindoc_09272011.pdf. [Google Scholar]
- 26.Adamson C, Kanu O, Mehta A, et al. Glioblastoma multiforme: a review of where we have been and where we are going. Expert Opin Investig Drugs. 2009;18(8):1061–83. doi: 10.1517/13543780903052764. [DOI] [PubMed] [Google Scholar]
- 27.Tatter S. The new WHO Classification of tumoros affecting the Central Nervous System. 2006. http://neurosurgery.mgh.harvard.edu/newwhobt.htm.
- 28.Villano JL, Koshy M, Shaikh H, et al. Age, gender, and racial differences in incidence and survival in primary CNS lymphoma. Br J Cancer. 2011;105(9):1414–18. doi: 10.1038/bjc.2011.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith M, Freidlin B, Gloeckler Ries L, Simon R. Trends in Reported Incidence of Primary Malignant Brain Tumors in Children in the United States. J Nat Cancer Inst. 1998;90(17):1269–77. doi: 10.1093/jnci/90.17.1269. [DOI] [PubMed] [Google Scholar]
- 30.Cote TR, Manns A, Hardy CR, et al. Epidemiology of brain lymphoma among people with or without acquired immunodeficiency syndrome. AIDS/Cancer Study Group. J Natl Cancer Inst. 1996;88:657–79. doi: 10.1093/jnci/88.10.675. [DOI] [PubMed] [Google Scholar]
- 31.Morris M, Hancock MS, Miller WC, et al. Prevalence of HIV infection among young adults in the United States: results from the Add Health Study. Am J Public Health. 2006;96:1091–97. doi: 10.2105/AJPH.2004.054759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346:1128–37. doi: 10.1056/NEJMsa012337. [DOI] [PubMed] [Google Scholar]
- 33.Brach PB, Schrag D, Brawley OW, et al. Survival of Blacks and Whites After a Cancer Diagnosis. JAMA. 2002;287(16):2106–13. doi: 10.1001/jama.287.16.2106. [DOI] [PubMed] [Google Scholar]
- 34.Sigrid H, Alofs L, Huiskes J, Wijnen-Meijer Ordering patterns for laboratory and radiology tests by students from different undergraduate medical curricula. BMC Med Educ. 2013;13:109. doi: 10.1186/1472-6920-13-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Owlia M, Deible C, Hughes MA, et al. Head CT scan overuse in frequently admitted medical patients. Am J Med. 2014;127(5):406–10. doi: 10.1016/j.amjmed.2014.01.023. [DOI] [PubMed] [Google Scholar]
- 36.Stern R. “Can we all get along?” Cooperative strategies to reduce imaging overuse. Am J Med. 2013;126(8):657–58. doi: 10.1016/j.amjmed.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 37.Braganza MZ, Kitahara CM, Berrington de Gonzalez A, et al. Ionizing radiation and the risk of brain and central nervous system tumors: a systematic review. Neuro Oncol. 2012;14(11):1316–24. doi: 10.1093/neuonc/nos208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kitahara CM, Linet MS, Brenner AV, et al. Personal History of Diabetes, Genetic Susceptibility to Diabetes, and Risk of Brain Glioma: A Pooled Analysis of Observational Studies. Cancer Epidemiol Biomarkers Prev. 2014;23(1):47–54. doi: 10.1158/1055-9965.EPI-13-0913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sorensen AG, Emblem KE, Polaskova P, et al. Increased Survival of Glioblastoma Patients Who Respond to Antiangiogenic Therapy with Elevated Blood Perfusion. Cancer Res. 2012;72(2):402–7. doi: 10.1158/0008-5472.CAN-11-2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.DeAngelis LM. Anaplastic Glioma: How to Prognosticate Outcome and Choose a Treatment Strategy. J Clin Oncol. 2009;27(25):5861–62. doi: 10.1200/JCO.2009.24.5985. [DOI] [PubMed] [Google Scholar]
- 41.Ponzoni M, Issa S, Batchelor TT, Rubenstein JL. Beyond high-dose methotrexate and brain radiotherapy: novel targets and agents for primary CNS lymphoma. Ann Oncol. 2014;25(2):316–22. doi: 10.1093/annonc/mdt385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rosenstock S, Whitman S, West JF, Balkin M. Racial Disparities in Diabetes Mortality in the 50 Most Populous US Cities. J Urban Health. 2014 doi: 10.1007/s11524-013-9861-4. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ali AN, Travers K, Athavale N, Al-Modoaris F. Malignant meningioma – a rare cause of temporal head lump. QJM. 2012 doi: 10.1093/qjmed/hcs009. Epub ahead of print. [DOI] [PubMed] [Google Scholar]