Abstract
Objective
Importance of fatty acid components and imbalances has emerged in coronary heart disease. In this study, we analyzed fatty acids and ankle-brachial index (ABI) in a Japanese cohort.
Methods
Peripheral arterial disease (PAD) was diagnosed in 101 patients by ABI ≤0.90 and/or by angiography. Traditional cardiovascular risk factors and components of serum fatty acids were examined in all patients (mean age 73.2±0.9 years; 81 males), and compared with those in 373 age- and sex-matched control subjects with no evidence of PAD.
Results
The presence of PAD (mean ABI: 0.71±0.02) was independently associated with low levels of gamma-linolenic acid (GLA) (OR: 0.90; 95% CI: 0.85–0.96; P = 0.002), eicosapentaenoic acid∶arachidonic acid (EPA∶AA) ratio (OR: 0.38; 95% CI: 0.17–0.86; P = 0.021), and estimated glomerular filtration rate (OR: 0.97; 95% CI: 0.96–0.98; P<0.0001), and with a high hemoglobin A1c level (OR: 1.34; 95% CI: 1.06–1.69; P = 0.013). Individuals with lower levels of GLA (≤7.95 µg/mL) and a lower EPA∶AA ratio (≤0.55) had the lowest ABI (0.96±0.02, N = 90), while the highest ABI (1.12±0.01, N = 78) was observed in individuals with higher values of both GLA and EPA∶AA ratio (P<0.0001).
Conclusion
A low level of GLA and a low EPA∶AA ratio are independently associated with the presence of PAD. Specific fatty acid abnormalities and imbalances could lead to new strategies for risk stratification and prevention in PAD patients.
Introduction
Peripheral arterial disease (PAD) is a manifestation of advanced systemic atherosclerosis, and is associated with high mortality and morbidity from cardiovascular disease (CVD) [1], [2]. The prevalence of PAD increases with age in both sexes, and affects 4.3% of individuals aged ≥40 years and 14.5% of those aged ≥70 years in the United States [3]. Although the prevalence of PAD in Japanese aged ≥40 years is lower, ranging from 1.5% to 2.7% [4], [5], [6], the number of Japanese PAD patients is anticipated to increase in the near future.
Traditional risk factors, such as diabetes mellitus (DM), hypertension, current smoking, and dyslipidemia, are strongly associated with the risk of PAD [3], [7]. In addition, other biomarkers, including homocysteine, C-reactive protein (CRP), lipoprotein(a), fibrinogen, and apolipoproteins (apo) A-I and B-100 have been examined for their association with PAD [7], [8], [9]. These biomarkers are not consistently and independently associated with the presence of PAD [8], [9], [10], [11]; therefore, it would be of great interest if new markers in addition to the traditional risk factors could contribute substantially to the diagnosis and the management of PAD.
A large scale cohort study spanning 30,829 person-years demonstrated that higher circulating components of omega-3 polyunsaturated fatty acid (PUFA) or the total omega-3 level were associated with lower total mortality, especially deaths from coronary heart disease in healthy older adults [12]. Likewise, low levels of omega-6 PUFAs (linoleic acid [13], [14] or dihomo-gamma-linolenic acid [15]) have been linked to the risk of coronary heart disease. Moreover, a reduced ratio of eicosapentaenoic acid (EPA), an omega-3 PUFA, to arachidonic acid (AA), an omega-6 PUFA (EPA∶AA), has recently been highlighted as a risk predictor for secondary cardiovascular events [16], [17], [18], [19]. Importantly, these abnormalities and/or imbalances can be a therapeutic target to prevent future cardiovascular events; however, some controversy still exists regarding the efficacy of individual fatty acids in the prevention of adverse cardiovascular outcomes. Recently, one large-scale randomized clinical trial, as well as a systemic review and meta-analyses of randomized clinical trials, failed to show any efficacy of omega-3 PUFA supplementation in reducing cardiovascular mortality [20], [21]. Overall, the effects of omega-3 and omega-6 PUFAs in the PAD population have remained elusive.
The Framingham risk score [22] and the NIPPON DATA80 [23] chart in Japan have been used to assess the 10-year risk of cardiovascular mortality in the general population; however, factors associated with the presence of PAD have not been completely identified. In addition, current management is not sufficient to fully minimize future cardiovascular events in PAD patients. In this case-controlled study, we examined well-known cardiovascular risk factors and fatty acid composition in PAD patients, in an attempt to provide a new strategy for their risk management.
Methods
1. Study design
This case-control study enrolled adults aged 40 years and older with PAD. PAD was defined if a patient had a resting ankle-brachial index (ABI) ≤0.90 in at least one limb, and/or in claudicant patients who had one or more stenoses >75% in at least one leg artery on angiography. Patients receiving oral EPA supplements and patients with chronic kidney disease (CKD) undergoing hemodialysis were excluded from the study. Laboratory analyses and ABI levels of the cases were compared with those of age- and sex-matched control subjects who had ABI levels between 0.90 and 1.40 or no evidence of PAD. The presence of comorbid diseases was determined as follows: 1) coronary heart disease was diagnosed by coronary angiography, 2) diabetes mellitus by an elevation of fasting blood glucose level (≥126 mg/dL) or use of antidiabetic agents, 3) dyslipidemia by the criteria of the Japan Atherosclerosis Society [24] or use of lipid-lowering agents, and 4) CKD by estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2. Levels of eGFR were calculated using the Modified Diet in Renal Disease study equation, modified for Japanese patients with CKD: eGFR = 194 × Serum creatinine−1.094×Age−0.287 (×0.739 if female) mL/min/1.73 m2 [25].
The ethics committee of Shinshu University School of Medicine approved the protocol. The study was performed in accordance with the Declaration of Helsinki and with Good Clinical Practice. Patients were managed according to the recommended guidelines of the Japanese Circulation Society.
2. Laboratory analyses
Levels of low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, triglycerides, hemoglobin A1c (HbA1c), CRP, and D-dimer were measured using routine laboratory tests in a hospital laboratory. Components of fatty acid levels were estimated from serum samples of fasting venous blood in all patients and control subjects using a gas chromatography technique, as described previously, at Special Reference Laboratories, Inc., Tokyo, Japan [16].
3. Measurements of ABI
Systolic blood pressures in upper extremities (brachial artery) and lower extremities (dorsalis pedis and posterior tibial artery) were measured using a FORM ABI/PWV device (Omron Colin Co., Tokyo, Japan), with the subjects resting in a supine position for at least 10 minutes. The ABI is the ratio of the systolic blood pressure in the leg to that in the arms. The ABI was calculated separately for each leg and the lower measurement was used in the analyses; an ABI ≤0.90 at rest represents clinically significant PAD.
4. Statistical analyses
Data were analyzed anonymously by using IBM SPSS Statistics version 18 (IBM Co.) and GraphPad Prism version 5.0f (GraphPad Software, San Diego, California). Throughout the analysis, a two-tailed P value <0.05 was considered to be statistically significant. Differences in continuous variables were assessed by Mann–Whitney U tests, and Fisher's exact tests were used to examine differences in categorical variables between the cases and the controls. Univariable logistic regression analyses were performed on the significant variables in the non-parametric tests, and followed by multivariable-adjusted logistic regression analyses for significant variables in the univariable model. Odds ratios (OR) and their 95% confidence intervals (CI) were calculated, with adjustments for age, sex, body mass index, eGFR, HbA1c, gamma-linolenic acid (GLA), EPA∶AA ratio, and docosahexaenoic∶arachidonic acid (DHA∶AA) ratio. Mann–Whitney U tests was used to calculate the distributions of GLA and EPA∶AA ratio according to ABI levels. The areas under the receiver operating characteristic (ROC) curve were used to identify the sensitivity and specificity of cut-off points for the detection of PAD. We next calculated cut-off points at which the value of “sensitivity + specificity – 1” was maximum (Youden's Index). One way ANOVA with Tukey's multiple comparison test was used to compare ABI levels in individuals with lower or higher proportions of GLA and EPA∶AA ratio using the cutoff values calculated from the ROC curve analyses.
Results
1. Characteristics of the study population
A total of 474 patients (101 cases with PAD and 373 controls without PAD) were enrolled. Among all subjects, mean age was 72.6±0.4 years, and 82% were male. The baseline characteristics are summarized in Table 1.
Table 1. Baseline characteristics of the study population.
| Patient's characteristics | PAD (N = 101) | Controls (N = 373) | P value* |
| Age (years) | 73.2±0.9 | 72.4±0.4 | 0.243 |
| Sex (male), N (%) | 81 (80) | 306 (82) | 0.665 |
| Body mass index (kg/m2) | 22.45±0.33 | 23.61±0.16 | 0.001 |
| Triglycerides (mg/dL) | 136.5±7.35 | 142.3±3.87 | 0.245 |
| LDL-C (mg/dL) | 95.67±3.26 | 98.76±1.57 | 0.245 |
| HDL-C (mg/dL) | 48.69±1.48 | 51.16±0.75 | 0.115 |
| LDL∶HDL ratio | 2.14±0.10 | 2.03±0.04 | 0.702 |
| ABI | 0.71±0.02 | 1.12±0.005 | <0.0001 |
| CRP (mg/dL) | 0.44±0.09 | 0.28±0.04 | 0.008 |
| D-dimer (µg/mL) | 2.16±0.33 | 1.80±0.19 | <0.0001 |
| HbA1c (%) | 6.45±0.12 | 6.02±0.07 | 0.002 |
| eGFR (mL/min/1.73 m2) | 49.96±2.37 | 64.57±0.95 | <0.0001 |
| Statin, N (%) | 48 (48) | 211 (57) | 0.1153 |
| Comorbidity, N (%) | |||
| Coronary heart disease | 61 (60) | 245 (66) | 0.349 |
| Diabetes mellitus | 46 (46) | 123 (33) | 0.025 |
| CKD (eGFR <60 mL/min/1.73 m2) | 59 (58) | 139 (37) | 0.0002 |
| Dyslipidemia | 18 (18) | 93 (25) | 0.146 |
Abbreviations: PAD, peripheral artery disease; LDL-C, low-density lipoprotein cholesterol; HDL-C high-density lipoprotein cholesterol; ABI, ankle-brachial index; CRP, c-reactive protein; HbA1c, hemoglobin A1c; eGFR, estimated glomerular filtration rate; CKD, chronic kidney disease. Data are given as mean ± standard error of mean or n (%);
*The Mann–Whitney U test and Fisher's exact test were used to analyze differences in continuous and categorical variables, respectively.
2. Conventional risk factors
There were no significant differences in the baseline serum levels of triglyceride, HDL cholesterol, LDL cholesterol, or LDL∶HDL cholesterol ratio between the cases and the controls. No significant differences were observed between the cases and the controls in the numbers of patients with coronary heart disease and dyslipidemia. There was significantly higher morbidity from diabetes (46% versus 33%, P = 0.025) and CKD (58% versus 37%, P = 0.0002) in the PAD patients than in the controls. Levels of D-dimer and CRP were also significantly elevated in PAD patients compared to controls (P<0.05) (Table 1).
3. Fatty acid components
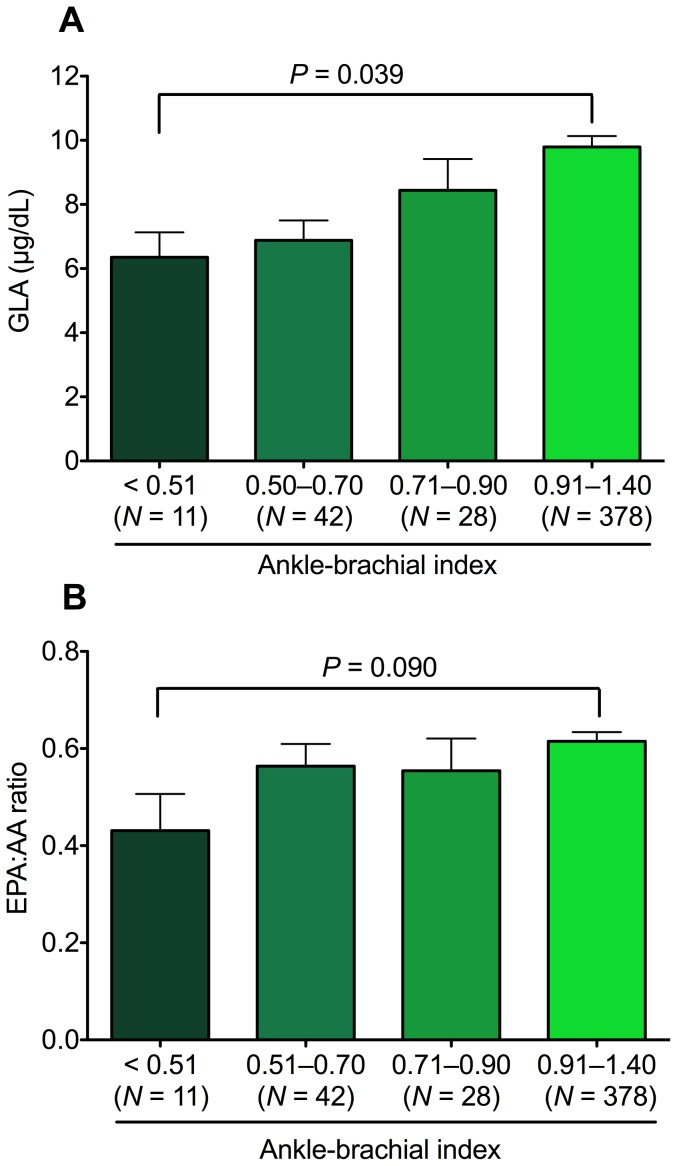
The PAD patients had significantly lower levels of docosapentaenoic acid (12%), docosahexaenoic acid (7%), GLA (27%), EPA∶AA ratio (10%), and DHA∶AA ratio (11%) than the controls. The lower levels of EPA∶AA ratio and DHA∶AA ratio were mainly due to lower EPA and DHA concentrations in PAD group. However, no significant differences were observed between the groups in saturated fatty acids, monosaturated fatty acids, alpha-linolenic acid, linoleic acid, dihomo-gamma-linolenic acid, AA, or eicosadienoic acid (Table 2). The distributions of GLA and EPA∶AA ratio according to ABI levels were further examined (N = 459). The GLA level in patients with ABI<0.51 was 6.35±0.77, significantly lower than the 9.79±0.33 in patients with ABI>0.90 and ≤1.40 (P = 0.039; Fig. 1A). The EPA∶AA ratio in patients with ABI<0.51 was 0.43±0.07, modestly lower than the 0.61±0.01 in patients with ABI>0.90 and ≤1.40 (P = 0.090; Fig. 1B).
Table 2. Serum fatty acid composition.
| Fatty Acids (µg/mL) | PAD (N = 101) | Controls (N = 373) | P value* |
| Saturated | |||
| Lauric acid (12∶0) | 5.1±1.45 | 4.45±0.40 | 0.644 |
| Myristic acid (14∶0) | 31.59±2.49 | 33.23±1.10 | 0.090 |
| Palmitic acid (16∶0) | 695.9±23.57 | 707.6±11.99 | 0.536 |
| Stearic acid (18∶0) | 205.3±6.04 | 215.4±3.19 | 0.111 |
| Behenic acid (22∶0) | 15.81±0.46 | 16.58±0.46 | 0.133 |
| Lignoceric acid (24∶0) | 15.30±0.45 | 15.87±0.64 | 0.588 |
| Monounsaturated | |||
| Arachidic acid (20∶0) | 7.14±0.17 | 7.23±0.08 | 0.704 |
| Palmitoleic acid (16∶1) | 70.60±4.12 | 69.65±1.97 | 0.833 |
| Eicosenoic acid (20∶1) | 6.53±0.29 | 6.72±0.22 | 0.627 |
| Olieic acid (18∶1) | 627.5±26.32 | 616.3±12.0 | 0.814 |
| Erucic acid (22∶1) | 1.90±0.13 | 1.97±0.26 | 0.299 |
| Polyunsaturated | |||
| Omega-3 | |||
| Alpha-linolenic acid (18∶3) | 28.71±1.64 | 30.11±0.86 | 0.284 |
| EPA (20∶5) | 76.71±4.32 | 89.81±2.44 | 0.008 |
| DHA (22∶6) | 150.2±7.11 | 163.7±3.01 | 0.012 |
| Docosapentaenoic acid (22∶5) | 21.60±0.98 | 23.77±0.51 | 0.024 |
| Omega-6 | |||
| Linoleic acid (18∶2) | 772.8±25.55 | 775.2±11.13 | 0.490 |
| GLA (18∶3) | 7.58±0.42 | 10.07±0.33 | < 0.0001 |
| Dihomo-gamma-linolenic acid (20∶3) | 31.66±1.23 | 33.57±0.65 | 0.128 |
| AA (20∶4) | 155.6±5.04 | 157.1±2.55 | 0.652 |
| Docosatetraenoic acid (22∶4) | 4.55±0.18 | 4.52±0.12 | 0.596 |
| Eicosadienoic acid (20∶2) | 5.92±0.20 | 5.88±0.09 | 0.612 |
| EPA∶AA ratio | 0.53±0.03 | 0.62±0.01 | 0.020 |
| DHA∶AA ratio | 1.01±0.04 | 1.10±0.02 | 0.020 |
Abbreviations: PAD, peripheral artery disease; EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid; GLA, gamma-linolenic acid; AA, arachidonic acid; EPA∶AA, eicosapentaenoic acid to arachidonic acid ratio; DHA∶AA, docosahexaenoic acid to arachidonic acid ratio. Numbers in parentheses show number of carbons and double bonds. Data are given as mean ± standard error of mean;
*Mann Whitney U test.
Figure 1. Distribution of levels of (A) gamma-linolenic acid (GLA) and (B) eicosapentaenoic acid∶arachidonic acid (EPA∶AA) ratio according to ankle-brachial index in 459 subjects.
4. Factors associated with the presence of PAD
Univariable logistic regression analyses showed that the presence of PAD was associated with low body mass index, reduced eGFR, elevated HbA1c and low levels of EPA, GLA, and EPA∶AA ratio (P<0.05). In analyses using multivariable-adjusted logistic regression, the presence of PAD was independently associated with reduced eGFR, elevated HbA1c, and low GLA levels and EPA∶AA ratio (P<0.05; Table 3). The areas under the ROC curve (95% CI) for eGFR, HbA1c, GLA, and EPA∶AA ratio were 0.67 (0.60–0.73, P<0.0001), 0.59 (0.53–0.66, P = 0.002), 0.63 (0.57–0.69, P<0.0001), and 0.58 (0.51–0.64, P = 0.020), respectively. Based on the ROC curve analyses, the optimal cutoff values for eGFR, HbA1c, GLA, and EPA∶AA ratio were 45.50 mL/min/1.73 m2 (42.0% sensitivity, 86.92% specificity), 6.05% (60.0% sensitivity, 58.45% specificity), 7.95 µg/mL (67.33% sensitivity, 58.49% specificity), and 0.55 (66.34% sensitivity, 47.18% specificity), respectively. Using the optimal cutoff values for GLA and EPA∶AA ratio, ABI levels were compared among the 4 subgroups (N = 90, GLA ≤7.95 µg/mL and EPA∶AA ratio ≤0.55; N = 161, GLA >7.95 µg/mL and EPA∶AA ratio ≤0.55; N = 123, GLA ≤7.95 µg/mL and EPA∶AA ratio >0.55; N = 78, GLA >7.95 µg/mL and EPA∶AA ratio >0.55; Fig. 2). There was a significant difference in ABI levels among the 4 subgroups (P<0.0001). Furthermore, the mean level of ABI (0.96±0.02) was lowest in individuals with a lower GLA (≤7.95 µg/mL) and a lower EPA∶AA ratio (≤0.55), as compared with the highest ABI levels (1.12±0.01) observed in individuals with higher values of both GLA and EPA∶AA ratio (P<0.001).
Table 3. Logistic regression analyses of 101 cases of PAD and 373 control subjects.
| Variables | Univariable analysis | Multivariable analyses* | ||
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Age | 1.01 (0.98–1.04) | 0.386 | 0.99 (0.95–1.02) | 0.532 |
| Sex (male) | 1.12 (0.64–1.96) | 0.672 | 1.06 (0.56–1.99) | 0.854 |
| Body mass index | 0.89 (0.83–0.96) | 0.002 | 0.92 (0.85–1.002) | 0.058 |
| eGFR | 0.96 (0.95–0.97) | <0.0001 | 0.97 (0.96–0.98) | <0.0001 |
| HbA1c | 1.28 (1.06–1.55) | 0.009 | 1.34 (1.06–1.69) | 0.013 |
| CRP | 1.19 (0.95–1.48) | 0.199 | ||
| D-dimer | 1.02 (0.96–1.08) | 0.397 | ||
| EPA | 0.99 (0.98–0.99) | 0.013 | ||
| DHA | 0.99 (0.99–1) | 0.051 | ||
| Docosapentaenoic acid | 0.97 (0.95–1) | 0.054 | ||
| GLA | 0.90 (0.85–0.95) | <0.0001 | 0.90 (0.85–0.96) | 0.002 |
| EPA∶AA ratio | 0.45 (0.22–0.91) | 0.026 | 0.38 (0.17–0.86) | 0.021 |
| DHA∶AA ratio | 0.57 (0.33–1.005) | 0.052 | 0.59 (0.31–1.11) | 0.104 |
Abbreviations: PAD, peripheral artery disease; eGFR, estimated glomerular filtration rate; HbA1c, hemoglobin A1c; CRP, c-reactive protein; EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid; GLA, gamma-linolenic acid; EPA∶AA, eicosapentaenoic acid to arachidonic acid ratio; DHA∶AA, docosahexaenoic acid to arachidonic acid ratio; OR, odds ratio; CI, confidence interval.
*Adjusted for age, sex, body mass index, eGFR, HbA1c, and GLA.
Figure 2. The association of serum levels of gamma-linolenic acid (GLA) and eicosapentaenoic acid∶arachidonic acid (EPA∶AA) ratio with ankle-brachial index levels.
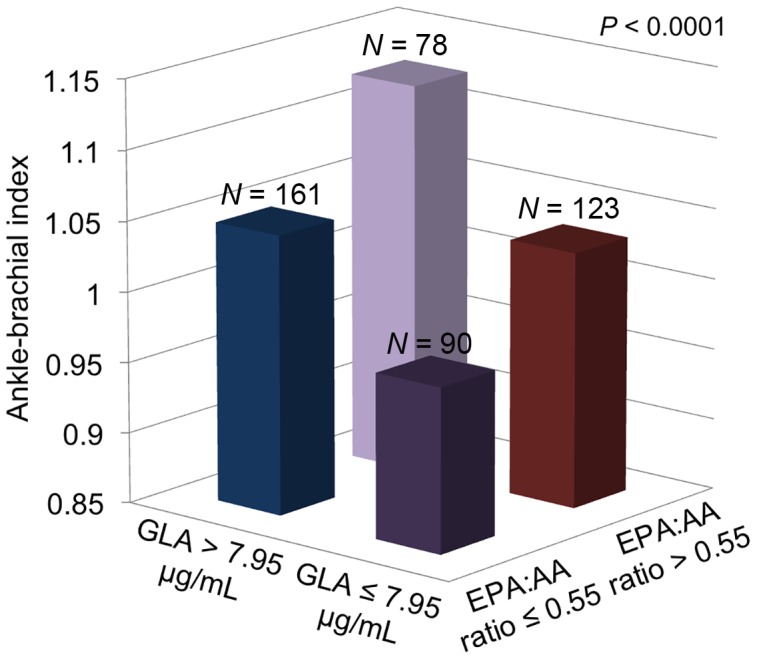
Study population was divided into 4 groups by using cut-off values at 7.95 µg/mL of GLA and at 0.55 of EPA∶AA ratio. There was an association of low levels of GLA and low EPA∶AA ratios with the presence of PAD.
Our results suggested that combined low GLA levels and low EPA∶AA ratios were correlated with a potentially more advanced status of PAD. There were 90 enrolled patients with combined low GLA levels (≤7.95 µg/mL) and low EPA∶AA ratios (≤0.55), and these patients had high morbidity of coronary heart disease (69%), chronic kidney disease (59%), diabetes mellitus (41%), and dyslipidemia (24%). Around 54% of these patients received statin treatment.
Discussion
This study showed that a low level of GLA and a low EPA∶AA ratio were independently associated with the presence of PAD. Moreover, individuals with both a low GLA level and a low EPA∶AA ratio had the lowest ABI levels, which suggest an advanced status of PAD. The areas under the ROC curves for CKD, DM, GLA, and EPA∶AA ratio were comparable, suggesting that these values had equally important associations with the presence of PAD. In contrast, neither the other components of fatty acids nor the DHA∶AA ratio had a significant association with PAD.
Background diseases in Japanese PAD patients have shown a high prevalence of DM (41.2%) [26] and CKD (60.7%) [27]. Similarly, high morbidities of DM and CKD were observed in PAD patients in this study. The average body mass index of our PAD patients was 5% lower than that of controls. This suggested the obesity paradox, which has been explained in part by malnutrition and/or systemic inflammation due to the severity of PAD and, more importantly, is associated with the high mortality of PAD patients [28], [29]. In fact, PAD patients in this study had significantly high CRP levels.
The following differences between EPA and AA have been demonstrated: EPA-derived eicosanoids decrease plasma triglyceride levels, improve endothelial function and arterial stiffness, inhibit platelet aggregation, attenuate inflammation, and stabilize atheromatous plaque [30], [31], [32], [33], [34], whereas AA derived eicosanoids are pro-inflammatory, pre-thrombotic, and vasoconstrictive [31], [32]. Importantly, the benefits of fish intake or dietary supplements of omega-3 PUFAs [16], [35], [36], [37] have emerged in large-scale randomized clinical trials with EPA that reduced coronary events by 19% in Japanese hypercholesterolemic patients [37]. Additive administration of EPA to increase the EPA∶AA ratio has reduced the incidence of secondary major adverse cardiovascular events in patients with either PAD [38] or established coronary artery disease [18].
Several findings that indicate favorable effects of GLA (omega-6 PUFA) on cardiovascular diseases have been reported. GLA supplementation was shown to have anti-inflammatory or immunomodulatory roles in patients with multiple sclerosis [39] or acne vulgaris [40], and also to promote vasodilation [41], reduce blood pressure [42], or to have an inhibitory effect on smooth muscle cell proliferation associated with the progression of atherosclerosis [43]. Combined treatment with GLA and EPA for 2 years in 120 patients with intermittent claudication showed a significant reduction in systolic blood pressure (P≤0.05), and modestly reduced non-fatal coronary events (10% vs. 15%, P>0.05) [44]. These reports could support the results of this study, which suggest a potential role of GLA in inflammation mediating the development of peripheral atherosclerosis.
Current clinical guidelines recommend consumption of omega-3 PUFAs for secondary prevention [45], [46]. Japan has one of the highest mean dietary omega-3 PUFAs intake in the world [47]. More cardiovascular benefits of omega-3 PUFAs would be expected in countries with low omega-3 PUFAs consumption. In addition to the benefits of omega-3 PUFAs, an American Heart Association Science Advisory has recommended the consumption of omega-6 PUFAs of at least 5% to 10% of energy to reduce the risk of coronary artery disease [48]. In fact, a recent case control study demonstrated that patients with a recent myocardial infarction had lower total PUFA levels of both omega-3 and omega-6, as compared with those in control subjects [49]. In our study, however, only GLA levels, but no other PUFA components of omega-6 or the total level, were significantly associated with PAD. Further studies are required to clarify whether a lower GLA level has an exclusive role in the pathophysiology of PAD.
Lipid-lowering therapies with strong statins have been shown to modify the concentrations of omega-3 and omega-6 PUFAs [50], [51]; however, we observed no significant difference in the proportion of individuals receiving statin therapy or in lipid profiles between PAD patients and control subjects (Table 1). In addition, neither GLA levels nor the EPA∶AA ratio were significantly correlated with lipid profiles in our study.
There are some limitations to our study. First, the sample size was relatively small and the study was performed at a single facility using hospital controls to minimize confounding variables. Second, the attending physician based the diagnosis of PAD on the diagnostic criteria, while their clinical manifestations and disease severity were not reported. Third, this study was not designed to calculate the amount of fish consumption and/or omega-3 intake in the study population. Fourth, the mechanisms of action of fatty acid components in PAD and therapeutic strategies utilizing specific components were beyond the scope of our study. Further research is encouraged to establish risk management and treatment strategies for patients with PAD.
In conclusion, we found that low levels of GLA and a low EPA∶AA ratio were significantly associated with the presence and advanced status of PAD in Japanese. These findings imply that, in addition to the well-known cardiovascular risk factors, abnormalities and imbalance of fatty acids play an important role in the pathophysiology of peripheral atherosclerosis, and may open new strategies for risk stratification and prevention for PAD patients.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within this paper.
Funding Statement
The authors have no support or funding to report.
References
- 1. Criqui MH, Langer RD, Fronek A, Feigelson HS, Klauber MR, et al. (1992) Mortality over a period of 10 years in patients with peripheral arterial disease. N Engl J Med 326: 381–386. [DOI] [PubMed] [Google Scholar]
- 2. Criqui MH, Ninomiya JK, Wingard DL, Ji M, Fronek A (2008) Progression of peripheral arterial disease predicts cardiovascular disease morbidity and mortality. J Am Coll Cardiol 52: 1736–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Selvin E, Erlinger TP (2004) Prevalence of and risk factors for peripheral arterial disease in the United States: results from the National Health and Nutrition Examination Survey, 1999–2000. Circulation 110: 738–743. [DOI] [PubMed] [Google Scholar]
- 4. Ohnishi H, Sawayama Y, Furusyo N, Maeda S, Tokunaga S, et al. (2010) Risk factors for and the prevalence of peripheral arterial disease and its relationship to carotid atherosclerosis: the Kyushu and Okinawa Population Study (KOPS). J Atheroscler Thromb 17: 751–758. [DOI] [PubMed] [Google Scholar]
- 5. Fujiwara T, Saitoh S, Takagi S, Ohnishi H, Ohata J, et al. (2004) Prevalence of asymptomatic arteriosclerosis obliterans and its relationship with risk factors in inhabitants of rural communities in Japan: Tanno-Sobetsu study. Atherosclerosis 177: 83–88. [DOI] [PubMed] [Google Scholar]
- 6. Usui T, Ninomiya T, Nagata M, Doi Y, Hata J, et al. (2011) Albuminuria as a risk factor for peripheral arterial disease in a general population: the Hisayama study. J Atheroscler Thromb 18: 705–712. [DOI] [PubMed] [Google Scholar]
- 7. Fabsitz RR, Sidawy AN, Go O, Lee ET, Welty TK, et al. (1999) Prevalence of peripheral arterial disease and associated risk factors in American Indians: the Strong Heart Study. Am J Epidemiol 149: 330–338. [DOI] [PubMed] [Google Scholar]
- 8. McDermott MM, Green D, Greenland P, Liu K, Criqui MH, et al. (2003) Relation of levels of hemostatic factors and inflammatory markers to the ankle brachial index. Am J Cardiol 92: 194–199. [DOI] [PubMed] [Google Scholar]
- 9. Ridker PM, Stampfer MJ, Rifai N (2001) Novel risk factors for systemic atherosclerosis: a comparison of C-reactive protein, fibrinogen, homocysteine, lipoprotein(a), and standard cholesterol screening as predictors of peripheral arterial disease. JAMA 285: 2481–2485. [DOI] [PubMed] [Google Scholar]
- 10. Folsom AR, Pankow JS, Tracy RP, Arnett DK, Peacock JM, et al. (2001) Association of C-reactive protein with markers of prevalent atherosclerotic disease. Am J Cardiol 88: 112–117. [DOI] [PubMed] [Google Scholar]
- 11. Erren M, Reinecke H, Junker R, Fobker M, Schulte H, et al. (1999) Systemic inflammatory parameters in patients with atherosclerosis of the coronary and peripheral arteries. Arterioscler Thromb Vasc Biol 19: 2355–2363. [DOI] [PubMed] [Google Scholar]
- 12. Mozaffarian D, Lemaitre RN, King IB, Song X, Huang H, et al. (2013) Plasma phospholipid long-chain ω-3 fatty acids and total and cause-specific mortality in older adults: a cohort study. Ann Intern Med 158: 515–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Harris WS, Poston WC, Haddock CK (2007) Tissue n-3 and n-6 fatty acids and risk for coronary heart disease events. Atherosclerosis 193: 1–10. [DOI] [PubMed] [Google Scholar]
- 14. Laaksonen DE, Nyyssönen K, Niskanen L, Rissanen TH, Salonen JT (2005) Prediction of cardiovascular mortality in middle-aged men by dietary and serum linoleic and polyunsaturated fatty acids. Arch Intern Med 165: 193–199. [DOI] [PubMed] [Google Scholar]
- 15. Wood DA, Butler S, Riemersma RA, Thomson M, Oliver MF, et al. (1984) Adipose tissue and platelet fatty acids and coronary heart disease in Scottish men. Lancet 2: 117–121. [DOI] [PubMed] [Google Scholar]
- 16. Ninomiya T, Nagata M, Hata J, Hirakawa Y, Ozawa M, et al. (2013) Association between ratio of serum eicosapentaenoic acid to arachidonic acid and risk of cardiovascular disease: the Hisayama Study. Atherosclerosis 231: 261–267. [DOI] [PubMed] [Google Scholar]
- 17. Fujihara M, Fukata M, Odashiro K, Maruyama T, Akashi K, et al. (2013) Reduced plasma eicosapentaenoic acid-arachidonic acid ratio in peripheral artery disease. Angiology 64: 112–118. [DOI] [PubMed] [Google Scholar]
- 18. Matsuzaki M, Yokoyama M, Saito Y, Origasa H, Ishikawa Y, et al. (2009) Incremental effects of eicosapentaenoic acid on cardiovascular events in statin-treated patients with coronary artery disease. Circ J 73: 1283–1290. [DOI] [PubMed] [Google Scholar]
- 19. Domei T, Yokoi H, Kuramitsu S, Soga Y, Arita T, et al. (2012) Ratio of serum n-3 to n-6 polyunsaturated fatty acids and the incidence of major adverse cardiac events in patients undergoing percutaneous coronary intervention. Circ J 76: 423–429. [DOI] [PubMed] [Google Scholar]
- 20. Roncaglioni MC, Tombesi M, Avanzini F, Barlera S, Caimi V, et al. (2013) n-3 fatty acids in patients with multiple cardiovascular risk factors. N Engl J Med 368: 1800–1808. [DOI] [PubMed] [Google Scholar]
- 21. Rizos EC, Ntzani EE, Bika E, Kostapanos MS, Elisaf MS (2012) Association between omega-3 fatty acid supplementation and risk of major cardiovascular disease events: a systematic review and meta-analysis. JAMA 308: 1024–1033. [DOI] [PubMed] [Google Scholar]
- 22.Eichler K, Puhan MA, Steurer J, Bachmann LM (2007) Prediction of first coronary events with the Framingham score: a systematic review. Am Heart J 153: : 722–31, 731. [DOI] [PubMed] [Google Scholar]
- 23. NIPPON DATA80 Research Group (2006) Risk assessment chart for death from cardiovascular disease based on a 19-year follow-up study of a Japanese representative population. Circ J 70: 1249–1255. [DOI] [PubMed] [Google Scholar]
- 24. Teramoto T, Sasaki J, Ishibashi S, Birou S, Daida H, et al. (2013) Executive summary of the Japan Atherosclerosis Society (JAS) guidelines for the diagnosis and prevention of atherosclerotic cardiovascular diseases in Japan -2012 version. J Atheroscler Thromb 20: 517–523. [DOI] [PubMed] [Google Scholar]
- 25. Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, et al. (2009) Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 53: 982–992. [DOI] [PubMed] [Google Scholar]
- 26. Yamazaki T, Goto S, Shigematsu H, Shimada K, Uchiyama S, et al. (2007) Prevalence, awareness and treatment of cardiovascular risk factors in patients at high risk of atherothrombosis in Japan. Circ J 71: 995–1003. [DOI] [PubMed] [Google Scholar]
- 27. Endo M, Kumakura H, Kanai H, Araki Y, Kasama S, et al. (2010) Prevalence and risk factors for renal artery stenosis and chronic kidney disease in Japanese patients with peripheral arterial disease. Hypertens Res 33: 911–915. [DOI] [PubMed] [Google Scholar]
- 28. Kumakura H, Kanai H, Aizaki M, Mitsui K, Araki Y, et al. (2010) The influence of the obesity paradox and chronic kidney disease on long-term survival in a Japanese cohort with peripheral arterial disease. J Vasc Surg 52: 110–117. [DOI] [PubMed] [Google Scholar]
- 29. Golledge J, Cronin O, Iyer V, Bradshaw B, Moxon J V, et al. (2013) Body mass index is inversely associated with mortality in patients with peripheral vascular disease. Atherosclerosis 229: 549–555. [DOI] [PubMed] [Google Scholar]
- 30. Thies F, Garry JMC, Yaqoob P, Rerkasem K, Williams J, et al. (2003) Association of n-3 polyunsaturated fatty acids with stability of atherosclerotic plaques: a randomised controlled trial. Lancet 361: 477–485. [DOI] [PubMed] [Google Scholar]
- 31. Mozaffarian D, Wu JHY (2011) Omega-3 fatty acids and cardiovascular disease: effects on risk factors, molecular pathways, and clinical events. J Am Coll Cardiol 58: 2047–2067. [DOI] [PubMed] [Google Scholar]
- 32. Schmitz G, Ecker J (2008) The opposing effects of n-3 and n-6 fatty acids. Prog Lipid Res 47: 147–155. [DOI] [PubMed] [Google Scholar]
- 33. Harris WS, Miller M, Tighe AP, Davidson MH, Schaefer EJ (2008) Omega-3 fatty acids and coronary heart disease risk: clinical and mechanistic perspectives. Atherosclerosis 197: 12–24. [DOI] [PubMed] [Google Scholar]
- 34. Tousoulis D, Plastiras A, Siasos G, Oikonomou E, Verveniotis A, et al. (2014) Omega-3 PUFAs improved endothelial function and arterial stiffness with a parallel antiinflammatory effect in adults with metabolic syndrome. Atherosclerosis 232: 10–16. [DOI] [PubMed] [Google Scholar]
- 35. GISSI-Prevenzione Investigators (1999) Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto miocardico. Lancet 354: 447–455. [PubMed] [Google Scholar]
- 36. Tavazzi L, Maggioni AP, Marchioli R, Barlera S, Franzosi MG, et al. (2008) Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet 372: 1223–1230. [DOI] [PubMed] [Google Scholar]
- 37. Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Saito Y, et al. (2007) Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet 369: 1090–1098. [DOI] [PubMed] [Google Scholar]
- 38. Ishikawa Y, Yokoyama M, Saito Y, Matsuzaki M, Origasa H, et al. (2010) Preventive effects of eicosapentaenoic acid on coronary artery disease in patients with peripheral artery disease. Circ J 74: 1451–1457. [DOI] [PubMed] [Google Scholar]
- 39. Rezapour-Firouzi S, Arefhosseini SR, Mehdi F, Mehrangiz E-M, Baradaran B, et al. (2013) Immunomodulatory and therapeutic effects of Hot-nature diet and co-supplemented hemp seed, evening primrose oils intervention in multiple sclerosis patients. Complement Ther Med 21: 473–480. [DOI] [PubMed] [Google Scholar]
- 40.Jung JY, Kwon HH, Hong JS, Yoon JY, Park MS, et al.. (2014) Effect of Dietary Supplementation with Omega-3 Fatty Acid and gamma-linolenic Acid on Acne Vulgaris: A Randomised, Double-blind, Controlled Trial. Acta Derm Venereol. [DOI] [PubMed]
- 41. Takai S, Jin D, Kawashima H, Kimura M, Shiraishi-Tateishi A, et al. (2009) Anti-atherosclerotic effects of dihomo-gamma-linolenic acid in ApoE-deficient mice. J Atheroscler Thromb 16: 480–489. [DOI] [PubMed] [Google Scholar]
- 42. Engler MM (1993) Comparative study of diets enriched with evening primrose, black currant, borage or fungal oils on blood pressure and pressor responses in spontaneously hypertensive rats. Prostaglandins Leukot Essent Fatty Acids 49: 809–814. [DOI] [PubMed] [Google Scholar]
- 43. Fan YY, Ramos KS, Chapkin RS (1995) Dietary gamma-linolenic acid modulates macrophage-vascular smooth muscle cell interactions. Evidence for a macrophage-derived soluble factor that downregulates DNA synthesis in smooth muscle cells. Arterioscler Thromb Vasc Biol 15: 1397–1403. [DOI] [PubMed] [Google Scholar]
- 44. Leng GC, Lee AJ, Fowkes FG, Jepson RG, Lowe GD, et al. (1998) Randomized controlled trial of gamma-linolenic acid and eicosapentaenoic acid in peripheral arterial disease. Clin Nutr 17: 265–271. [DOI] [PubMed] [Google Scholar]
- 45. Smith SC, Benjamin EJ, Bonow RO, Braun LT, Creager MA, et al. (2011) AHA/ACCF Secondary Prevention and Risk Reduction Therapy for Patients with Coronary and other Atherosclerotic Vascular Disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation. Circulation 124: 2458–2473. [DOI] [PubMed] [Google Scholar]
- 46. Perk J, De Backer G, Gohlke H, Graham I, Reiner Z, et al. (2012) European Guidelines on cardiovascular disease prevention in clinical practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). Eur Heart J 33: 1635–1701. [DOI] [PubMed] [Google Scholar]
- 47. Micha R, Khatibzadeh S, Shi P, Fahimi S, Lim S, et al. (2014) Global, regional, and national consumption levels of dietary fats and oils in 1990 and 2010: a systematic analysis including 266 country-specific nutrition surveys. BMJ 348: g2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Harris WS, Mozaffarian D, Rimm E, Kris-Etherton P, Rudel LL, et al. (2009) Omega-6 fatty acids and risk for cardiovascular disease: a science advisory from the American Heart Association Nutrition Subcommittee of the Council on Nutrition, Physical Activity, and Metabolism; Council on Cardiovascular Nursing; and Council on Epidemiology and Prevention. Circulation 119: 902–907. [DOI] [PubMed] [Google Scholar]
- 49. Marangoni F, Novo G, Perna G, Perrone Filardi P, Pirelli S, et al. (2014) Omega-6 and omega-3 polyunsaturated fatty acid levels are reduced in whole blood of Italian patients with a recent myocardial infarction: the AGE-IM study. Atherosclerosis 232: 334–338. [DOI] [PubMed] [Google Scholar]
- 50. Harris JI, Hibbeln JR, Mackey RH, Muldoon MF (2004) Statin treatment alters serum n-3 and n-6 fatty acids in hypercholesterolemic patients. Prostaglandins Leukot Essent Fatty Acids 71: 263–269. [DOI] [PubMed] [Google Scholar]
- 51. Nozue T, Yamamoto S, Tohyama S, Fukui K, Umezawa S, et al. (2013) Effects of statins on serum n-3 to n-6 polyunsaturated fatty acid ratios in patients with coronary artery disease. J Cardiovasc Pharmacol Ther 18: 320–326. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within this paper.